Introduction
In China, hepatocellular carcinoma (HCC) is a
common, malignant tumor with poor prognosis (1). The mortality rate of HCC is the 3rd
highest among all malignant tumors (1). Metastasis and recurrence are the main
causes of poor prognosis (2). The
invasion and metastasis of HCC is a complex multi-gene, multi-step
and multi-factor process involving interactions between cancer
cells, and between cancer cells and the host microenvironment
(3). As multiple biological factors
are involved in HCC metastasis, HCC models with different
metastatic potentials constructed by the Hepatocellular Carcinoma
Institute of Fudan University have become an effective means to
study the metastatic behavior of HCC (4). For example, the highly metastatic HCC
cell line MHCC97-H has a lung metastasis rate of 100% (5). Therefore, this cell line is the most
effective model for studying HCC lung metastasis (6).
The development of stem cell technology has provided
novel data for the occurrence, progress and treatment of HCC. Stem
cell technology can be divided into normal stem cell technology and
cancer stem cell (CSC) technology. The CSC concept states that
tumor growth, analogous to the renewal of healthy tissues, is
fueled by small numbers of dedicated stem cells (7). Normal stem cell technology refers to
the use of human normal stem cells for tumor intervention
techniques in order to observe their role on tumors or tumor cells
(8). Autologous bone marrow
mesenchymal stem cells (BMSCs) transplantation is a promising tool
for tumor treatment (9). In liver
disease, for example liver cirrhosis, autologous BMSCs generate
hepatocytes and bile duct cells, which can repair damaged liver
tissue and therefore prevent liver transplantation (10). This method has become the point of
focus in research for HCC biotherapy due to its advantages,
including easy collection, ability to avoid rejection reactions,
simple and easy transplantation process, low treatment cost and
absence of ethical concerns (11,12).
Current research focuses mainly on the biological
intervention of BMSCs for the metastatic potential of HCC, which
provides a basis for finding suitable biotherapeutic targets
(13,14). However, there are still numerous
uncertainties regarding the efficacy of BMSCs in tumor
intervention, including stem cell tumorigenicity, long term
interventional efficacy and the influence of stem cells on the
biological behavior of tumors, which all require further research
and observation (8).
In a previous study, autologous BMSCs were
genetically modified to upregulate osteopontin (OPN) and
transforming growth factor β1 (TGFβ1)
gene expression in order to observe the effect of stem cells on HCC
cells with high (MHCC97-H) and low (MHCC97-L) metastatic
potentials. OPN promotes tumor metastasis following interaction
with integrin αvβ3 and induces cell movement by altering the
cytoskeleton, promoting angiogenesis and cell adhesion, and
preventing cell apoptosis (15).
Exogenous OPN secretion by BMSCs serves a role in promoting tumor
invasion by inhibiting the secretion of endogenous OPN in MHCC97-H
cells, mainly by activating matrix metalloproteinase-2(15). Previous studies have also
demonstrated that TGFβ1 is associated with tumor
proliferation and has different effects on liver cancer cells with
high and low metastatic potentials (16), namely, the ability of BMSCs with
TGFβ1 to promote MHCC97-L invasion was higher than that
for MHCC97-H (17) and MSCs may be
capable of enhancing the angiogenesis of HCC, which may be partly
due to the involvement of TGFβ1(18). In the relationship between TGFβ1, OPN
and integrin αvβ3, OPN mediates the adhesion
of integrin to the extracellular matrix (ECM) and becomes a
critical factor in tumor metastasis, while TGFβ1
activates protein kinase B by regulating the expression of integrin
linked kinase. TGFβ1 promotes the phosphorylation of
focal adhesion kinase tyrosine and activates downstream signaling
molecules to directly or indirectly participate in the adhesion of
integrin and ECM, which promotes HCC invasion and metastasis
(19,20).
Based on previous findings, the current study was
designed to determine the effect of reduced OPN and
TGFβ1 levels on MHCC97-H metastasis. Therefore, the
metastasis-associated gene OPN and the tumor growth-related
gene TGFβ1 were silenced using small interfering
RNAs (siRNAs) in MHCC97-H cells and the change in metastatic
potential was evaluated. Furthermore, the effect of BMSCs on
MHCC97-H following gene silencing was also evaluated.
Materials and methods
Instruments and reagents
Instruments used included: a frozen microtome (HM525
NX; Thermo Fisher Scientific, Inc.), an upright fluorescence
microscope (BX43; Olympus Corporation), a refrigerator (BCD-211KD3;
TCL Corporation), a rotary microtome (RM2235; Leica Microsystems
GmbH), a pathological tissue drift Bakeware (Tec 2500; Changzhou
Haosilin Instrument Equipment Co., Ltd.), a rotary table scanning
confocal microscope (DSU; Olympus Corporation), an electric
thermostatic blast drying oven (101-3, Shanghai Jinping Instrument
Co., Ltd.), a water-proof constant temperature incubator
(PYX-DHS500BS-II; Shanghai Yuejin Medical Devices Co., Ltd.), an
electronic balance (FA1104; Shanghai Tianping Instrument Factory),
an ultra-low temperature refrigerator (BS-812; Qingdao Haier Co.,
Ltd.) and a purification bench (SW-CJ-2D; Shanghai Xinmiao Medical
Instrument Manufacturing Co., Ltd.).
Reagents used included: mouse polyclonal integrin
αvβ3 antibody (L2206; Santa Cruz Animal
Health; 1:50), donkey anti-rabbit fluorescent secondary antibody
(15316; Thermo Fisher Scientific, Inc.; 1:800; excitation/emission
wavelength: 490/520 nm); citric acid antigen recovery solution (pH
6.0; Fuzhou Maixin Biotechnology Co., Ltd.), xylene (Chengdu Kelong
Chemical Reagent Factory), anhydrous ethanol (Chengdu Kelong
Chemical Reagent Factory) and DAPI (Sigma-Aldrich; Merck KGaA;
absorption wavelength/emission wavelength: 358/461 nm). DMEM
medium, trypsin and 0.02% EDTA were purchased from Gibco, Thermo
Fisher Scientific, Inc. Phosphate buffered saline (PBS) was
purchased from China Pharmaceutical Chemicals Co., Ltd. Fetal
bovine serum (FBS) was purchased from Biological Industries, blue
streptomycin (100 x double antibody) from Hangzhou Haotian
Biotechnology Co., Ltd., and L-Glutamine medium from Shanghai
Ruibosai Biological Technology Co., Ltd.
Cell experiment and culture
methods
MHCC97-H were provided by the Institute of Liver
Cancer at Fudan University. MHCC97-H cells were incubated with 10%
FBS with 1X DMEM medium at 37˚C in a 5% CO2 incubator.
Cells were subcultured by washing with PBS, followed by
dissociation with 0.25% trypsin and 0.02% EDTA. BMSCs were provided
by Saiye Biotechnology Co., Ltd., (cat. no. HUXMA-90011). BMSCs
were derived from the bone marrow of healthy adults (18-45 years),
purchased from Saiye Biotechnology Co., Ltd., (cat. no.
HUXMA-90011). BMSCs were cultured and passaged to the 2nd
generation in vitro and then stored frozen. BMSCs activity
was tested before each experiment, include BMSCs growth state,
differentiation ability and cluster differentiation tests. BMSCs
were incubated with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
+ 1X MSCM (Beijing Yuhengfeng Technology Co., Ltd.) + L-Glutamine
medium at 37˚C in a 5% CO2 incubator. When cells
confluence reached 90%, BMSCs were passaged by washing with PBS,
followed by dissociation with 0.25% Trypsin and 0.02% EDTA.
Animal experiments and feeding
conditions
Animal experiments were approved by the Ethics
Committee of the Fourth Medical Center of the Chinese PLA General
Hospital. A total of 32 male BALB/c nude mice, aged 4-5 weeks, were
provided by Shanghai SLAC Experimental Animal Co., Ltd. The
production license no. was SCXK 2012-0002, SPF grade and the animal
quality certification no. was 0205939. At the time of purchase,
each animal weighed 20±2 g. Sterilized ultrapure drinking water was
provided to the mice and the quality of drinking water met the
provisions of the People's Republic of China National Standard for
Drinking Water Hygiene (GB5749-2006). The animal maintenance feed
was provided by Shanghai SLAC Experimental Animals Co., Ltd., and
the standard GB14924.3-2010 ‘Nutrient Components of Compound Feeds
for Experimental Animals’ protocol was implemented (21). The laboratory animal room use permit
no. was SYXK 2015-0008. The mice were housed at temperatures of
20-25˚C and the relative humidity range was 40-70%. Animal lighting
10-20 Lux, light and dark cycle 10/14 h, work lighting 100-200 Lux.
Nude mice were adapted for 6 days in the animal room environment
before testing. Following animal experiments, the tumor-bearing
animals were euthanized.
MHCC97-H gene silencing
Previous results have demonstrated that MHCC97-H
overexpresses the metastasis-associated gene OPN and the
proliferation-related gene TGFβ1 (5). Therefore, the current study separately
interfered with these genes.
Construction of adenoviral vectors:
TGFβ1 siRNA1 sequence (GTGGAGCTGTACCAGAAAT) and
OPN siRNA1 sequence (GAGGAGTTGAATGGTGCATAC) were used to
synthesize oligo sequences purified from a PAGE gel, and gene
sequences obtained from GeneBank (http://ncbi.nlm.nih.gov/GenBank/). Vectors were
digested with BamH I and EcoR I (New England Biolabs,
Inc.) and recovered by cutting. Following annealing at 50˚C for 30
sec, single strands of short hairpin RNA (shRNA) 3' and 5'
fragments were obtained. The shRNA of the target fragment was
ligated with the vector and transformed into TGFβ1. The
oligonucleotide fragment was diluted 100 times with water for use,
and Annealing Buffer (Beijing Solarbio Science and Technology Co.,
Ltd.) for Oligos (5x) was added. Annealing was performed at 95˚C
for 5 min, then naturally cooled to room temperature. The ligation
reaction solution was as follows: T4 DNA ligase 5 U, linearized
carrier 2 µl, diluted oligonucleotide 2 µl and 10X ligase buffer 1
µl, made up to 10 µl with water. Consecutive reactions were
performed according to the manufacturer's instructions.
Recombinant adenovirus
TGFβ1 and OPN shRNA adenoviruses
Recombinant plasmids were prepared and packaged with
recombinant adenoviral vectors. The Lentivirus was collected and
amplified in ~107 cells at an inoculating cell density
of 2-5x104 293T cells/cm2 in a 75
cm2 square bottle containing DMEM +10% FBS. A total of
10 ml of the virus supernatant was added to infect the cells. After
3-4 days, the cells almost became round and half of the cells were
in suspension. At this point, all of the cells were collected and
centrifuged at 500 x g, 37˚C for 60 min and the supernatant was
discarded. TGFβ1 and OPN shRNA adenovirus
assays were performed and Green Fluorescent protein (GFP; Abcam)
was used as a marker gene. PCR was used to identify recombinant
viruses. A total of 5 µl virus supernatant was taken and 10 µl
proteinase K was added, incubated at 55˚C for 1 h, then boiled for
5 min. After a further centrifugation at 4000 x g, 37˚C, 5 min, 1-2
µl was used for PCR. The virus was then used to infect MHCC97-H
cells. GFP was used as the marker gene. Expression levels of
TGFβ1 and OPN in MHCC97-H cells were measured before and
after gene interference using reverse transcription quantitative
(RT-q)PCR. RT-qPCR was performed using homo OPN forward,
AGGAGGAGGCAGAGCACA and reverse, CTGGTATGGCACAGGTGATG; and homo
TGFβ1 forward, GGCGATACCTCAGCAACCG and reverse,
CTAAGGCGAAAGCCCTCAAT. A total of 1×106 MHCC97-H cells
were collected, lysed and centrifuged (4˚C, 12,000 x g, 20 min)
using a TRIzol®-spin column (Invitrogen; Thermo Fisher
Scientific, Inc.). RNA purity was verified by placing 2 µl of RNA
solution on the Micro-volume Spectrophotometer SMA4000 (Merinton
Instrument, Inc.). RNA was reverse transcribed to cDNA using a
High-Capacity RNA-to-cDNA™ kit (Invitrogen; Thermo Fisher
Scientific, Inc.) according to operating instructions. β-actin was
used as the internal control. The PCR reaction conditions were:
93˚C for 2 min, then 93˚C for 1 min, and 55˚C for 2 min, for a
total of 40 cycles. The blank control group of OPN and
TGFβ1 were used as the relative quantitative PCR expression
levels and were normalized by the internal control (22). Relative expression levels were
calculated using the 2-ΔΔCq method.
MHCC97-H cell and BMSCs co-culture
migration experiment
The Transwell method was used to evaluate the
metastatic ability at the cellular level, as it simulates the
basement membrane structure of HCC tissue and basement membrane
breakthrough (23). MHCC97-H blank
was set as the control group (Blank control; BC) and MHCC97-H gene
interference was the negative control group (Native Contrast; NC),
in which GFP gene was transfected as marker gene into the
two groups. The experimental groups comprised of the MHCC97H
TGFβ1 gene interference and MHCC97-H OPN
gene interference groups, with both containing the GFP gene
as reporter gene. MHCC97-H cells in log phase were harvested and
dissociated with 0.25% Trypsin and 0.02% EDTA. Cells were counted
under microscope and 24-well Transwell plates (5.0 µm) were seeded
in DMEM medium at densities of 5x104 cells/well in the
upper chamber and 2x103 cells/well in the lower chamber.
The plates were incubated at 37˚C in a 5% CO2 incubator.
After 48 h of incubation, the wells were washed once with PBS and
the cells were fixed with 40 g/l paraformaldehyde for 10 min at
37˚C. The paraformaldehyde was then removed and the non-migrating
cells in the upper chamber were wiped off with a cotton swab. The
Transwell inserts were removed, inverted, air dried and rinsed
twice with pre-cooled PBS (4˚C) and fixed with pre-cooled 100%
methanol (4˚C) for 10 min. The methanol was removed, and the
inserts were incubated for 10 min at room temperature in 200 µl of
0.1% crystal violet (Abcam) that was added to the bottom of each
well. Images of three randomly selected fields were taken using an
inverted fluorescence microscope (x200; Olympus Corporation; MF53)
and cell counts were performed.
MHCC97-H xenograft model
MHCC97-H cells in logarithmic growth phase were
collected, centrifuged at 1,000 rpm for 5 min and the cell
concentration was adjusted to 2.5x107 cells/ml to
prepare a single cell suspension in DMEM medium. Nude mice were
subcutaneously inoculated with 0.2 ml cell suspension
(5x106 cells/mouse) of the corresponding group in the
right side of the armpit. Light pressure was put on the injection
site for 30 sec following injection to prevent leakage of the cell
suspension. Anesthesia was performed by intraperitoneal injection
of 1% sodium pentobarbital. The dose for nude mice was ~0.3 ml. The
method of euthanasia was spinal dislocation.
BMSCs intervention in the MHCC97-H
xenograft model and experimental methods
A total of 32 nude mice were randomly divided into
four groups: MHCC97-H blank control (BC; n=8), MHCC97-H-NC gene
silencing negative control (NC; n=8), MHCC97-H-OPN gene
silencing (n=8) and MHCC97-H-TGFβ1 gene silencing
(n=8) groups. After the nude mice were inoculated for 14 days and
tumor volume was allowed to grow to ~50 mm3, prior to
the initiation of the intratumoral injection of BMSCs
(1x105 cells/tumor) biweekly for 4 weeks. Pathological
observations of tumor tissue were performed using the HE staining
method. Sample fixation: 95% ethanol fixation for 20 min and PBS
wash twice, 1 min each time. Staining nucleus: Hematoxylin staining
for 2-3 min and washing with water. Staining cytoplasm: Immerse in
Eosin staining for 1 min and wash with water. The cells were then
air dried and sealed with neutral gum. Observations were performed
under a general optical microscope, magnification 200x.
Analysis of lung metastasis in
MHCC97-H xenograft model
The lung metastasis animal model of MHCC97-H was
performed according to a previous method (24). Briefly, cell lines in the logarithmic
growth phase were collected, centrifuged at 1,000 rpm for 5 min and
cell concentrations were adjusted to 2.5x107 cells/ml to
prepare a single cell suspension. Nude mice were intravenously
inoculated via tail vein with 0.2 ml (5x106 cells/mouse)
of prepared cell suspensions. Light pressure was applied to the
injection site for 30 sec after injection to prevent leakage of the
cell suspension.
Analysis of MHCC97-H metastatic lung
tumor pathology
The lungs were excised at the experimental end-point
(14 day) and fixed in 4% formaldehyde fixation for 3-5 days. The
fixed tissues were processed through serial ethanol gradients (50,
70, 80 and 95%) and xylene, and then embedded in paraffin. The
lungs were sectioned (6 µm) and stained with DAPI for 10 min at
37˚C, followed by cover slipping with glycerol (cat. no. 228220;
Shanghai Canspec Scientific Instruments Co., Ltd.) and PBS. HCC
lung metastasis nodules were observed under a fluorescence
microscope (magnification, 400). Differences in fluorescence
intensity were analyzed using Image J software (v1.52; National
Institutes of Health) and the integrated optical density (IOD)
value was used as a semi-quantitative measurement index.
Analysis of expression of integrin
αvβ3 in nude mice by immunofluorescence
The excised lung tissues underwent xylene dewaxing
at 37˚C, gradient alcohol rehydration with xylene twice for 20 min
each at room temperature, 100% ethanol twice for 5 min each, 95%
ethanol for 5 min, 80% ethanol for 5 min and then washed three
times with PBS for 3 min each at room temperature. Samples were
rinsed with 0.001 M PBST (Beijing Solarbio Science and Technology
Co., Ltd.) for 5 min, 3 times, and blocked with 10% BSA (Thermo
Fisher Scientific, Inc.) in a humidified chamber for 30 min. Slides
were placed in 0.01 M citrate buffer (pH 6.0) in the microwave for
10 min and allowed to cool to room temperature, followed by three
PBS rinses for 3 min for antigen retrieval. Primary antibodies were
added to the slides in a dropwise manner and the slides were
incubated at 4˚C overnight. The slides were then washed three times
for 3 min each with PBS. Secondary antibodies were added drop by
drop and the slides were incubated at 37˚C for 60 min and then
rinsed three times with PBS for 5 min each. DAPI staining was
performed at room temperature for 10 min. The slides were cover
slipped with glycerol and PBS, and observed using a confocal
microscope (magnification, x150). Immunofluorescence exhibited a
positive green expression. Quantitative analysis of integrin
αvβ3 expression by positive pixel levels in
the metastatic hepatocellular carcinoma cells of lung tissue was
analyzed with Image J software (v1.52; National Institutes of
Health).
Analysis of animal model test
index
Each group of nude mice was weighed 1 week following
cell inoculation and tumor volume was measured. Three times a week,
the longest diameter (a) and the shortest diameter (b) of the tumor
were measured with Vernier calipers and tumor volume (V) was
calculated: V(cm3)=1/2ab2=0.5ab2,
and a tumor growth curve was plotted. Furthermore, the animals were
weighed three times a week, and the animal tumor weight was
calculated as: total animal weight - tumor volume (assuming a tumor
density of 1). The following calculations were used for analysis:
tumor volume inhibition rate (%) = (1-average tumor volume in
experimental group/mean tumor volume in control group) x100%; tumor
weight inhibition rate (%) = (1-average tumor weight in
experimental group/mean tumor weight in control group) x100%.
Statistical analysis
Experimental data are expressed as mean ± SD. The
data were analyzed using SPSS statistical software (v16.0; IBM
Corp.). The IOD value quantitative comparison between cell counts
was performed using one-way ANOVAs with post-hoc LSD tests, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
TGFβ1 and OPN expression
differences before and after gene silencing in MHCC97-H cells
The relative quantitative results are shown in
Table I. OPN and
TGFβ1 expression were clearly decreased in the
MHCC97-H cells following gene silencing.
 | Table ITGFβ1 and OPN expression in MHCC97-H
cells before and after siRNA (2-ΔΔCt). |
Table I
TGFβ1 and OPN expression in MHCC97-H
cells before and after siRNA (2-ΔΔCt).
| Item |
Proliferation-related gene TGFβ1 |
Metastasis-associated gene OPN |
|---|
| MHCC97-H before
gene interference | 1.000±0.026 | 1.000±0.135 |
| MHCC97-H after gene
interference | 0.557±0.062 | 0.473±0.095 |
Cell counts from Transwell assay
experiments on MHCC97-H cells
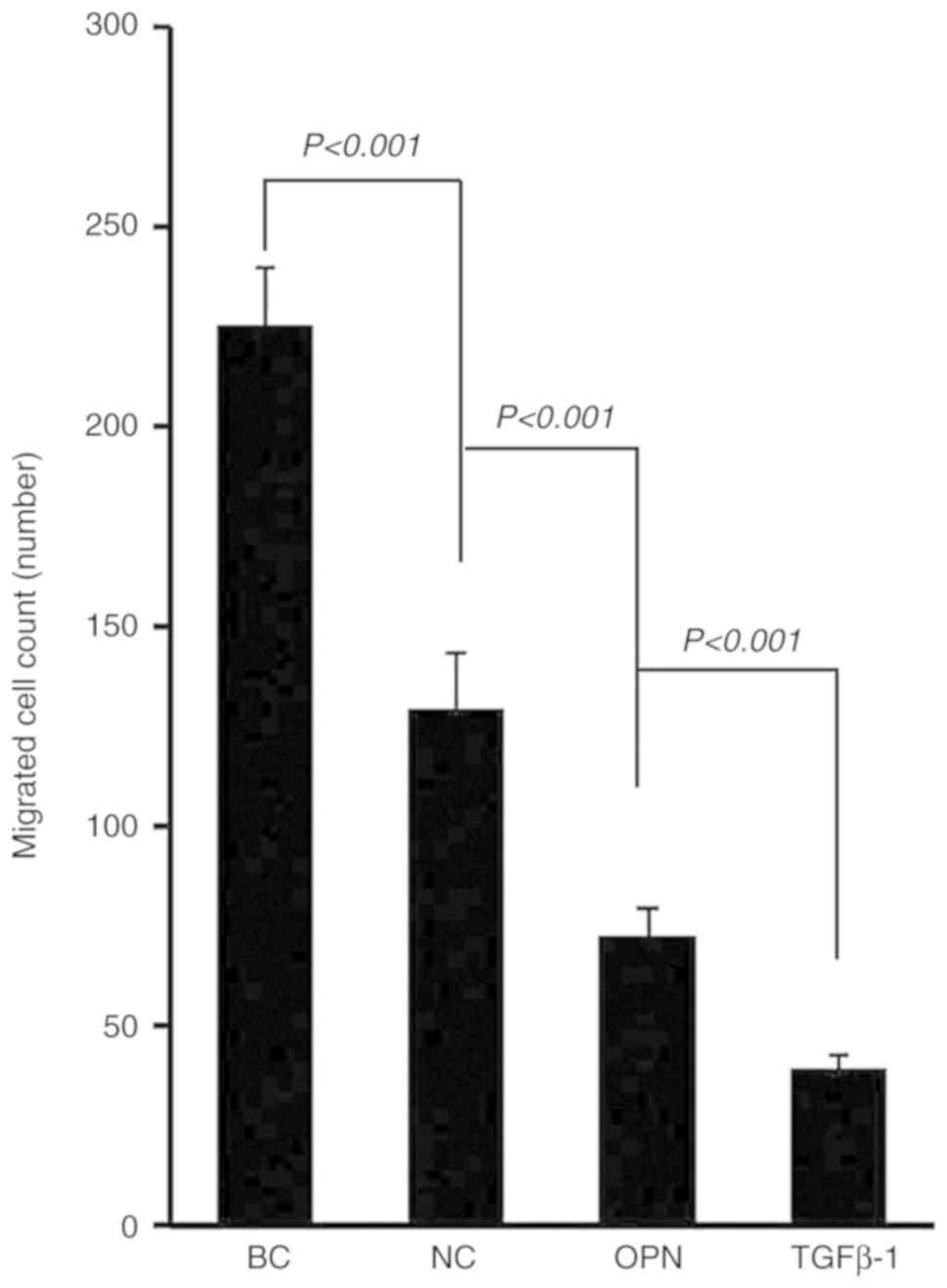
The results demonstrated that there was a
significant difference between the control group compared with the
other groups in the number of migrated cells (Fig. 1; F=7.461; P<0.001). This indicated
that the number of migrating cells following gene interference was
significantly reduced, particularly in the MHCC97-H OPN and
MHCC97-H TGFβ1 interference groups
(P<0.001).
BMSC intervention in the MHCC97-H
animal model
After the nude mice were inoculated for 14 days and
the tumor volume was >50 mm3, the intratumoral
injection of human BMSCs was initiated for intervention. The BMSC
intervention groups are shown in Table
II. At the end of the experiment, each group of mice was
sacrificed and tumors were excised. It was found that the boundary
between tumors and surrounding tissues was clear, with slight
adhesion between the skin and subcutaneous connective tissue for
all mice bearing-tumor. Additionally, capsules were intact.
 | Table IIComparison of tumor volume and weight
in hepatocellular carcinoma animal models following 28 days of BMSC
cell intervention. |
Table II
Comparison of tumor volume and weight
in hepatocellular carcinoma animal models following 28 days of BMSC
cell intervention.
| Group | Tumor volume
(mm3) | Tumor weight
(g) |
|---|
| MHCC97-H BC |
3064.90±821.78a,b | 20.52±3.32 |
| MHCC97-H NC |
2776.62±704.36a,b | 18.90±1.87 |
| MHCC97-H OPN |
1200.25±181.59a | 23.72±2.53 |
| MHCC97-H TGFβ1 | 595.83±343.83 | 23.34±2.23 |
Comparison of the tumor volumes revealed that the
MHCC97-H gene-silenced group had statistically significantly
smaller compared with the BC group (P<0.05), indicating that
BMSCs had a significant inhibitory effect on tumor volume in the
gene-silenced group. Comparison of tumor weights demonstrated that
BMSCs had no significant inhibitory effect on tumor weight
following gene silencing in the MHCC97-H tumor model.
MHCC97-H tissue inhibition rate was calculated using
the inhibition rates of tumor volume inhibition and tumor weight.
The results are shown in Table
III. BMSCs had mild inhibitory effects on the tumor volume and
tumor weight in the MHCC97-H NC group compared with the MHCC97-H BC
group. Compared with the MHCC97-H BC and NC groups, BMSCs had a
significant inhibitory effect on both tumor volume and tumor weight
in the MHCC97-H OPN-silenced (volume inhibition rate 60.84%,
weight inhibition rate 47.23%) and MHCC97-H
TGFβ1-silenced groups (volume inhibition rate
80.56%, weight inhibition rate 84.08%).
 | Table IIIAnalysis of the inhibition rate of
hepatocellular carcinoma before and after gene modification in
animal models. |
Table III
Analysis of the inhibition rate of
hepatocellular carcinoma before and after gene modification in
animal models.
| Group | Tumor volume
inhibition rate (%) | Tumor weight
inhibition rate (%) |
|---|
| MHCC97-H BC | 0.00 | 0.00 |
| MHCC97-H NC | 9.41 | 4.99 |
| MHCC97-H OPN | 60.84 | 47.23 |
| MHCC97-H TGFβ1 | 80.56 | 84.08 |
Pathological results of BMSC
intervention in MHCC97-H xenograft model before and after gene
modification
Increased tumor necrosis was observed in the
MHCC97-H BC and MHCC97-H NC groups compared to the MHCC97-H group
with OPN and TGFβ1 genes silenced groups (Fig. 2). Fibroproliferation was evident and
there was an increased number of mitoses in all groups.
Furthermore, there was relatively less fibrosis and mitoses in the
MHCC97-H OPN-silencing group, while the MHCC97-H
TGFβ1-silenced group exhibited significantly more fibrosis
and mitoses, when compared with the control group.
Lung metastasis animal model
fluorescence imaging results of MHCC97-H
The results demonstrated that there were more tumor
cells in the lung tissues of the BC and NC groups (Fig. 3A and B). There were fewer tumor cells in the
OPN- and TGFβ1-silenced groups in the lung
tissue compared with the BC group (Fig.
3C and D), indicating that the
lung metastasis of gene-silenced MHCC97-H tumor potentially
declined.
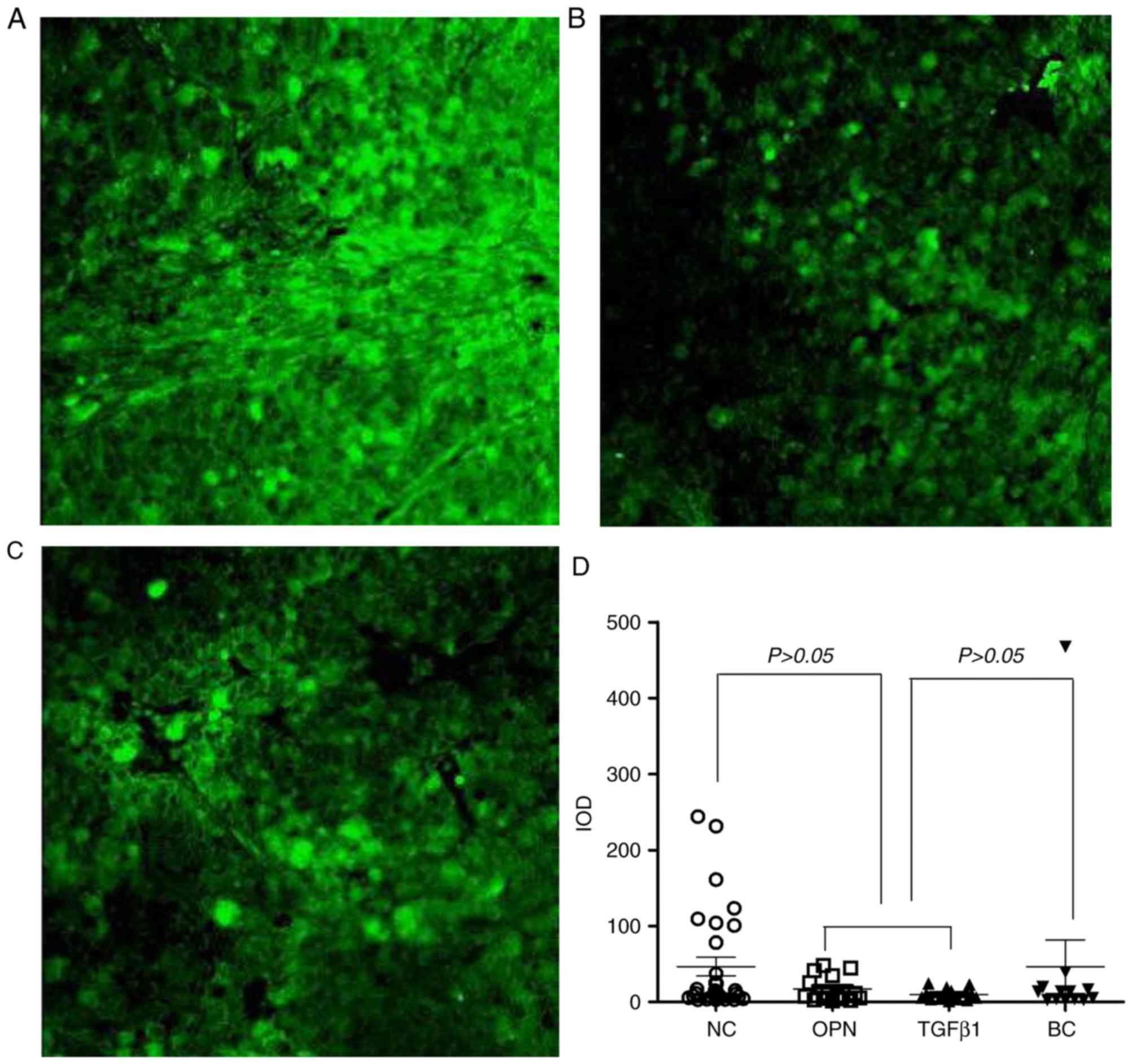
 | Figure 3Fluorescence imaging of the MHCC97-H
tumor lung metastasis animal model. The tumor cells are red and the
nuclei of the lungs are blue. Magnification, x200. (A) Blank
control group, (B) gene-negative control group, (C) TGFβ1
interference group, (D) OPN interference group, and (E)
quantitative analysis of fluorescence imaging of metastatic
hepatocellular carcinoma cells in lung tissue. MHCC97-H, high
metastatic potential hepatocellular carcinoma; IOD, integrated
optical density; NC, negative control group; OPN, osteopontin gene
silencing group; TGFβ1, transforming growth factor β1 gene
silencing group |
To further analyze the metastasis of HCC cells in
lung tissue, quantitative analysis was performed on the
fluorescence intensity of MHCC97-H in pathological sections using
Image J software. IOD was used as a quantitative indicator
(Fig. 3E). The IOD values of the
MHCC97-H cells were lower in the OPN- and
TGFβ1-silenced groups compared with the BC and NC
groups. This difference was statistically significant (P<0.05),
indicating that the lung metastasis ability of MHCC97-H was reduced
following interference by OPN and TGFβ1
genes, which was consistent with the results observed using a
microscope.
Immunofluorescence of
metastasis-associated integrin αvβ3
expression
The positive staining for integrin
αvβ3 demonstrated a strong green color
(Fig. 4A-C). The density of green
fluorescent MHCC97-H cells was significantly reduced in the gene
interference groups compared with the control group. The
fluorescence intensity of integrin αvβ3
expressed in MHCC97-H cells in the lung was quantitatively analyzed
using Image J software (Fig. 4D).
The results demonstrated that the IOD value of migrated cells was
not statistically different among the three groups (P>0.05),
indicating that neither OPN nor TGFβ1 gene
interference in MHCC97-H altered the ability of the cells to
express integrin αvβ3.
Discussion
In the present study, two genes were silenced in
MHCC97-H cells. OPN is a type of secreted phosphorylated
glycoprotein and its molecular structure contains an RGD
(Arg-Gly-Asp) polypeptide sequence. By combining with integrin
αvβ3 or CD44, OPN participates in important
biological processes, such as cell adhesion and signal
transduction. Furthermore, it promotes tumor cell metastasis
(25). A previous study demonstrated
that OPN is significantly overexpressed in MHCC97-H cells,
suggesting that OPN is involved in the metastatic behavior of HCC
(26). Furthermore, the metastatic
behavior of HCC can be delayed by blocking the expression of OPN
(27).
TGFβ1 is an important biological factor
involved in tumor growth, invasion and metastasis, and in the
occurrence and progression of HCC in terms of proliferation and
metastasis (28). TGFβ1
and integrin αvβ3 receptors are associated
with tumor metastasis-related signaling pathways (29). Integrin and TGFβ1
receptor-mediated signal transduction pathways share certain
signaling molecules, such as Rac1 and ERK, and interact to exert a
variety of biological effects, such as cell collagen hyperplasia
and cell fibrogenesis (30).
Therefore, based on previous studies, the current study silenced
OPN and TGFβ1 genes in MHCC97-H. Changes
in metastasis and proliferation ability in MHCC97-H cells were
observed following BMSC treatment in vitro and in
vivo. The Transwell method was used to evaluate the metastatic
ability at the cellular level, as it simulates the basement
membrane structure of HCC tissue and basement membrane
breakthrough, which is a critical step in metastasis (25). Animal models were used to evaluate
metastatic ability in a lung metastasis model of MHCC97-H. MHCC97-H
cells were injected before and after gene interference in order to
increase the concentrations of BMSCs in tumors and the lung
metastasis of HCC was observed after 28 days.
The results of the cytology experiments and the
animal model indicated that BMSCs migration ability following gene
silencing in MHCC97-H cells was significantly decreased, especially
following TGFβ1 silencing. This indicated that
biological factors associated with OPN and TGFβ1 were involved in
HCC metastasis. Furthermore, the results suggested that BMSCs are
more effective at reducing metastasis in gene-silenced MHCC97-H
cells. Therefore, the TGFβ1 gene may be the best
target for BMSCs to interfere with metastatic behavior in HCC.
Previous studies (31,32) have demonstrated that
TGFβ1 overexpression may lead to excessive
suppression of immune cells, including T and B lymphocytes, by
reducing the immune surveillance function of the host, immune
response and clearance to invading pathogens and by increasing
tumor cell elimination, leading to the occurrence and metastasis of
tumors. This mechanism may explain the reason
TGFβ1 gene silencing is more conducive to
inhibiting the metastasis of HCC. Further previous studies have
demonstrated that low doses of TGFβ1 (≤0.25 ng/ml)
increases BMSC proliferation, while higher doses of TGFβ1 (≥1
ng/ml) inhibit their proliferation (33). Additionally, there are data that
indicate that low doses of TGFβ signaling alongside BMSCs may lower
their immunomodulatory potential (34). Therefore, with low TGFβ1
expression in HCC, BMSCs may be better at inhibiting the
proliferation and metastasis of HCC.
The results for tumor weight and volume indicated
that BMSCs have a positive effect on the inhibition of HCC tissues
growth after gene silencing. The lack of significant change in
tumor weight may be explained by the pathological results, as tumor
necrosis and fibrosis increased, and number of mitoses decreased in
the experimental groups compared with the control group. Therefore,
the reason that the tumor weight did not change significantly was
associated with the increase in fibrous tissue in tumors with
necrotic components. Furthermore, solid tumor weight inhibition
ratios were different from tumor weight measurements. When the
tumor inhibition rate was calculated, the weight of the animal
itself was taken into account. Because of excessive nutrient
consumption of tumor-bearing nude mice, the total weight of the
animals reduced (35). However,
changes in tumor volume inhibition were consistent with the trend
of simply measuring volume changes. Moreover, OPN silencing
promoted cell apoptosis, delayed tumor cell proliferation and
promoted fibrous tissue proliferation, a result that is consistent
with that of Zhu et al (36).
Following TGFβ1 silencing, the promotion of tumor
cell proliferation was weakened and replaced by fibrous tissue
proliferation, which was consistent with the function of
TGFβ1 reported in the literature (37). The ability of metastasis and
proliferation of gene-silenced MHCC97-H cells and animal models
following BMSC intervention was markedly reduced. These data were
confirmed by the lung metastasis fluorescence imaging in the
MHCC97-H animal model.
In order to further explore the metastatic potential
changes in gene-silenced MHCC97-H, the expression of the key
biological molecule integrin αvβ3 in HCC
tissue with lung metastasis was analyzed. However, the experimental
results revealed no significant changes in the expression of
integrin αvβ3 in MHCC97-H cells in the lungs
of the OPN- and
TGFβ1-gene-silenced groups compared
with the control group. This may be related to the expression of
integrin αvβ3. The vascular endothelium, as
well as the cancer tissue, is also involved in the expression of
integrin αvβ3 (38). Thus, the decrease in metastatic
potential following gene silencing in MHCC97-H was not associated
with integrin αvβ3 expression, but was
related to the decrease in OPN and TGFβ1 expression. At
lower levels, OPN and TGFβ1 do not bind to integrin
αvβ3 (39).
Therefore, it is possible that low OPN and TGFβ1
expression in MHCC97-H may reduce metastasis by other mechanisms.
Previous findings have suggested that OPN promotes tumor
proliferation and metastasis by mediating
TGFβ1-dependent mesenchymal stem cell-to-fibrocyte
transformation mechanisms (40).
Another previous study investigated the relationship between
TGFβ1 and MHCC97-H metastasis and the results
demonstrated that high TGFβRII expression may inhibit the
proliferation, migration and invasion of MHCC97-H (41).
BMSCs have different efficacies for HCC
interventions, such as influencing tumor progression, and these
differences may be related to the tumor microenvironment (13). Numerous factors may be involved in
BMSC intervention in tumors. MCP-3 can increase the migration of
liver cancer cells with different metastatic potential and StTRAIL
can promote apoptosis of liver cancer cells (42,43). In
the present study, TGFβ1 and OPN were involved in the
proliferation and metastasis of tumors. The results of the current
study indicated that the efficacy of BMSCs intervention in MHCC97-H
following gene silencing was significantly increased compared with
the experimental control group.
In conclusion, the current study employed gene
silencing methods and the results revealed that the reduction of
OPN and TGFβ1 expression led to reduced
metastatic potential in MHCC97-H. The metastatic potential and
proliferative potential of MHCC97-H following BMSC intervention
were significantly reduced both in vitro and in vivo,
particularly in the TGFβ1-silenced group. The
reduced metastatic potential following gene silencing in MHCC97-H
was not related to integrin αvβ3 expression.
Therefore, OPN and TGFβ1 factors may be considered to be
targets for BMSC intervention, of which TGFβ1 may be the
best targets. Since the regulation mechanism of MHCC97-H metastatic
potential was not further studied, research into stem cells in
metastasis mechanisms in HCC is necessary. The current study
provides a basis for treatment of HCC using BMSCs. Future research
should involve searching for a suitable gene that inhibits the
metastasis and proliferation of HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by Chinese National
Natural Science Foundation (grant no. 81271607).
Availability of data and materials
The datasets used and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
TL designed the experimental trial, acquired and
analyzed the data, and drafted the manuscript. BZ designed the
clinical trial, collected experimental data and analyzed the data.
LS and YZ acquired data and drafted the manuscript. YF contributed
to the conception and design, administrative support. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by the Ethics
Committee of the Fourth Medical Center of the Chinese PLA General
Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao P, Dai M, Chen W and Li N: Cancer
trends in China. Jpn J Clin Oncol. 40:281–285. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ni J and Hua HQ: Progress in the treatment
of primary hepatocellular carcinoma by sorafenib combined with
traditional Chinese medicine. Mil Med J Southeast China.
17:175–177. 2015.
|
|
3
|
Han TS, Ban HS, Hur K and Cho HS: The
epigenetic regulation of HCC metastasis. Int J Mol Sci.
19(3978)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li Y, Tang ZY, Ye SL, Liu YK, Chen J, Xue
Q, Chen J, Gao DM and Bao WH: Establishment of cell clones with
different metastatic potential from the metastatic hepatocellular
carcinoma cell line MHCC97. World J Gastroenterol. 7:630–636.
2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li Y, Tang Y, Ye L, Liu B, Liu K, Chen J
and Xue Q: Establishment of a hepatocellular carcinoma cell line
with unique metastatic characteristics through in vivo selection
and screening for metastasis-related genes through cDNA microarray.
J Cancer Res Clin Oncol. 129:43–51. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li GC, Ye QH, Dong QZ, Ren N, Jia HL and
Qin LX: TGF beta1 and related-Smads contribute to pulmonary
metastasis of hepatocellular carcinoma in mice model. J Exp Clin
Cancer Res. 31(93)2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Batlle E and Clevers H: Cancer stem cells
revisited. Nat Med. 23:1124–1134. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu WH, Song FQ, Ren LN, Guo WQ, Wang T,
Feng YX, Tang LJ and Li K: The multiple functional roles of
mesenchymal stem cells in participating in treating liver diseases.
J Cell Mol Med. 19:511–520. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vakhshiteh F, Atyabi F and Ostad SN:
Mesenchymal stem cell exosomes: A two-edged sword in cancer
therapy. Int J Nanomedicine. 14:2847–2859. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tsuchiya A, Takeuchi S, Watanabe T,
Yoshida T, Nojiri S, Ogawa M and Terai S: Mesenchymal stem cell
therapies for liver cirrhosis: MSCs as ‘conducting cells’ for
improvement of liver fibrosis and regeneration. Inflamm Regen.
39(18)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Guo XZ, Liu X, Wang D, Zhao JJ, Li YH,
Shao XD and Ren LN: The effect of autologous bone marrow stem cell
transplantation on liver cirrhosis with different causes. Chin J
Digestion. 31:53–54. 2011.
|
|
12
|
Vainshtein JM, Kabarriti R, Mehta KJ,
Roy-Chowdhury J and Guha C: Bone marrow-derived stromal cell
therapy in cirrhosis: Clinical evidence, cellular mechanisms, and
implications for the treatment of hepatocellular carcinoma. Int J
Radiat Oncol Biol Phys. 89:786–803. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yin Z, Jiang K, Li R, Dong C and Wang L
and Wang L: Multipotent mesenchymal stromal cells play critical
roles in hepatocellular carcinoma initiation, progression and
therapy. Mol Cancer. 17(178)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu Y, Ren H, Zhou Y, Shang L, Zhang Y,
Yang F and Shi X: The hypoxia conditioned mesenchymal stem cells
promote hepatocellular carcinoma progression through YAP mediated
lipogenesis reprogramming. J Exp Clin Cancer Res.
38(228)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li TR, Cai LJ, Zhao SH, Wei ZM, Huo TL, Lu
GM and Song B: Effect of OPN transfected BMSCs on MHCC97-H. Chin J
Gastroenterol Hepatol. 24:1057–1061. 2015.
|
|
16
|
Li GC, Ye QH, Xue YH, Sun HJ, Zhou HJ, Ren
N, Jia HL, Shi J, Wu JC, Dai C, et al: Human mesenchymal stem cells
inhibit metastasis of a hepatocellular carcinoma model using the
MHCC97-H cell line. Cancer Sci. 101:2546–2553. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li TR, Du XK, Song B, Ye YK, Wei ZM and
Huo TL: Experimental study of TGFβ 1 transfected hMSC intervene
MHCC97-H. Chin J Gastroenterol Hepatol. 22:615–619. 2013.
|
|
18
|
Li GC, Zhang HW, Zhao QC, Sun LI, Yang JJ,
Hong L, Feng F and Cai L: Mesenchymal stem cells promote tumor
angiogenesis via the action of transforming growth factor β 1.
Oncol Lett. 11:1089–1094. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang R, Pan X, Huang Z, Weber GF and
Zhang G: Osteopontin enhances the expression and activity of MMP-2
via the SDF-1/CXCR4 axis in hepatocellular carcinoma cell lines.
PLoS One. 6(e23831)2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Weber CE, Kothari AN, Wai PY, Li NY,
Driver J, Zapf MA, Franzen CA, Gupta GN, Osipo C, Zlobin A, et al:
Osteopontin mediates an MZF1-TGF-β 1-dependent transformation of
mesenchymal stem cells into cancer-associated fibroblasts in breast
cancer. Oncogene. 34:4821–4833. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
General Administration of Quality
Supervision, Inspection and Quarantine of the People’s Republic of
China, and Standardization Administration of the People’s Republic
of China: Laboratory animals-Nutrients for formula feeds (GB
14924.3-2010) issued by National Standards of People’s Republic of
China, December 23, 2010. http://www.gb688.cn/bzgk/gb.
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Marshall J: Transwell® invasion
assays. Methods Mol Biol. 769:97–110. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kang Y: Imaging TGFβ signaling in mouse
models of cancer metastasis. Methods Mol Biol. 1344:219–232.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Icer MA and Gezmen-Karadag M: The multiple
functions and mechanisms of osteopontin. Clin Biochem. 59:17–24.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ye QH, Qin LX, Forgues M, He P, Kim JW,
Peng AC, Simon R, Li Y, Robles AI, Chen Y, et al: Predicting
hepatitis B virus-positive metastatic hepatocellular carcinomas
using gene expression profiling and supervised machine learning.
Nat Med. 9:416–423. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Huang H, Zhang XF, Zhou HJ, Xue YH, Dong
QZ, Ye QH and Qin LX: Expression and prognostic significance of
osteopontin and caspase-3 in hepatocellular carcinoma patients
after curative resection. Cancer Sci. 101:1314–1319.
2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Budhu A and Wang XW: The role of cytokines
in hepatocellular carcinoma. J Leukoc Biol. 80:1197–1213.
2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tang J, Gifford CC, Samarakoon R and
Higgins PJ: Deregulation of negative controls on TGF-β 1 signaling
in tumor progression. Cancers (Basel). 10(159)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hayashida T, Jones JC, Lee CK and Schnaper
HW: Loss of beta1-integrin enhances TGF-beta1-induced collagen
expression in epithelial cells via increased alphavbeta3-integrin
and Rac1 activity. J Biol Chem. 285:30741–30751. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li MO, Wan YY, Sanjabi S, Robertson AK and
Flavell RA: Transforming growth factor-beta regulation of immune
responses. Annu Rev Immunol. 24:99–146. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Prasad P, Tiwari AK, Kumar KM, Ammini AC,
Gupta A, Gupta R and Thelma BK: Association of TGFbeta1, TNFalpha,
CCR2 and CCR5 gene polymorphisms in type-2 diabetes and renal
insufficiency among Asian Indians. BMC Med Genet.
8(20)2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li D, Liu Q, Qi L, Dai X, Liu H and Wang
Y: Low levels of TGF-β 1 enhance human umbilical cord-derived
mesenchymal stem cell fibronectin production and extend survival
time in a rat model of lipopolysaccharide-induced acute lung
injury. Mol Med Rep. 14:1681–1692. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
de Araújo Farias V, Carrillo-Gálveza AB,
Martína F and Andersona P: TGF-β and mesenchymal stromal cells in
regenerative medicine, autoimmunity and cancer. Cytokine Growth
Factor Rev. 43:25–37. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Molfino A, Amabile MI and Muscaritoli M:
Nutrition support for treating cancer-associated weight loss: An
update. Curr Opin Support Palliat Care. 12:434–438. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhu Y, Gao XM, Yang J, Xu D, Zhang Y, Lu
M, Zhang Z, Sheng YY, Li JH, Yu XX, et al: C-C chemokine receptor
type 1 mediates osteopontin-promoted metastasis in hepatocellular
carcinoma. Cancer Sci. 109:710–723. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Neuzillet C, de Gramont A,
Tijeras-Raballand A, de Mestier L, Cros J, Faivre S and Raymond E:
Perspectives of TGF-β inhibition in pancreatic and hepatocellular
carcinomas. Oncotarget. 5:78–94. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhou JP and Zhou WP: Expression of VEGF,
integrin αV and MVD in viable residual tumor tissues from large
hepatocellular carcinoma after TACE. Mil Med J Southeast China.
12:206–208. 2010.
|
|
39
|
Li TR, Yu MH, Huang XB, Yang ZJ, Lu GM and
Li YJ: Magnetic resonance Gd-RGD imaging study of hepatocellular
carcinoma with high and low metastatic potential before and after
human bone marrow-derived mesenchymal stem cell intervention. Chin
Med J (Engl). 130:2591–2600. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hayashida T, Wu MH, Pierce A, Poncelet AC,
Varga J and Schnaper HW: MAP-kinase activity necessary for
TGFbeta1-stimulated mesangial cell type I collagen expression
requires adhesion-dependent phosphorylation of FAK tyrosine 397. J
Cell Sci. 120:4230–4240. 2007.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li Y, Liu G, Li X, Dong H, Xiao W and Lu
S: Long non-coding RNA SBF2-AS1 promotes hepatocellular carcinoma
progression through regulation of miR-140-5p-TGFBR1 pathway.
Biochem Biophys Res Commun. 503:2826–2832. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wen X, Yao M, Lu Y, Chen J, Zhou J, Chen
X, Zhang Y, Lu W, Qian X, Zhao J, et al: Integration of prealbumin
into child-pugh classification improves prognosis predicting
accuracy in HCC patients considering curative surgery. J Clin
Transl Hepatol. 6:377–384. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Deng Q, Zhang Z, Feng X, Li T, Liu N, Lai
J, Shuai L, Xiong Q, Fu C, Zou H, et al: TRAIL-secreting
mesenchymal stem cells promote apoptosis in heat-shock-treated
liver cancer cells and inhibit tumor growth in nude mice. Gene
Ther. 21:317–327. 2014.PubMed/NCBI View Article : Google Scholar
|