Introduction
Blunt chest trauma leads to death in up to 60% of
patients after car accidents (1).
Small and large animal models of blunt chest trauma are necessary
to develop novel therapeutic approaches. Previously, a blast wave
generator was developed to consistently induce chest traumata in
rats (2). In the last decade, mice
have primarily been used as experimental animals for studies
investigating blunt chest trauma in order to explore the abundant
immunological and genetic tools in this species. A previous study
reported the physiological and immunological characterization of a
long-term model of blunt chest trauma in mice using a blast wave
generator (3). Another approach for
inducing blunt chest trauma is dropping a weight from a defined
height, which has been used in multiple rat and murine trauma
models (4-6).
In the present study, a device was used to simulate
the frontal impact of a car on the human body. A block of metal of
varying weight dropped from different heights was used to induce
defined trauma, ranging from contusions up to severe lesions. In
contrast to non-elastic material, real bodies display post mortem
recoil after impact. The present device was developed to measure
the maximum depth of impression. To illustrate the effect of the
maximum depth of impression on blunt trauma against thoracic
organs, including the heart, lungs and big vessels, a schematic
drawing is presented in Fig. 1. The
device was tested on plasticine dummies as well as on mouse
cadavers. Cadavers were used to obtain preliminary results to
minimize the use of living animals. The present study aimed to
demonstrate that the new device developed was able to generate
reproducible impact trauma, which may be calculated and physically
defined. This device may aid in subsequent impact studies and
improve the current knowledge on the effect of maximum depth of
impression on blunt chest trauma.
Materials and methods
Blunt chest trauma device
The device used in the present study was a block of
metal of defined weight that was dropped from a defined height on
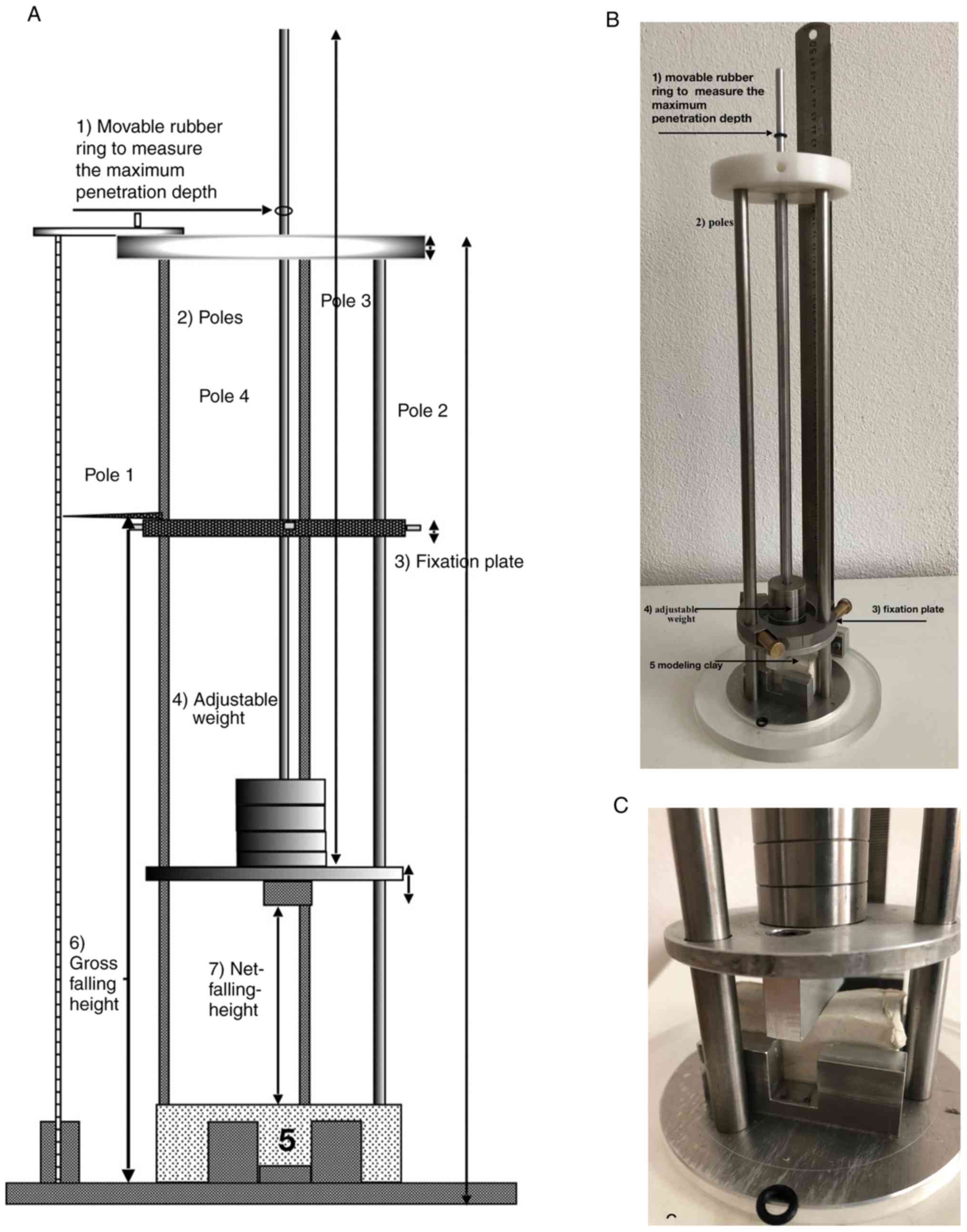
the dummies or mouse cadavers. A schematic of the construction is
presented in Fig. 2A and an image
of the device is presented in Fig.
2B and C. The device was built
using different materials, including V2A steel (rods), aluminum
(ground plate), teflon (upper plate), acrylic glass (ring for
ground plate), rubber (rings) and brass (fixing plate and screws).
Further details may be requested from the corresponding author. The
device was constructed in several steps in order to avoid handling
and reproducibility problems. Dummies made of plasticine (300 for
development of the device and 15 used in the reported experiments)
were tested to reach the required reproducibility standard.
Standard drop heights were 100, 200 and 300 mm. The effective drop
heights were calculated using measurements of the top edge of the
plasticine blocks or mouse cadavers, respectively. Standard
formulas were used for the calculation of energy, velocity, impact
and deceleration. Recoil was measured using rings of rubber on top
of the rods. The rings indicate the difference between the maximum
depth of impression and the depth of impression after recoil from
the impact (Fig. 2C).
Test blocks
Test blocks (19.5x19.1x61.1 mm) were made of
plasticine (Pelikan Vertriebsgesellschaft mbH & Co. KG) using a
form made of acrylic glass and had a mean weight of 25±1 g
each.
Mouse cadavers
Mouse cadavers (9; female C57BL/6 mice; weight,
23-26 g; age, 12 weeks; Charles River Laboratories, Inc.) were
subjected to blunt chest trauma using the novel impact device by
dropping weights from different heights within 30 min post mortem
(at room temperature). While alive, the mice were housed in
Macrolon® cages on a 12-h day/night cycle with free
access to chow and water. The mice were euthanized using an
overdose of pentobarbital (intraabdominal injection; 800 mg per kg
body weight; death confirmed by absence of breathing, heartbeat and
corneal reflex) and the trauma was induced within 30-min
postmortem. After trauma induction, chest X-rays were performed
using a Bruker Skyscan (Bruker Corp.). Following imaging, the
chests of the cadavers was carefully opened to investigate the
chest organs. The cadavers were obtained following experiments that
will be presented separately and were from the same control group
(protocol no. 36/2015, Animal Ethical Committee of the Saarland,
Homburg/Saarbrücken, Germany).
Results
Test blocks
Energy, maximum velocity (Vmax), impact, depth of
impression and recoil of the plasticine blocks from the standard
drop heights of 100, 200 and 300 mm are presented in Table I. As expected, the plasticine
dummies exhibited no recoil.
 | Table IData for plasticine blocks. |
Table I
Data for plasticine blocks.
| Gross falling-height
(mm) | Effect. falling
height (mm) | Weight (g) | Recoil (mm) | Mean recoil (mm) | SD recoil (mm) | max.
penetration-depth (mm) | Mean max. penetration
depth (mm) | SD max. penetration
depth (mm) | Potential energy
(J) | Impact (Impulse) (kg
m/sec) | Max. velocity
(m/sec) |
|---|
| 100 | 45 | 241 | 0.45 | 0.37 | 0.045 | 2.03 | 1.85 | 0.137 | 1.06 | 0.23 | 0.94 |
| | 46 | | 0.35 | | | 1.91 | | | 1.09 | | 0.95 |
| | 45 | | | | | 1.74 | | | 1.07 | | 0.94 |
| | 46 | | | | | 1.69 | | | 1.09 | | 0.95 |
| | 46 | | | | | 1.88 | | | 1.09 | | 0.95 |
| 200 | 145 | 190 | 0.45 | 0.45 | 0,035 | 3.03 | 3.15 | 0.186 | 2.71 | 0.32 | 1.69 |
| | 146 | | 0.5 | | | 3.19 | | | 2.72 | | |
| | | | 0.4 | | | 3.23 | | | 2.72 | | |
| | | | 0.45 | | | 3.38 | | | 2.72 | | |
| | | | 0.45 | | | 2.9 | | | 2.7 | | |
| 300 | 246 | 139 | 0.5 | 0.49 | 0.022 | 3.39 | 3.51 | 0.180 | 3.35 | 0.3 | 2.2 |
| | 245 | | 0.5 | | | 3.43 | | | 3.33 | | |
| | 246 | | 0.45 | | | 3.53 | | | 3.35 | | |
| | 245 | | 0.5 | | | 3.4 | | | 3.34 | | |
| | 246 | | 0.5 | | | 3.82 | | | 3.34 | | |
Mouse cadavers
A standard falling height of 100 mm (effective or
net falling heights of 44-46 mm, Fig.
2A), was used for the mouse cadavers to achieve impact energies
from 0.13 to 0.23 kg m/sec using falling weights of 138, 190 and
241 g. Impact (or impulse) and other data, as well as the various
depths of impression are listed in Table II. The impact ranged from 0.13 to
0.23 kg m/sec and depths of impression were between 12.4 and 14.7
mm. Mouse cadaver recoils were between 0.6 and 1 mm. In the cadaver
experiments, no fractures or dislocations of the ribs were observed
at any drop height, as shown in representative X-rays (Fig. 3A). By contrast, soft-tissue injuries
were observed with varying characteristics: A net falling height of
45 mm and a drop weight of 241 g resulted in massive hemorrhage
within the chest cavity. Furthermore, perforation/rupture of the
heart right atrium was observed (Fig.
3B and C). Hemorrhage was also
observed in the lung lobes. Following impact with an effective or
net falling height (from lowest part of the falling weight to the
highest part of the animal thorax) of 44 mm (gross falling height
100 mm, for details, Fig. 2A) and a
drop weight of 139 g, the heart was normal but the lower lobe of
the right lung exhibited a cleft (Fig.
3D); however, another cadaver (44 mm/139 g) had no cleft or
hemorrhage after the impact, but contusions/impressions from the
ribs on the left and right lungs were observed (Fig. 3E and F). After an impact of medium force (48
mm/191 g), one of the three mice in this experimental group
exhibited a hemothorax and rupture of the right atrium, whereas the
other two mice displayed no ruptures or clefts (images not shown),
whereas one of them had contusions from the ribs in the right
lung(images not shown but similar to those presented in Fig. 3F and E). None of the mice exhibited any visible
injuries to abdominal organs.
 | Table IIData for mouse cadavers. |
Table II
Data for mouse cadavers.
| Effective
falling-height (mm) | Mouse weight (g) | Falling weight
(g) | Recoil (mm) | Mean recoil (mm) | SD recoil (mm) | max.
penetration-depth (mm) | Mean max. penetration
depth (mm) | SD max. penetration
depth (mm) | Potential energy
(J) | Impact (Impulse) (kg
m/sec) | Max. velocity
(m/sec) | Figure no. |
|---|
| 44 | 25 | 138 | 1.2 | 0.97 | 0.21 | 14.38 | 13.93 | 1.05 | 0.60 | 0.13 | 0.93 | 3D |
| 44 | 23 | | 0.8 | | | 12.73 | | | 0.61 | | | |
| 44 | 25 | | 0.9 | | | 14.69 | | | 0.60 | | | 3EF |
| 44 | 24 | 190 | 1.3 | 0.98 | 0.33 | 13.67 | 13.53 | 0.68 | 0.83 | 0.18 | 0.93 | |
| 44 | 27 | | 1.0 | | | 14.13 | | | 0.83 | | 0.93 | |
| 45 | 23 | | 0.65 | | | 12.8 | | | 0.85 | | 0.94 | |
| 46 | 26 | 241 | 0.5 | 0.62 | 0.10 | 12.42 | 13.44 | 0.91 | 1.09 | 0.23 | 0.95 | |
| 44 | 25 | | 0.65 | | | 14.19 | | | 1.05 | 0.22 | 0.93 | |
| 45 | 24 | | 0.7 | | | 13.70 | | | 1.06 | 0.23 | 0.94 | 3A-C |
Discussion
In the present study, a novel device for the
induction of blunt chest traumata in mice was developed with the
aim of improving measurement of maximal penetration depth and
recoil variable energies in comparison to use of blast wave
generator. In the present mouse model, no bone fractures were
observed, even after large impacts. Following impact, potentially
lethal injuries were observed, including rupture of the right
atrium and the consecutive hemothorax. In addition, a cleft in a
lung lobe would, in most cases, lead to death. To the best of our
knowledge, rib-induced impressions on or contusions of the lungs
may be compatible with acute survival but may also cause lung
hemorrhage and lung edema, leading to death hours or days
post-impact.
A noteworthy limitation of the present study is the
lack of comparability between live animals and cadavers. However,
the cadaver study yielded useful results. For instance, the mouse
cadavers in the present study subjected to impact with an energy of
~1 J incurred severe lesions, which would have likely been fatal in
live animals. Data from a previous study using living rats
demonstrated that sustaining a dropping weight impact of 2.7 J
resulted in a mortality rate of 33% (6).
The present study concluded that an impact with an
energy of 0.5 J would induce a severe, but probably not lethal,
thorax trauma in mice. This would occur in the case of a drop
height of 100 mm (~44 mm effectively) and a drop weight of ~110 g.
However, to the best of our knowledge, no previous study has
demonstrated the lethality of an impact with an energy of 0.5 J in
live mice. The data obtained in the present study provided a
further interesting observation, namely with regard to the maximum
penetration depth and recoil. There was no difference in the
maximum penetration depth of the lightest and the heaviest drop
weight in the cadaver experiments. This suggests that a threshold
value or maximum of penetration depth had been reached. By
contrast, the recoil tended to be reduced with heavier drop weights
and would likely be running to zero with very heavy weights. The
recoil forces of the animals tissues (skeleton, muscles, organs)
are limited. Since a significant impact leads to a contusion of the
organs underneath, the severity of the contusion of heart, lung and
vessels is a complex result of the following: i) The impact energy
and, ii) the maximum penetration depth. When comparing different
trauma-inducing models the impact energy and the maximum
penetration depth are both important in the view of the
authors.
Another factor that should be taken into
consideration is the shape of the impact block. If a rounded or
bullet-like block is used instead of a plain block, this could
influence the severity of organ lesions. The present study
simulated the frontal impact such as that which would be
experienced by a human struck by a car. To simulate a side impact
of a vehicle on a human, the animal may be easily turned to the
right side or the left side. For further translational research, it
may be necessary to adapt this device for sheep, pigs or, in the
case of cadaver research, even in humans. However, any device for
the induction of experimental thoracic trauma and injury will only
ever have a model character, as motor vehicle collisions or
injuries incurred in other situations are always complex and
unique. The presented model may be of use in establishing thoracic
traumata for the testing of potential treatments for such trauma.
Several drugs are potentially applicable for the treatment of
severe but not fatal blunt chest traumata, including adrenomedullin
(7,8) and interleukin-6 antagonists (9), which are both suitable for the
prevention of secondary lesions, such as inflammation and
edema.
Overall, the device presented in the present study
is a useful option complementary to the blast wave generator
because maximal penetration depth and recoil can be measured and
the trauma energy can be adjusted to mortality.
Acknowledgements
The authors thank the following members of the
Department of Anatomy and Cell Biology (Saarland University, Campus
Homburg): Mr. Jörg Sauerbaum, Mr. Christoph Neuhardt and Mr. Ronald
Dollwett for building the device, Ms. Helga Meyer for providing the
materials and Ms. Ann Söther for language editing.
Funding
No funding was received for the study itself, but
Open Access Publishing was supported by the DFG and the Saarland
University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DH, MWL, NTV, RK and TT developed the device. TT, TP
and RK conducted the study. DH and CK performed the experiments. DH
wrote the draft and all authors worked on the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Mouse cadavers were taken from terminated
experiments (protocol no. 36/2015, Animal Ethical Committee of the
Saarland, Homburg/Saarbrücken, Germany).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eghbalzadeh K, Sabashnikov A, Zeriouh M,
Choi YH, Bunck AC, Mader N and Wahlers T: Blunt chest trauma: A
clinical chameleon. Heart. 104:719–724. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jaffin JH, McKinney L, Kinney RC,
Cunningham JA, Moritz DM, Kraimer JM, Graeber GM, Moe JB, Salander
JM and Harmon JW: A laboratory model for studying blast
overpressure injury. J Trauma. 27:349–356. 1987.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hafner S, Wagner K, Wepler M, Matallo J,
Gröger M, McCook O, Scheuerle A, Huber-Lang M, Frick M, Weber S, et
al: Physiological and immune-biological characterization of a
long-term murine model of blunt chest trauma. Shock. 43:140–147.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fitschen-Oestern S, Lippross S, Klueter T,
Weuster M, Varoga D, Tohidnezhad M, Pufe T, Rose-John S, Andruszkow
H, Hildebrand F, et al: A new multiple trauma model of the mouse.
BMC Musculoskelet Disord. 18(468)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Neunaber C, Oestern S, Andruszkow H,
Zeckey C, Mommsen P, Kutter D, Stöfen M, Krettek C and Hildebrand
F: Cytokine productive capacity of alveolar macrophages and Kupffer
cells after femoral fracture and blunt chest trauma in a murine
trauma model. Immunol Lett. 152:159–166. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Raghavendran K, Davidson BA, Helinski JD,
Marschke CJ, Manderscheid P, Woytash JA, Notter RH and Knight PR: A
rat model for isolated bilateral lung contusion from blunt chest
trauma. Anesth Analg. 101:1482–1489. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Muller HC, Witzenrath M, Tschernig T,
Gutbier B, Hippenstiel S, Santel A, Suttorp N and Rosseau S:
Adrenomedullin attenuates ventilator-induced lung injury in mice.
Thorax. 65:1077–1084. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Müller-Redetzky HC, Will D, Hellwig K,
Kummer W, Tschernig T, Pfeil U, Paddenberg R, Menger MD, Kershaw O,
Gruber AD, et al: Mechanical ventilation drives pneumococcal
pneumonia into lung injury and sepsis in mice: Protection by
adrenomedullin. Crit Care. 18(R73)2014.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Kang S, Tanaka T and Kishimoto T:
Therapeutic uses of anti-interleukin-6 receptor antibody. Int
Immunol. 27:21–29. 2015.PubMed/NCBI View Article : Google Scholar
|