Introduction
As pancreatic carcinoma (PC) is a rapidly
progressive, fatal malignant tumor, patients with PC have been
found to exhibit the poorest prognosis among all major carcinoma
types (1). PC is the fourth leading
cause of cancer-associated mortality with ~48,960 new cases and
~40,560 deaths estimated in 2015 (2,3) and it
has been projected to be the second leading cause of death in the
USA by 2030(4). Considerable
progress has been made in the diagnosis and treatment of PC in
recent decades; however, no significant improvement in its high
mortality rate has been observed, with a 5-year survival rate of
only ~8% in patients with PC (5).
Poor patient prognosis has been primarily attributed to the
advanced stage at which the disease is diagnosed, with the majority
of patients developing locally advanced and distant metastases and
succumbing to cancer metastasis (6-8).
Autopsy studies have revealed that ~90% of PC cases are complicated
by distant metastases (9), which
have also been associated with ~90% of cancer-related mortality
(10). Therefore, the in-depth
study of molecular interactions during the tumorigenesis and
progression of PC may aid in the identification of therapeutic
targets and diagnostic strategies.
MicroRNAs (miRNAs/miRs) are small non-protein coding
RNAs that have been associated with tumorigenesis, cell cycle
regulation, proliferation, apoptosis, invasiveness, metastasis and
chemoresistance in multiple cancers, including PC (11). miRNAs modulate post-transcriptional
gene expression by binding to the complementary regions in the
3'-untranslated (3'-UTR) region of their targets, and subsequently
inducing degradation or inhibition of the translation of the target
RNA (12). In the past decade,
various miRNAs have been associated with numerous types of human
cancer. For example, miR-373 has been found to enhance the
proliferative and metastatic abilities of oral squamous cell
carcinoma (13), while miR-146b-5p
has been revealed to inhibit the progression of non-small cell lung
carcinoma (14) and miR-495 has
been reported to suppress the biological activities of gastric
cancer cells by targeting Twist family bHLH transcription factor
1(15). Certain miRNAs, including
miR-381, miR-340 and miR-359, have also been associated with the
progression of PC (16-18).
Several studies have confirmed that the aberrant expression of
miR-23a in numerous human malignancies. miR-23a expression has been
previously found to be decreased in acute erythroid leukemia
(19), whilst it was revealed
increased in prostate cancer (20)
and osteosarcoma (21).
Furthermore, miRNA microarray analysis revealed that miR-23a
expression was increased in PC compared with that in normal
pancreatic tissues of patients (22). Additional studies have revealed that
miR-23a modulated the biological functions of PC via targeting
specific genes. For example, miR-23a has been found to promote the
development of a tumor by targeting forkhead box P2 (FOXP2) gene in
ductal adenocarcinoma (23).
Furthermore, miR-23a has been revealed to promote PC cell
proliferation by directly targeting apoptotic peptidase-activating
factor 1 (APAF1) (24) and enhance
the metastatic ability of PC cells by targeting epithelial splicing
regulatory protein 1(12).
Tissue factor pathway inhibitor (TFPI)-2, which is
also known as placental protein, has been identified as a key
molecule in angiogenesis, intravascular fibrinolysis, plasmin
transfer, tumor invasion and trypsin-induced activation of matrix
metalloproteinase zymogens (25).
Previous studies have revealed that downregulated TFPI-2 and
upregulated matrix metalloproteinase-2 protein expression was
associated with angiogenesis, lymph node metastasis, perineural
invasion and early postoperative recurrence of patients with PC
(26,27). In the current study, a critical
function of miR-23a in PC progression was identified, namely that
miR-23a promoted PC progression by negatively targeting TFPI-2. In
summary and to the best of our knowledge, this is the first study
to report this function of miR-23a, which may provide novel
strategies for the diagnosis and treatment of PC.
Materials and methods
Cell lines and culture
The hTERT-HPNE normal pancreatic ductal epithelial
cell line and Panc-1, MiaPaCa2 and Aspc-1 PC cell lines were
obtained from American Type Culture Collection. All cell lines were
cultured in DMEM (PAN-Biotech GmbH) supplemented with 10% FBS
(Invitrogen; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37˚C in a humidified incubator with 5% CO2.
Transfection
miR-23a mimics, negative control (NC) mimics,
miR-23a inhibitor, NC inhibitor, small interfering RNA against
TFPI-2 (si-TFPI-2) and the corresponding NC (TFPI-2 NC) were
purchased from Shanghai GenePharma Co., Ltd. Panc-1 and MiaPaCa2
cells were transfected with the appropriate constructs using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
transfection concentrations of the miR-23a mimics and inhibitor
were 50 and 100 nmol/l, respectively, and the concentrations of
si-TFPI-2 and TFPI-2 NC were 50 nmol/l. At 48 h post-transfection,
cells were collected for subsequent experiments. The sequences were
as follows: miR-23a mimics, 5'-AUCACAUUGCCAGGGAUUUCC-3'; miR-23a
inhibitor, 5'-GGAAAUCCCUGGCAAUGUGAU-3'; mimics NC,
5'-CGUAAGGCAAUCAAUGCCCUU-3'; inhibitor NC,
5'-AAUCUGAUACAUAUUGAGACC-3'; si-TFPI-2, 5'-GCCAAUGUGACUCGCUAUUAT-3'
and TFPI-2 NC, 5'-AAUCCUACUGACUUAUCGCGU-3'.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from Panc-1 and MiaPaCa2
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. RNA
purity was determined using a DU 800 UV/Visible Spectrophotometer
(Beckman Coulter, Inc.), and RNA was reverse transcribed into cDNA
using ReverTra Ace-α® kit (Toyobo Life Science)
according to manufacturer's instructions, using the following
temperature protocol: 16˚C for 30 min, 42˚C for 30 min and 85˚C for
5 min. qPCR was performed using the SYBR Green™
Real-Time PCR Master Mix (Toyobo Life Science) on an ABI 7900
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific
Inc.). U6 and GAPDH were used to normalize the relative expression
of miR-23a and TFPI-2 mRNA, respectively. qPCR was conducted in a
thermocycler using the following conditions: 94˚C for 4 min,
followed by 40 cycles of 94˚C for 30 sec, 56˚C for 30 sec and 72˚C
for 25 sec. The 2-∆∆Cq method
(28) was used to calculate the
relative expression levels of miRNA or mRNA. The associated primers
are presented in Table I.
 | Table IPrimer sequences used for reverse
transcription-quantitative PCR. |
Table I
Primer sequences used for reverse
transcription-quantitative PCR.
| Primer name | Primer sequences
(5'-3') |
|---|
| TFPI-2, F |
GAATTCTATGGACCCCGCTCGCCCC |
| TFPI-2, R |
AGTCGACTTAAAATTGCTTCTTCCG |
| microRNA-23a,
F |
CAGGCGGGTAGTAGATG |
| microRNA-23a,
R |
AGGGACGGGCATGGAAAGG |
| U6 small nuclear
RNA, F |
TCGTCTATCGCAGCACATAGTCG |
| U6 small nuclear
RNA, R |
GCGATTCACGAATTTGCCCGAC |
| GAPDH, F |
CGACCAGCCGACGGGTGCAG |
| GAPDH, R |
AGCTCGCCTACACCGAACGT |
Cell viability assay
MTT assay was used to determine the viability of
Panc-1 cells following miR-23a knockdown and MiaPaCa2 cells
following miR-23a overexpression. The cells were seeded into
96-well plates at a density of 5x103 cells/well. Panc-1
and MiaPaCa2 cells were subsequently transfected with miR-23a
inhibitor, NC inhibitor, miR-23a mimics or NC mimics using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Panc-1 and MiaPaCa2
cells were treated with or without Lipofectamine to observe the
effects of Lipofectamine on cell viability. Panc-1 cells were
treated with Lipofectamine and NC inhibitor in the NC group.
MiaPaCa2 cells were treated with Lipofectamine and NC mimics in the
NC group. MTT assays were conducted every 24 h for 3 days
post-transfection, the purple formazan was dissolved by adding DMSO
and the absorbance was determined at 570 nm using a microplate
reader (Synergy™ H4 Hybrid; BioTek Instruments,
Inc.).
Apoptosis assay
Panc-1 and MiaPaCa2 cells (1x106
cells/ml) were seeded into a 6-well plate and transfected as
aforementioned. The cells were washed with PBS at 24 h
post-transfection and subsequently stained with Annexin V-FITC and
propidium iodide (both Dojindo Molecular Technologies, Inc.),
according to the manufacturer's protocol. Apoptosis was evaluated
using a flow cytometer BD FACSCalibur™ (BD Biosciences) and BD
FACS™ software (v1.0.0.650; BD Biosciences).
Migration and invasion assays
Transwell plates (pore size, 8.0 µm) were used to
assess the invasive and migratory abilities of PC cells. For the
invasion assay, the membranes were pre-coated with Matrigel at 37˚C
for 4 h. Transwell plates and Matrigel were obtained from Corning
Life Sciences. At 48 h post-transfection with miR-23a inhibitor,
mimics or the corresponding NC, Panc-1 and MiaPaCa2 cells
(3x104 cells/well) were seeded into the upper chamber
with serum-free DMEM medium in a 24-well plate, respectively, while
the lower chamber was supplemented with medium containing 10% FBS.
The plates were incubated for 24 (migration assay) and 48 h
(invasion assay) at 37˚C. The cells on the upper membrane were
removed, and the invading cells on the lower membrane were fixed
with 4% formaldehyde for 30 min at room temperature and stained
with 0.2% crystal violet for 20 min, at room temperature. The cells
in five random fields were counted using an inverted light
microscope (magnification, x200; Olympus Corporation).
Wound healing assay
Panc-1 and MiaPaCa2 cells (2x106
cells/well) were cultured in a six-well plate until ~100%
confluence was achieved. The cell monolayers were scratched using a
200-µl sterile pipette tip and then washed with PBS. The cells were
subsequently cultured in serum-free DMEM and images were captured
at 0 and 24 h time points, using an inverted light microscope
(magnification, x200).
Target prediction
To investigate the association between miR-23a and
TFPI-2, TargetScan (version 7.2; http://www.targetscan.org/vert_72/) and miRanda
(august 2010; https://microrna.org) online
databases were used to predict the binding sites of miR-23a within
the 3'-UTR of TFPI-2, according to the manufacturer's protocol.
Plasmid construction
TFPI-2 wild-type (TFPI-2-WT) or mutant (TFPI-2-MUT)
reporter plasmids were constructed by synthesizing the putative
miR-23a-WT target binding sequences within TFPI-2 and cloning these
into a luciferase reporter plasmid. The overlap extension PCR
method (29) was performed to
obtain the TFPI-2-MUT 3'-UTR sequence. Subsequently, MUT and WT
target sequences were inserted into the psiCHECK-2 vector, and
Sanger sequencing was performed by Sangon Biotech Co., Ltd. to
confirm the sequences. All vectors were purchased from Promega
Corporation.
Dual-luciferase reporter assays
Panc-1 cells were seeded into a 24-well plate
(1x105 cells/well) and then co-transfected with 100 nM
TFPI-2-WT or TFPI-2-MUT plasmids, with 50 nM miR-23a or NC mimics
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. Cells were
harvested after 48 h, and Dual-Luciferase® Reporter
Assay system (Promega Corporation) was used to compare firefly
luciferase and Renilla luciferase activities.
Western blot analysis
Total protein extraction from Panc-1 and MiaPaCa2
cells was performed using RIPA Buffer (Beyotime Institute of
Biotechnology) according to the manufacturer's instructions. The
protein concentrations were quantified using a bicinchoninic acid
assay (Beyotime Institute of Biotechnology). SDS-PAGE (10%) was
used to separate equal amounts of protein (30 µg/sample), which
were subsequently transferred to a PVDF membrane. The membranes
were blocked with 5% skimmed milk for 60 min at room temperature,
followed by incubation with anti-TFPI-2 antibody (dilution,
1:1,000; cat. no. ab86933; Abcam) or GAPDH (dilution, 1:1,000; cat.
no. 5174S; CST Biological Reagents Co., Ltd.), which was used as
the internal control, overnight at 4˚C. The membranes were
subsequently washed with TBS-Tween-20 buffer and incubated with the
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (dilution, 1:5,000; cat. no. LK2001; Sungene Biotech Co.,
Ltd.) at 20˚C for 60 min. The protein bands were visualized using
an ECL kit [Hangzhou Multi Sciences (Lianke) Biotech Co., Ltd.] and
ImageJ software (v1.8.0; National Institutes of Health) was used
for densitometry analysis.
Statistical analysis
The data are presented as the mean ± standard
deviation and all experiments were performed three times in
triplicate. SPSS v25.0 software (IBM Corp.) was used to analyze the
experimental data. Statistical differences were analyzed using
unpaired Student's t-test (for two groups) or one-way ANOVA
followed by Tukey's or Dunnett's post hoc test (for ≥3 groups).
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-23a expression is higher in PC
cells compared with that in normal pancreatic cells
To determine whether miR-23a was associated with the
development of PC, RT-qPCR was performed to quantify the mRNA
expression levels of miR-23a in three PC cell lines (Panc-1,
MiaPaCa2 and Aspc-1) and a normal pancreatic ductal epithelial cell
line (hTERT-HPNE). Compared with that in the control, the results
indicated that miR-23a expression level was significantly increased
in the PC cell lines (Fig. 1). In
the three PC cell lines, the expression level of miR-23a in Panc-1
was relatively high, whilst that in MiaPaCa2 was relatively low.
Panc-1 and MiaPaCa2 cells were therefore subsequently selected for
further experimentation.
miR-23a promotes PC cell viability and
inhibits apoptosis
To delineate the functions of miR-23a in PC
progression, miR-23a mimics and inhibitor (and the respective NCs)
were used to determine the biological effects of miR-23a in Panc-1
and MiaPaCa2 cells. RT-qPCR was performed to assess the
transfection efficiency of miR-23a mimics and inhibitor and the
results revealed that miR-23a expression was increased in Panc-1
cells following transfection with miR-23a mimics (Fig. 2A), while it was downregulated
following transfection with miR-23a inhibitor (Fig. 2B). Similar results were obtained in
MiaPaCa2 cells transfected with miR-23a mimics or inhibitor
(Fig. 2C and D, respectively). Subsequently, the
viability of Panc-1 cells following miR-23a knockdown and MiaPaCa2
cells followingmiR-23a overexpression were determined using MTT
assay. As presented in Fig. 2E and
F, miR-23a inhibition significantly
decreased Panc-1 cell viability compared with that in the
respective NC, whilst MiaPaCa2 viability was significantly
increased by miR-23a overexpression. In addition, the flow
cytometry data revealed that compared with that in the respective
NC, the apoptotic rate of Panc-1 cells was significantly increased
following transfection with miR-23a inhibitor, while that of
MiaPaCa2 cells was significantly decreased following transfection
with miR-23a mimics (Fig. 2G-I).
These findings indicated that miR-23a promoted PC cell viability
and inhibited apoptosis.
miR-23a inhibition suppresses PC cell
migration and invasion
To further investigate the functions of miR-23a in
PC metastasis, the migratory and invasive abilities of Panc-1 and
MiaPaCa2 cells were evaluated using Transwell assays or without
Matrigel pre-coating, respectively. The results indicated that
miR-23a inhibition significantly suppressed the migration of Panc-1
and MiaPaCa2 cells (Fig. 3A and
B). Furthermore, the invasive
ability of Panc-1 and MiaPaCa2 cells was also inhibited following
transfection with miR-23a inhibitor, compared with that in cells
transfected with NC (Fig. 3C and
D). In addition, a wound-healing
assay also confirmed the inhibitory effect of miR-23a inhibitor on
the migration of Panc-1 and MiaPaCa2 cells (Fig. 3E and F). Taken together, these results indicated
that miR-23a inhibition suppressed the migratory and invasive
abilities of PC cells.
miR-23a directly targets TFPI-2 and
negatively modulates the expression of TFPI-2
To determine the downstream targets of miR-23a,
which may serve crucial roles in the development of PC, the
TargetScan online tool predicted TFPI-2 to be the target gene of
miR-23a (Fig. 4A). To confirm this,
TFPI-2-WT and TFPI-2-MUT 3'-UTR plasmids were constructed and
co-transfected with miR-23a mimics or NC into Panc-1 cells, and the
reporter activities were subsequently evaluated using a
dual-luciferase reporter assay. As presented in Fig. 4B, miR-23a mimics significantly
inhibited the luciferase activity of TFPI-2-WT 3'-UTR in Panc-1
cells compared with that in the NC group; however, no significant
difference was observed in the TFPI-2-MUT group. Western blot and
RT-qPCR analyses were also performed and revealed that miR-23a
inhibition significantly increased the TFPI-2 protein (Fig. 4C and D) and mRNA (Fig. 4E) expression levels in Panc-1 cells,
compared with that in the control groups, and similar results were
observed in the MiaPaCa2 cell line (Fig. 4F-H). The present results indicated
that miR-23a directly targeted TFPI-2 and negatively modulated its
expression.
TFPI-2 knockdown rescues the effects
on PC cell apoptosis, viability, migration and invasion, which are
induced by miR-23a downregulation
Finally, rescue experiments were performed to
determine whether miR-23a affects the apoptosis, proliferation,
migratory and invasive abilities of PC cells by regulating the
expression level of TFPI-2. RT-qPCR was used to determine the
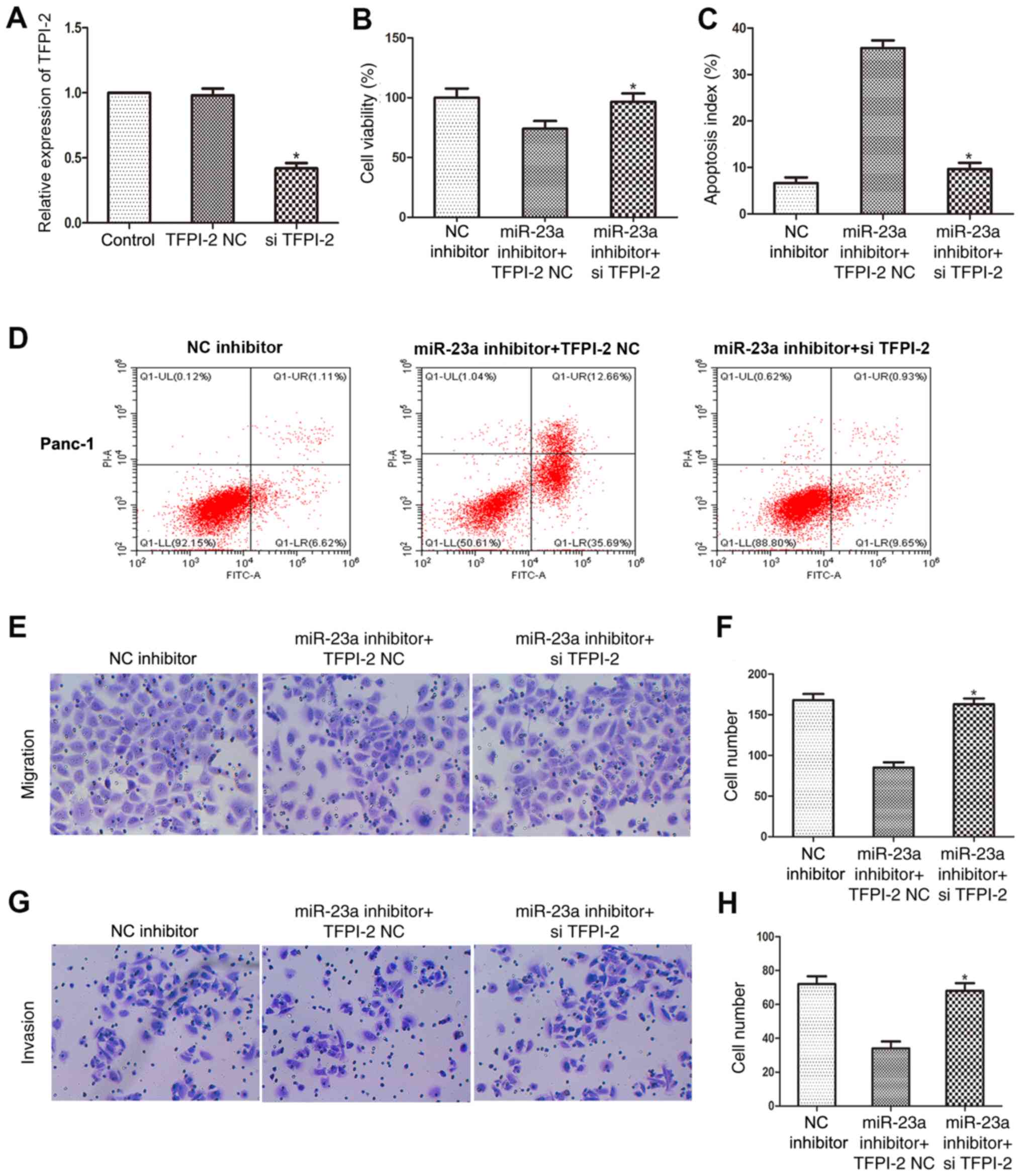
transfection efficiency of si-TFPI-2 and the results revealed that
TFPI-2 mRNA expression level was significantly downregulated in
Panc-1 cells following si-TFPI-2 transfection (Fig. 5A). An MTT assay indicated that
si-TFPI-2 restored the viability of Panc-1 cells transfected with
miR-23a inhibitor (Fig. 5B). In
addition, flow cytometry analysis found that si-TFPI-2 attenuated
the increase in Panc-1 cell apoptosis following transfection with
miR-23a inhibitor (Fig. 5C and
D). Furthermore, the migratory
(Fig. 5E and F) and invasive (Fig. 5G and H) abilities of Panc-1 cells transfected
with miR-23a inhibitor were restored by TFPI-2 knockdown, as
determined using Transwell and Matrigel assays. These data provided
additional evidence that miR-23a inhibited apoptosis and promoted
the proliferation, migration and invasiveness of PC cells by
targeting TFPI-2.
Discussion
Previous studies on different types of cancer,
including non-small cell lung carcinoma, liver cancer and glioma,
have revealed that miRNAs may regulate the migratory and invasive
ability of cells by targeting specific genes (30-32).
miR-499a has been shown to target ADAM metallopeptidase domain 10
in non-small cell lung carcinoma, whilst miR-99a has been
previously demonstrated to targets homeobox A1 in liver cancer and
miR-374b targets epidermal growth factor receptor in glioma
(30-32).
miR-23a has been reported to be a critical regulator of
tumorigenesis and serve distinct roles in different types of human
cancer. For example, several studies have reported that miR-23a
promoted the progression of a variety of cancers, including gastric
cancer, colorectal and liver carcinoma (33-35).
By contrast, miR-23a was found to serve as a cancer suppressor in
melanoma and osteosarcoma, as the overexpression of miR-23a
inhibited osteosarcoma cell proliferation, migration and invasive
ability (36), and suppressed
melanoma cell proliferation, migration and invasion in mice
(37). The results of the current
study revealed that miR-23a mRNA expression level was upregulated
in PC cell lines (MiaPaCa2, Panc-1 and Aspc-1) compared with that
in a normal pancreatic ductal cell line (hTERT-HPNE), suggesting
that miR-23a may be associated with the tumorigenesis and
progression of PC. The results of the MTT assay also indicated that
miR-23a knockdown inhibited PC cell proliferation. Furthermore,
wound-healing, Transwell and Matrigel assays revealed that miR-23a
knockdown decreased PC cell migration and invasion, while flow
cytometry demonstrated the pro-apoptotic effects of miR-23a
knockdown. These data support those of a previous study (24), indicating that miR-23a acts as a
tumor-promoting factor in PC cells, and may be utilized as a
biomarker for PC diagnosis and treatment.
Extracellular matrix (ECM) proteolysis has been
revealed to serve a key role in tumor metastasis (38,39),
therefore protecting the structural integrity of the ECM may be an
important aspect to inhibit cancer cell metastasis. TFPI-2 has been
considered as an effective cancer suppressor, which maintains ECM
structural integrity in various types of cancer, including breast
cancer, amelanotic melanoma and lung cancer (40-42).
A previous study revealed that TFPI-2 protein expression was
decreased in PC compared with that in normal pancreatic tissues,
and that overexpression of TFPI-2 prevented a PC malignant
phenotype both in vitro and in vivo (25). Several studies have reported that
TFPI-2 is regulated by miRNAs. For example, TFPI-2 expression was
found to be negatively regulated by miR-616, thereby inducing the
androgen-dependent proliferation of PC cells (43). Furthermore, miR-494 has been
demonstrated to indirectly upregulate TFPI-2 by modulating the
transcription factors, aryl hydrocarbon receptor and ETS-related
transcription factor Elf-1 in breast cancer cells (44). However, in-depth studies on TFPI-2
have been rarely reported in PC, and the upstream and downstream
regulators of this gene remain largely unknown, to the best of our
knowledge. miR-23a has been reported to affect the progression of
PC by regulating specific genes. For example, miR-23a was found to
inhibit PC development by negatively regulating
serine/threonine-protein kinase PLK1(45), and miR-23a overexpression was found
to promote PC progression by suppressing FOXP2 mRNA expression
levels (23). miR-23a has also been
reported to act as an oncogene in PC via downregulating
APAF1(24).
Numerous studies have demonstrated the important
role of miR-23a in PC tumorigenesis; however, other potential
molecular mechanisms such as the role of miR-23a in ECM proteolysis
and the detailed function of miR-23a, remain unclear. In the
present study, bioinformatics analysis was used to predict the
interaction between miR-23a and TFPI-2, and this interaction was
confirmed using a luciferase reporter assay. Upregulation of TFPI-2
expression was also evaluated in PC cells following miR-23a
knockdown. The results indicated that miR-23a knockdown increased
the protein and mRNA expression level of TFPI-2, and that miR-23a
may be involved in PC progression by negatively modulating TFPI-2
expression. Furthermore, rescue experiments demonstrated the
interdependent relationship between miR-23a and TFPI-2 in PC cells.
These results confirmed that downregulating TFPI-2 rescued the
effects on PC cell apoptosis, proliferation, migration and
invasion, which were induced by the inhibition of miR-23a.
There are also limitations in the present study. For
example, the endogenous mRNA expression levels of miR-23a in human
or animal tissues were not examined. In addition, functional
studies investigating the inhibition of miR-23a indicated the role
of miR-23a in inducing cell migration and invasion; however,
functional studies using miR-23a mimics are missing.
In summary, the results of the present study
indicated that by directly targeting TFPI-2, miR-23a exerted an
oncogenic effect in PC cells, promoting cell proliferation,
migration and invasiveness and suppressing apoptosis. The
aforementioned results suggested that miR-23a may potentially be
utilized as a useful target and/or biomarker for the diagnosis and
treatment of PC.
Acknowledgements
The authors would like to thank Dr Hongmin Sun
(School of Public Health, Anhui Medical University; Hefei, China)
for his assistance in statistical analysis.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WW and JZN were responsible for conception and
design of the study, data collection and analysis and contributed
to writing the manuscript. ZGT designed the study, performed
critical revision and supervised all phases of the study. YH and
LCY performed the invasion and migration assays and analyzed the
data. LY and LW performed the cell viability and apoptosis assays
and analyzed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Klein AP: Identifying people at a high
risk of developing pancreatic cancer. Nat Rev Cancer. 13:66–74.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016.
|
|
3
|
Moutinho-Ribeiro P, Coelho R, Giovannini M
and Macedo G: Pancreatic cancer screening: Still a delusion?
Pancreatology. 17:754–765. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Talmadge JE and Fidler IJ: AACR centennial
series: The biology of cancer metastasis: Historical perspective.
Cancer Res. 70:5649–5669. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pang EJ, Yang R, Fu XB and Liu YF:
Overexpression of long non-coding RNA MALAT1 is correlated with
clinical progression and unfavorable prognosis in pancreatic
cancer. Tumor Biol. 36:2403–2407. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li Y, Vandenboom TG II, Wang Z, Kong D,
Ali S, Philip PA and Sarkar FH: miR-146a suppresses invasion of
pancreatic cancer cells. Cancer Res. 70:1486–1495. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kamisawa T, Isawa T, Koike M, Tsuruta K
and Okamoto A: Hematogenous metastases of pancreatic ductal
carcinoma. Pancreas. 11:345–349. 1995.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wu G, Li ZH, Jiang P, Zhang X, Xu YQ, Chen
K and Li XW: MicroRNA-23a promotes pancreatic cancer metastasis by
targeting epithelial splicing regulator protein 1. Oncotarget.
8:82854–82871. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang XJ, Jin Y, Song JL and Deng F:
MiR-373 promotes proliferation and metastasis of oral squamous cell
carcinoma by targeting SPOP. Eur Rev Med Pharmacol Sci.
23:5270–5276. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li Y, Zhang H, Dong Y, Fan Y, Li Y, Zhao
C, Wang C, Liu J, Li X, Dong M, et al: MiR-146b-5p functions as a
suppressor miRNA and prognosis predictor in non-small cell lung
cancer. J Cancer. 8:1704–1716. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu C, Jian M, Qi H and Mao WZ:
MicroRNA-495 inhibits proliferation and metastasis and promotes
apoptosis by targeting twist1 in gastric cancer cells. Oncol Res.
27:389–397. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Qiao G, Li J, Wang J, Wang Z and Bian W:
miR-381 functions as a tumor suppressor by targeting ETS1 in
pancreatic cancer. Int J Mol Med. 44:593–607. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhou Y, Li Z, Ding Y, Zhang P and Wang J:
MicroRNA-340 suppresses pancreatic cancer growth by targeting
BICD2. Pancreatology. 19:30556–30563. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yu H, Gao G, Cai J, Song H, Ma Z, Jin X,
Ji W and Pan B: MiR-539 functions as a tumor suppressor in
pancreatic cancer by targeting TWIST1. Exp Mol Pathol. 108:143–149.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Su R, Dong L, Zou D, Zhao H, Ren Y, Li F,
Yi P, Li L, Zhu Y, Ma Y, et al: MicroRNA-23a, -27a and -24
synergistically regulate JAK1/Stat3 cascade and serve as novel
therapeutic targets in human acute erythroid leukemia. Oncogene.
35:6001–6014. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cai S, Chen R, Li X, Cai Y, Ye Z, Li S, Li
J, Huang H, Peng S, Wang J, et al: Downregulation of microRNA-23a
suppresses prostate cancer metastasis by targeting the PAK6-LIMK1
signaling pathway. Oncotarget. 6:3904–3917. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang G, Li B, Fu Y, He M, Wang J, Shen P
and Bai L: miR-23a suppresses proliferation of osteosarcoma cells
by targeting SATB1. Tumour Biol. 36:4715–4721. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bloomston M, Frankel WL, Petrocca F,
Volinia S, Alder H, Hagan JP, Liu CG, Bhatt D, Taccioli C and Croce
CM: MicroRNA expression patterns to differentiate pancreatic
adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA.
297:1901–1908. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Diao H, Ye Z and Qin R: miR-23a acts as an
oncogene in pancreatic carcinoma by targeting FOXP2. J Investig
Med. 66:676–683. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu N, Sun YY, Zhang XW, Chen S, Wang Y,
Zhang ZX, Song SW, Qiu GB and Fu WN: Oncogenic miR-23a in
pancreatic ductal adenocarcinogenesis via inhibiting APAF1. Dig Dis
Sci. 60:2000–2008. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tang Z, Geng G, Huang Q, Xu G, Hu H, Chen
J and Li J: Expression of tissue factor pathway inhibitor 2 in
human pancreatic carcinoma and its effect on tumor growth,
invasion, and migration in vitro and in vivo. J Surg Res.
167:62–69. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhai LL, Wu Y, Huang DW and Tang ZG:
Increased matrix metalloproteinase-2 and reduced tissue factor
pathway inhibitor-2 expression correlate with angiogenesis and
early postoperative recurrence of pancreatic carcinoma. Am J Transl
Res. 7:2412–2422. 2015.PubMed/NCBI
|
|
27
|
Zhai LL, Wu Y, Cai CY and Tang ZG:
Upregulated matrix metalloproteinase-2 and downregulated tissue
factor pathway inhibitor-2 are risk factors for lymph node
metastasis and perineural invasion in pancreatic carcinoma. Onco
Targets Ther. 8:2827–2834. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2012.
|
|
29
|
Hussain H and Chong NF: Combined overlap
extension PCR method for improved site directed mutagenesis. Biomed
Res Int. 2016(8041532)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Meng H, Huang Q, Zhang X, Huang J, Shen R
and Zhang B: MiR-449a regulates the cell migration and invasion of
human non-small cell lung carcinoma by targeting ADAM10. Onco
Targets Ther. 12:3829–3838. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tao C, Sun H, Sang W and Li S: miRNA-99a
inhibits cell invasion and migration in liver cancer by directly
targeting HOXA1. Oncol Lett. 17:5108–5114. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pan DS, Cao P, Li JJ, Fan D and Song ZQ:
MicroRNA-374b inhibits migration and invasion of glioma cells by
targeting EGFR. Eur Rev Med Pharmacol Sci. 23:4254–4263.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhu LH, Liu T, Tang H, Tian RQ, Su C, Liu
M and Li X: MicroRNA-23a promotes the growth of gastric
adenocarcinoma cell line MGC803 and downregulates interleukin-6
receptor. FEBS J. 277:3726–3734. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang Z, Wei W and Sarkar FH: miR-23a, a
critical regulator of ‘migR’ation and metastasis in colorectal
cancer. Cancer Discov. 2:489–491. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Huang S, He X, Ding J, Liang L, Zhao Y,
Zhang Z, Yao X, Pan Z, Zhang P, Li J, et al: Upregulation of
miR-23a approximately 27a approximately 24 decreases transforming
growth factor-beta-induced tumor-suppressive activities in human
hepatocellular carcinoma cells. Int J Cancer. 123:972–978.
2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
He Y, Meng C, Shao Z, Wang H and Yang S:
MiR-23a functions as a tumor suppressor in osteosarcoma. Cell
Physiol Biochem. 34:1485–1496. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lu S and Xu Q: MicroRNA-23a inhibits
melanoma cell proliferation, migration, and invasion in mice
through a negative feedback regulation of SDCBP and the MAPK/ERK
signaling pathway. IUBMB Life. 71:587–600. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Eble JA and Niland S: The extracellular
matrix in tumor progression and metastasis. Clin Exp Metastasis.
36:171–198. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chang TT, Thakar D and Weaver VM:
Force-dependent breaching of the basement membrane. Matrix Biol.
57-58:178–189. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang G, Zeng Y, Chen S, Li D, Li W, Zhou
Y, Singer RH and Gu W: Localization of TFPI-2 in the nucleus
modulates MMP-2 gene expression in breast cancer cells. Sci Rep.
7(13575)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Konduri SD, Tasiou A, Chandrasekar N,
Nicolson GL and Rao JS: Role of tissue factor pathway inhibitor-2
(TFPI-2) in amelanotic melanoma (C-32) invasion. Clin Exp
Metastasis. 18:303–308. 2000.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Iochmann S, Bléchet C, Chabot V, Saulnier
A, Amini A, Gaud G, Gruel Y and Reverdiau P: Transient RNA
silencing of tissue factor pathway inhibitor-2 modulates lung
cancer cell invasion. Clin Exp Metastasis. 26:457–467.
2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ma S, Chan YP, Kwan PS, Lee TK, Yan M,
Tang KH, Ling MT, Vielkind JR, Guan XY and Chan KW: MicroRNA-616
induces androgen-independent growth of prostate cancer cells by
suppressing expression of tissue factor pathway inhibitor TFPI-2.
Cancer Res. 71:583–592. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Andresen MS, Stavik B, Sletten M, Tinholt
M, Sandset PM, Iversen N and Skretting G: Indirect regulation of
TFPI-2 expression by miR-494 in breast cancer cells. Sci Rep.
10(4036)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chen B, Zhu A, Tian L, Xin Y, Liu X, Peng
Y, Zhang J, Miao Y and Wei J: miR 23a suppresses pancreatic cancer
cell progression by inhibiting PLK 1 expression. Mol Med Rep.
18:105–112. 2018.PubMed/NCBI View Article : Google Scholar
|