Introduction
Sepsis is a type of organ dysfunction that poses a
threat to human life and is induced by the imbalanced response of
the host to confirmed or suspected infection (1). Septic shock, which is a subtype of
sepsis, has been recognized as a specific process of sepsis
development (2). There are millions
of new sepsis patients every year worldwide. Among these patients,
~1/4 die (1,3). Sepsis is characterized by high cost of
medical treatment, high mortality and high morbidity, and it
frequently results in organ dysfunction. Specifically, the heart is
the organ frequently affected by sepsis. Cardiac dysfunction
induced by sepsis is the leading cause of death (4). Of note, cardiac dysfunction usually
occurs in sepsis patients, which gives rise to reduced systemic
perfusion of the organ and in turn enhances disease progression
(5,6). For patients who die due to septic
shock and severe sepsis, most succumb due to damage to the
cardiovascular system, and ~40% of patients who survive sepsis
develop myocardial damage (7).
Furthermore, these conditions worsen when cardiac dysfunction
occurs earlier, along with a higher death rate (8). Thus, it would be favorable to
accurately identify sepsis-induced cardiac dysfunction early for
improving the prognosis of these patients.
Transthoracic echocardiography (TTE) has become a
vital approach to evaluate the cardiac function of patients in the
clinic. In addition, it is the leading technique for the diagnosis
of cardiac insufficiency (9,10).
According to previous studies, a left ventricular ejection fraction
(LVEF) of <50% may be utilized to determine sepsis-induced
cardiac dysfunction (11,12). However, other investigators have
discovered that ~1/2 of patients with sepsis and cardiac
dysfunction exhibit a normal ejection fraction based on an LVEF of
≤50% (6). At present, N-terminal
pro-brain natriuretic peptide (NT-proBNP) is used as the ‘gold
standard’ for the diagnosis of heart failure (HF) in adults
(13). The serum level of NT-proBNP
is altered with changes in cardiac function. As suggested by
previous studies, for patients with sepsis, cardiac insufficiency
is a type of reversible functional alteration (14,15).
Thus, NT-proBNP may serve as an indicator for the differentiation
of patients with normal LVEF from patients with sepsis. However,
apart from cardiac function, elevated NT-proBNP levels with sepsis
are impacted by various factors in such patients. Sepsis-induced
cardiac dysfunction is generally diagnosed on the basis of clinical
signs, symptoms and assisted examination, combined with LVEF or
NT-proBNP. As a marker for myocardial damage, heart-type fatty
acid-binding protein (H-FABP) has recently become a research
hotspot. H-FABP is better than conventional cardiac markers for the
early diagnosis of acute myocardial damage due to higher
sensitivity and specificity (16,17).
Furthermore, H-FABP has a close correlation with the severity of
sepsis and may serve as a candidate cardiac marker for the
diagnosis of sepsis-induced cardiac dysfunction (17-19).
In the present study, TTE, as well as the dynamic changes of H-FABP
and NT-proBNP, were explored in patients with sepsis to determine
the precise time of the occurrence of sepsis-induced cardiac
dysfunction and the clinical significance of H-FABP for
diagnosis.
Materials and methods
Study subjects
Between October 2016 and February 2018, a total of
94 inpatients with sepsis aged ≥18 years were recruited from the
intensive care unit (ICU) of the First Affiliated Hospital of
Shihezi University (Shihezi, China) in accordance with Sepsis
3.0(20). Among these patients, 80
were finally enrolled in the present study as the sepsis group. A
total of 30 healthy volunteers who had normal physical examinations
were included as the control group. None of the patients screened
had arrhythmia, organic heart disease (HD), recent cardiovascular
or cerebrovascular diseases or chronic renal insufficiency. In
addition, none of the enrolled patients were hospitalized for <3
days for any different reasons. For these patients, organic HDs
referred to the following diseases: Ischemic cardiomyopathy, old
myocardial infarction (MI) and acute MI; primary cardiomyopathy
(including restrictive, hypertrophic and dilated cardiomyopathy);
metabolic cardiomyopathy (e.g., alcoholic, hyperthyroid, anemic and
uremic cardiomyopathy); congenital HD; valvular HD; and infective
endocarditis and myocarditis (Fig.
1).
Diagnostic standards
The diagnostic standards for sepsis were as follows:
i) Patients at the ICU with suspected or confirmed sepsis infection
with a Sequential Organ Failure Assessment (SOFA) score of ≥2
points were diagnosed as having sepsis (1) (Table
SI); and ii) in patients without suspected or confirmed ICU
infection, the quick SOFA score (including a systolic blood
pressure of ≤100 mmHg, an altered conscious state and a respiratory
rate of ≥22 beats/min) was assessed and sepsis was diagnosed when
the patient was positive for at least two items.
The diagnostic standards for cardiac dysfunction
were as follows: By an experienced clinician, cardiac insufficiency
was determined according to the imaging data and the signs and
clinical symptoms of patients combined with the LVEF or NT-proBNP
values. As recommended in the 2017 Guidelines for Heart Failure
Management by the American Heart Association (AHA), American
College of Cardiology (ACC) and Heart Failure Society Of America
(HFSA) (13), acute HF was
diagnosed in the case of NT-pro BNP >1,800 pg/ml (>75 years),
NT-pro BNP >900 pg/ml (50-75 years) and NT-pro BNP >450 pg/ml
(<50 years); acute HF was excluded when NT-pro BNP was <300
pg/ml; chronic HF was excluded when NT-pro BNP was <125 pg/ml;
and systemic dysfunction was suspected when LVEF was <50%.
According to the 2016 American Society of Echocardiography (ASE)
and the European Association of Cardiovascular Imaging (EACVI)
guidelines for diastolic function of the left ventricle examined by
echocardiography, an E/A of <0.8 was considered as an indicator
of diastolic dysfunction in the left ventricle (21).
Variables in the study
The baseline patient characteristics, including
gender and age, were recorded. At the same time, the 24 small-order
SOFA and the Acute Physiology and Chronic Health Evaluation II
(APACHE II) scores of patients with sepsis were determined upon
admission (22), according to the
results of the laboratory tests and the clinical symptoms. The SOFA
and APACHE II scores were recorded.
These sepsis patients were hospitalized at the
Department of Clinical Laboratory of the First Affiliated Hospital
of Shihezi University (Shihezi, China) for 1, 3, 7 and 10 days.
Healthy subjects were assigned to the control group and their
relevant information was collected on the day of the physical
examination. A CELL-DYN Ruby was utilized to measure the neutrophil
and white blood cell (WBC) percentages. Furthermore, the level of
creatine kinase-MB (CK-MB) was determined using the 2700 automatic
biochemical analyzer (Olympus Corporation), and the level of
cardiac troponin I (cTnI) was measured by the automatic enzyme
immunoassay device AIA-360 (Tosoh Corporation) using a fluorescence
magnetic microparticle enzyme technique. In addition, the NT-proBNP
and procalcitonin (PCT) contents were measured using the Roche
Cobas 8000 by electrochemiluminescence. Next, cubital venous
samples were collected at specific time-points after fasting. ELISA
was performed with the ELX800 microplate reader at the Key
Laboratory of Xinjiang Endemic and Ethnic Diseases (Shihezi,
China). H-FABP was measured using the H-FABP test kit (cat. no.
E-EL-H1431c; Elabscience), according to the manufacturer's
protocol. To obtain the results for the H-FABP test, the optical
density value at 450 nm was determined using the ELX800 microplate
reader (Bioelisa; Biokit). Finally, the serum levels of H-FABP were
acquired.
Heart ultrasound was performed using the Philips
IE33 (Philips Healthcare) by one experienced sonographer who was
trained at TTE, and the following indicators were recorded:
End-systolic volume (ESV), end-diastolic volume (EDV), LVEF,
cardiac output (CO), stroke volume (SV), mitral atrial systolic
peak velocity (A), mitral early diastolic peak velocity (E), E/A
ratio (E/A), diastolic pulmonary venous peak velocity (D), systolic
pulmonary venous peak velocity (S) and the S/D ratio (S/D).
Clinical evaluation
A total of 80 patients with sepsis were monitored
for a period of 28 days following enrollment. In the present study,
the endpoints were deemed survival and death, and these were
examined on days 1, 3, 7, 10 and 28 during the study process.
Statistical analysis
SPSS 20.0 (IBM Corp.) was utilized for all
statistical analyses. The measurement values are presented as the
mean ± standard deviation, while abnormally distributed values are
presented as the median (interquartile range). Normality
distribution of the variables was tested using the 1-sample
Kolmogorov-Smirnov test. Continuous variables were compared using
Student's t-test or the Mann-Whitney U test between groups,
depending on distribution. Categorical variables were analyzed
using the χ2 test. The data were compared between two
groups and a t-test was performed in the presence of homogeneous
variance in the measurement data, while the Wilcoxon rank-sum test
was utilized in the case of heterogeneous variance. For data with
heterogeneous variance, the Kruskal-Wallis test was used for
comparison of multiple groups, followed by Dunn's post-hoc test,
while the Mann-Whitney U-test was used for comparison of two
groups. Categorical data were examined by the χ2 test.
Repeated-measures analysis of variance was employed to analyze the
repeated measurement data. Spearman's rank correlation was applied
in the correlation analysis. Furthermore, binary logistic
regression was used to assess cardiac dysfunction and determine the
28-day death rate. At the same time, the best threshold was
confirmed using receiver operating characteristic (ROC) curve
analysis. P<0.05 was considered to indicate statistical
significance.
Results
General patient features
There were no significant differences in age or
gender between the control group and the sepsis group. The APACHE
II scores of patients in the sepsis group were significantly higher
than those in the control group (Table
I).
 | Table IGeneral characteristics of all
patients of the present study. |
Table I
General characteristics of all
patients of the present study.
| Variable | Control (n=30) | Sepsis (n=80) |
t/χ2 | P-value |
|---|
| Sex
(male/female) | 14/16 | 51/29 | 3.738 | 0.053 |
| Age (years) | 65.90±12.17 | 71.11±14.24 | 1.775 | 0.079 |
| APACHEII score | 4.00±1.53 | 15.19±5.95 | 10.123 | <0.001 |
| SOFA score | - | 5.54±3.62 | - | - |
The sepsis group was further divided into two groups
based on the presence or absence of cardiac insufficiency: The
sepsis without cardiac dysfunction group and the sepsis with
cardiac dysfunction group. The common features, including age and
gender, were not statistically significant between these two
groups. In addition, compared with patients with sepsis and normal
heart function, the 28-day death rate and APACHE II score were
increased in patients with sepsis and cardiac dysfunction, but
there was no statistically significant difference (P>0.05).
Compared with patients with no cardiac dysfunction, the SOFA score
increased among patients with sepsis-induced cardiac dysfunction
(P=0.037). However, the difference in infection source was not
statistically significant between these two groups (P=0.384;
Table II).
 | Table IIGeneral characteristics of patients
with sepsis by cardiac dysfunction in the present study. |
Table II
General characteristics of patients
with sepsis by cardiac dysfunction in the present study.
| Variables | Sepsis with cardiac
dysfunction (n=50) | Sepsis without
cardiac dysfunction (n=30) |
t/χ2 | P-value |
|---|
| Age (years) | 71.52±15.496 | 70.43±12.091 | 0.328 | 0.743 |
| Sex
(male/female) | 29/21 | 8/22 | 1.908 | 0.231 |
| APACHEII score
(1st day ICU) | 15.98±6.57 | 13.83±4.56 | 1.575 | 0.119 |
| SOFA score
(1st day ICU) | 6.12±4.09 | 4.57±2.45 | 2.126 | 0.037 |
| 28-day
mortality | 13 (26.0) | 3 (10.0) | 1.870 | 0.143 |
| Source of
infection | | | 5.269 | 0.384 |
|
Abdominal | 8 | 3 | | |
|
Pulmonary | 35 | 25 | | |
|
Urinary | 3 | 0 | | |
|
Central
nervous system | 0 | 1 | | |
|
Blood | 1 | 0 | | |
|
Skin or soft
tissue | 3 | 1 | | |
Laboratory parameters
The WBC, neutrophil ratio (N%), PCT, NT-proBNP, cTnI
and H-FABP of patients in the sepsis group were higher than those
of healthy subjects in the control group (P<0.05) on days 1, 3,
7 and 10. Furthermore, the serum CK-MB was higher than that in the
control group on days 1 and 3 (P<0.05), but there was no
significant difference between groups on days 7 and 10 (P>0.05;
Table III).
 | Table IIIComparison of laboratory parameters
between control and sepsis groups. |
Table III
Comparison of laboratory parameters
between control and sepsis groups.
| | Sepsis | |
|---|
| Laboratory
parameter | Normal
ranges/limits | Control | 1st
day | 3rd
day | 7th
day | 10th day | χ2 |
|---|
| WBC
(x109/l) | (3.5, 9.5) | 6.61±1.53 |
11.79±6.83a |
9.89±4.87a |
9.02±4.42a,b |
9.39±3.93a | 21.130 |
| N% | (40, 75) | 6.61±1.53 |
82.81±12.77a |
80.33±9.52a |
80.33±9.52a-c |
74.81±11.16a-c | 83.231 |
| PCT (ng/ml) | <0.05 | 0.017±0.030 |
18.717±44.700a |
7.919±21.570a |
1.149±3.654a,b |
0.883±2.339a,b | 89.617 |
| NT-pro BNP
(pg/ml) | <450 | 53.6 (38.0,
65.7) | 2099.5 (692.0,
6998.5)a | 1225.0 (441.0,
5117.0)a | 825.0 (285.0,
3410.0)a,b | 653 (274.5,
2648.0)a,b | 80.024 |
| cTnI (ng/ml) | (0, 0.12) | 0.010±0.002 |
0.404±1.583a |
0.405±1.083a |
0.101±0.186a |
0.106±0.225a-c | 57.446 |
| CK-MB (U/l) | (0, 25) | 16.59±3.18 |
27.19±26.30a |
20.71±17.15a |
15.48±6.69b |
16.41±13.13b | 17.308 |
| H-FABP | (0.6, 10) | 8.68±3.93 |
38.43±14.38a |
35.47±11.58a |
33.80±12.48a |
28.60±14.31a,b | 27.242 |
The following results were obtained from the
repeated-measures analysis of the laboratory test data for sepsis
with or without cardiac dysfunction in these two groups. The
H-FABP, WBC, CK-MB and N% at various time-points were higher in
patients with cardiac dysfunction than in patients without cardiac
dysfunction (P<0.05). Regardless of the time-point, the H-FABP,
CK-MB and N% of patients with sepsis-induced cardiac dysfunction
were higher than in those without cardiac dysfunction (P<0.05);
the WBC increased among patients with sepsis-induced cardiac
dysfunction, but the difference was not statistically significant
(P>0.05), and the WBC, N%, CK-MB and H-FABP exhibited no
significant differences among the different groups and the
different time-points (P>0.05). For various groups and at
various time-points, PCT increased in patients with sepsis-induced
cardiac dysfunction (P<0.05), and the differences were
significant (P<0.05). Furthermore, the cTnI levels at various
time-points and among various groups increased in patients with
sepsis-induced cardiac dysfunction, but the difference was not
statistically significant (P>0.05), and no significant
difference was detected among the different groups and different
time-points (P>0.05). Furthermore, PCT exhibited significant
differences among the different groups and different time-points
(P<0.05), but the other indicators were not significantly
different among the different groups and different time-points
(P>0.05; Table IV). NT-proBNP
was increased among patients with sepsis-induced cardiac
dysfunction in various groups and at various time-points
(P<0.001; Table V).
 | Table IVRepeated-measures analysis of
laboratory indicators of sepsis with or without cardiac
dysfunction. |
Table IV
Repeated-measures analysis of
laboratory indicators of sepsis with or without cardiac
dysfunction.
| Group/day | WBC
(x109/l) | N % | PCT (ng/ml) | cTnI (ng/ml) | CK-MB (U/l) | H-FABP (ng/ml) |
|---|
| Sepsis with cardiac
dysfunction |
|
1 | 12.32±7.12 | 84.12±13.94 |
26.705±54.08a | 0.515±1.974 |
32.84±36.82a |
41.08±11.75a |
|
3 | 10.71±5.10 |
82.34±8.08a | 11.54±26.50 | 0.533±1.327 | 22.98±16.81 |
38.17±10.32a |
|
7 | 9.37±4.68 |
79.00±8.89a | 1.97±4.47 | 0.125±0.207 | 16.11±7.80 |
36.21±10.32a |
|
10 | 9.80±4.39 | 76.11±10.76 | 1.13±2.82 | 0.124±0.240 | 18.42±16.08 |
32.84±14.29a |
| Sepsis without
cardiac dysfunction |
|
1 | 10.90±6.34 | 80.62±10.39 | 5.40±14.40 | 0.219±0.429 | 17.77±8.01 | 34.02±17.27 |
|
3 | 8.52±4.17 | 76.96±10.85 | 1.89±4.58 | 0.191±0.379 | 16.93±17.32 | 30.97±12.33 |
|
7 | 8.44±3.98 | 71.73±11.29 | 0.70±1.25 | 0.062±0.141 | 14.43±4.14 | 29.78±13.21 |
|
10 | 8.70±2.96 | 72.65±11.16 | 0.467±1.05 | 0.075±0.199 | 13.05±3.68 | 21.54±11.40 |
| Statistical
comparison: Time |
|
F | 7.253 | 15.306 | 8.736 | 2.775 | 6.580 | 11.584 |
|
P-value | 0.002 | <0.001 | 0.003 | 0.091 | 0.003 | <0.001 |
| Statistical
comparison: Time x group |
|
F | 10.110 | 0.880 | 4.049 | 0.680 | 2.449 | 0.758 |
|
P-value | 0.651 | 0.432 | 0.042 | 0.440 | 0.097 | 0.501 |
| Statistical
comparison: Group |
|
F | 2.512 | 6.973 | 4.639 | 1.444 | 5.868 | 17.303 |
|
P-value | 0.117 | 0.010 | 0.034 | 0.233 | 0.018 | <0.001 |
 | Table VComparison of N-terminal pro-brain
natriuretic peptide (pg/ml) between sepsis with cardiac function
group and sepsis without cardiac dysfunction group. |
Table V
Comparison of N-terminal pro-brain
natriuretic peptide (pg/ml) between sepsis with cardiac function
group and sepsis without cardiac dysfunction group.
| Day | Sepsis with cardiac
dysfunction | Sepsis without
cardiac dysfunction | Z | P-value |
|---|
|
1 | 5505 (2189.0,
8020.0) | 671 (353.0,
813.0) | -7.067 | <0.001 |
|
3 | 2197.5 (1317.0,
6261.0) | 441 (229.0,
640.5) | -5.965 | <0.001 |
|
7 | 2753.5 (826.0,
4976.0) | 279.5 (119.6,
300.5) | -6.285 | <0.001 |
| 10 | 2044.1 (589.8,
4541.0) | 284.3 (153.0,
316.0) | -5.588 | <0.001 |
Echocardiography
According to the echocardiographic indicators of the
sepsis group at different time-points, the ultrasound indicators of
EDV, SV and EF at days 7 and 10; CO and E/A at day 10; and E at day
7 in the sepsis group were compared with those in the control
group, and the differences were not statistically significant
(P>0.05). However, for the other time-points, the differences
were statistically significant (P<0.05; Table VI).
 | Table VIComparison of echocardiographic
indicators between control and sepsis groups. |
Table VI
Comparison of echocardiographic
indicators between control and sepsis groups.
| | Sepsis | |
|---|
|
Echocardiography | Control | 1st
day | 3rd
day | 7th
day | 10th
day | χ2 |
|---|
| EDV (ml) | 117.62±30.11 | 137.19±41.84 | 130.01±22.31 |
124.03±30.43a |
115.23±20.78a | 33.08 |
| ESV (ml) | 42.55±16.07 | 61.54±33.10 | 55.91±17.30 | 51.50±22.10 | 44.88±9.701 | 47.211 |
| SV (ml) | 76.72±17.71 | 79.15±28.55 | 78.28±16.88 |
77.03±17.46a |
70.35±15.66a | 8.571 |
| EF (%) | 64.76±7.09 | 57.49±9.97 | 60.32±7.38 |
62.25±8.09a |
61.32±5.33a | 26.464 |
| CO (l/min) | 5.81±1.47 | 7.10±2.81 | 7.12±1.45 | 6.65±2.07 |
5.75±1.54a | 27.245 |
| E (m/sec) | 0.710±0.121 | 0.749±0.100 | 0.795±0.101 |
0.700±0.146a | 0.815±0.265 | 138.184 |
| A (m/sec) | 0.740±0.092 | 0.954±0.196 | 1.032±0.100 | 1.026±0.177 | 1.033±0.303 | 113.440 |
| E/A | 0.959±0.050 | 0.866±0.127 | 0.875±0.118 | 0.770±0.164 |
0.879±0.289a | 137.240 |
| S (m/sec) | 0.590±0.122 | 0.413±0.058 | 0.405±0.036 | 0.401±0.079 | 0.420±0.073 | 80.389 |
| D (m/sec) | 0.440±0.113 | 0.530±0.077 | 0.522±0.072 | 0.500±0.085 | 0.476±0.772 | 74.266 |
| S/D | 1.34±0.013 | 0.78±0.07 | 0.79±0.12 | 0.81±0.14 | 0.91±0.24 | 105.339 |
According to the repeated-measures analysis of the
echocardiography results, the differences in EF, ESV, CO, SV, E/A,
A, E, S/D, D and S at various time-points were statistically
significant between the sepsis with cardiac dysfunction group and
the sepsis without cardiac dysfunction group (P<0.05).
Furthermore, the differences in EF, ESV, CO, SV, D and S were
statistically significant between groups, regardless of the
time-point (P<0.05). In addition, there were significant
differences among ESV, EDV, D, S, A and S/D at the different
time-points and between the different groups (P<0.05), while
none were obtained for EF, SV, E, CO and E/A (P>0.05; Tables VII and VIII).
 | Table VIIRepeated-measures analysis of
variance of the echocardiography data of patients with sepsis with
or without cardiac dysfunction. |
Table VII
Repeated-measures analysis of
variance of the echocardiography data of patients with sepsis with
or without cardiac dysfunction.
| | Sepsis with cardiac
dysfunction | Sepsis without
cardiac dysfunction |
|---|
|
Echocardiography | 1st
day | 3rd
day | 7th
day | 10th
day | 1st
day | 3rd
day | 7th
day | 10th
day |
|---|
| EDV (ml) | 135.48±48.82 | 129.68±26.99 | 126.65±29.97 |
106.75±16.70a | 140.04±26.99 | 130.57±22.46 | 119.67±31.19 | 129.36±19.35 |
| ESV (ml) | 63.26±39.12 |
60.43±18.52a |
57.03±23.87a |
43.06±10.88a | 58.69±19.62 | 48.39±11.94 | 42.28±15.06 | 47.90±6.45 |
| SV (ml) | 72.22±22.68 |
73.80±13.17a |
74.77±13.94a |
63.69±12.30a | 81.35±20.49 | 82.18±11.71 | 77.39±20.21 | 81.46±14.44 |
| EF (%) | 56.57±10.96 |
57.99±8.13a |
59.51±7.81a | 61.30±6.23 | 59.02±7.98 | 64.21±3.40 | 66.80±6.41 | 62.36±3.45 |
| CO (l/min) | 6.70±2.37 |
6.79±1.50a | 6.54±1.83 |
5.15±1.40a | 7.77±3.36 | 7.68±1.20 | 6.84±2.44 | 6.75±1.23 |
| E (m/sec) | 0.754±0.099 | 0.787±0.099 | 0.677±0.138 | 0.789±0.280 | 0.742±0.105 | 0.809±0.106 | 0.737±0.153 | 0.809±0.242 |
| A (m/sec) |
1.022±0.211a | 1.040±0.078 |
0.996±0.147a | 1.031±0.316 | 0.841±0.088 | 1.019±0.129 | 1.076±0.210 | 1.037±0.286 |
| E/A | 0.769±0.204 | 0.755±0.068 | 0.704±0.246 |
0.740±0.071a | 0.879±0.106 | 0.802±0.110 | 0.728±0.317 | 0.831±0.304 |
| S (m/sec) |
0.377±0.031a |
0.393±0.024a |
0.421±0.070a | 0.417±0.063 | 0.472±0.041 | 0.425±0.044 | 0.369±0.084 | 0.424±0.087 |
| D (m/sec) |
0.482±0.052a |
0.536±0.066a |
0.523±0.074a |
0.449±0.066a | 0.610±0.032 | 0.496±0.076 | 0.463±0.090 | 0.520±0.075 |
| S/D | 0.788±0.048 |
0.739±0.071a | 0.808±0.127 | 0.947±0.216 | 0.779±0.094 | 0.876±0.139 | 0.806±0.161 | 0.800±0.268 |
 | Table VIIIEchocardiography repeated measures
analysis of variance statistical results. |
Table VIII
Echocardiography repeated measures
analysis of variance statistical results.
| | Time | Time x group | Group |
|---|
|
Echocardiography | F | P-value | F | P-value | F | P-value |
|---|
| EDV (ml) | 7.974 | <0.001 | 4.263 | 0.013 | 1.444 | 0.233 |
| ESV (ml) | 8.306 | <0.001 | 3.615 | 0.031 | 4.663 | 0.034 |
| SV (ml) | 5.511 | 0.002 | 1.246 | 0.294 | 11.852 | 0.001 |
| EF (%) | 8.512 | <0.001 | 2.365 | 0.082 | 17.110 | <0.001 |
| CO (l/min) | 9.515 | <0.001 | 1.861 | 0.142 | 9.951 | 0.002 |
| E (m/sec) | 6.792 | 0.002 | 0.813 | 0.433 | 0.488 | 0.487 |
| A (m/sec) | 5.917 | 0.003 | 6.936 | 0.001 | 0.956 | 0.331 |
| E/A | 8.438 | 0.001 | 0.265 | 0.693 | 0.028 | 0.868 |
| S (m/sec) | 4.488 | 0.007 | 23.142 | <0.001 | 7.242 | 0.009 |
| D (m/sec) | 12.145 | <0.001 | 32.123 | <0.001 | 11.185 | 0.001 |
| S/D | 8.102 | 0.001 | 7.927 | 0.001 | 0.102 | 0.750 |
The trends for ESV, EDV, CO, SV, E/A and EF
suggested that ESV and EDV decreased, which was distinct on day 10
among patients with sepsis-induced cardiac dysfunction. These two
indicators reached their minimum on day 7 in patients with sepsis
and no cardiac dysfunction but increased on day 10. The SV among
patients with sepsis-induced cardiac dysfunction reached its
maximum on day 7 but decreased on day 10. The EF gradually
increased among patients with sepsis-induced cardiac dysfunction.
The EF reached its maximum on day 7 among patients with sepsis and
no cardiac dysfunction but decreased to the initial level on day
10. The E/A ratios in the two groups decreased. These levels
reached a minimum on day 7 and were further elevated on day 10. The
E/A among patients with sepsis-induced cardiac dysfunction did not
increase to the normal level on day 10 (Fig. 2).
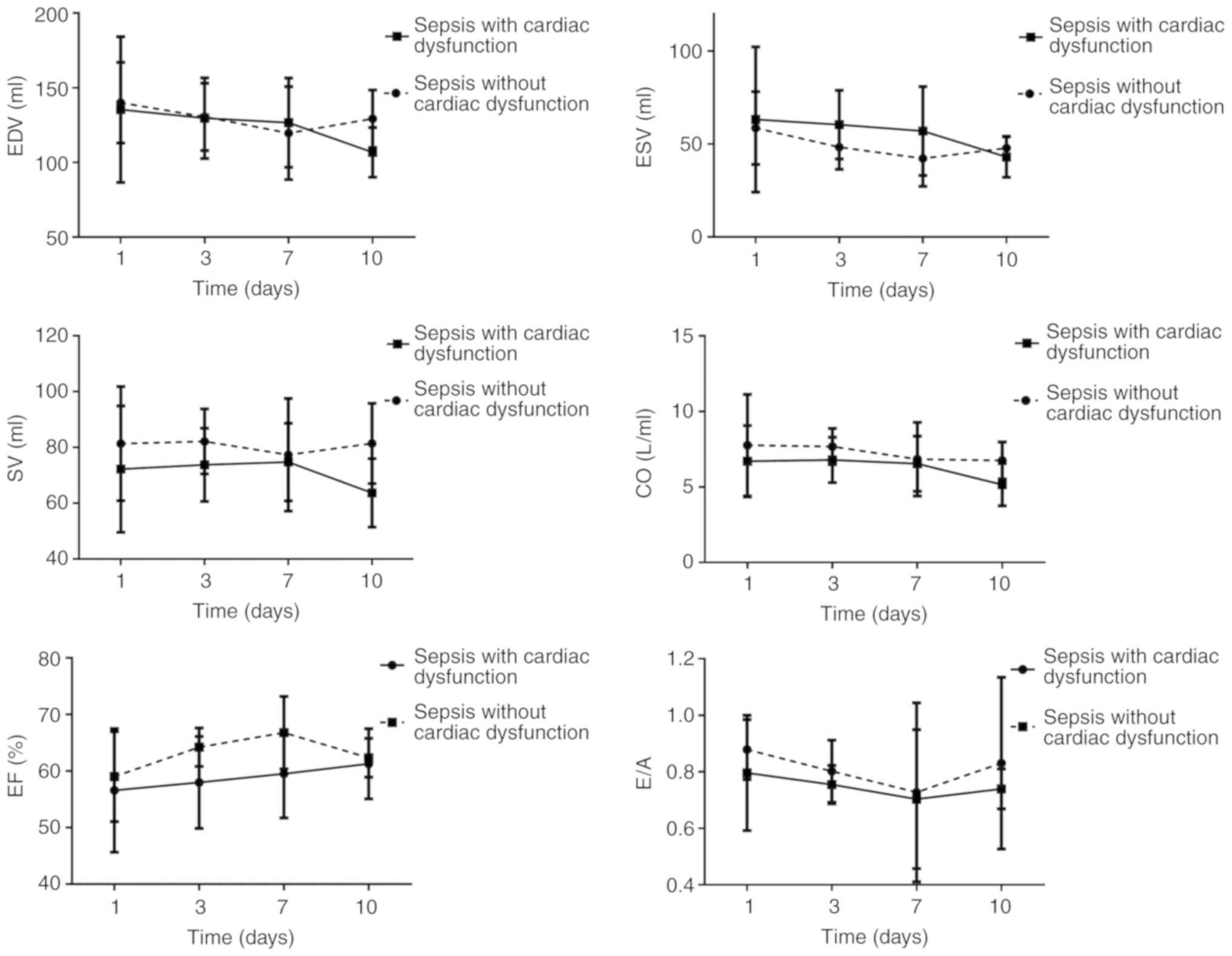 | Figure 2Changes in ultrasound indexes EDV,
ESV, SV, CO, EF and E/A over time. EDV, end-diastolic volume; ESV,
end-systolic volume; SV, stroke volume; EF, left ventricle ejection
fraction; CO, cardiac output; E, mitral early diastolic peak
velocity; A, mitral atrial systolic peak velocity; E/A, ratio of
E/A. |
Correlation analysis
The following results were acquired through the
correlation analysis (Fig. S1).
NT-proBNP was positively correlated with H-FABP (r=0.403;
P<0.001) and cTnI (r=0.0.573; P<0.001) but was negatively
correlated with EF (r=-0.495; P<0.001) and E/A (r=-0.195;
P=0.001). H-FABP was positively correlated with cTnI (r=0.252;
P<0.001) but was negatively correlated with EF (r=-0.202;
P=0.001) and E/A (r=-0.141; P=0.019).
ROC curve and binary logistic
regression analyses for the 28-day mortality rate among patients
with sepsis
Each ultrasound or laboratory indicator was analyzed
and the occurrence of cardiac dysfunction was also determined. The
results of the logistic regression analysis suggested that CK-MB,
A, HF and H-FABP were associated with the 28-day death rate of
patients with sepsis (P<0.05). However, the differences in the
AUC of CK-MB and A were not statistically significant, based on the
ROC curve analysis (P>0.05). The threshold for H-FABP was 35.7
ng/ml, with a specificity and sensitivity of 53.00 and 76.60%. An
HF value >0 indicated that the patient had HF. Specificity and
sensitivity values were 40.1 and 80.9% (Fig. 3; Table
IX).
 | Table IXBinary logistic regression analysis
for parameters affecting 28-day mortality. |
Table IX
Binary logistic regression analysis
for parameters affecting 28-day mortality.
| Variable | b | SE | Wald
χ2 | P-value | OR | 95% CI |
|---|
| A (m/sec) | 1.784 | 0.859 | 4.317 | 0.038 | 5.957 | (1.106,
32.069) |
| PCT (ng/ml) | -0.280 | 0.019 | 2.282 | 0.131 | 0.972 | (0.937, 1.008) |
| CK-MB (U/l) | 0.016 | 0.008 | 4.036 | 0.045 | 1.016 | (1.000, 1.032) |
| H-FABP (ng/ml) | 0.063 | 0.015 | 17.272 | <0.001 | 1.065 | (1.034, 1.097) |
| NT-proBNP
(pg/ml) | 0.000 | 0.000 | 3.647 | 0.056 | 0.999 | (0.999, 1.000) |
| HF | 1.090 | 0.445 | 5.999 | 0.014 | 2.975 | (1.243, 7.120) |
| Constant | -6.528 | 1.226 | 28.349 | <0.001 | 0.001 | - |
Diagnosis of cardiac dysfunction
induced by sepsis
In the ROC curve analysis for NT-proBNP to identify
cardiac dysfunction, the best threshold level was determined as
861.7 pg/ml (specificity, 89.22%; sensitivity, 83.62%; 95% CI,
0.876, 0.945; AUC, 0.915). For EF, the best threshold was
determined as 58.8% (specificity, 89.20%; sensitivity, 62.10%; 95%
CI, 0.698, 0.802; AUC, 0.753), and for H-FABP, the best threshold
was determined as 30.3 ng/ml (specificity, 61.76%; sensitivity,
76.27%; 95% CI, 0.615, 0.728; AUC, 0.673; Fig. 4).
Discussion
In the present study, alterations in
echocardiography results were compared among patients with sepsis
with or without cardiac dysfunction. These results suggested that
patients with sepsis have an increased risk of cardiac
insufficiency on days 7-10 after treatment. H-FABP was identified
as a useful tool to predict the prognosis of patients with sepsis
in the short-term. In addition, an H-FABP of >30.3 ng/ml was
able to determine the occurrence of cardiac dysfunction among
patients with sepsis.
The present results suggest that CO, EF and SV
decrease in patients with sepsis-induced cardiac dysfunction when
compared with patients without cardiac dysfunction. In addition,
the differences in EF on days 1 and 10 and in CO on days 1 and 7
were not statistically significant between these two groups. This
indicates that heart function compensation occurs after active
treatment in patients in an early stage of sepsis, and this is
associated with early cardiac sepsis through a self-regulatory
mechanism. This is referred to as the Frank-Starling mechanism,
which is a filling force mechanism based on a positive association
between the distension of the ventricular chamber and its force of
ejection (23,24). Furthermore, the increase in venous
return dilates the ventricle(s), stretches the myocardium and
increases the contractility of the muscle, efficiently regulating
the stroke volume. Furthermore, the sympathetic-adrenal axis is
excited to release excessive catecholamines, and the secretion of
renin-angiotensin is increased, along with enhanced heart pumping
function and myocardial contractility (25), finally attaining normal or greater
cardiac output (26). Patients with
sepsis-induced cardiac insufficiency receive active treatment to
maintain the normal CO level and retain systemic organ perfusion,
but their cardiac functions are not evidently enhanced. When heart
function is not persistently improved, the disease in patients with
sepsis is aggravated, they gradually enter the refractory phase and
the systolic residual blood volume in the left ventricle and
peripheral resistance decrease, while EF returns to the normal
level or increases. The present study revealed that the E/A among
patients with sepsis-induced cardiac dysfunction was minimized on
day 7 and had not returned to the normal level on day 10,
suggesting that the diastolic function of the left ventricle in
patients with sepsis transitioned on day 7 and the recovery of
diastolic function may be slow when compared with that of systolic
function. Parker et al (27)
examined the results of radionuclide scanning of 20 patients with
septic shock and discovered that cardiac function was temporarily
lowered in 13 patients with normal initial myocardial function. Of
note, the myocardial function recovery pattern on days 7-10 was of
crucial importance to survival. Thus, the cardiac function recovery
time is expected to be on days 7-10 for patients with sepsis, which
is associated with the prognosis of these patients.
NT-proBNP has become a vital indicator to assess
changes in cardiac function. In addition, NT-proBNP is a critical
factor in the evaluation and serological diagnosis according to
global HF guidelines (13). Khoury
et al (28) suggested that
NT-proBNP may serve as an independent factor to predict the
short-term or long-term death rate of inpatients with sepsis. Lin
et al (29) performed a
study on 21 pediatric patients with sepsis and reported that an
NT-proBNP of >1,268 ng/l may be used to predict the incidence of
cardiac insufficiency in such patients. Furthermore, NT-proBNP may
be an important reference for the diagnosis of sepsis in patients
with cardiac insufficiency. FABP is a cytosolic protein that has a
vital role in the metabolism, transport and uptake of fatty acids
(30). Typically, H-FABP is
specific for myocardial cells and is detected in serum in the case
of myocardial damage due to different reasons (31). In addition, H-FABP may predict even
small amounts of myocardial damage (32,33).
These present findings suggest that H-FABP >35.7 ng/ml may
predict a dismal prognosis for patients with sepsis in the short
term, which is consistent with the results reported by other
studies. performed a study on 295 patients with sepsis and reported
that H-FABP may serve as an independent factor to predict the
28-day death rate and organ dysfunction in such patients. Jo et
al (34) investigated 99
patients with sepsis and reported that H-FABP Chen and Li (18) >40 ng/ml was an independent factor
that predicted the death of patients with sepsis, and H-FABP >40
ng/ml also increased the risk of death by 5.57-fold at the 28-day
follow-up. Thus, H-FABP is closely correlated with the severity of
sepsis, which is a risk factor for a dismal prognosis of patients
with sepsis (17,18). Fan et al (35) reported that the serum H-FABP levels
in mice with sepsis-induced myocardial injury evidently increased
compared with those in mice with no myocardial injury. Furthermore,
the H-FABP level decreased as cardiac function improved. However,
no existing study has been performed to examine the association
between sepsis-induced cardiac dysfunction and H-FABP in
humans.
According to the present results, H-FABP exhibited a
positive correlation with NT-proBNP (r=0.403; P<0.001) and cTnI
(r=0.252; P<0.001). Furthermore, the H-FABP, cTnI and NT-proBNP
levels in patients with sepsis-induced cardiac dysfunction were
increased relative to those in patients with sepsis without cardiac
dysfunction. These results suggest that patients with sepsis and
severe myocardial damage have an increased risk of cardiac
insufficiency. On the other hand, the present results indicate that
the H-FABP, cTnI and NT-proBNP levels in patients with sepsis and
cardiac insufficiency peaked on the first day. Subsequently, the
H-FABP gradually decreased, regardless of the presence or absence
of cardiac dysfunction. On the 10th day, the H-FABP in sepsis
patients without HF evidently increased when compared with that in
patients without HF, suggesting that various changes in heart
function occurred in patients with sepsis on days 7-10. The cTnI
and H-FABP levels in patients with sepsis were higher on day 10 of
hospitalization, which indicates that a longer time is required to
recover the damage to myocardial cells following myocardial injury
in patients with sepsis, regardless of the improved heart
function.
Patients with sepsis and cardiac insufficiency have
a poor response to liquid volume (36). Hence, increased fluid resuscitation
is required during the retreatment process, as well as the use of
vasoactive drugs. Therefore, patients with sepsis and cardiac
insufficiency have a heavy volume load, which increases the
concentration of NT-proBNP in the blood. The present study also
revealed that inflammatory indicators were higher in the sepsis
with cardiac dysfunction group than in the sepsis with normal heart
function group, indicating that sepsis with heart dysfunction has a
more serious inflammatory response and that inflammatory cells and
mediators directly or indirectly induce cardiac toxicity, causing
NT-proBNP to increase (26,37). A study performed with the use of
myocardial radionuclides in patients with septic shock revealed
that the 7- to 10-day repair time of myocardial injury is important
for the prognosis of patients with sepsis due to myocardial injury
caused by septic shock. This indicates that the recovery time for
myocardial injury in patients with sepsis may be within 7-10 days.
Therefore, NT-proBNP may still be high in patients with sepsis and
cardiac insufficiency at 10 days.
The alterations in cardiac markers and the
echocardiography results of patients with sepsis suggest that such
patients have an increased risk of cardiac insufficiency on days
7-10 of hospitalization. Lorts et al (38) suggested that patients with sepsis
have the highest risk of cardiac dysfunction on days 5-7 of
hospitalization. Therefore, day 7 of hospitalization is a vital
time-point to examine the changes in heart function in patients
with sepsis.
According to the present results, ~58.8% of patients
with sepsis (n=47) suffered from diastolic dysfunction in the left
ventricle, while only 3 (3.8%) had systolic dysfunction in the
ventricle and 10 (12.5%) had diastolic and systolic dysfunction in
the left ventricle. This suggests that patients with sepsis have a
higher risk of diastolic dysfunction than of systolic dysfunction.
Furthermore, studies suggested that the serum H-FABP concentration
in patients with sepsis is significantly higher than that in
healthy subjects. Regardless of the type of cardiac dysfunction
that occurred in patients with sepsis, the serum H-FABP
concentration at various time-points was higher in patients with
sepsis with cardiac dysfunction than in patients with sepsis
without cardiac dysfunction. The correlation analysis revealed that
H-FABP was negatively correlated with EF (r=-0.202, P=0.001) and
E/A (r=-0.141, P=0.019), further indicating that the level of
H-FABP increased in the case of either systolic dysfunction or
diastolic dysfunction. These results suggest that the serum levels
of H-FABP may be used to assess dysfunction in the left ventricle
in patients with sepsis.
Kandil et al (39) investigated 49 patients with sepsis
and discovered that the BNP content in those patients was
apparently increased when compared with that in patients with early
sepsis with positive BNP, indicating that the BNP level in patients
with sepsis increases during circulatory dysfunction. Furthermore,
Landesberg et al (40)
investigated 256 patients with septic shock and severe sepsis and
discovered that NT-proBNP and high-sensitive troponin T (hs-TnT)
were markedly increased in groups of patients with sepsis that
survived or died, with data in the latter group being significantly
higher compared with former group. In addition, relative to
patients with normal diastolic and systolic functions, patients
with an e'-wave of <8 cm/sec, EF ≤50% or e'-wave <8 cm/sec
and EF ≤50% combined had higher levels of NT-proBNP and hs-TnT.
Furthermore, the cTnI and NT-proBNP levels were apparently
increased in patients with sepsis-induced cardiac dysfunction
compared with those in patients without cardiac dysfunction. The
results of the ROC curve analysis for the diagnosis of
sepsis-induced cardiac dysfunction revealed that HF may be
considered to be present in patients with sepsis and NT-proBNP
>861.7 pg/ml. The specificity, sensitivity and AUC (with 95% CI)
were 89.22, 83.62% and 0.915 (0.876, 0.945), respectively. In
addition, the specificity, sensitivity and AUC (with 95% CI) for
the diagnosis of cardiac dysfunction in patients with sepsis and EF
≤58.8% were 89.20, 62.10% and 0.753 (0.698, 0.826), respectively.
EF and NT-proBNP have higher specificity but lower sensitivity in
the diagnosis of sepsis-induced cardiac dysfunction. Thus, these
results have clinical significance in the detection of cardiac
dysfunction in patients with sepsis. A diagnosis of sepsis-induced
cardiac dysfunction may be indicated if H-FABP >30.3 ng/ml, and
the specificity, sensitivity and AUC (with 95% CI) were 61.7,
76.27% and 0.673 (0.615, 0.728), respectively. The specificity and
sensitivity of H-FABP in the diagnosis of sepsis-induced cardiac
dysfunction remain poor, which may be ascribed to the increased fat
catabolism and glycogen at sepsis onset. The free fatty acid levels
increased, which may directly result in elevated H-FABP levels
(41). In addition, H-FABP is
mainly excreted via renal excretion (42). As a result, in the case of decreased
renal function associated with sepsis, the H-FABP level was
elevated. Thus, patient conditions should be comprehensively
assessed when H-FABP is applied in the diagnosis of cardiac
insufficiency for patients with sepsis.
Studies have indicated that the occurrence of HF is
a risk factor for 28-day mortality in patients with sepsis, with an
odds ratio of 2.975 (95% CI: 1.243, 7.120), indicating that
patients with cardiac insufficiency have a poor short-term
prognosis. This may be because early fluid resuscitation is
recommended for sepsis treatment and patients with cardiac
insufficiency have poorer fluid response, heavier fluid load and
receive more vasoactive drugs, but systemic tissue perfusion may
not significantly improve during treatment. Furthermore, organ
function is continuously impaired and even fails, which in turn
causes multiple organ dysfunction syndrome, making the treatment of
patients with sepsis more difficult and worsening the prognosis.
However, studies have suggested that, although the mortality of
patients with sepsis and cardiac insufficiency is higher than that
of normal cardiac function, the difference was not statistically
significant and this is considered to be associated with the small
sample size of the present study.
Acute systolic HF is inherent in patients with
sepsis, but diastolic HF almost never occurs unless HF has occurred
in the past. Of note, in the present study, the EF values were
normal, while the E/A values were abnormal in the patients with
sepsis and HF, and in those with sepsis and no HF, but the
difference was not significant. According to the Evaluation of Left
Ventricular Diastolic Function by Echocardiography guidelines from
the American Society of Echocardiography and the European
Association of Cardiovascular Imaging (43), the E peak gradually decreases with
age, while the A peak gradually increases with age and the E/A
ratio gradually decreases. The Echocardiography Examination
Guideline for patients of Chinese ethnicities in 2018 also revealed
similar results (44), which may be
ascribed to the normal aging-induced increase in left ventricular
wall stiffness, the reduction in myocardial elongation length and
the weakened or non-weakened early diastolic aspiration. As a
result, the heart filling pressure is upregulated, ventricular
diastolic function is reduced, the ventricular isovolumic
relaxation time is extended and the E peak decreases. Accordingly,
to maintain the normal level of cardiac output, the late
ventricular diastolic filling volume increases and the A peak
becomes elevated. In addition, the cause of such serial changes may
be CAD or other subclinical diseases, especially in elderly
subjects with sub-health status. Therefore, the elderly cardiac
function pattern may manifest as mild diastolic dysfunction when
compared with that of young patients. Furthermore, the guidelines
(43) suggested that E/A ≥0.8, E/
early diastolic mitral annular velocity (e') <10, peak tricuspid
regurgitation (TR) velocity (m/sec) <2.8 and left atrial volume
index at the normal range may possibly indicate normal ventricular
diastolic function. The subjects enrolled in the present study were
older but had little healthcare-associated knowledge, and they did
not undergo regular physical examinations. Thus, it was not
possible to definitely exclude potential CADs. E/A<1 was
obtained in the sepsis with normal cardiac function group, which
may be associated with aggressive liquid resuscitation and the use
of anti-infective agents during the sepsis treatment process, in
addition to the age factor. Furthermore, 7-10 days of
anti-infective treatment was recommended in the Sepsis Diagnosis
and Treatment Guideline for patients with sepsis or septic shock,
which is similar to the result that 7-10 days is the transition
point of cardiac function in patients with sepsis (1). This is considered to be associated
with the alleviation or elimination of inflammation suppression or
injury on myocardial cells, thereby achieving an effective
anti-infective treatment. Similarly, certain studies reported that
patients with sepsis may develop simple diastolic dysfunction
(45); that is, patients have a
normal EF while E/A<1. At the same time, the physical
examination results suggest that 0.8<E/A<1. Accordingly,
patients with sepsis may have a normal EF value when E/A<1.
Certain limitations of the present study should be
pointed out. First, the sample size of the present study was small
due to its single-center nature. Furthermore, the patients with
sepsis had unstable vital signs and were critically ill upon
admission. Hence, the patients always need emergent treatment and
it was difficult to collect heart ultrasound data prior to
treatment. Furthermore, the alterations in cardiac markers and
heart ultrasound findings in patients with sepsis were monitored
and more precise and creditable results were obtained when compared
to those acquired from prior studies, which pertain to the cardiac
function of patients with sepsis. In addition, prior studies have
not sufficiently focused on sepsis-induced cardiac dysfunction and
H-FABP in patients. Instead, these studies mainly focused on
disease severity and patient prognosis.
In conclusion, patients with sepsis have an
increased risk of cardiac insufficiency on days 7-10. H-FABP may
serve as an indicator to estimate the prognosis of patients with
sepsis in the short term, which is of certain significance in the
diagnosis of sepsis-induced cardiac dysfunction.
Supplementary Material
The correlation analysis of NT-proBNP,
EF, E/A, cTnI and H-FABP.
Sequential (sepsis-associated) organ
failure assessment score.
Acknowledgements
Not applicable.
Funding
This work was supported by the Science and
Technology Development Plan Project of the Xinjiang Production and
Construction Corps, China (grant no. KC0038).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GW screened the cases and performed statistical
analysis. PT performed laboratory analyses of the patient samples.
XY performed cardiac echocardiography. QC designed the study and
majorly contributed to the writing of the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The current study protocol was approved by the
Ethics Committee of the First Affiliated Hospital of Medical
College, Shihezi University (no. 250457) and written informed
consent was obtained from each participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rhodes A, Evans LE, Alhazzani W, Levy MM,
Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally
ME, et al: Surviving sepsis campaign: International guidelines for
management of sepsis and septic shock: 2016. Intensive Care Med.
43:304–377. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shankar-Hari M, Phillips GS, Levy ML,
Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD and
Singer M: Sepsis Definitions Task Force. Developing a new
definition and assessing new clinical criteria for septic shock:
For the third international consensus definitions for sepsis and
septic shock (Sepsis-3). JAMA. 315:775–787. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhou J, Qian C, Zhao M, Yu X, Kang Y, Ma
X, Ai Y, Xu Y, Liu D, An Y, et al: Epidemiology and outcome of
severe sepsis and septic shock in intensive care units in mainland
China. PLoS One. 9(e107181)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zanotti-Cavazzoni SL and Hollenberg SM:
Cardiac dysfunction in severe sepsis and septic shock. Curr Opin
Crit Care. 15:392–397. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chong J, Dumont T, Francis-Frank L and
Balaan M: Sepsis and septic shock: A review. Crit Care Nurs Q.
38:111–120. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sanfilippo F, Corredor C, Fletcher N,
Landesberg G, Benedetto U, Foex P and Cecconi M: Erratum to:
Diastolic dysfunction and mortality in septic patients: A
systematic review and meta-analysis. Intensive Care Med.
41:1178–1179. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Brennan LM, Widder MW, McAleer MK, Mayo
MW, Greis AP and van der Schalie WH: Preparation and testing of
impedance-based fluidic biochips with RTgill-W1 cells for rapid
evaluation of drinking water samples for toxicity. J Vis Exp,
2016.
|
|
8
|
Werdan K, Schmidt H, Ebelt H, Zorn-Pauly
K, Koidl B, Hoke RS, Heinroth K and Müller-Werdan U: Impaired
regulation of cardiac function in sepsis, SIRS, and MODS. Can J
Physiol Pharmacol. 87:266–274. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Rudski LG, Lai WW, Afilalo J, Hua L,
Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK and
Schiller NB: Guidelines for the echocardiographic assessment of the
right heart in adults: A report from the American society of
echocardiography endorsed by the European association of
echocardiography, a registered branch of the European society of
cardiology, and the canadian society of echocardiography. J Am Soc
Echocardiogr. 23:685–713. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Group CMAUMBE. Chinese adult
echocardiography examination and measurement guide. Chin J
Ultrasonography. 25:645–666. 2016.(In Chinese).
|
|
11
|
Pulido JN, Afessa B, Masaki M, Yuasa T,
Gillespie S, Herasevich V, Brown DR and Oh JK: Frequency and
clinical spectrum of myocardial dysfunction in severe sepsis and
septic shock. Mayo Clin Proc. 87:620–628. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sato R, Kuriyama A, Takada T, Nasu M and
Luthe SK: Prevalence and risk factors of sepsis-induced
cardiomyopathy: A retrospective cohort study. Medicine (Baltimore).
95(e5031)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yancy CW, Jessup M, Bozkurt B, Butler J,
Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC,
Givertz MM, et al: 2017 ACC/AHA/HFSA focused update of the 2013
ACCF/AHA guideline for the management of heart failure: A report of
the American college of cardiology/American heart association task
force on clinical practice guidelines and the heart failure society
of America. J Am Coll Cardiol. 70:776–803. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kakihana Y, Ito T, Nakahara M, Yamaguchi K
and Yasuda T: Sepsis-induced myocardial dysfunction:
Pathophysiology and management. J Intensive Care.
4(22)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sato R and Nasu M: A review of
sepsis-induced cardiomyopathy. J Intensive Care.
3(48)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Luo JQ, LI JC and Long ZH: The clinical
application of heart-fatty acid binding protein in AMI early
diagnosis. Chin J Clin Pathologist. 1:22–25. 2016.(In Chinese).
|
|
17
|
Zhang ZC, Dai HW, Yu YH, Yang JD and Hu
CB: Usefulness of heart-type fatty acid-binding protein in patients
with severe sepsis. J Crit Care. 27:e413–e415.e418. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chen YX and Li CS: The prognostic and
risk-stratified value of heart-type fatty acid-binding protein in
septic patients in the emergency department. J Crit Care.
29:512–516. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
You HJ, Kim K, Lee JH, Rhee JE, Lee JH,
Kang KW, Rim KP, Hwang SS and Park HM: Heart-type fatty
acid-binding protein as a prognostic factor in patients with severe
sepsis and septic shock. Am J Emerg Med. 30:1749–1755.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nagueh SF, Smiseth OA, Appleton CP, Byrd
BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC,
Klein AL, Lancellotti P, et al: Recommendations for the evaluation
of left ventricular diastolic function by echocardiography: An
update from the American society of echocardiography and the
European association of cardiovascular imaging. J Am Soc
Echocardiogr. 29:277–314. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Knaus WA, Draper EA, Wagner DP and
Zimmerman JE: APACHE II: A severity of disease classification
system. Crit Care Med. 13:818–829. 1985.PubMed/NCBI
|
|
23
|
Holubarsch C, Ruf T, Goldstein DJ, Ashton
RC, Nickl W, Pieske B, Pioch K, Ludemann J, Wiesner S, Hasenfuss G,
et al: Existence of the Frank-Starling mechanism in the failing
human heart Investigations on the organ, tissue, and sarcomere
levels. Circulation. 94:683–689. 1996.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shiels HA and White E: The frank-starling
mechanism in vertebrate cardiac myocytes. J Exp Biol.
211:2005–2013. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Redfors B, Shao Y and Omerovic E: Male
rats are more prone to develop Takotsubo-like cardiac dysfunction
in response to high-dose catecholamine than are female rats. Eur
Heart J. 34(P5047)2013.
|
|
26
|
Chaui-Berlinck JG and Monteiro LHA:
Frank-Starling mechanism and short-term adjustment of cardiac flow.
J Exp Biol. 220:4391–4398. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Parker MM, Shelhamer JH, Bacharach SL,
Green MV, Natanson C, Frederick TM, Damske BA and Parrillo JE:
Profound but reversible myocardial depression in patients with
septic shock. Ann Intern Med. 100:483–490. 1984.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Khoury J, Arow M, Elias A, Makhoul BF,
Berger G, Kaplan M, Mashiach T, Ismael-Badarneh R, Aronson D and
Azzam ZS: The prognostic value of brain natriuretic peptide (BNP)
in non-cardiac patients with sepsis, ultra-long follow-up. J Crit
Care. 42:117–122. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lin CW, Tang W, Wen F, Chen JJ, Zeng XL
and Chen ZG: Diagnostic accuracy of NT-ProBNP for heart failure
with sepsis in patients younger than 18 years. PLoS One.
11(e0147930)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Storch J and Thumser AE: The fatty acid
transport function of fatty acid-binding proteins. Biochim Biophys
Acta. 1486:28–44. 2000.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Smathers RL and Petersen DR: The human
fatty acid-binding protein family: Evolutionary divergences and
functions. Hum Genomics. 5:170–191. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang Y, Guo Z and Huang L: Value of
different biochemical markers in early diagnosis of acute
myocardial infarction. Nan Fang Yi Ke Da Xue Xue Bao. 34:1347–1350.
2014.PubMed/NCBI(In Chinese).
|
|
33
|
Cubranic Z, Madzar Z, Matijevic S, Dvornik
S, Fisic E, Tomulic V, Kunisek J, Laskarin G, Kardum I and
Zaputovic L: Diagnostic accuracy of heart fatty acid binding
protein (H-FABP) and glycogen phosphorylase isoenzyme BB (GPBB) in
diagnosis of acute myocardial infarction in patients with acute
coronary syndrome. Biochem Med (Zagreb). 22:225–236.
2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jo YH, Kim K, Lee JH, Rhee JE, Lee JH,
Kang KW, Rim KP, Hwang SS and Park HM: Heart-type fatty
acid-binding protein as a prognostic factor in patients with severe
sepsis and septic shock. Am J Emerg Med. 30:1749–1755.
2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fan TT, Feng XY, Yang YZ, Gao F and Liu Q:
Downregulation of PI3K-g in a mouse model of sepsis-induced
myocardial dysfunction. Cytokine. 96:208–216. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hartemink KJ, Twisk JW and Groeneveld AB:
High circulating N-terminal pro-B-type natriuretic peptide is
associated with greater systolic cardiac dysfunction and
nonresponsiveness to fluids in septic vs nonseptic critically ill
patients. J Crit Care. 26:e101–e108. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tomaru Ki K, Arai M, Yokoyama T, Aihara Y,
Sekiguchi Ki K, Tanaka T, Nagai R and Kurabayashi M:
Transcriptional activation of the BNP gene by lipopolysaccharide is
mediated through GATA elements in neonatal rat cardiac myocytes. J
Mol Cell Cardiol. 34:649–659. 2002.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lorts A, Burroughs T and Shanley TP:
Elucidating the role of reversible protein phosphorylation in
sepsis-induced myocardial dysfunction. Shock. 32:49–54.
2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kandil E, Burack J, Sawas A, Bibawy H,
Schwartzman A, Zenilman ME and Bluth MH: B-type natriuretic
peptide: A biomarker for the diagnosis and risk stratification of
patients with septic shock. Arch Surg. 143:242–246. 2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Landesberg G, Levin PD, Gilon D, Goodman
S, Georgieva M, Weissman C, Jaffe AS, Sprung CL and Barak V:
Myocardial dysfunction in severe sepsis and septic shock: No
correlation with inflammatory cytokines in real-life clinical
setting. Chest. 148:93–102. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yan GT, Lin J, Hao XH, Xue H, Zhang K and
Wang LH: Heart-type fatty acid-binding protein is a useful marker
for organ dysfunction and leptin alleviates sepsis-induced organ
injuries by restraining its tissue levels. Eur J Pharmacol.
616:244–250. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tanaka T, Hirota Y, Sohmiya K, Nishimura S
and Kawamura K: Serum and urinary human heart fatty acid-binding
protein in acute myocardial infarction. Clin Biochem. 24:195–201.
1991.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Nagueh SF, Smiseth OA, Appleton CP, Byrd
BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC,
Klein AL, Lancellotti P, et al: Recommendations for the evaluation
of left ventricular diastolic function by echocardiography: An
update from the American society of echocardiography and the
European association of cardiovascular imaging. Eur Heart J
Cardiovasc Imaging. 17:1321–1360. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Echocardiography Group of Ultrasound
Medicine Branch of Chinese Medical Association. Guide for Chinese
adult echocardiography examination and measurement. Chin J
Ultrasonogr. 25:645–666. 2016.
|
|
45
|
Landesberg G, Gilon D, Meroz Y, Georgieva
M, Levin PD, Goodman S, Avidan A, Beeri R, Weissman C, Jaffe AS and
Sprung CL: Diastolic dysfunction and mortality in severe sepsis and
septic shock. Eur Heart J. 33:895–903. 2012.PubMed/NCBI View Article : Google Scholar
|


















