Introduction
Scald burns are common accidental injuries usually
caused by exposure to hot liquids or metals (1). The degree of skin damage caused by
scald burns is primarily associated with the temperature and
duration of exposure and depth is determined by pathological
examination (2,3). The determination of depth is
consistent with the clinical ‘three degree quartering' method
(4). Furthermore, second-degree
scald burns may be divided into shallow and deep burns (4). Deep second-degree scald burns are
characterized by a deep dermal layer burn and the healing process
is comprised of various biological processes involving numerous
cell and tissue components, including inflammatory response, cell
proliferation and migration and wound remodeling, as three
overlapping processes (5,6).
At present, antibiotics and silver salt are the
major components of chemical drugs used to treat scald burns
(7). However, chemical drugs have a
single mechanism of action and have low efficacy (7). Recently, antibiotic abuse and
bacterial drug resistance have increased, leading to complications
with the use of chemical drugs in the treatment of burns and scald
(8,9). Due to this, gene targeting therapy for
scald burns may be a focus of interest.
MicroRNAs (miRNAs or miRs) are endogenous,
eukaryotic non-coding RNAs with various regulatory functions
(10). By regulating the expression
of target genes, miRNAs participate in important physiological
processes, including growth, development, differentiation and
metabolism, and serve an important biological function (11). Abnormal miRNA expression has been
frequently reported in malignant tumors in humans, including in
lung cancer and prostate cancer, in which they serve roles in
proliferation, apoptosis and invasion (12-14).
Furthermore, recent studies have demonstrated that multiple miRNAs
are involved in scald burn wound healing (15,16).
miR-27b is abnormally expressed in liver cancer and
kidney cancer, and has been reported to be associated with
angiogenesis (17,18). However, the role of miR-27b in the
wound healing process of scald burns has not been previously
reported in the literature. Therefore, the present study evaluated
the effect of miR-27b on skin healing by constructing a rat model
of deep second-degree scald burns. In addition, the effect of
miR-27b on the proliferation and migration of BJ human skin
fibroblast cells was investigated using an in vitro model.
In short, the present study investigated the role and mechanism of
action of miR-27b in the healing of scald burns in rats and in BJ
fibroblast cells, providing a novel strategy for the treatment of
scald burns.
Materials and methods
Animals
A total of 72 male Sprague Dawley rats (weight,
230-250 g; age, 8-9 weeks) were purchased from the Jinan Pengyue
Experimental Breeding Co., Ltd. with license no. SCXK (lu)
2014-0007. All rats were housed at temperatures of 22-25˚C with a
humidity of 50-60% and 12 h light/dark cycles. Rats had free access
to drinking water and food for 1 week. The current study was
approved by the Committee on Animal Protection and Use of the
Affiliated Yantai Yuhuangding Hospital of Qingdao University
(Yantai, China) and was conducted in strict accordance with the
National Institute of Health guidelines (pub. no. 85-23; revised
1996).
Establishment of the deep
second-degree scald burn rat model
The control group did not receive a scald burn. The
other 5 groups of rats were used to establish a deep second-degree
scald burn model. The rats were administered deep anesthesia via
intraperitoneal injection of 40 mg/kg 3% pentobarbital sodium
following fasting for 12 h. Hair was removed from the back (area,
3x3 cm) using 8% sodium sulfide (Zhejiang Shiyan Medicine Co.,
Ltd.) and 10 ml water at 80˚C was applied for 10 sec to induce a
scald wound (area, 2.5x2.5 cm) and establish the model. Rats were
then intraperitoneally injected with 30 ml/kg of Ringer's solution
(Zhejiang Shiyan Medicine Co., Ltd.) and the scald site was treated
with a sterile gauze (19).
Following modeling, rats in each group were
subcutaneously injected (depth, 2.5-3 mm) with corresponding
therapeutic agents around of the trauma site every day for 21 days
and controls were injected with equal amounts of normal saline.
miR-27b mimic control reagent (cat. no. mir1n0000001-1-5), miR-27b
mimics (cat. no. MIMAT0000419), miR-27b inhibitor control reagent
(cat. no. mir2n00000000419-1-5) and miR-27b inhibitor reagent (cat.
no. mir20000419-1-5) were all obtained from Guangzhou Ruibo
Biological Co., Ltd. The mimics, inhibitors and control reagents
(100 µg) were dissolved in 100 µl ddH2O.
No animals were lost during the experiment. A total
of 72 rats were randomly divided into 6 groups (n=12). The controls
were injected with equal amounts of saline. Following the
establishment of the deep second-degree scald burn model, the
remaining 5 groups were treated as follows: The model group, which
was injected subcutaneously with equal volumes of saline; the
miR-27b mimics (miR27b) group, which was injected subcutaneously
with 4 µg/kg of miR-27b mimics; the miR-27b mimics control (MC)
group, which was injected subcutaneously with 4 µg/kg miR-27b
mimics control reagent; miR-27b inhibitor (inhibitor) group, which
was injected subcutaneously with 4 µg/kg of miR-27b inhibitor; and
the miR-27b inhibitor control (IC) group, which was injected with 4
µg/kg miR-27b inhibitor control reagent.
Measurement of wound healing rate
The rats were intraperitoneally anesthetized with 40
mg/kg 1% pentobarbital sodium (Zhejiang Shiyan Medicine Co., Ltd.)
on days 0, 3, 7, 14 and 21 following modeling and images of the
scald wounds were captured. The wound was covered with sterile
plastic film and the unhealed area of the wound was traced in order
to measure the area of unhealed skin and the wound healing rate was
calculated at each time-point as follows: Wound healing rate =
[(original wound area - unhealed wound area)/original wound area]
x100%.
The rats were anesthetized via intraperitoneal
injection of 40 mg/kg 1% pentobarbital sodium (Zhejiang Shiyan
Medicine Co., Ltd.) 2 h after the end of treatment on day 21 days
post-modelling. Following anesthesia, the rats were sacrificed by
cervical dislocation. Wound tissue or regenerated tissue (~1x1 cm)
from the middle or the side of the wound was excised and were
either fixed in 4% paraformaldehyde (Beijing Solarbio Science &
Technology Co., Ltd.) for H&E and Masson staining or cultured
in liquid nitrogen (Beijing Solarbio Science & Technology Co.,
Ltd.) and transferred to the refrigerator at -80˚C for reverse
transcription-quantitative PCR (RT-qPCR) and western blot
analysis.
H&E staining
Tissues from the healing scald wounds of the rats
were excised and fixed with 4% paraformaldehyde at 37˚C for 48 h
(Beijing Solarbio Science & Technology Co., Ltd.). Fixed
tissues were dehydrated, cleared and embedded in paraffin. The
tissues were cut into 5-µm sections and then dried, dewaxed and
dehydrated with graded series of ethanol (Zhejiang Shiyan Medicine
Co., Ltd.; 100% ethanol for 5 min, 95% ethanol for 5 min, 90%
ethanol for 5 min, 80% ethanol for 5 min and 70% ethanol for 5
min). Hematoxylin was used for staining for 5 min at room
temperature and then eosin for 2 min at room temperature. Skin
tissue morphology was observed at a magnification of x400 with an
optical microscope (Olympus BX51; Olympus Corp.).
Masson staining
Skin tissue sections were stained with lignin
(AR1069; Wuhan Boster Biological Technology, Ltd.) for 5 min at
25˚C and treated with acidic ethanol differentiation solution
(Wuhan Boster Biological Technology, Ltd.). Following staining with
Masson bluing solution, lichun red magenta dyeing solution (G1340;
Beijing Solarbio Science & Technology Co., Ltd.) was used for
staining at 25˚C for 10 min. Following leaching with 2% glacial
acetic acid solution (Wuhan Boster Biological Technology, Ltd.) at
25˚C for 2 min, the sections were washed with phosphomolybdate
solution (Wuhan Boster Biological Technology, Ltd.) for 3 min and
then stained with aniline blue staining solution for 5 min at 25˚C.
The slices were dehydrated with 95% ethanol and anhydrous ethanol,
and permeabilized with xylene and sealed with neutral gum. Collagen
fibrosis in skin tissues was observed at a magnification of x400
using an optical microscope (Olympus BX51; Olympus Corp.).
BJ cell culture and grouping
BJ cells (CRL-2522; American Type Culture
Collection) were cultured in Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc.) and supplemented with 1%
penicillin-streptomycin (Beijing Solarbio Science & Technology
Co., Ltd.) and 10% fetal bovine serum (Sigma-Aldrich; Merck KGaA).
Cells were incubated in a cell incubator (Thermo Fisher Scientific,
Inc.) at 37˚C with 5% CO2 and saturated humidity.
BJ cells in the logarithmic phase were seeded into
6-well plates (2x105/well) and inoculated for 24 h. The
wells were divided into the control, miR-27b, MC, inhibitor and IC
groups and Lipofectamine™ 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) was used to transfect corresponding
reagents into cells for 12 h to construct BJ cells exhibiting
miR-27b overexpression or inhibition.
MTT assay
BJ cell proliferation was determined via MTT assay.
A cell suspension of total of 1x104/ml was inoculated
into 96-well plates (100 µl/well) and cultured in 5% CO2
and saturated humidity at 37˚C for 24, 48, 72 and 96 h.
Subsequently, 20 µl MTT solution (5 mg/ml) was added to each well,
followed by incubation at 37˚C for 4 h. The supernatant was removed
and 200 µl DMSO (Sigma-Aldrich; Merck KGaA) was added to each well.
The cells were blown evenly and the optical density was measured at
490 nm using the 190 SpectraMax spectrophotometer (Eppendorf).
Wound-healing assay
BJ cell migration was evaluated with a wound-healing
assay. Cells from each group were suspended at a concentration of
3x105/ml and were inoculated into 6-well plates and the
cell layer was cultured to cover the 6-well plate. Cells were
scratched with a 10-µl pipette tip and cell debris was removed. An
inverted microscope (Olympus Corp.) was used to capture images and
at time-points 0 and 24 h following scratching and wound healing
rate was calculated using ImageJ software (version 1.46r; National
Institutes of Health,).
RT-qPCR analysis
Total RNA from skin tissue and BJ cells was
extracted using a Total RNA Extraction kit (Invitrogen; Thermo
Fisher Scientific, Inc.) and RNA was reverse-transcribed into cDNA
using SuperScript III Reverse Transcriptase (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. SYBR
Green PCR kit (Qiagen) was used as the fluorophorem according to
the manufacturer's protocol. RT-qPCR was performed using 2 µl cDNA
as a template. The thermocycling conditions were 95˚C for 10 min,
95˚C for 15 sec and 60˚ for 30 sec for a total of 40 cycles.
β-actin was used as the reference gene and relative expression
levels were calculated according to the
2-ΔΔCq method (20). The sequences of all primers
(Shanghai Shenggong Biology Engineering Technology & Services
Co., Ltd.) were as follows: Collagen I forward,
5'-CCAGTCACCTGCGTACAGAACG-3' and reverse,
5'-GCCAGTGTCTCCTTTGGGTCC-3'; collagen III forward,
5'-AGGCAACAGTGGTTCTCCTG-3' and reverse, 5'-GAC
CTCGTGCTCCAGTTAGC-3', matrix metalloproteinase-1 (MMP-1) forward,
5'-CCGAGATCTCATGCACAGCTTTCCT CCACT-3' and reverse,
5'-CGGTTAACCGTCAATTTTTCC TGCAGTTG-3', α-smooth muscle actin (α-SMA)
forward, 5'-CCACCGCAAATGCTTCTAAGT-3' and reverse, 5'-GGC
AGGAATGATTTGGAAAGG-3'; and β-actin forward,
5'-GATCATTGCTCCTCCTGAGC-3' and reverse, 5'-CACCT
TCACCGTTCCAGTTT-3'.
Western blot analysis
Total protein from skin tissue and BJ cells was
extracted using RIPA buffer (Beyotime Institute of Biotechnology)
and then quantified using a Pierce™ BCA Protein assay kit (cat. no.
23225; Thermo Fisher Scientific, Inc.). A total of 40 µg
protein/lane was separated via SDS-PAGE (Mini-Protean-3; Bio-Rad
Laboratories, Inc.) and transferred to polyvinylidene difluoride
membranes (EMD Millipore). The membrane was blocked with 5% skimmed
milk powder solution for 1 h and then incubated with the following
primary antibodies at 4˚C overnight: Rabbit anti-collagen I
antibody (cat. no. ab34710; 1:2,000 dilution; Abcam), rabbit
anti-collagen III antibody (cat. no. ab7778; 1:5,000 dilution;
Abcam), rabbit anti-MMP-1 antibody (cat. no. ab137332; 1:1,000
dilution; Abcam), rabbit anti-α-SMA antibody (cat. no. YM-H0645;
1:500 dilution; Shanghai Yuan Mu Biotechnology Co., Ltd.) and
rabbit anti-β-actin antibody (cat. no. ab8227; 1:2,00 dilution;
Abcam). The protein bands were then incubated with secondary
antibody goat anti-rabbit IgG (cat. no. ab6721; 1:2,000 dilution;
Abcam) at room temperature for 2 h and treated with enhanced
chemiluminescence solution (Thermo Fisher Scientific, Inc.).
β-actin was used as the internal reference and the gray values of
protein bands were quantitatively analyzed by ImageJ software
(version 1.46r; National Institutes of Health).
Statistical analysis
All data were expressed as the mean ± standard
deviation and SPSS software (version 19.0; IBM Corp.) was used to
analyze data. One-way analysis of variance and Tukey's post hoc
test was used. All experiments were performed in triplicate.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Determination of skin healing rate in
rats
A rat model of deep second-degree scald burn was
established and the wound healing rate of each group was determined
(Fig. 1A and B). The degree of scald healing in each
group demonstrated different degrees of recovery in a
time-dependent manner following modelling. There was no significant
difference in recovery between the MC, IC and model groups.
However, at days 7, 14 and 21 following modelling, the healing rate
was significantly higher in the miR-27b inhibitor group and
significantly lower in the miR-27b group compared with the model
group, respectively (P<0.05). These results indicated that
miR-27b inhibition significantly accelerated the degree of scald
burn healing in rats, while overexpression of miR-27b had the
opposite effect.
Observation of tissue morphology and
collagen fibrosis in rats
HE staining was used to observe tissue morphology of
scald burn skin in rats in each group 21 days post-operation
(Fig. 2A). The skin tissue cells of
controls were organized and exhibited non-inflammatory cell
infiltration. The model, MC and IC groups exhibited disordered
cells and a certain degree of epidermal epithelialization.
Furthermore, cells in the inhibitor group were in an ordered
arrangement, were observed to increase in number and exhibited
re-epithelialization compared with the model group. The miR-27b
group demonstrated increased inflammatory cell infiltration and was
observed to exhibited a higher degree of pathological damage
compared with the model group.
Masson staining was used to observe the degree of
collagen fibrosis in the skin tissues of rats in each group
(Fig. 2B). The collagenous fibers
in the control group were organized, while the model, MC and IC
groups exhibited disordered fibers and low levels of collagen
synthesis. The number of fibroblasts observed to be stained with
Masson bluing solution in the inhibitor group was markedly higher
compared with the model group and the cells were arranged in an
orderly manner. Furthermore, the number of fibroblasts in the
miR-27b group was observably lower than that in the model group and
the collagen fibers were bulky.
MMP-1, α-SMA, collagen I and collagen
III expression in rat skin tissue
MMP-1, α-SMA, collagen I and collagen III expression
in skin tissues was determined using RT-qPCR and western blot
analysis. The mRNA and protein expression of these proteins in the
model, MC and IC groups were significantly lower compared with the
control group; however, there was no significant difference between
those groups (Fig. 3A and B; P<0.05). The expression of these
proteins in the inhibitor group was significantly increased
compared with the model group (P<0.05) and the reverse effect
was observed in the miR-27b group compared with the model group
(P<0.05). According to these results, miR-27b inhibition
upregulated MMP-1, α-SMA, collagen I and collagen III expression
in vivo, thereby accelerating the healing of scald burn of
the skin in rats.
Effects of miR-27b on the
proliferation and migration of BJ cells
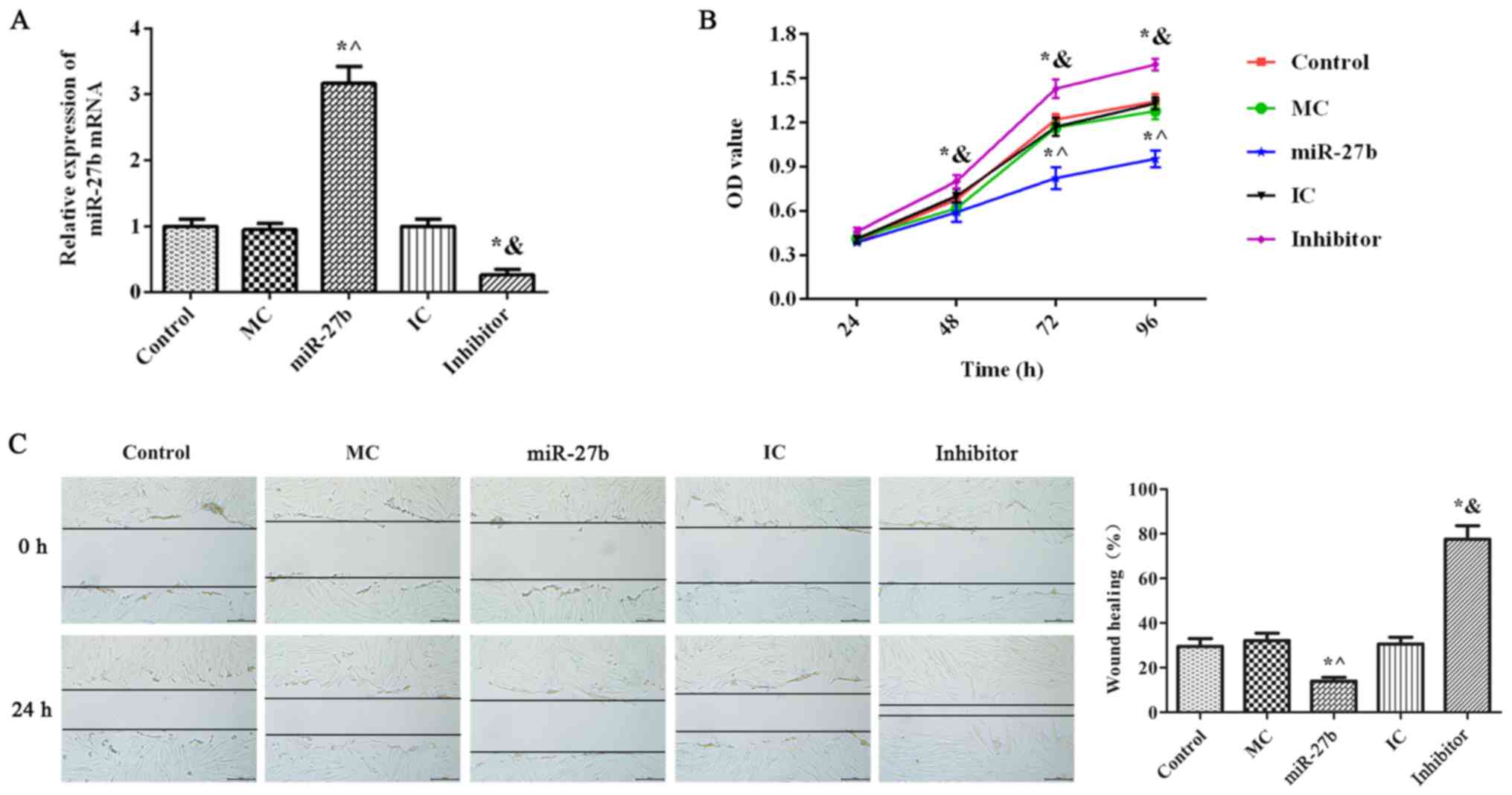
The RT-qPCR results demonstrated that there was no
significant difference in miR-27b expression between the MC, IC and
control groups (Fig. 4A;
P<0.05). miR-27b expression in the miR-27b group was
significantly increased compared with the MC and control groups
(P<0.05). Furthermore, miR-27b expression in the inhibitor group
was significantly decreased compared with the control and IC groups
(P<0.05), indicating the successful transfection of miR-27b
mimics and miR-27b inhibitors. Furthermore, there were no
significant differences in BJ cell proliferation and migration in
the MC and IC groups compared with the control group (Fig. 4B and C; P<0.05). BJ cell proliferation and
migration in the inhibitor group were significantly increased
compared with those in the IC group (P<0.05), whereas the
opposite effect was observed in the miR-27b group (P<0.05).
These results indicated that miR-27b inhibition significantly
increased BJ cell proliferation and migration.
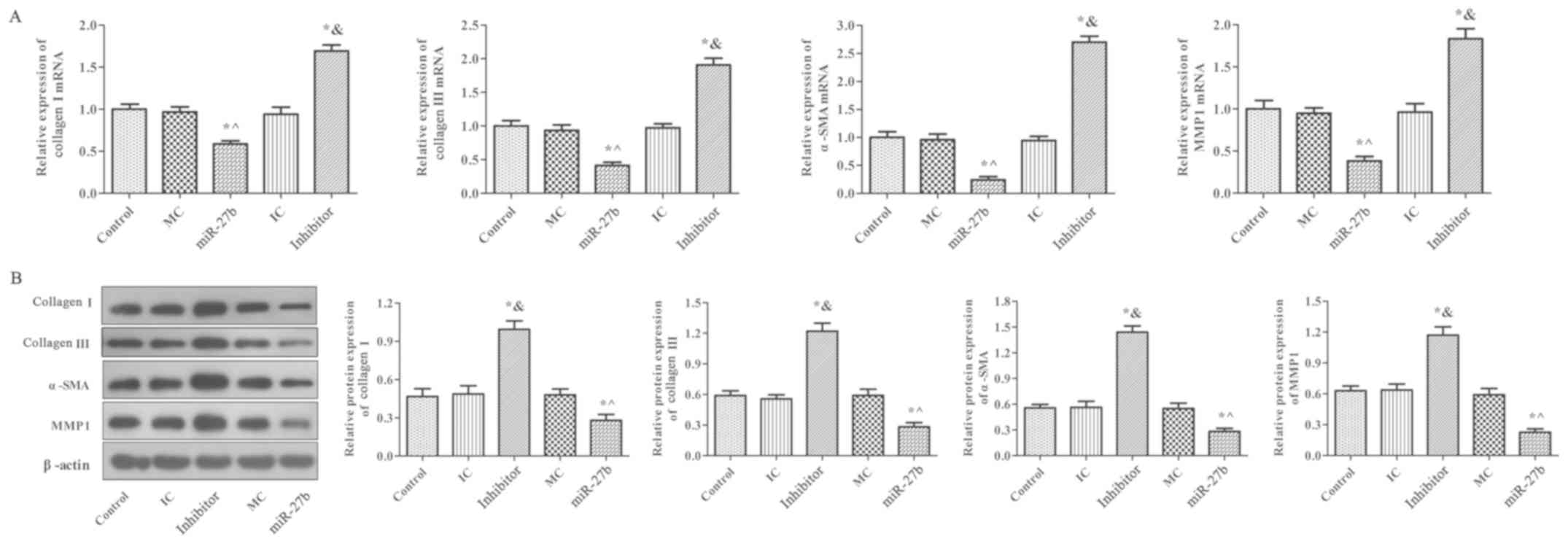
Effect of miR-27b on MMP-1, α-SMA,
collagen I and collagen III expression in BJ cells
MMP-1, α-SMA, collagen I and collagen III expression
in BJ cells was determined using RT-qPCR and western blot analysis
(Fig 5.). There was no significant
difference between the MC, IC and control groups (P﹥0.05).
Furthermore, mRNA and protein expression of these proteins in the
inhibitor group were significantly increased compared with the
control group (P<0.05) and the opposite effect was reported in
the miR-27b group (P<0.05). These results demonstrated that
miR-27b inhibition significantly upregulated MMP-1, α-SMA, collagen
I and collagen III expression in BJ cells. These results were
consistent with the results obtained in rats.
Discussion
Scald burns are a common type of injury in clinical
practice and wound management is an important part of scald
treatment (2,21). Infections are likely to occur during
wound healing, which cause the wound to deepen (22). Therefore, the effectiveness of
topical drug application to wounds directly affects the treatment
course (23). The healing rate and
time of scald burns may be used as direct, objective and effective
evaluation indexes of wound healing and measuring the wound healing
area may be used to further investigate the effect of drugs on
wound healing (24).
In the present study, the effects of miR-27b on the
healing rate of scalded skin were evaluated by establishing rat
models of deep second-degree scald burns and injecting agents such
as miR-27b mimics and miR-27b inhibitors into the wounds. The
results demonstrated that miR-27b inhibition observably improved
the healing rates of scald burns in rats, while miR-27b
overexpression had the opposite effect. This indicated that miR-27b
inhibition significantly accelerated the degree of scald healing in
rats and may be an effective therapeutic target to promote scald
repair.
Deep second-degree scald burns involve the deep
dermis and a small amount of residual deep skin attachments
(25). The fibroblasts proliferate
to fill the wound and the epidermal stem cells proliferate and
differentiate to form new epidermis and complete repair (26). In the present study, H&E and
Masson staining demonstrated that miR-27b inhibition on day 21
post-modelling significantly increased the number of fibroblasts
and collagen in the wound surface and reduced the infiltration of
inflammatory cell. Furthermore, in the wounds injected with miR-27b
mimics, a large number of inflammatory cell infiltration, low
amounts of new collagen and bulky collagen fiber morphology in
scalded skin tissues were observed. The results also indicated that
miR-27b inhibition observably improved the morphology of scalded
skin tissue and the degree of collagen fibrosis in rats.
Repaired skin tissue and cells require fibroblast
proliferation for the healing of scald burns (27). Fibroblasts, the primary cells
involved in wound healing, serve a vital role by multiplying,
synthesizing and secreting collagen fibers and matrix components to
improve wound healing (28). A
previous study confirmed that increasing fibroblast proliferation
and migration effectively promotes the healing speed of scald burns
(29). Due to this, the present
study hypothesized that inhibiting miR-27b expression may promote
BJ cell proliferation and migration, thereby accelerating the
healing of scald burns.
Scald wound healing involves angiogenesis,
granulation tissue generation, extracellular matrix (ECM) protein
synthesis, collagen storage and tissue remodeling (30). Collagen is an extracellular fibrin
that promotes the formation of the intracellular matrix and repair
of damaged cell structures (31).
Type I and III collagen are the major components of collagen in the
ECM and serve a major role in wound healing (32). The results of the present study
demonstrated that miR-27b inhibition significantly upregulated the
mRNA and protein expression of collagen I and III in scalded skin
tissues of rats and in BJ cells, which may be associated with the
accelerated healing of scalded skin in rats.
α-SMA is a biomarker of activated fibroblasts and
participates in the synthesis of type Ⅰ and Ⅲ collagen, and
promotes fibrosis (33). A previous
study reported that increased α-SMA expression promotes the
transformation of fibroblasts into myofibroblasts, accelerates
wound contraction and shortens the healing time (27). Furthermore, MMPs are a group of
proteases secreted by effector cells and are involved in ECM
degradation (34). MMPs serve
important roles in early wound clearance of necrotic tissue and are
required for epithelial cell migration to infiltrate the wound
surface (35). As the repair
process progresses, MMPs are primarily located in the basal
membrane expressed in fibroblasts in the late stages of tissue
healing (36). MMP-1 is
fibroblast-type collagenase, which promotes the clearance of
necrotic tissue and serves an important role in ECM degradation
(37). The results of the current
study revealed that miR-27b inhibition significantly increased the
mRNA and protein expression of α-SMA and MMP-1 in scalded skin
tissues of rats and in BJ cells. It has been previously reported
that human adipose mesenchymal stem cells promote the
differentiation and proliferation of fibroblasts by upregulating
MMP-1, α-SMA, collagen I and collagen III expression in skin wound
tissues, thereby accelerating skin wound repair (38). Therefore, the current study
suggested that inhibiting miR-27b expression may be a strategy to
promote the healing of scalded skin in rats by upregulating the
expression of MMP-1, α-SMA, collagen I and collagen III.
In summary, the results of the present study
demonstrated that miR-27b inhibition significantly promoted the
growth, proliferation and collagen synthesis of cultured BJ cells
in vitro. These results were consistent with the
observational in vivo animal experiments, which revealed
that miR-27b inhibition increased the wound healing rate and
promoted wound collagen production. Therefore, in conclusion, the
present study posits that promoting fibroblast proliferation and
ECM synthesis is a possible method of inhibiting miR-27b expression
in order to promote skin wound healing in rats.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL conducted the experiments, data collection and
interpretation. XW participated in study design, coordination of
the experiments and data collection. QB participated in study
design, data collection and data analysis and prepared the
manuscript. FS participated in study design, data analysis, data
interpretation and wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Committee on
Animal Protection and Use of the Affiliated Yantai Yuhuangding
Hospital of Qingdao University (approval no. 2018-081303) and was
conducted in strict accordance with the National Institute of
Health guidelines (pub. no. 85-23; revised 1996).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kleina HT, Pádua T, Jacomino AP and May De
Mio LL: Postharvest quality of plums in response to the occurrence
of leaf scald disease. Postharvest Biol Technol. 143:102–111.
2018.
|
|
2
|
Andrews CJ, Kimble RM, Kempf M and Cuttle
L: Evidence-based injury prediction data for the water temperature
and duration of exposure for clinically relevant deep dermal scald
injuries. Wound Repair Regen. 25:792–804. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu X, Zhao Y, Gao Y, Li D, Hu G, Zhu M,
Yi K and Shao J: Modulations of the plasma scald on the downstream
beam. Opt Commun. 355:290–295. 2015.
|
|
4
|
Zhou J, Gao Z, Sun Y, Chen X, Wu X and
Wang F: Effects of hypertonic sodium saline resuscitation on the
liver damage of rats at early stage of severe scald. Zhonghua Shao
Shang Za Zhi. 33:31–36. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
5
|
Ma M, Jiang T, Li N, Aliya A and Tuhan A:
Treatment and mechanism of BMMSCs on deep II degree scald of
hamster skin. Genet Mol Res. 14:8244–8251. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xi P, Li Y, Ge X, Liu D and Miao M: Effect
of nano-silver hydrogel coating film on deep partial thickness
scald model of rabbit. Saudi J Biol Sci. 25:797–800.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li D, Shang Y, Shen C, Li L, Zhao D, Ma L
and Yu Y: Effects of Exendin-4 on pancreatic islets function in
treating hyperglycemia post severe scald injury in rats. J Trauma
Acute Care Surg. 85:1072–1080. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liao X, Luo X, Dai L, Huang H and Guo X:
Experimental study on adipose derived stem cells combined with
chitosan chloride hydrogel for treating deep partial thickness
scald in rats. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi.
33:101–109. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
9
|
Shen C and Li D: 113 Effects of Exendin-4
on Pancreatic Islets Function in Treating Hyperglycemia Post Severe
Scald Injury in Rats. J Burn Care Res. 40 (Suppl. 1):S72–S73.
2019.
|
|
10
|
Thomou T, Mori MA, Dreyfuss JM, Konishi M,
Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R,
Grinspoon SK, et al: Adipose-derived circulating miRNAs regulate
gene expression in other tissues. Nature. 542:450–455.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Vidigal JA and Ventura A: The biological
functions of miRNAs: Lessons from in vivo studies. Trends Cell
Biol. 25:137–147. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Michael JV, Wurtzel JGT, Mao GF, Rao AK,
Kolpakov MA, Sabri A, Hoffman NE, Rajan S, Tomar D, Madesh M, et
al: Platelet microparticles infiltrating solid tumors transfer
miRNAs that suppress tumor growth. Blood. 130:567–580.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Colden M, Dar AA, Saini S, Dahiya PV,
Shahryari V, Yamamura S, Tanaka Y, Stein G, Dahiya R and Majid S:
MicroRNA-466 inhibits tumor growth and bone metastasis in prostate
cancer by direct regulation of osteogenic transcription factor
RUNX2. Cell Death Dis. 8(e2572)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu L, Bi N, Wu L, Ding X, Men Y, Zhou W,
Li L, Zhang W, Shi S, Song Y, et al: MicroRNA-29c functions as a
tumor suppressor by targeting VEGFA in lung adenocarcinoma. Mol
Cancer. 16(50)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li D and Landén NX: MicroRNAs in skin
wound healing. Eur J Dermatol. 27S:S12–S14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Soliman AM, Das S, Abd Ghafar N and Teoh
SL: Role of MicroRNA in Proliferation Phase of Wound Healing. Front
Genet. 9(38)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Veliceasa D, Biyashev D, Qin G, Misener S,
Mackie AR, Kishore R and Volpert OV: Therapeutic manipulation of
angiogenesis with miR-27b. Vasc Cell. 7(6)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mu W, Hu C, Zhang H, Qu Z, Cen J, Qiu Z,
Li C, Ren H, Li Y, He X, et al: miR-27b synergizes with anticancer
drugs via p53 activation and CYP1B1 suppression. Cell Res.
25:477–495. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bader A, Ebert S, Giri S, Kremer M, Liu S,
Nerlich A, Günter CI, Smith DU and Machens HG: Skin regeneration
with conical and hair follicle structure of deep second-degree
scalding injuries via combined expression of the EPO receptor and
beta common receptor by local subcutaneous injection of nanosized
rhEPO. Int J Nanomedicine. 7:1227–1237. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sahu SA, Agrawal K and Patel PK: Scald
burn, a preventable injury: Analysis of 4306 patients from a major
tertiary care center. Burns. 42:1844–1849. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ramirez JI, Thomas DM, Neal DJ and Maguina
P: A new injury prevention target: Summer hair braids. J Burn Care
Res. 39:911–914. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhong L, Zhan JH, Luo JH and Cheng X:
Effects of astragalus polysaccharide on cardiac dysfunction in
rabbits with severe scald injury. Zhonghua Shao Shang Za Zhi.
33:668–676. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
24
|
Johnson BL III, Rice TC, Xia BT, Boone KI,
Green EA, Gulbins E and Caldwell CC: Amitriptyline usage
exacerbates the immune suppression following burn injury. Shock.
46:541–548. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gao H, Liu Q, Zhang N, Wang H, Liu H and
Sun H: Effect of Fenghua Scald Ointment on the Content of TNF-α and
IL-10 in Rats with II-degree Deep Burn. Xiandai Shengwu Yixue
Jinzhan. 2015(6)2015.
|
|
26
|
de Campos EP, Trombini LN, Rodrigues R,
Portella DL, Werner AC, Ferraz MC, de Oliveira RVM, Cogo JC,
Oshima-Franco Y, Aranha N, et al: Healing activity of Casearia
sylvestris Sw. in second-degree scald burns in rodents. BMC Res
Notes. 8(269)2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xu HL, Chen PP, ZhuGe D-L, Zhu Q-Y, Jin
B-H, Shen B-X, Xiao J and Zhao Y-Z: ZhuGe DL, Zhu QY, Jin BH, Shen
BX, Xiao J and Zhao YZ: Liposomes with Silk Fibroin Hydrogel Core
to Stabilize bFGF and Promote the Wound Healing of Mice with Deep
Second-Degree Scald. Adv Healthc Mater. 6(1700344)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu R, Wang S-M, Li Z-Y, Yu W, Zhang H-P
and Zhou F-Q: Pyruvate in reduced osmolarity oral rehydration salt
corrected lactic acidosis in sever scald rats. J Surg Res.
226:173–180. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pelizzo G, Avanzini MA, Mantelli M, Croce
S, Maltese A, Vestri E, De Silvestri A, Percivalle E and Calcaterra
V: Granulation tissue-derived mesenchymal stromal cells: A
potential application for burn wound healing in pediatric patients.
J Stem Cells Regen Med. 14:53–58. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang XG, Li XM, Zhou XX, Wang Y, Lai WY,
Liu Y, Luo YC and Zhang JQ: The Wound Healing Effect of
Callicarpa nudiflora in Scalded Rats. Evid Based Complement
Alternat Med. 2019(1860680)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhan DC, Shen YS, Zhao YR and Meng FJ:
Efficacy and safety of basic fibroblast growth factor in the
treatment of burns: Protocol for a systematic review and
meta-analysis of randomized controlled trials. Medicine
(Baltimore). 98(e15102)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Baptista VIA, Quintana HT, Lazzarin MC,
Benfato ID, De Carvalho FP, Le Sueur-Maluf L, De Oliveira CAM,
Baptista JDS and De Oliveira F: Short time insulin treatment post
burn improves elastic-collagen rearrangement and reepithelization.
Connect Tissue Res. 60:230–239. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yen YH, Pu CM, Liu CW, Chen YC, Chen YC,
Liang CJ, Hsieh JH, Huang HF and Chen YL: Curcumin accelerates
cutaneous wound healing via multiple biological actions: The
involvement of TNF-α, MMP-9, α-SMA, and collagen. Int Wound J.
15:605–617. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yang ML, Li YH, Tan Q, Li JT and Que LL:
Effect of hydrocinnamoyl-L-valyl pyrrolidine on healing quality of
deep partial-thickness scald wound in mice. Zhonghua Shao Shang Za
Zhi. 32:658–666. 2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
35
|
Rocha J, Eduardo-Figueira M, Barateiro A,
Fernandes A, Brites D, Pinto R, Freitas M, Fernandes E, Mota-Filipe
H and Sepodes B: Erythropoietin reduces acute lung injury and
multiple organ failure/dysfunction associated to a scald-burn
inflammatory injury in the rat. Inflammation. 38:312–326.
2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li XF, Zhang XJ, Zhang C, Wang LN, Li YR,
Zhang Y, He TT, Zhu XY, Cui LL and Gao BL: Ulinastatin protects
brain against cerebral ischemia/reperfusion injury through
inhibiting MMP-9 and alleviating loss of ZO-1 and occludin proteins
in mice. Exp Neurol. 302:68–74. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kim JY, Lee SH, Bae IH, Shin DW, Min D,
Ham M, Kim KH, Lee TR, Kim HJ, Son ED, et al: Pyruvate Protects
against Cellular Senescence through the Control of Mitochondrial
and Lysosomal Function in Dermal Fibroblasts. J Invest Dermatol.
138:2522–2530. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang L, Hu L, Zhou X, Xiong Z, Zhang C,
Shehada HMA, Hu B, Song J and Chen L: Exosomes secreted by human
adipose mesenchymal stem cells promote scarless cutaneous repair by
regulating extracellular matrix remodelling. Sci Rep.
7(13321)2017.PubMed/NCBI View Article : Google Scholar
|