Introduction
Flexible bronchoscopy (FB) was developed in the
1960s. The principle mechanism is that light, originating from an
external source, is transmitted into the airway by optical
waveguide fiber (1,2). The endoscopic field can be observed by
eye, or in the case of modern bronchoscopic devices, on a monitor.
The condition of trachea and bronchia can be clearly observed and
determined by observation. FB functions as an important diagnostic
tool to identify the etiology of respiratory diseases (3). Some abnormalities, such as
tracheal-malacia or stenosis, which cannot be diagnosed by
radiological images, can be confirmed by FB (4). It can also be used to move foreign
bodies in the respiratory tract. Currently, FB is widely used in
the clinical practice of pediatrics (Table I). It has demonstrated fundamental
value in clinical diagnoses and treatment (5,6).
However, as an invasive procedure, the use of FB is limited due to
concerns regarding the tolerance of the procedure and the possible
complications in neonatal units. The purpose of the present study
was to evaluate the safety and efficacy of FB in the neonatal
intensive care unit (NICU).
 | Table IApplications of flexible
bronchoscopy. |
Table I
Applications of flexible
bronchoscopy.
| 1 | Tracheal, bronchial,
pulmonary dysplasia or abnormity. |
| | Primary alterations
include laryngomalacia, tracheobronchomalacia, laryngeal stenosis,
tracheobronchial stenosis and esophagotracheal fistula. |
| | Secondary alterations
include tracheobronchial compression caused by subglottic
hemangioma, pulmonary artery sling, vascular ring, cardiac
dilatation, and stenosis caused by intubation. |
| 2 | Atelectasis; using
bronchoscopy to check and/or offer bronchoalveolar lavage |
| 3 | Hemoptysis or
blood-stained sputum, using bronchoscopy to investigate the
pathogen and perform pathological examination |
| 4 | Chronic cough and
recurrent respiratory infection |
| 5 | Recurrent or
persistent stridor or wheezing |
| 6 | Diffused or focal
lesions in the lungs |
| 7 | Pulmonary
tuberculosis |
| 8 | Removal of foreign
bodies |
| 9 | Assistance in
diagnosis in some thoracic surgery |
| 10 | Assistance in
difficult intubations |
| 11 | Other treatments
including cryotherapy, interventional therapy and balloon
dilatation therapy |
Subjects and methods
Neonates and criteria
A total of 54 FB neonates admitted to the NICU of
Shanghai Children's Hospital (Shanghai, China) between January 2012
and December 2016 were enrolled in the present study according to
the Pediatric Bronchoscopy Guidelines (6). Among the 54 FB neonates in
experimental group, 37 (68.5%) were male and 17 (31.5%) were
female. Gestational age ranged from 207 to 290 days (261.87±19.72
days). The birth weight ranged from 1,300 to 4,400 g
(2,889.63±675.55 g). The postnatal age ranged from 2 to 290 days
(29.2±27.79 days). Inclusion criteria were neonates with recurrent
dyspnea or suspicious respiratory tract anomaly; with recurrent
pulmonary infection or atelectasis in the same lung lobe; with
suspicious tracheal stenosis in radiological image [X-ray or
computed tomography (CT) scan]; inability to be extubated without
clear reason; or confirmed congenital esophageal atresia to clarify
the presence and position of esophagobronchial fistula before
surgery (Fig. 1). Exclusion
criteria were multiple organ dysfunction; severe respiratory
diseases with high values of ventilation; severe congenital heart
disease or cardiac function failure; coagulopathy; present
hyperthermia; or preterm weight <1,500 g. Data were recorded in
detail, including gestational age, postnatal age, birth weight,
corrected gestational age while receiving FB, hospital stays,
ventilation status and outcomes. Vital signs reflecting FB
procedure, such as breathing rate, heart rate and oxygen saturation
(SaO2), were monitored. Bronchoalveolar lavage (BAL) was
performed when signs of endobronchitis were identified, and BAL
fluid was sampled for laboratory tests including cell count,
biochemistry and culture. Indicators including blood gas, CBC, CRP
and X-ray at 1 h before and 1 h after FB were ascertained. Patient
breathing rate, temperature and blood pressure following FB were
recorded. Another 54 neonates who required nebulization treatment
and tracheal secretion suction were set as the control group. The
neonates were also admitted to the NICU of Shanghai Children's
Hospital (Shanghai, China) between January 2012 and December 2016.
Among the 54 FB neonates, 36 (66.7%) were male and 18 (33.3%) were
female. The birth weight ranged from 1,250 to 4,500 g
(2,921.26±743.96 g). The postnatal age ranged from 4 to 282 days
(31.45±25.67 days). The present study was reviewed and approved by
the Ethics Committee of Shanghai Children's Hospital, China
(approval no. 2011-231). All families who participated in the
present study voluntarily signed informed consent. The primary
diseases diagnosed in the control group were pneumonia, neonatal
respiratory distress syndrome and wet lung.
The Olympus BFXP40 bronchoscope (Olympus Corp.) was
used for FB. The outer diameter of the probe was 2.8 mm, with a 1.2
mm operation tunnel. In addition to using the probe to observe the
trachea and bronchia, the operation tunnel, through which oxygen
suction can be conducted, was employed for BAL. Patients did not
eat from 4 h before FB to prevent vomiting and aspiration during
the procedure. In case of complications, such as laryngeal edema,
laryngospasm, or pneumothorax, the FB procedure was performed in
the resuscitation unit of the NICU. In addition to a bedside oxygen
inhalation tube, a tracheal tube, a resuscitation mask and a
suction machine were made ready. Midazolam was used as sedation,
with 0.1-0.3 mg/kg intravenous injection adopted prior to the FB
procedure. During the FB, midazolam 0.1 mg/kg/h intravenous
infusion was administered, and lidocaine hydrochloride mucilage was
administered as local anesthesia. The patients received oxygen by
nasal tube during the procedure.
Statistical analysis
All the statistical analyses were performed using
SPSS 17.0 software (SPSS, Inc.). Unpaired Student's t-test was used
for the comparison between twogroups and χ2 test was
used for enumeration data. P<0.05 was considered to indicate a
statistically significant difference.
Results
General data
A total of 56 FB procedures were conducted with 54
neonates, among whom two received FB twice. Among the 54 neonates,
33 (61.1%) used ventilation and 12 (22.2%) used nasal continuous
positive airway pressure; 8 cases received the FB procedure with
intubation. No significant differences in sex, gestational age,
birth weight or postnatal age were observed between the
experimental and the control groups (P>0.05; Table II).
 | Table IIGeneral data. |
Table II
General data.
| Characteristics | Experimental group
(n=54) | Control group
(n=54) | χ2 or
t-test | P-value |
|---|
| Sex
(male/female) | 37/17 | 34/20 | 0.37 | 0.54 |
| Gestational age
(days) | 261.87±19.72 | 263.56±18.31 | 0.46 | 0.65 |
| Birth weight
(g) | 2889.63±675.55 | 2915.72±688.31 | 0.2 | 0.84 |
| Postnatal age
(days) | 29.2±27.79 | 28.36±27.45 | 0.16 | 0.87 |
Results of FB
Among the 54 FB patients, 44 (81.5%) were identified
with varying degrees of airway abnormality. Endobronchitis was
observed in 9 cases (16.7%), while there was only 1 case (1.8%)
without any abnormalities. During the procedure, it was noticed
that in 3 patients the probe of FB could not pass through the
glottis; one case presented severe epiglottis malacia and severe
glottic stenosis was detected in 2 cases. There were 27 patients
(50%) with different degrees of respiratory tract malacia and 18
(33.3%) exhibited different degrees of respiratory tract stenosis.
In addition, 9 cases (16.7%) were diagnosed with both malacia and
stenosis while 6 cases (11.1%) had esophagotracheal fistula
(Figs.
2-7).
Outcomes
In the experiment group, 44 neonates (81.4%) were
discharged with improved condition, 5 (9.3%) succumbed and 5 (9.3%)
abandoned the treatment and left the hospital. Among the patients
who were discharged, 3 were treated with a tracheostomy and went
home with trachea cannula and 1 presented with epiglottic anomaly
and the FB could not pass the vocal cord. The other 2 cases
presented laryngomalacia combined with trachobronchomalacia. Among
the 5 mortalities, 1 was identified with severe glottic stenosis
and the FB could not pass, 1 was observed with
multiple-malformations of bones, annular stenosis at the middle of
trachea, stenosis at the opening of bilateral main bronchi and
severe stenosis at left lower bronchus. Severe endobronchitis with
tracheal stenosis, mild bronchomalacia with endobronchitis,
bronchomalacia combined with stenosis at the left main bronchus
were observed in the other 3 cases, respectively. These last 3
cases were all intubated, but succumbed as parents decided to
relinquish all medical treatments. In all, 34 cases were examined
by X-ray or CT scan prior to and following FB and improvement on
the radiological images was observed in 21 cases (61.8%).
BAL
Among the 54 patients, 28 received BAL. BAL fluid
samples were collected for cell counts, biochemistry and culture
test. For the BAL fluid culture, 24 cases tested as positive
(85.7%) while 4 were negative (14.3%). Consistent results between
the BAL fluid culture and respiratory secretion culture or tracheal
tube culture were identified in 21 cases (75%; Fig. 8).
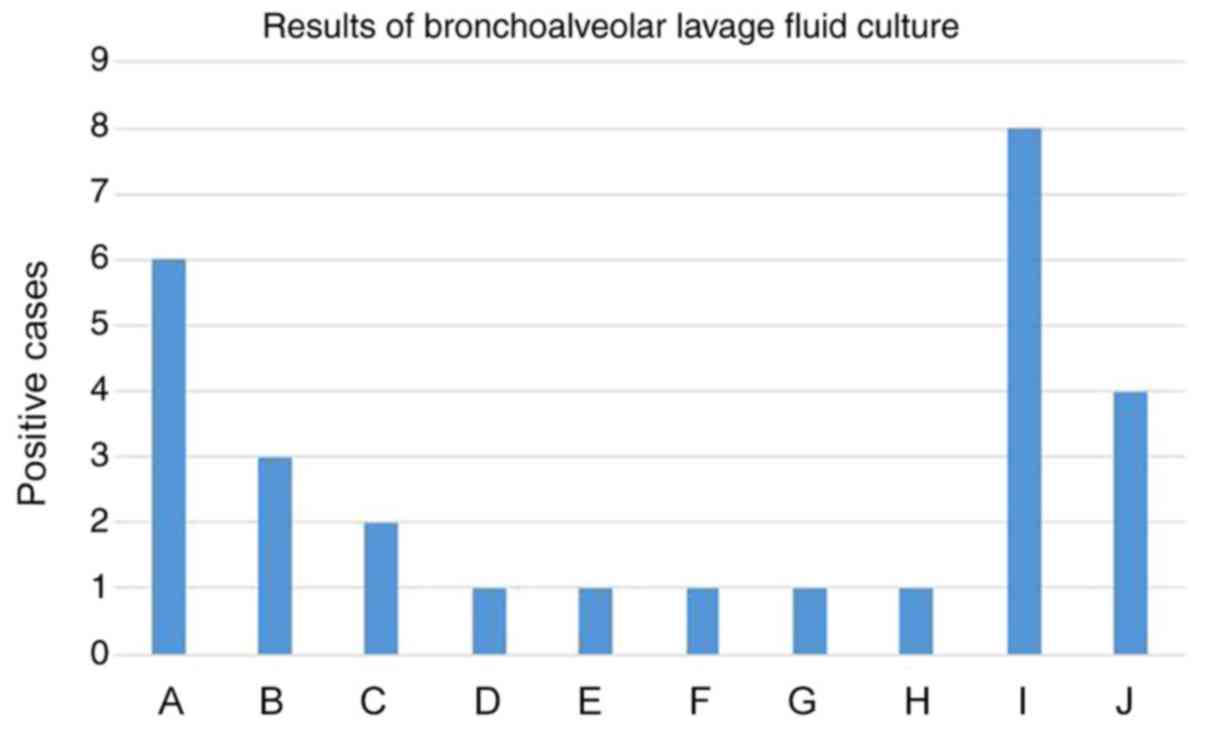 | Figure 8Results of bronchoalveolar lavage
fluid culture. A, Acinetobactor baumanii; B, Klebsiella
pneumoniae; C, Pseudomonas aeruginosa; D, Klebsiella
oxytoca; E, Escherichia coli; F, Staphylococcus
haemolyticus; G, Stenotrophomonas maltophilia; H,
Enterobacter cloacae; I, Streptococcus viridans
and/or Neisseria; J, Negative. |
Safety of FB
Blood gases were detected at 1 h before and 1 h
after FB for the patients in the experimental group. The results
demonstrated that there were no statistical differences in the
values of pH, partial pressure of carbon dioxide (PCO2)
and HCO3-. However, partial pressure of
oxygen (PO2) was significantly higher following FB
procedure compared with that prior to FB (P<0.05; Table III). Blood gases were also tested
at 1 h before and 1 h after atomization and secretion suction in
the patients of the control group. The results demonstrated that
there were no significant differences in pH, PCO2,
PO2 and HCO3- (Table IV). In comparison between the
experimental group and control group, no significant difference in
pH, PCO2, PO2 and HCO3-
was observed (Table V). No
statistical differences were observed in white blood cell count,
hemoglobin, platelet count and neutrophil ratio prior to and
following FB. Notably, CRP was significantly decreased following FB
(P<0.05; Table VI).
 | Table IIIBlood gas analysis before and after
flexible bronchoscopy. |
Table III
Blood gas analysis before and after
flexible bronchoscopy.
| Blood gas | Before | After | t-test | P-value |
|---|
| pH | 7.39±0.08 | 7.40±0.07 | -0.627 | 0.535 |
| PCO2
(mmHg) | 49.03±9.95 | 47.78±11.39 | 0.791 | 0.434 |
| PO2
(mmHg) | 46.83±13.36 | 58.32±24.10 | -2.688 | 0.011 |
|
HCO3- (mmol/l) | 28.77±5.07 | 29.19±5.00 | -0.787 | 0.436 |
 | Table IVBlood gas analysis before and after
atomization and secretion suction. |
Table IV
Blood gas analysis before and after
atomization and secretion suction.
| Blood gas | Before | After | t-test | P-value |
|---|
| pH | 7.35±0.10 | 7.36±0.09 | -1.477 | 0.148 |
| PCO2
(mmHg) | 47.93±12.47 | 45.65±10.66 | 1.253 | 0.218 |
| PO2
(mmHg) | 44.95±19.88 | 48.3±17.22 | -1.556 | 0.128 |
|
HCO3- (mmol/l) | 25.70±3.29 | 25.63±3.40 | 0.175 | 0.862 |
 | Table VBlood gas analysis comparison between
experimental and control groups. |
Table V
Blood gas analysis comparison between
experimental and control groups.
| Blood gas | Experimental
group | Control group | t-test | P-value |
|---|
| pH | -0.01±0.09 | -0.02±0.07 | 0.426 | 0.672 |
| PCO2
(mmHg) | 1.25±9.74 | 2.28±11.22 | -0.380 | 0.706 |
| PO2
(mmHg) | -11.5±26.37 | -3.35±13.28 | -1.707 | 0.096 |
|
HCO3- (mmol/l) | -0.43±3.33 | 0.07±2.41 | -0.756 | 0.455 |
 | Table VIComplete blood count and CRP before
and after flexible bronchoscopy. |
Table VI
Complete blood count and CRP before
and after flexible bronchoscopy.
| Complete blood
count | Before | After | t-test | P-value |
|---|
| White blood cells
(109/l) | 14.31±5.62 | 13.42±5.84 | 1.363 | 0.179 |
| Hemoglobin
(g/l) | 135.28±29.36 | 131.44±32.28 | 1.397 | 0.169 |
| Platelets
(109/l) | 382.94±170.24 | 385.42±158.37 | -0.187 | 0.852 |
| Neutrophils
(%) | 54.62±14.15 | 50.86±16.57 | 1.987 | 0.053 |
| CRP (mg/l) | 6.06±10.90 | 4.04±6.29 | 2.335 | 0.024 |
Complications
During the 56 FB procedures performed on 54
patients, SaO2 decreased to ~80% in 13 cases (23.3%).
After a transient pause, oxygen suction was provided through the
operation tunnel and SaO2 increased quickly in all 13
cases. The patients in all 13 cases could tolerate the intervention
until FB was completed. Mild tracheal or bronchial mucosa
hemorrhage occurred in 13 cases (23.3%), but no case presented
severe bleeding. No pneumothorax, shock or other severe
complications, which might impede the procedure were identified. No
fever or diffused pneumonia was observed following FB.
Discussion
Flexible bronchoscopy (FB) has developed rapidly in
pediatrics in recent years (7). In
addition to the value of observation and diagnosis as endoscopy, FB
can be used to remove foreign bodies, provide guidance for
bronchoalveolar lavage (BAL), intubation and balloon dilatation
surgery and as a tool for treatments (5,6). At
present, there are four different models of bronchoscope in
pediatrics. The inner diameters are 4.9, 3.5, 2.8 and 2.2 mm,
respectively, with optional operation tunnels. The smallest one is
the 2.2-mm diameter probe with no operation tunnel, which can only
be used to observe and diagnose. The 2.8-mm diameter probe is
widely used in neonatal wards, because it can be operated through a
3.5 or 4 mm tracheal tube and can be accompanied with an operation
tunnel for oxygen supply. Continuous or recurrent dyspnea and
stridor frequently occur in some patients in NICU. Occasionally the
confirmatory diagnosis cannot be made by regular radiological
examination. For these patients, FB presents an alternative to
detect anomalies of the respiratory tract. He et al
(8) reported that pathological
changes were identified in 73 cases out of 82 patients (89.0%), in
which 24 cases were diagnosed with congenital respiratory tract
malformations (39.3%). In the present study, respiratory
construction problems were identified in 44 cases out of 54
patients (81.5%). In NICU, due to different reasons, many
clinically indicated neonates cannot receive FB examination: It may
lead to misdiagnosis and affect the treatment outcomes, resulting
in a high positive rate of anomalies.
Respiratory tract malacia, including laryngomalacia,
tracheomalacia and bronchomalacia, represents the most common
respiratory malformation (9). In
neonates and children with stridor, the morbidity of laryngonalacia
is 60-70% (10). The majority of
patients with laryngomalacia do not require intervention. However,
some severe cases can progress to pulmonary hypertension and
pulmonary heart disease due to a lack of appropriate treatments. As
laryngonalacia is poorly tolerated in 10% of cases, assessment and
surgical management as well as management of any associated
gastro-esophageal reflux are often required to effectively control
symptoms (11). For instance, 5-20%
of children with severe or refractory disease may require a more
aggressive intervention, most commonly in the form of transoral
supraglottoplasty (12). Erdem
et al (13) reported that in
109 infants with stridor, 37 cases were identified with isolated
laryngomalacia, 54 cases with laryngomalacia with secondary airway
lesions, including tracheomalacia, bronchomalacia and
tracheobronchomalacia. Only 19 patients received surgery, of which
12 had a tracheostomy. The present study identified 14 patients
with laryngomalacia, of which three received tracheostomy. However,
Olney et al (14)
recommended that FB should not be routinely performed in
laryngomalacia; only if there is evidence of concomitant airway
lesion. The symptoms of tracheobronchomalacia are not distinctive,
but it is the most common cause of recurrent stridor and cough in
infants. According to its etiology, tracheomalacia can be divided
into two types: Primary tracheomalacia and secondary
tracheomalacia. Primary tracheomalacia is a congenital condition.
It may represent an isolated finding or be associated with other
congenital anomalies, such as cleft palate, choanal atresia and
esophageal atresia. Secondary tracheomalacia is always caused by
trauma, extratracheal compression, positive pressure ventilation,
respiratory tract infection or inflammation (15). In preterms with bronchopulmonary
dysplasia, secondary tracheomalacia is very common (16). The present study identified 19 cases
of tracheomalacia, bronchomalacia or tracheobronchomalacia out of
54 patients (35.2%). Neonates with respiratory tract malacia will
progress to obstruction with secretion following airway infection.
Malacia can lead to dyspnea, atelectasis, recurrent respiratory
infection and difficulty in extubation. Therefore, the positive
rate of respiratory tract malacia is high in the indicated neonates
of FB. The majority of these patients require only supportive
treatments, but some, who have severe symptoms of obstruction, may
require surgery (17-19).
Congenital heart disease is the main reason for secondary
tracheomalacia and tracheal stenosis. Lee et al (20) reported that in the children with
congenital heart disease and respiratory obstructive problems, 67%
of them were diagnosed by FB with tracheal stenosis due to
extratracheal compression. The present study identified two cases
with tracheal stenosis caused by extratracheal compression
associated with a large ventricular septal defect. FB is also
important to surgical departments. It offers useful assistance for
thoracic, otolarynological and some general surgeries (21-24).
BAL can remove the secretion in the respiratory
tract to remove obstructions. BAL fluid culture provides
confirmatory identification of the specific pathogen and guides the
anti-infection treatment. The testing of BAL fluid is an important
method to assist clinic diagnosis and predict outcomes (25-27).
Currently the research on BAL has moved from the cellular to the
molecular level (28-30).
There remains a safety concern about FB in neonatal
units and complications of FB are frequently identified, including
laryngeal edema, laryngeal spasm, hemorrhage, pneumothorax or
mediastinal emphysema, hypoxia and fever (31). However, in pediatric clinical
practice, severe complications rarely happen (32). It is even safe for patients who
receive extracorporeal membrane oxygenation treatment (33). Soong et al (34) reported that the oxygen supplied by
using nasopharyngeal catheter during FB is a simple and
cost-effective method to maintain appropriate SaO2. In
line with the previous findings, the present study illustrated the
favorable improvements due to FB in neonates, although there were a
number of side effects during the procedure. Overall, 44 neonates
(81.4%) were discharged with an improved condition, 5 cases (9.3%)
succumbed and 5 patients (9.3%) abandoned the treatment and left
the hospital. Overall, no significant difference in terms of pH,
PCO2, PO2 and HCO3- was
identified between the experimental and control groups. However,
PO2 was significantly increased, whereas CRP was
significantly reduced following FB procedure compared with prior to
FB (P<0.05). No fever or diffused pneumonia was observed
following FB. A limitation in the present study was that the
clinical safety requires further validation with a larger number of
patients in NICU. As for the sedation during the FB procedure,
midazolam in combination with fentanyl or alfentanil is the first
choice (28). More studies are
required to examine the best way to decrease damage caused by FB in
neonates.
In conclusion, FB possesses important value in
diagnosis and differentiation in neonatal respiratory diseases. BAL
can be a useful treatment in atelectasis. FB is relatively safe in
clinical practice in NICU and severe complications rarely
occur.
Acknowledgements
The authors would like to thank Dr Lu Min, Dr Shu
Linhua and Dr Gu Haoxiang of the Respiratory Department, Shanghai
Children's Hospital, Shanghai, China.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CY and YH contributed to the conception and design
of the study and performed the experiments. GQ, XG and DE
contributed to acquisition, analysis and interpretation of data. CY
drafted the manuscript and revised it critically for important
intellectual content and gave final approval of the version to be
published. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Ethics Committee of Shanghai Children's Hospital, Shanghai, China
(approval no. 2011-231). All families participated in the present
study voluntarily and signed informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pereira W, Kovnat DM, Khan MA, Iacovino
JR, Spivack ML and Snider GL: Fever and pneumonia after flexible
fiberoptic bronchoscopy. Am Rev Respir Dis. 112:59–64.
1975.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang W, Yang YX, Yu W and Qi SH: Cough
Suppression during flexible bronchoscopy using transcutaneous
electric acupoint stimulation: A randomized controlled study. Evid
Based Complement Alternat Med. 2019(5650413)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ndilanha DA, Shayo GA, Hassan R,
Byomuganyizi M and Lema LEK: Diagnoses from lung specimen collected
through flexible bronchoscopy from patients in a tertiary hospital
in Dar es Salaam Tanzania: A retrospective cross-sectional study.
BMC Pulm Med. 19(214)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sachdev A, Chhawchharia R, Gupta D and
Gupta N: Flexible fiberoptic bronchoscopy directed interventions in
neonatal intensive care unit. Indian Pediatr. 56:563–565.
2019.PubMed/NCBI
|
|
5
|
The Pediatric Bronchoscopy Collaborative
Group, the Subspecialty Group of Respiratory Diseases, the Society
of Pediatrics, Chinese Medical Association. Guide to pediatric
bronchoscopy (2009 edition). Chin J Pediatr. 47:740–744.
2009.PubMed/NCBI
|
|
6
|
Pérez-Frías J, Moreno Galdó A, Pérez Ruiz
E, Barrio Gómez De Agüero MI, Escribano Montaner A and Caro
Aguilera P: Sociedad Española de Neumología y Cirugía Torácica.
Pediatric bronchoscopy guidelines. Arch Bronconeumol. 47:350–360.
2011.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
7
|
Hysinger EB, Hart CK, Burg G, De Alarcon A
and Benscoter D: Differences in flexible and rigid bronchoscopy for
assessment of tracheomalacia. Laryngoscope: Apr 13, 2020 (Epub
ahead of print). doi: 10.1002/lary.28656.
|
|
8
|
He SR, Sun YX, Yu YH, Liu YM, Zhong J,
Liang SX, et al: The applications of flexible fiberoptic
bronchoscopy in NICU. J Clin Pediatr. 27:18–21. 2009.
|
|
9
|
Pan W, Peng D, Luo J, Liu E, Luo Z, Dai J,
Fu Z, Li Q and Huang Y: Clinical features of airway malacia in
children: A retrospective analysis of 459 patients. Int J Clin Exp
Med. 7:3005–3012. 2014.PubMed/NCBI
|
|
10
|
Daniel SJ: The upper airway: Congenital
malformations. Paediatr Respir Rev. 7 (Suppl 1):S260–S263.
2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ayari S, Aubertin G, Girschig H, Van Den
Abbeele T, Denoyelle F, Couloignier V and Mondain M: Management of
laryngomalacia. Eur Ann Otorhinolaryngol Head Neck Dis. 130:15–21.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Thorne MC and Garetz SL: Laryngomalacia:
Review and summary of current clinical practice in 2015. Paediatr.
Respir Rev. 17:3–8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Erdem E, Gokdemir Y, Unal E, Ersu R,
Karadag B and Karakoc F: Flexible bronchoscopy as a valuable tool
in the evaluation of infants with stridor. Eur Arch
Otorhinolaryngol. 270:21–25. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Olney DR, Greinwald JH Jr, Smith RJ and
Bauman NM: Laryngomalacia and its treatment. Laryngoscope.
109:1770–1775. 1999.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Majid A, Fernandez L, Bussy SF, Herth F
and Ernst A: Tracheobronchomalacia. Arch Bronconeumol. 46:196–202.
2010.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
16
|
Hysinger EB and Panitch HB: Paediatric
Tracheomalacia. Paediatr Respir Rev. 17:9–15. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wright CD: Treatment of congenital
tracheal stenosis. Thorac Cardiovasc Surg. 21:274–277.
2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jaquiss RD: Management of pediatric
tracheal stenosis and tracheomalacia. Semin Thorac Cardiovasc Surg.
16:220–224. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fraga JC, Jennings RW and Kim PC:
Pediatric tracheomalacia. Semin Pediatr Surg. 25:156–164.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lee S, Cheung YF, Leung MP, Ng YK and Tsoi
NS: Airway obstruction in children with congenital heart disease:
Assessment by flexible bronchoscopy. Pediatr Pulmonol. 34:304–311.
2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang ZY, Liang L, Liu WJ, Zhang Q, Jin DQ,
Jiang R, et al: Application of fiberoptic bronchoscopy on diagnosis
and treatment of respiratory comorbidity in children with
congenital heart disease. J Appl Clin Pediatr. 26:260–261.
2011.
|
|
22
|
Sun YX, He SR, Liang SX, Zhong J, Liu YM,
Ge PJ, et al: Dilation guided by fiberoptic bronchoscope treating
for subglottic stenosis in infants. J Appl Clin Pediatr.
25:1758–1761. 2010.
|
|
23
|
Atzori P, Lacobelli BD, Bottero S,
Spirydakis J, Laviani R, Trucchi A, Braguglia A and Bagolan P:
Preoperative tracheobronchoscopy in newborns with esophageal
atresia: Does it matter? J Pediatr Surg. 41:1054–1057.
2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Parolini F, Boroni G, Stefini S, Agapiti
C, Bazzana T and Alberti D: Role of preoperative
tracheobronchoscopy in newborns with esophageal atresia: A review.
World J Gastrointest Endosc. 6:482–487. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Antabak A, Luetic T, Caleta D and Romic I:
H-type tracheoesophageal fistula in a newborn: Determining the
exact position of fistula by intra-operative guidewire placement. J
Neonatal Surg. 3(36)2014.PubMed/NCBI
|
|
26
|
Finke MD: Transtracheal wash and
bronchoalveolar lavage. Top Companion Anim Med. 28:97–102.
2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Torre O and Harari S: The diagnosis of
cystic lung diseases: A role for bronchoalveolar lavage and
transbronchial biopsy? Respir Med. 104 (Suppl 1):S81–S85.
2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Palomares LJ, Juan JM, Izquierdo LG,
Dominguez AC, Becerra ER and Panadero FR: Bronchoalveolar lavage
findings in patients with diffuse interstitial lung disease:
Prospective study of a cohort of 562 patients. Arch Bronconeumol.
45:115–121. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Noël-Georis I, Bernard A, Falmagne P and
Wattiez R: Database of bronchoalveolar lavage fluid proteins. J
Chromatogr B Analyt Technol Biomed Life Sci. 771:221–236.
2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wattiez R and Falmagne P: Proteomics of
bronchoalveolar lavage fluid. J Chromatogr B Analyt Technol Biomed
Life Sci. 815:169–178. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tang LF, Xu YC, Wang YS, Wang CF, Zhu GH,
Bao XE, Lu MP, Chen LX and Chen ZM: Airway foreign body removal by
flexible bronchoscopy: Experience with 1027 children during
2000-2008. World J Pediatr. 5:191–195. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen WM, Lu GP, Lu ZJ and Zhang LE: The
application of bronchofibroscope in PICU. Chin Pediatr Emergency
Med. 18:129–132. 2011.
|
|
33
|
Kamat PP, Popler J, Davis J, Leong T,
Piland SC, Simon D, Harsch A, Teague WG and Fortenberry JD: Use of
flexible bronchoscopy in pediatric patients receiving
extracorporeal membrane oxygenation (ECMO) Support. Pediatr
Pulmonol. 46:1108–1113. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Soong WJ, Lee YS, Tsao PC, Yang CF and
Jeng MJ: Comparison of oxygenation among different supplemental
oxygen methods during flexible bronchoscopy in infants. J Chin Med
Assoc. 74:556–560. 2011.PubMed/NCBI View Article : Google Scholar
|
















