Introduction
Diabetes mellitus (DM) has now become the third most
common non-communicable disease after cardiovascular diseases and
malignant tumors, that seriously threatens human life and health
(1). WHO research data show that,
with the improvement of living standards and changes in living
behavior, the number of global diabetes patients is predicted to
increase from 415 million in 2015 to 642 million by 2040, bringing
a severe problem in prevention and control of the disease (2). Diabetes peripheral neuropathy (DPN) is
one of the most common complications of type 2 diabetes, and it is
also the most common clinical cause of neuropathy (3). The main clinical manifestations of DPN
are burning pain in the distal limbs; however the early symptoms
are not obvious. If not properly controlled, the symptoms gradually
worsen and reversal in the later stages is difficult, eventually
resulting in progressive aggravation of pain and serious impact on
the quality of life of patients. According to the International
Diabetes Federation Survey (4), the
prevalence of DPN in patients with DM is as high as 30-50%, with
diabetes foot (DF) being the most common DPN manifestation in
severe cases, resulting in foot ulcers, gangrene and even
amputations. If early evaluation, diagnosis and treatment of
patients with DPN can be performed, the incidence of lower
extremity ulcers and amputations would be effectively reduced. The
incidence of risk could be reduced by 60 and 85%, respectively
(4); therefore, early prevention
and control is extremely important. DPN pathological changes often
occur when they are asymptomatic, thus early assessment of their
risk factors and active personalized nursing interventions are the
key to prevent and reduce the incidence of DPN (5). This research statistically analyzed
the general data parameters and related clinical data parameters of
patients with DM. Multivariate logistic regression was used to
analyze the independent risk factors for DPN. Individualized
comprehensive nursing intervention was applied for patients with
DPN in order to provide theoretical basis for comprehensive
prevention and treatment of DPN.
Patients and methods
Patients and clinical materials
One hundred and thirty patients with type 2 diabetes
mellitus (T2DM) in the community surrounding Weifang People's
Hospital from January 2017 to June 2018 were selected as the
research subjects. They were divided into a DPN group (62 cases)
and non-DPN group (68 cases) according to the presence or absence
of peripheral neuropathy. A unified questionnaire was used to
investigate the general clinical data of patients, including sex,
age, body mass index (BMI), comorbidities, blood pressure and life
history. Patient biochemical indexes were regularly tested
according to research requirements. Inclusion criteria were as
follows: i) T2DM and DPN diagnostic criteria were in line with the
2013 American Diabetes Association (ADA) Guidelines and the ADA
Position Statement on Peripheral Diabetes Neuropathy (2017)
(6); ii) Peripheral neuropathy
included symmetrical peripheral neuropathy and asymmetric
neuropathy. Symmetrical peripheral neuropathy refers to multiple
peripheral neuropathy with symmetrical distal limbs, distal muscle
weakness and muscular atrophy, reduction or disappearance of tendon
reflexes, and may also be accompanied by impairment of autonomic
nerve function. Asymmetric neuropathy included early onset of
proximal weakness of one leg and muscular atrophy, about half of
them gradually involved the proximal pelvic girdle muscles of both
legs, manifested as difficulty in standing up, walking and stepping
on stairs, often accompanied by sharp pain in the deep thigh and
lumbosacral area; iii) Patients were without barrier in language or
communication, with clear awareness of information; iv) Patients
agreed to participate in the research and cooperated with
researchers. Exclusion criteria were as follows: i) Patients
without the ability to act independently and unable to communicate
normally, and who did not cooperate with the researcher; ii)
Patients with type 1 diabetes; iii) Patients with secondary
diabetes; iv) patients with peripheral nerve pain caused by
infectious or other metabolic diseases; v) Patients with other
orthopedic and neurological diseases, or severe heart, liver and
kidney dysfunction. There was no significant difference in age, sex
and other general data between the two groups (P>0.05), which
was comparable. This study was approved by the Ethics Committee of
Weifang People's Hospital (Slunlh:20161223).
Methods
Laboratory index inspection
Biochemical indexes (blood glucose, blood lipids,
creatinine) and urine microalbumin were detected by Siemens
automatic biochemical analyzer ADVIA2400 (Siemens), and reagents
for blood glucose, blood lipids, creatinine and urine microalbumin
were provided by Siemens. HbAlc was detected by the G8 glycosylated
hemoglobin analyzer (Japan Tosoh Co., Ltd.). Body mass index
(BMI)=weight (kg)/the square of the height (m). Biochemical
indicators were evaluated every three months.
Intervention methods
Due to the convenience of community health services,
patients were followed up via various forms (telephone,
door-to-door follow-up, visits to the community service
center).
Intervention measures
Patients in both groups were given routine care. The
DPN group received targeted personalized comprehensive nursing
intervention on the basis of routine care for six months.
Comprehensive interventions were implemented, such as health
education, diet intervention, exercise intervention, medication
intervention and prevention of complications for diabetes patients.
First, health education for patients included oral education,
brochures, multimedia promotion, and patient interaction. Multiple
modes are used to spread knowledge of DNP, allowing patients to
fully understand the risk factors, hazards, and the importance of
prevention of DPN. The patients must receive strengthened
psychological care and self-intervention management (7). Second, diet intervention is the basis
to control blood glucose (8). A
personalized diet plan should be developed according to the
specific circumstances of the patient. According to the patient's
age, physique and condition, nursing staff should calculate
calories and nutrients every day, reduce high-calorie and
high-sugar foods, and add foods with rich coarse fiber. The
proportion of the three meals should be reasonable, and the
calories for breakfast, lunch and dinner are 1/5, 2/5 and 2/5,
respectively. Patients should quit alcohol and tobacco, change poor
lifestyle and behavior habits, develop good eating habits, so as to
control blood sugar, which can significantly reduce the occurrence
of chronic complications of T2DM (9). Third, the goals of medication, correct
medication methods and medication precautions should be explained
to the patients. The patients should be guided to improve the
awareness in blood sugar self-testing and self-management ability.
Fourth, diabetes foot is a common type of diabetes peripheral
neuropathy (10), thus patients
should strengthen foot care and exercise, promote blood
circulation, improve the peripheral nerve nutrition of the
patient's limbs, which could significantly reduce the incidence of
diabetes foot. Diabetic foot ulcers should be treated as soon as
possible. Fifth, exercise can reduce obesity, insulin resistance,
and improve the body's regulation of blood glucose (11). Therefore, patients should be
encouraged to exercise according to their own conditions,
especially distal muscle strength training and endurance training,
so as to reduce the incidence of complications (12). All of the above interventions are
helpful to prevent and delay the occurrence and development of
diabetic peripheral neuropathy.
Evaluation
The SF-36 quality of life table (13) was used for quality of life
evaluation. There are 36 items in the SF-36 quality of life table:
10 items for Physiological function (PF), 4 items for Role physical
(RP), 3 items for Emotional function (RE), 6 items for General
health (GH), 2 items for Social function (SF), 5 items of Mental
health (MH), 2 items of Body pain (BP), 4 items of Vigor (VT), and
patients were evaluated in 8 dimensions. The higher the score, the
better the patient's physical function and mental and psychological
status.
Statistical methods
SPSS 25.00 statistical software (IBM Corp.) was used
for data processing. The continuous variables were expressed as
mean ± standard deviation. t-test was used for the comparison
between groups, and χ2 test was used for the comparison
between all categorial variable groups. DPN or non-DPN were
regarded as dependent variables, and statistically significant
parameters in univariate analysis were used as independent
variables. Multivariate logistic regression analysis was used to
analyze the peripheral nerves in type 2 diabetes mellitus.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Single factor analysis of general data
and laboratory data of the two groups of patients
Statistical analysis was performed on general
clinical data of the two groups of patients, including sex, age,
diastolic blood pressure, systolic blood pressure, body mass index
(BMI), duration of diabetes, smoking history, drinking history, and
laboratory data including glomerular filtration rate (GFR), 24 h
urinary microalbumin excretion (24hmALB), blood creatinine, fasting
blood glucose (FBG), postprandial blood glucose (PBG), glycated
hemoglobin (HbA1c), triglyceride (TG), total cholesterol (TC),
high-density lipoprotein cholesterol (HDL-C) and low-density
lipoprotein cholesterol (LDL-C). As shown in Table I, the results revealed that when
comparing the general clinical data between the DPN group and the
control group, there was no significant difference in sex, age, and
diastolic blood pressure (P>0.05); the body mass index (BMI) of
the DPN group was significantly larger than that of the control
group, with statistically significant difference (P<0.05); the
duration of diabetes in the DPN group was significantly longer than
that of the control group, and the difference was statistically
significant (P<0.001); the population of smoking and drinking
history in the DPN group was significantly increased compared with
the control group (P<0.05); the number of people with a family
history of diabetes in the DPN group was significantly higher
compared with the control group (P<0.05), and the systolic blood
pressure in the DPN group was significantly higher than that in the
control group (P<0.05). When comparing the DPN group with the
control group in terms of laboratory data, the DPN group was unable
to effectively control the long-term blood glucose, which caused
damage to the kidney glomerulus to varying degrees, resulting in a
significant reduction in glomerular filtration rate (GFR) and 24-h
urine microvolume. The albumin excretion volume (24hmALB) was
significantly increased, and the difference was statistically
significant compared with the control group (P<0.001). The serum
creatinine was significantly increased compared with the control
group (P<0.05). Patients in the DNP group were at high blood
glucose levels for an extended period of time, with impaired
glucose tolerance. The fasting blood glucose (FBG) was
significantly increased compared with the control group
(P<0.05). Postprandial blood glucose (PBG) and glycated
hemoglobin (HbA1c) were significantly increased compared with the
control group (P<0.001). Triglycerides (TG) in blood lipids were
significantly increased compared with the control group
(P<0.05), HDL cholesterol (HDL-C) was significantly decreased
compared with the control group (P<0.001).
 | Table IUnivariate analysis of general data of
the two groups of patients. |
Table I
Univariate analysis of general data of
the two groups of patients.
| Variable | DPN group (n=62) | Non-DPN group
(n=68) | χ2/t
value | P-value |
|---|
| Sex (n) |
|
Male | 34 | 37 |
χ2=0.291 | 0.590 |
|
Female | 28 | 31 | | |
| Age (years) | 65.32±5.79 | 54.12±5.37 | t=1.554 | 0.171 |
| BMI
(kg/m2) | 27.16±2.25 | 21.78±1.69 | t=2.986 | 0.020 |
| Course of disease
(years) | 15.25±1.43 | 8.67±1.54 | t=4.230 | <0.001 |
| Smoking history [n
(%)] | 35 (56.45) | 24 (38.71) |
χ2=5.857 | 0.016 |
| Drinking history [n
(%)] | 36 (58.06) | 26 (38.24) |
χ2=5.112 | 0.024 |
| Family history of
diabetes [n (%)] | 30 (48.39) | 20 (29.41) |
χ2=6.660 | 0.010 |
| Blood pressure
(mmHg) |
|
Systolic
pressure | 135.21±4.34 | 122.41±3.48 | t=2.521 | 0.045 |
|
Diastolic
pressure | 85.36±1.37 | 82.45±1.24 | t=1.727 | 0.135 |
| 24hmALB (mg/24
h) | 298.67±15.37 | 165.43±10.29 | t=7.860 | <0.001 |
| GFR [ml/(min/1.73
m2)] | 65.38±10.24 | 104.69±12.37 | t=-4.679 | <0.001 |
| Serum creatinine
(µmol/l) | 91.97±13.64 | 62.51±11.54 | t=2.423 | 0.049 |
| FBG (mmol/l) | 7.97±0.86 | 5.55±0.54 | t=2.842 | 0.029 |
| PBG (mmol/l) | 12.35±1.06 | 7.23±0.78 | t=5.262 | <0.001 |
| HbAlc (%) | 11.78±1.44 | 7.72±0.85 | t=5.712 | <0.001 |
| TG (mmol/l) | 3.45±0.84 | 1.77±0.51 | t=2.608 | 0.045 |
| TC (mmol/l) | 4.67±0.86 | 3.66±0.67 | t=1.015 | 0.349 |
| HDL-C (mmol/l) | 0.86±0.11 | 1.32±0.13 | t=4.689 | <0.001 |
| LDL-C (mmol/l) | 3.79±0.75 | 3.25±0.78 | t=0.547 | 0.604 |
Logistic regression analysis of risk
factors related to peripheral neuropathy in type 2 diabetes
mellitus
With DPN as the dependent variable, the
statistically significant parameters in the univariate analysis
were included in multivariate logistic regression analysis as
independent variables, including patient body mass index (BMI),
duration of diabetes, smoking history, drinking history, family
history of diabetes, systolic blood pressure, 24-h urine
microalbumin excretion (24hmALB), glomerular filtration rate (GFR),
blood creatinine, fasting blood glucose (FBG), postprandial blood
glucose (PBG), glycated hemoglobin (HbA1c), triglyceride (TG) and
high-density lipoprotein cholesterol (HDL-C). Multivariate logistic
regression analysis was included. As shown in Table II, the results revealed that the
duration of diabetes, PBG, HbA1c, HDL-C, 24hmALB, and GFR were
independent risk factors for DPN.
 | Table IILogistic regression analysis of
DPN-related risk factors. |
Table II
Logistic regression analysis of
DPN-related risk factors.
| Variable | B | SE | Wald | P-value | OR | 95% CI |
|---|
| BMI | 0.459 | 0.533 | 3.756 | 0.061 | 1.236 | 0.925-1.364 |
| Duration of
diabetes | 1.035 | 0.532 | 14.325 | <0.001 | 3.567 | 1.472-5.631 |
| Smoking
history | 0.674 | 0.369 | 3.012 | 0.097 | 1.110 | 0.567-1.964 |
| Drinking
history | 0.236 | 0.297 | 2.531 | 0.101 | 2.036 | 0.712-2.036 |
| Family history of
diabetes | 0.179 | 0.269 | 3.826 | 0.055 | 1.278 | 0.539-2.115 |
| FBG | 0.486 | 0.452 | 1.378 | 0.214 | 1.634 | 0.768-3.214 |
| PBG | 0.765 | 0.129 | 12.306 | <0.001 | 2.354 | 1.563-2.896 |
| HbAlc | 1.032 | 0.345 | 13.256 | <0.001 | 2.798 | 1.563-3.015 |
| TG | 0.4355 | 0.502 | 0.821 | 0.436 | 1.587 | 0.586-2.124 |
| HDL-C | 0.869 | 0.236 | 11.967 | <0.001 | 1.896 | 1.335-2.036 |
| Systolic
pressure | 0.642 | 0.521 | 0.621 | 0.524 | 1.253 | 0.657-1.962 |
| 24hmALB | 0.596 | 0.306 | 13.675 | <0.001 | 2.015 | 1.764-3.125 |
| GFR | 1.158 | 0.234 | 14.012 | <0.001 | 3.269 | 1.564-4.326 |
Comparison of the quality of life of
patients in the DPN group before and after comprehensive nursing
intervention
Six months after individualized comprehensive
nursing intervention, the quality of life indexes (PF, RP, RE, GH)
of patients in the DPN group were significantly higher than these
indexes before intervention (t=7.829, t=4.018, t=3.028, t=6.048;
P<0.001, P=0.001, P=0.015, P<0.001) (all P<0.05) (Fig. 1A). The quality of life indexes (SF,
MH, BP, VT) after intervention were higher than those before the
intervention (t=4.258, t=3.856, t=4.233, t=6.278; P=0.001, P=0.003,
P=0.001, P<0.001) (Fig. 1B).
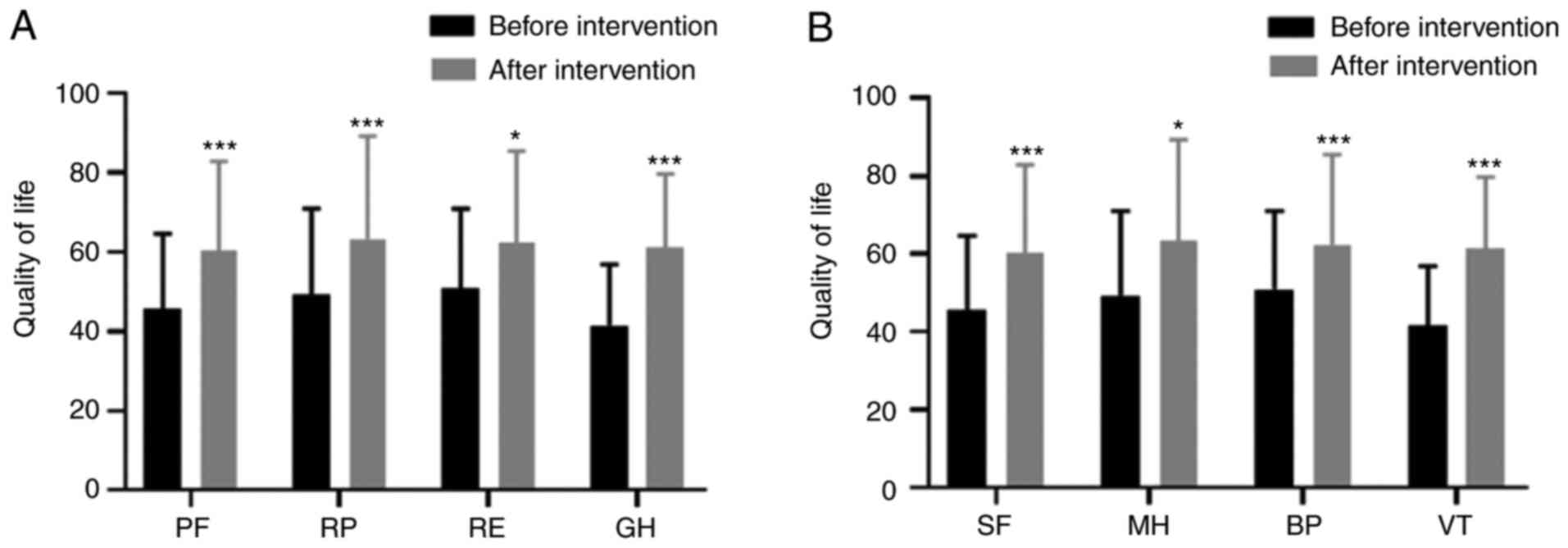 | Figure 1Comparison of life quality in the DPN
group before and after comprehensive nursing intervention. (A) The
quality of life indexes (PF, RP, RE, GH) of patients in the DPN
group were significantly higher than these indexes before
intervention (t=7.829, t=4.018, t=3.028, t=6.048; P<0.001,
P=0.001, P=0.015, P<0.001). (B) The quality of life indexes (SF,
MH, BP, VT) after intervention were higher than those before the
intervention (t=4.258, t=2.856, t=4.233, t=6.278; P=0.001, P=0.023,
P=0.001, P<0.001). *P<0.05 and
***P<0.001. PF, physiological function; RP,
role-physical; RE, role-emotional; GH, general health; SF, social
function; MH, mental health; BP, bodily pain; VT, vitality. |
Quality of life after comprehensive
nursing intervention in the DPN group compared with non-DPN control
group
There was no significant difference in the quality
of life (PF, RP, RE, GH, SF, MH, BP, VT) between the DPN group and
non-DPN group (t=0.103, t=0.208, t=0.234, t=0.318, t=0.391,
t=0.233, t=0.086, t=0.278; P=0.901, P=0.835, P=0.823, P=0.761,
P=0.709, P=0.824, P=0.934, P=0.792) (all P>0.05) (Table III).
 | Table IIIComparison of life quality between
the DPN group and the non-DPN group after intervention. |
Table III
Comparison of life quality between
the DPN group and the non-DPN group after intervention.
| Project | DPN group after
intervention | Non-DPN group | t value | P-value |
|---|
| PF | 66.82±14.53 | 64.42±11.98 | 0.103 | 0.901 |
| RP | 61.74±14.31 | 57.16±13.35 | 0.208 | 0.835 |
| RE | 56.37±13.01 | 59.83±13.69 | 0.234 | 0.823 |
| GH | 58.62±13.97 | 55.47±12.85 | 0.318 | 0.761 |
| SF | 60.25±14.29 | 56.80±13.57 | 0.391 | 0.709 |
| MH | 63.42±16.34 | 60.24±14.64 | 0.233 | 0.824 |
| BP | 62.37±14.59 | 64.12±13.30 | 0.086 | 0.934 |
| VT | 61.255±14.66 | 57.82±13.78 | 0.276 | 0.792 |
Discussion
At present, the pathogenesis of diabetes peripheral
neuropathy (DPN) is unclear, and scholars believe that factors such
as metabolic disorders, vascular endothelial damage, chronic
microinflammatory response, and oxidative stress are involved in
the pathogenesis (14). Toronto
Diabetes Neuropathy Committee (15)
believes that long-term hyperglycemia, cardiovascular risk and
other factors result in metabolic disorders and microvascular
changes, leading to chronic, symmetrical sensorimotor
polyneuropathy. DPN, as the most common diabetes mellitus (DM)
neuropathy today, has certain influence on the peripheral nervous
system. Symmetry of numbness, paresthesia and pain are the typical
clinical manifestation of DPN. If not controlled, DPN tends to
develop toward the center of body, then sock-like sensation loss
occurs in the feet, above the ankles, and in both hands (16). As a result, muscle sensory
abnormalities and proprioceptive dysfunction in patients may cause
joint disease, ulcers, and even gangrene in severe cases (17). Veves et al (18) found that quality of life scores,
such as behavior ability, physiological function, vigor, social
function and mental health, were significantly reduced because of
the impaired ability of action and balance caused by peripheral
nerve pain. DPN is also one of the main causes of non-traumatic
lower limb amputation. Therefore, early assessment of risk factors
and targeted care interventions are important to improve the
quality of life of these patients (19).
Metabolic disorders, vascular injury, cytokine
abnormalities, oxidative stress, neurotrophic factor deficiency,
and immune factors all play roles in the development of DPN
(20), of which long-term
hyperglycemia caused by metabolic disorders is the main cause of
DPN (21). Long-term substandard
blood glucose control leads to increased levels of non-enzymatic
glycosylation products in peripheral nerve peripheral neurons,
which can increase the flux of the polyphenol pathway, activate
protein kinase C and stimulate oxidative stress, enhance the
formation of late glycosylation end products, and cause
corresponding clinical symptoms such as peripheral nerve damage
(22). The duration of diabetes is
an independent risk factor for DPN. Studies have found that the
risk of developing DPN increases by 6.70% each year after the onset
of diabetes. The incidence of DPN in patients with DM increases
with a gradient of 30.00 (5 years), 60.00 (10 years) and 90.00% (20
years), respectively (23). The
glycated hemoglobin (HbA1c) level reflects the blood glucose levels
in patients with DM for 8-12 weeks, thus HbA1c can accurately
reflect the recent blood glucose control. Research found that for
every 1% increase in HbAlc, the risk of DPN increased by 5.30%,
indicating that HbA1c is also a risk factor for DPN (24). Type 2 diabetes mellitus (T2DM) is
caused by insufficient or relatively insufficient insulin
secretion, which causes high blood sugar. Sustained high blood
sugar can cause toxic effects on nerve cells and neurological
dysfunction, leading to peripheral neuropathy. The results of the
present study demonstrated that with the extension of the duration
of diabetes and unqualified glycemic control, the prevalence of DPN
increases significantly. Thus, the fundamental measures for the
prevention of DPN is to control blood sugar.
Abnormal blood lipid metabolism is also a risk
factor for DPN. Studies have found that patients with DPN have
higher peripheral blood TG, TC content, and lower HDL-C levels
(25). Wiggin et al
(26) explored the degree of DPN
neuropathy and blood lipids through nerve biopsy technology, and
found that a decrease in HDL-C levels in patients with DM can
accelerate the course of DPN. Padilla et al (27) found that palmitic acid, an important
component of blood lipids, is a long-chain saturated fatty acid,
which is involved in the occurrence of DPN, further suggesting a
close correlation between lipid levels and DPN. In addition, high
blood lipids could cause blood viscosity, and indirectly cause
peripheral blood vessel blockage. Peripheral blood supply is
reduced, and consequently nutrients do not reach peripheral nerve
cells through peripheral blood vessels, finally leading to DPN
(28). Research has confirmed that
diabetes nephropathy and DPN are strongly associated with low HDL-C
levels and have been proven to be independent risk predictors of
lower limb amputation and wound-related death in diabetes foot
ulcers (28). Multivariate logistic
regression results showed that HDL-C is an independent risk factor
for DPN among lipid metabolism-related parameters. In addition, it
has been demonstrated that microvascular complications also play an
important role in the development of DPN (29). Diabetes nephropathy is one of the
diabetes microvascular complications. A large amount of proteinuria
indicates that patients have a high risk of developing DPN, and a
low level of GFR [<60 ml/min/1.73 m2)] is an
important risk factor for DPN (30). This research also confirmed that
high levels of microalbuminuria and low levels of GFR were
independent risk factors for DPN. Although smoking history,
drinking history and BMI were not independent risk factors for DPN
after logistic regression in this study, smoking and drinking are
closely related to atherosclerosis. Atherosclerosis may increase
the risk of peripheral neuropathy by increasing vascular
endothelial dysfunction (31). In
addition, obesity is inextricably linked to insulin resistance,
which is usually exacerbated by fat accumulation. With the increase
in BMI, individuals are prone to insulin resistance (32). The insulin-mediated diastolic
function of vascular endothelial cells could be impaired, and the
risk of diabetic peripheral neuropathy is increased. The occurrence
and development of DPN are associated with multiple risk factors,
thus it is necessary to involve multiple risk factors in the early
comprehensive evaluation of DPN.
Community health services are integrated with
prevention, medical treatment, health care, rehabilitation and
health education. Community nursing has the characteristics of
convenience, economy and flexibility. In particular, there are
obvious advantages in nursing interventions for chronic diseases
(33), thus we should make full use
of the advantages of community health services. This research
showed that the quality of life of the DPN group intervention was
significantly improved compared with that before intervention, and
the effect was remarkable.
In conclusion, analyzing the risk factors of
developing DPN and implementing comprehensive and reasonable
interventions in the community can significantly improve the
quality of life of patients. There are certain limitations to this
study. The intervention time of this study was only 6 months. In
the future, we will make full use of the advantages of community
services to promote health education, and improve the level of
community health services.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL designed the study and drafted the manuscript. XL
and BX were responsible for the collection and analysis of the
experimental data. SW, TG and HL revised the manuscript critically
for important intellectual content. SW and TG were responsible for
the inspection of biochemical indexes (blood glucose, blood lipids,
creatinine) and urine microalbumin. HL was responsible for the
quality of life evaluation.. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Weifang People's Hospital, (Weifang, Shandong, China). Patients who
participated in this research, signed an informed consent and had
complete clinical data. Signed written informed consents were
obtained from all patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ang L, Cowdin N, Mizokami-Stout K and
Pop-Busui R: Update on the management of diabetic neuropathy.
Diabetes Spectr. 31:224–233. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Popkin BM: Nutrition transition and the
global diabetes epidemic. Curr Diab Rep. 15(64)2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Albers JW and Pop-Busui R: Diabetic
neuropathy: Mechanisms, emerging treatments, and subtypes. Curr
Neurol Neurosci Rep. 14(473)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hewston P and Deshpande N: Falls and
balance impairments in older adults with Type 2 diabetes: Thinking
beyond diabetic peripheral neuropathy. Can J Diabetes. 40:6–9.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Themistocleous AC, Ramirez JD, Shillo PR,
Lees JG, Selvarajah D, Orengo C, Tesfaye S, Rice AS and Bennett DL:
The pain in neuropathy study (PiNS): A cross-sectional
observational study determining the somatosensory phenotype of
painful and painless diabetic neuropathy. Pain. 157:1132–1145.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pop-Busui R, Boulton AJ, Feldman EL, Bril
V, Freeman R, Malik RA, Sosenko JM and Ziegler D: Diabetic
neuropathy: A position statement by the American Diabetes
Association. Diabetes Care. 40:136–154. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schillinger D, Grumbach K, Piette J, Wang
F, Osmond D, Daher C, Palacios J, Sullivan GD and Bindman AB:
Association of health literacy with diabetes outcomes. JAMA.
288:475–482. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Callaghan BC, Little AA, Feldman EL and
Hughes RA: Enhanced glucose control for preventing and treating
diabetic neuropathy. Cochrane Database Syst Rev.
13(CD007543)2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Miller CK, Edwards L, Kissling G and
Sanville L: Nutrition education improves metabolic outcomes among
older adults with diabetes mellitus: Results from a randomized
controlled trial. Prev Med. 34:252–259. 2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Im S, Kim SR, Park JH, Kim YS and Park GY:
Assessment of the medial dorsal cutaneous, dorsal sural, and medial
plantar nerves in impaired glucose tolerance and diabetic patients
with normal sural and superficial peroneal nerve responses.
Diabetes Care. 35:834–839. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lu B, Yang Z, Wang M, Yang Z, Gong W, Yang
Y, Wen J, Zhang Z, Zhao N, Zhu X, et al: High prevalence of
diabetic neuropathy in population-based patients diagnosed with
type 2 diabetes in the Shanghai downtown. Diabetes Res Clin Pract.
88:289–294. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liebreich T, Plotnikoff RC, Courneya KS
and Boulé N: Diabetes NetPLAY: A physical activity website and
linked email counselling randomized intervention for individuals
with type 2 diabetes. Int J Behav Nutr Phys Act.
6(18)2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Campolina AG and Ciconelli RM: SF-36 and
the development of new assessment tools for quality of life. Acta
Reumatol Port. 33:127–133. 2008.(In Portuguese). PubMed/NCBI
|
|
14
|
Qu GB, Wang LL, Tang X, Wu W and Sun YH:
The association between vitamin D level and diabetic peripheral
neuropathy in patients with type 2 diabetes mellitus: An update
systematic review and meta-analysis. J Clin Transl Endocrinol.
9:25–31. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Javed S, Alam U and Malik RA: Burning
through the pa in: Treatments for diabetic neuropathy. Diabetes
Obes Metab. 17:1115–1125. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tesfaye S and Selvarajah D: Advances in
the epidemiology, pathogenesis and management of diabetes
peripheral neuropathy. Diabetes Metab Res Rev. 28:8–14.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Djibril AM, Mossi EK, Djagadou AK, Balaka
A, Tchamdja T and Moukaila R: Epidemiological, diagnostic,
therapeutic and evolutionary features of diabetic foot: A study
conducted at the medico-surgical clinic, University Hospital
Sylvanus Olympio in Lomé. Pan Afr Med J 30: 4, 2018 (In
French).
|
|
18
|
Veves A, Backonja M and Malik RA: Painful
diabetic neuropathy: Epidemiology, natural history, early
diagnosis, and treatment options. Pain Med. 9:660–674.
2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kim SS, Won JC, Kwon HS, Kim CH, Lee JH,
Park TS, Ko KS and Cha BY: Prevalence and clinical implications of
painful diabetes peripheral neuropathy in type 2 diabetes mellitus:
Results from a nationwide hospital-based research of diabetes
neuropathy in Korea. Diabetes Res Clin Pract. 103:522–529.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Al-Mahroos F and Al-Roomi K: Diabetic
neuropathy, foot ulceration, peripheral vascular disease and
potential risk factors among patients with diabetes in Bahrain: A
nationwide primary care diabetes clinic-based study. Ann Saudi Med.
27:25–31. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pai YW, Lin CH, Lee IT and Chang MH:
Variability of fasting plasma glucose and the risk of painful
diabetic peripheral neuropathy in patients with type 2 diabetes.
Diabetes Metab. 44:129–134. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ziegler D, Strom A, Lobmann R, Reiners K,
Rett K and Schnell O: High prevalence of diagnosed and undiagnosed
polyneuropathy in subjects with and without diabetes participating
in a nationwide educational initiative (PROTECT study). J Diabetes
Complications. 29:998–1002. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Katulanda P, Ranasinghe P, Jayawardena R,
Constantine GR, Sheriff MH and Matthews DR: The prevalence,
patterns and predictors of diabetic peripheral neuropathy in a
developing country. Diabetol Metab Syndr. 4(21)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
McCarter RJ, Hempe JM and Chalew SA: Mean
blood glucose and biological variation have greater influence on
HbA1c levels than glucose instability: An analysis of data from the
diabetes control and complications trial. Diabetes Care.
29:352–355. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li G, Sun C, Wang Y, Liu Y, Gang X, Gao Y,
Li F, Xiao X and Wang G: A clinical and neuropathological study of
Chinese patients with diabetic peripheral neuropathy. PLoS One.
9(e91772)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wiggin TD, Sullivan KA, Pop-Busui R, Amato
A, Sima AA and Feldman EL: Elevated triglycerides correlate with
progression of diabetes neuropathy. Diabetes. 58:1634–1640.
2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Padilla A, Descorbeth M, Almeyda AL, Payne
K and De Leon M: Hyperglycemia magnifies Schwann cell dysfunction
and cell death triggered by PA-induced lipotoxicity. Brain Res.
1370:64–79. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ikura K, Hanai K, Shinjyo T and Uchigata
Y: HDL cholesterol as a predictor for the incidence of lower
extremity amputation and wound-related death in patients with
diabetic foot ulcers. Atherosclerosis. 239:465–469. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Isak B, Oflazoglu B, Tanridag T, Yitmen I
and Us O: Evaluation of peripheral and autonomic neuropathy among
patients with newly diagnosed impaired glucose tolerance. Diabetes
Metab Res Rev. 24:563–569. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Hämäläinen H, Rönnemaa T, Halonen JP and
Toikka T: Factors predicting lower extremity amputations in
patients with type 1 or type 2 diabetes mellitus: A
population-based 7-year follow-up research. J Intern Med.
246:97–103. 1999.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wiggin TD, Sullivan KA, Pop-Busui R, Amato
A, Sima AAF and Feldman EL: Elevated triglycerides correlate with
progression of diabetic neuropathy. Diabetes. 58:1634–1640.
2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li L, Chen J, Wang J and Cai D: Prevalence
and risk factors of diabetic peripheral neuropathy in type 2
diabetes mellitus patients with overweight/obese in Guangdong
province, China. Prim Care Diabetes. 9:191–195. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Davies B, Edwards N, Ploeg J and Virani T:
Insights about the process and impact of implementing nursing
guidelines on delivery of care in hospitals and community settings.
BMC Health Serv Res. 8(29)2008.PubMed/NCBI View Article : Google Scholar
|















