Introduction
Circadian clock genes (CCGs) are indispensable
regulators responsible for governing host rhythmic activities
according to the 24-h solar cycle. Mechanistically, CCGs control
circadian clock-dependent behaviors by modulating a wide range of
physiological processes such as sleeping, appetite regulation,
hormone secretion or cellular metabolism (1-3).
To date, several CCGs have been identified, including CLOCK, Bmal1,
Period family, Cry1/2, CKIε and TIM (4,5).
Period2 (Per2) belongs to the Period family and serves as a crucial
regulator of the mammalian circadian clock (6). Per2 deletion in mice causes severe
arrhythmicity (7). Besides its
roles in controlling the circadian rhythm, emerging evidence has
demonstrated that Per2 regulates the biological behaviors of tumor
cells. For example, Per2 induce mouse lung and breast cancer cell
apoptosis (8). Moreover, Per2
deficient mice are more prone to γ radiation-triggered tumor
development (9). However, the
effects of Per2 on human cancer are not completely understood and
require further investigation.
Chronic myeloid leukemia (CML) is a
myeloproliferative disease that has an incidence of 1-2/100,000
individuals worldwide (10). The
most common etiology of CML is fusion of the BCR gene with the ABL
gene resulting from chromosome translocation (11). Identification of novel molecular
targets that control the malignant behavior of CML cells may aid
the diagnosis and treatment of the disease.
In the present study, we investigated the expression
patterns of Per2 in patients with CML, acute myeloid leukemia (AML)
or chronic lymphocytic leukemia (CLL), as well as the role of Per2
on CML cell function both in vitro and in a mouse CML
model.
Materials and methods
Human specimens
Peripheral blood samples were collected from 30
patients with CML patients (21 male patients and 9 female patients;
age, 19-86 years; average age, 56 years) and 30 healthy donors (18
male donors and 12 female donors, age: 21 to 77 years, 51 for
average) from Yantai Yuhuangding Hospital between September 2016
and March 2018. Neutrophils were isolated from the peripheral blood
samples using the Human Neutrophil Isolation kit (Tianjin Haoyang
Biological Co., Ltd.). All patients provided written informed
consent. The experimental protocol was approved by the Ethic
Committee of The Affiliated Yantai Yuhuangding Hospital of Qingdao
University (approval no. 2016-185).
Cell culture
KCL22 cells (American Type Culture Collection) were
cultured in DMEM (Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (Hangzhou Sijiqing Biological Engineering Materials Co.,
Ltd.) at 37˚C in a 5% CO2 incubator.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from patient blood neutrophils, KCL22
cells and mouse tumor tissues was extracted using TRIzol (Thermo
Fisher Scientific, Inc.). Total RNA was reverse transcribed into
cDNA using a PrimeScript RT Reagent kit (Takara Bio, Inc.) using
the following parameters: 37˚C for 30 min and 85˚C for 3 min.
Subsequently, qPCR was performed on a ABI 7500 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using SYBR-Green
reagent (Kangwei), according to the manufacturer's protocol. The
thermocycling conditions were as follows: 95˚C for 10 min, followed
by 40 cycles of 95˚C for 15 sec and 60˚C for 60 sec. The primer
sequences used for qPCR are listed in Table I. mRNA expression levels were
quantified using the 2-∆∆Cq method (12) and normalized to the internal
reference gene β-actin.
 | Table IPrimer sequences used for quantitative
PCR. |
Table I
Primer sequences used for quantitative
PCR.
| Gene | Sequence (5'→3') |
|---|
| Per2 | F:
TTGGACAGCGTCATCAGGTA |
| | R:
TCCGCTTATCACTGGACCTT |
| c-Myc | F:
CAACCCTTGCCGCATCCAC |
| | R:
CCTCCTCGTCGCAGTAGAA |
| Cyclin D1 | F:
CCCTCGGTGTCCTACTTCA |
| | R:
CTCCTCGCACTTCTGTTCCT |
| Wee1 | F:
TGTGGTGGTGTGCTGCTTAT |
| | R:
TTCAAAGGGAGGGTATGTCTG |
| β-actin | F:
CATGTACGTTGCTATCCAGGC |
| | R:
CTCCTTAATGTCACGCACGAT |
Lentiviral transduction
Per2-encoding and control lentiviruses were
constructed by Shanghai SunBio Biotechnology Co., Ltd. A total of
5x105 KCL22 cells were seeded into 6-well plates with
lentiviral particles (MOI=40) and polybrene (5 µg/ml). Following
incubation for 24 h at 37˚C, the culture medium was replaced with
fresh RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
containing 2 µg/ml puromycin. Cells were cultured for 15 days at
37˚C to obtain stably transfected KCL22 cells and were then used
for the following experiments.
Western blotting
KCL22 cells were lyzed in RIPA lysis buffer
(Beyotime Institute of Biotechnology) on ice for 50 min. The amount
of protein was determined using an Ads Pierce™ BCA Protein Assay
Kit (Thermo Fisher Scientific). A total of 20 µg/lane protein was
separated via 10% SDS-PAGE and transferred to PVDF membranes. The
membranes were blocked with 5% fat-free milk at room temperature
(18-25˚C) for 90 min. Subsequently, the membranes were incubated
with anti-Per2 (Abcam; ab179813; 1:1,000) or anti-GAPDH (Abcam;
ab181602; 1:3,000) primary antibodies at 4˚C overnight. Following
primary incubation, the membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (Abcam; ab205718;
1:2,000) at room temperature (18-25˚C) for 60 min. The bands were
visualized using Pierce™ ECL Western Blotting Substrate (Thermo
Fisher Scientific, Inc.) on a ChemiDoc machine (Bio-Rad
Laboratories, Inc.).
Cell Counting Kit-8 (CCK-8) assay
A total of 4x103 KCL22 cells were seeded
into 96-well plates and cultured at 37˚C overnight. Subsequently,
10 µl CCK-8 solution (Beyotime Institute of Biotechnology) was
added to each well and incubated at 37˚C for 3.5 h. The absorbance
of each well was measured every 24 h for a total of 72 h at a
wavelength of 450 nm using a microplate reader.
Lactate dehydrogenase (LDH) release
assay
A total of 2x104 KCL22 cells were seeded
into a 96-well plate and cultured at 37˚C overnight. The
concentration of LDH in culture supernatant was measured using a
LDH Assay kit (Abcam), according to the manufacturer's protocol.
The absorbance (OD value) was read on a Microplate Reader (Promega
Corporation) at the wavelength of 450 nm.
Tumor model
A total of 54 nude male mice (age, 6-8 weeks;
weight, 20-25 g) were purchased from Shanghai SLAC Laboratory
Animal Center) and were housed in specific pathogen free
conditions. The housing conditions included a temperature of
23±2˚C, 50% humidity and 12 h light/dark cycle. Mice were given
food and water ad libitum. The mice received a subcutaneous
injection of KCL22 cells (1x106) in PBS into the left
flank. The mice were divided into three groups: The PBS group in
which mice were injected with PBS only, the Lv-scramble (scr) group
in which mice were injected with KCL22 cells transduced with Lv-scr
and the Lv-Per2 group in which mice were injected with KCL22 cells
transduced with Lv-Per2 (n=6 mice per group). Tumor volume was
measured and calculated using a caliper every 4-5 days. Tumor
volume was calculated as: V = length x width2/2. On day
20-22, when the mean tumor volume in the control group reached
~1,200 mm3 (maximum tumour volume was ~1,400-1,500
mm3), mice were sacrificed by cervical dislocation.
Death was verified by cessation of the heartbeat and lack of
movement. Subsequently, the tumors were removed to evaluate tumor
weight and gene expression. Multiple tumours were not observed in
any of the mice. The animal experimental protocols were approved by
the Qingdao University Ethics Committee (approval no.
QD20161183).
Cell cycle detection
KCL22 cells were centrifuged at 300 x g and 4˚C for
5 min, followed by permeabilization in 70% ethanol and fixation at
4˚C overnight. Subsequently, cells were washed with PBS and
incubated with 1 ml PBS containing 40 µl RNase and 20 µl propidium
iodide (Beyotime Institute of Biotechnology) at 37˚C in the dark
for 10 min. Cell cycle distribution was detected by flow cytometry
using a FACSCalibur flow cytometer (BD Biosciences). The results
were analyzed using FlowJo software (version VX1; Tree Star).
Cell apoptosis detection
KCL22 cells were washed with ice-cold PBS.
Subsequently, 2x105 cells were incubated in 200 µl
binding buffer containing 5 µl 7-AAD and 5 µl APC-Annexin V
(Biolegend Inc.) at room temperature for 10 min. Following
centrifugation at 300 x g, 4˚C for 5 min, cell pellets were
resuspended in PBS and assessed by flow cytometry on a FACSCalibur
flow cytometer (BD Biosciences). The percentages of early and late
apoptotic cells (Annexin V+, 7-AAD+ and
Annexin V+7-AAD+) were analyzed using FlowJo
software (version VX10; Tree Star).
GEO datasets
The numbers and URL links of the two Gene Expression
Omnibus (GEO) datasets analyzed were: GDS2643 (ncbi.nlm.nih.gov/gds?LinkName=geoprofiles_gds&from_uid=36949877)
(13), in which gene expression
profiles between chronic lymphocytic leukemia patients and healthy
controls were analyzed, and GDS4407 (ncbi.nlm.nih.gov/gds?LinkName=geoprofiles_gds&from_uid=88969842)
(14), in which gene expression
profiles between acute myeloid leukemia patients and healthy
controls were compared.
Statistical analysis
Data are presented as the mean ± standard deviation.
All experiments were performed in triplicated. Statistical analyses
were performed using SPSS software (version 17.0; SPSS, Inc.).
Comparisons among multiple groups were analyzed using one-way ANOVA
followed by Tukey's post hoc test. Spearman's rank correlation test
was performed to investigate the relationship between Per2
expression and c-Myc, cyclin D1 or WEE1 G2 checkpoint kinase (Wee1)
expression. P<0.05 was considered to indicate a statistically
significant difference.
Results
Per2 expression is decreased in
neutrophils from patients with CML
First, the expression of Per2 in peripheral
neutrophils isolated from patients with CML and healthy controls
was assessed. Patients with CML displayed significantly lower Per2
expression compared with healthy controls. In terms of cell
cycle-associated genes, c-Myc and cyclin D1 expression levels were
significantly increased, whereas Wee1 expression was significantly
decreased in patients with CML compared with healthy controls
(Fig. 1A). Moreover, in patients
with CML, there was a significant negative correlation between Per2
and c-Myc mRNA expression levels (Fig.
1B). However, Per2 expression was not significantly correlated
with cyclin D or Wee1 expression. The results indicated that CML
development might be associated with reduced Per2 expression.
Per2 overexpression suppresses CML
cell proliferation in vivo and in vitro
To investigate the role of Per2 during CML tumor
development, KCL22 cells that stably overexpressed Per2 were
generated by lentiviral transduction [lentivirus (Lv)-Per2 KCL22].
The RT-qPCR and western blotting results indicated successful Per2
overexpression in KCL22 cells (Fig.
2A and B). Subsequently,
Lv-Per2 KCL22 cells and control KCL22 cells were subcutaneously
injected into nude mice. By monitoring tumor volume, the results
indicated that when compared mice injected with PBS or Lv-scr KCL22
cells, Per2 overexpression significantly suppressed KCL22 cell
proliferation in mice (Fig. 2C).
Consistently, tumor weight was significantly reduced in mice
injected with Per2-overexpression KCL22 cells when compared mice
injected with PBS or Lv-scr KCL22 cells (Fig. 2D). As expected, Per2 expression was
significantly enhanced in Lv-Per2 tumors compared with controls and
Lv-scr) tumors (Fig. 2E).
Furthermore, the expression levels of proliferative genes c-Myc and
cyclin D were significantly reduced in Lv-Per2 tumors compared with
controls and Lv-scr tumors (Fig.
2F). To further investigate the role of Per2 on CML cell
proliferation in vitro, the CCK-8 assay was performed. The
results suggested that compared with controls and
Lv-scr-transfected cells, Per2 overexpression significantly
suppressed KCL22 cell proliferation at the 24, 48 and 72 h time
points (Fig. 2G). Moreover, the
levels of c-Myc, cyclin D1 and Wee1 were significantly decreased in
Lv-Per2 cells compared with controls and Lv-scr cells (Fig. 2H). The results suggested that Per2
may serve as a suppressor of in vivo and in vitro CML
cell proliferation.
 | Figure 2Per2 overexpression inhibits KCL22
cell proliferation in vivo and in vitro. Per2
overexpression was induced in KCL22 cells by lentiviral
transduction. Per2 (A) mRNA and (B) protein expression levels. (C)
Nude mice were subcutaneously injected with 1x106 KCL22
cells and tumor growth was monitored. Representative tumors on day
22 are presented (scale bar, 1 cm). (D) Tumor weight was measured
on day 22. The expression of (E) Per2, (F) c-Myc, cyclin D1 and
Wee1 in tumor tissues. (G) KCL22 cell proliferation was measured by
performing the Cell Counting Kit-8 assay. (H) The expression of
c-Myc, cyclin D1 and Wee1 in KCL22 cells. *P<0.05,
**P<0.01, ***P<0.001 vs. the Lv-Per1
group. Per2, Period2; Wee1, WEE1 G2 checkpoint kinase; Lv,
lentivirus; scr, scramble; OD, optical density. |
Per2 does not regulate human CML cell
apoptosis
Subsequently, whether the antiproliferative role of
Per2 on KCL22 cells was associated with a proapoptotic effect of
Per2 was investigated. The percentage of apoptotic cells was
comparable among controls, Lv-scr and Lv-Per2 cells, which
indicated that Per2 did not regulate CML cell apoptosis (Fig. 3A). Additionally, the LDH assay
demonstrated that compared with controls and Lv-scr cells, Lv-Per2
cells did not display significantly altered levels of cell
apoptosis (Fig. 3B). Therefore, the
results suggested that the antiproliferative effect of Per2 was not
due to enhanced cell apoptosis.
Per2 induces cell cycle arrest in
human CML cells
Based on the aforementioned result that Per2
overexpression reduced the expression of cell cycle-related genes
such as cyclin D1, it was hypothesized that Per2 regulated the cell
cycle of CML cells. To investigate the hypothesis, PI staining was
performed. The percentage of G1-phase cells was
significantly higher in Lv-Per2 KCL22 cells compared with controls
and Lv-scr KCL22 cells. By contrast, the percentage of
S/G2-phase cells was significantly decreased in Lv-Per2
KCL22 cells compared with controls and Lv-scr KCL22 cells (Fig. 4A and B). The ratio of G1-phase cells
to S/G2-phase cells was significantly increased by Per2
overexpression compared with controls and Lv-scr cells (Fig. 4C). Therefore, the results suggested
that Per2 induced G1/S cell cycle arrest in human CML
cells.
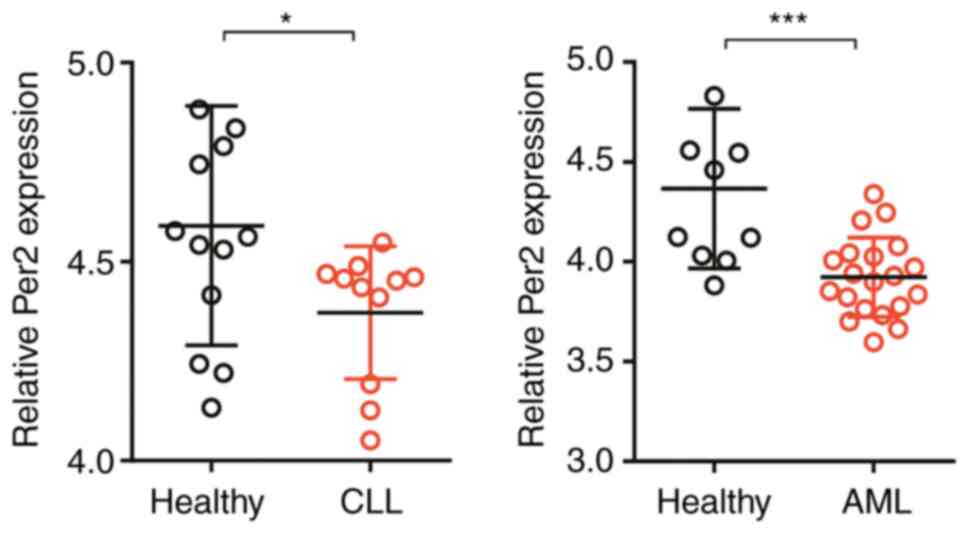
Per2 expression is reduced in patients
with AML and CLL
Finally, Per2 expression was assessed in two other
types of leukemia, AML and CLL. To assess Per2 expression in
patients with AML and CLL, data mining from the Gene Expression
Omnibus database was performed. Consistent with the results
obtained for patients with CML, the expression level of Per2 was
significantly reduced in peripheral blood mononuclear cells (PBMCs)
isolated from patients with AML compared with healthy donors.
Similarly, compared with healthy controls, B cells from patients
with CLL displayed significantly lower Per2 expression (Fig. 5). The results indicated that Per2
may be involved in regulating multiple kinds of leukemia.
Discussion
CCGs are ubiquitously expressed in the vast majority
of mammalian cells (15). In
addition to the involvement in controlling circadian rhythm,
increasing evidence has indicated that CCGs are involved in
modulating tumor development (16,17).
Moreover, abnormal Per2 expression was observed in skin, breast and
gastric cancer, as well as head and neck squamous cell carcinoma
(18-21).
In the present study, the expression levels of Per2 were
downregulated in patients with CML compared with healthy
individuals, which suggested that Per2 may serve as a diagnostic
marker for CML. A previous study had also reported lower expression
of Per2 in PBMCs isolated from patients with CML (22); however, PBMCs also contain a large
proportion of non-myeloid cells such as T and B cells. Therefore,
in the present study, in order to obtain more reliable results,
peripheral neutrophils were enriched instead of PBMCs to examine
Per2 expression. The results also indicated that Per2 expression
was negatively correlated with c-Myc expression, an oncogene whose
overexpression often leads to cell hyperproliferation (23). By contrast, Per2 overexpression
inhibited CML cell proliferation both in vitro and in
vivo. Mechanistically, Per2 overexpression facilitated cell
cycle arrest at G1 phase. Based on the suppressive role
of Per2 in CML cell proliferation, it was speculated that Per2 may
serve as a detrimental factor in diseases such as granulocytopenia.
Moreover, the detailed molecular mechanisms underlying the
antiproliferative function of Per2 require further
investigation.
In addition to the in vitro results, the
results also suggested that compared with mice inoculated with PBS
or Lv-scr-KCL22 cells, Per2 overexpression significantly reduced
CML tumor growth in mice. Furthermore, Per2 overexpression reduced
the expression levels of c-Myc, cyclin D1 and Wee1 in tumor
tissues, compared with tumor tissues from mice inoculated with PBS
or Lv-scr-KCL22 cells. The in vivo results suggested that
targeting Per2 may serve as a potential therapeutic strategy for
CML.
On the other hand, the finding that Per2 did not
alter cell apoptosis was contradictory to a previous study, which
reported that Per2 overexpression induced LLC mouse lung cancer
cell and EMT6 mouse breast cancer cell apoptosis (5). A potential explanation for the
inconsistency could be that the apoptosis-inducing role of Per2 is
cell-type specific; therefore, whether Per2 affects CML cell
apoptosis under certain stress conditions (such as hypoxia or
chemotherapy treatment) requires further investigation.
Furthermore, Per2 expression was also decreased in patients with
AML and CLL, which indicated that Per2 may also have diagnostic
value in other kinds of leukemia as well as in CML. However, the
exact impacts of Per2 on AML and CLL require further investigation.
For example, a mouse model of AML or CLL could be used to
investigate whether Per2 also suppresses AML or CLL tumor growth
and how Per2 alters the expression of oncogenes such as c-Myc. From
a clinical view, the relationship between Per2 expression and the
clinical characteristics of patients with AML or CLL, such as tumor
stage or patient prognosis, requires further investigation.
Moreover, the molecular mechanisms underlying the regulatory roles
of Per2 on leukemia also need to be investigated in future studies.
Due to the crucial function of Per2 in modulating circadian
clock-dependent behaviors, it is possible that circadian rhythmic
activities may impact the development of leukemia.
Collectively, the present study identified Per2 as a
candidate tumor suppressor, which may have potential diagnostic or
therapeutic values in CML.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Shandong Province (grant no.
ZR2015HM073).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. Furthermore, the GEO datasets analyzed during the current
study are available at: ncbi.nlm.nih.gov/gds?LinkName=geoprofiles_gds&from_uid=36949877
and ncbi.nlm.nih.gov/gds?LinkName=
geoprofiles_gds&from_uid=88969842.
Authors' contributions
CS conceived and designed the present study. MM and
XW collected the clinical specimens. NW, MM and XW performed the
experiments and analyzed the data. NW and CS drafted the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocol was approved by the Ethics
Committee of The Affiliated Yantai Yuhuangding Hospital of Qingdao
University (approval no. 2016-185). Written informed consent was
obtained from all patients. The animal experimental protocols were
approved by the Qingdao University Ethics Committee (approval no.
QD20161183).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Panda S: Circadian physiology of
metabolism. Science. 354:1008–1015. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Andreani TS, Itoh TQ, Yildirim E, Hwangbo
DS and Allada R: Genetics of circadian rhythms. Sleep Med Clin.
10:413–421. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Roenneberg T and Merrow M: The circadian
clock and human health. Curr Biol. 26:R432–R443. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xie Y, Tang Q, Chen G, Xie M, Yu S, Zhao J
and Chen L: New insights into the circadian rhythm and its related
diseases. Front Physiol. 10(682)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rijo-Ferreira F and Takahashi JS: Genomics
of circadian rhythms in health and disease. Genome Med. 11:82–92.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yoo SH, Yamazaki S, Lowrey PL, Shimomura
K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, et al:
PERIOD2:LUCIFERASE real-time reporting of circadian dynamics
reveals persistent circadian oscillations in mouse peripheral
tissues. Proc Natl Acad Sci USA. 101:5339–5346. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zheng B, Albrecht U, Kaasik K, Sage M, Lu
W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, et al:
Nonredundant roles of the mPer1 and mPer2 genes in the mammalian
circadian clock. Cell. 105:683–694. 2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hua H, Wang Y, Wan C, Liu Y, Zhu B, Yang
C, Wang X, Wang Z, Cornelissen-Guillaume G and Halberg F: Circadian
gene mPer2 overexpression induces cancer cell apoptosis. Cancer
Sci. 97:589–596. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fu L, Pelicano H, Liu J, Huang P and Lee
C: The circadian gene Period2 plays an important role in tumor
suppression and DNA damage response in vivo. Cell. 111:41–50.
2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Baccarani M and Dreyling M: ESMO
Guidelines Working Group: Chronic myelogenous leukemia: ESMO
clinical recommendations for diagnosis, treatment and follow-up.
Ann Oncol. 20 (Suppl 4):105–107. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Apperley JF: Chronic myeloid leukaemia.
Lancet. 385:1447–1459. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-ΔΔC(T)) method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gutiérrez NC, Ocio EM, de Las Rivas J,
Maiso P, Delgado M, Fermiñán E, Arcos MJ, Sánchez ML, Hernández JM
and San Miguel JF: Gene expression profiling of B lymphocytes and
plasma cells from Waldenström's macroglobulinemia: Comparison with
expression patterns of the same cell counterparts from chronic
lymphocytic leukemia, multiple myeloma and normal individuals.
Leukemia. 21:541–549. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bacher U, Schnittger S, Macijewski K,
Grossmann V, Kohlmann A, Alpermann T, Kowarsch A, Nadarajah N, Kern
W, Haferlach C, et al: Multilineage dysplasia does not influence
prognosis in CEBPA-mutated AML, supporting the WHO proposal to
classify these patients as a unique entity. Blood. 119:4719–4722.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang R, Lahens NF, Ballance HI, Hughes ME
and Hogenesch JB: A circadian gene expression atlas in mammals:
Implications for biology and medicine. Proc Natl Acad Sci USA.
111:16219–16224. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shostak A: Circadian clock, cell division,
and cancer: From molecules to organism. Int J Mol Sci.
18(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Savvidis C and Koutsilieris M: Circadian
rhythm disruption in cancer biology. Mol Med. 18:1249–1260.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lengyel Z, Lovig C, Kommedal S, Keszthelyi
R, Szekeres G, Battyáni Z, Csernus V and Nagy AD: Altered
expression patterns of clock gene mRNAs and clock proteins in human
skin tumors. Tumour Biol. 34:811–819. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hu ML, Yeh KT, Lin PM, Hsu CM, Hsiao HH,
Liu YC, Lin HY, Lin SF and Yang MY: Deregulated expression of
circadian clock genes in gastric cancer. BMC Gastroenterol.
14(67)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen ST, Choo KB, Hou MF, Yeh KT, Kuo SJ
and Chang JG: Deregulated expression of the PER1, PER2 and PER3
genes in breast cancers. Carcinogenesis. 26:1241–1246.
2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hsu CM, Lin SF, Lu CT, Lin PM and Yang MY:
Altered expression of circadian clock genes in head and neck
squamous cell carcinoma. Tumour Biol. 33:149–155. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yang MY, Chang JG, Lin PM, Tang KP, Chen
YH, Lin HY, Liu TC, Hsiao HH, Liu YC and Lin SF: Downregulation of
circadian clock genes in chronic myeloid leukemia: Alternative
methylation pattern of hPER3. Cancer Sci. 97:1298–1307.
2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Reavie L, Buckley SM, Loizou E, Takeishi
S, Aranda-Orgilles B, Ndiaye-Lobry D, Abdel-Wahab O, Ibrahim S,
Nakayama KI and Aifantis I: Regulation of c-Myc ubiquitination
controls chronic myelogenous leukemia initiation and progression.
Cancer Cell. 23:362–375. 2013.PubMed/NCBI View Article : Google Scholar
|