Introduction
Pulmonary hypertension (PH) is a rare disease that
is characterized by the progressive elevation of pulmonary vascular
resistance and pressure (1). PH is
associated with poor prognosis, with a 5-year survival rate of 30%
and estimated prevalence in different registries of between 15 and
26 cases per million population >14 years (2,3). The
progression of PH leads to right ventricular dysfunction and
right-sided heart failure, which culminates in heart palpitations,
dyspnea, syncope and ascites (4-7).
The methods used to diagnose PH and evaluate the pathological
conditions of patients with PH have dramatically improved due to
development of new imaging equipment, including echocardiography,
X-ray, CT and MRI, in addition to the identification of novel
biomarkers (8). In particular,
prostacyclin analogues, phosphodiesterase-5 inhibitors, guanylate
cyclase stimulators, prostacyclin receptor agonists and endothelin
receptor antagonists, coupled with novel diagnostic and prognostic
advances, have revolutionized treatment of PH (4). Although exercise tolerance, New York
Heart Association (NYHA) functional class, hemodynamic parameters
such as mean right atrial pressure (RAP) and cardiac index are all
currently associated with PH prognosis, other factors are proposed
to be involved (4). Of the number
of factors that are hypothesized to contribute to PH pathology,
pulmonary arterial endothelial cell dysfunction serves an important
role. Endothelial cell dysfunction results from the overproduction
of vasoconstrictors and proliferative factors, such as endothelin-1
(ET-1), and the reduced expression of vasodilators and
antiproliferative factors, such as prostacyclin and nitric oxide
(9-12).
ET-1 is a vasoconstrictor peptide produced by vascular endothelial
cells, which has been previously found to correlate positively with
the severity PH (13-17).
Therefore, ET-1 antagonists are frequently applied as a therapeutic
agent for pulmonary hypertension (16). It has been reported that
AMP-activated protein kinase (AMPK) deficiency promotes vascular
endothelial cell dysfunction in association with ET-1(18). AMPK is a serine/threonine kinase
expressed in various tissues, and consists of a catalytic subunit,
α, and two regulatory subunits, β and γ; it contributes to the
maintenance of intracellular energy homeostasis, and it exhibits
antiapoptotic effects in endothelial cells and anti-remodeling
effects in vascular smooth muscle cells (19,20).
The anti-remodeling and antiapoptotic effects of AMPK are thought
to attenuate the onset and progression of PH (18,21).
Ibe et al (18) demonstrated
that AMPKα1 and AMPKα2 had differential roles
in the survival of pulmonary arterial smooth muscle cells under
hypoxia and hypoxia-induced PH. In addition, Omura et al
(22) demonstrated that long-term
treatment of endothelial-specific AMPK-knockout mice with the AMPK
activator, metformin, significantly attenuated hypoxia-induced PH.
Thus, metformin may represent a novel therapeutic agent for PH. The
present study aimed to evaluate the therapeutic effects of
metformin treatment in rats with monocrotaline (MCT)-induced PH
using echocardiography and invasive pulmonary artery pressure (PAP)
measurements, as well as evaluating biomarkers and
histopathology.
Materials and methods
Animal studies
This study was approved by the Animal Experimental
Subcommittee of Tokyo University of Agriculture and Technology
(Tokyo, Japan). All animal experiments were conducted in accordance
with the Regulations on Animal Experiments of Tokyo University of
Agriculture and Technology, and with the Guide for the Care and Use
of Laboratory Animals (23). A
total of 36 male Wistar rats (age, 12 weeks; weight, 370-550 g;
Oriental yeast, Co., Ltd.) were housed at 22˚C, 40-70% relative
humidity in a 21% O2 room with a 12-h light/dark cycle
and provided with free access to standard laboratory rat chow and
water. Rats were randomly divided into the following three groups:
i)Saline-injected group (sham; n=9); ii) MCT-injected group (PH;
n=19); iii) MCT-injected and metformin-treated group (MT; n=8).
Nine rats from the PH group died between the second and third weeks
of drug administration due to drug-related complications: From the
results of autopsy, the rats died from acute lung injury due to
severe pulmonary arteritis (24,25).
MCT is an 11-member macrocyclic pyrrolizidine alkaloid that induces
pulmonary arteritis in rats, which gradually progresses into PH
(26-28).
Previous studies have reported that the progression to PH occurs ~4
weeks following MCT administration (26-32).
Rats in all groups, except for the sham group, were subjected to an
intraperitoneal injection of 60 mg/kg MCT (Wako Pure Chemical
Industries, Ltd.) to induce PH. MCT was dissolved in 1 M HCl, and
the pH was adjusted to 7.4 with 1 M NaOH.
Metformin (Metformin hydrochloride; Pfizer, Inc.)
was delivered orally (100 mg/kg/day) to the MT group through the
drinking water, starting the day after MCT injection (15,16).
This solution was changed every day for 28 days. Four weeks after
MCT injection, cardiac ultrasonography, invasive hemodynamic
measurements and blood extraction were performed to evaluate the
effects of the treatment. Rats were euthanized by exsanguination
under anesthesia (the method of anesthesia was the same method as
described for anesthesia for echocardiography) in accordance with
the Regulations on Animal Experiments of Tokyo University of
Agriculture and Technology and with the Guide for the Care and Use
of Laboratory Animals. Following euthanasia, the lungs and hearts
of all rats were excised for histopathological evaluation.
Right-sided heart functional
evaluation by echocardiography
Echocardiography was performed under isoflurane
anesthesia after 2.5 mg/kg butorphanol and 2.5 mg/kg midazolam were
injected intramuscularly (33-36).
The isoflurane concentration was adjusted between 1.0 and 1.5% to
maintain the heart rate within the range of 300-350 beats/min
(33). The thoraxes of the rats
were shaved, and the rats were positioned in right and left lateral
recumbency. Echocardiography was performed using a ProSound F75
PremierCV (Hitachi Aloka Medical, Ltd.), with a 7.5-MHz transducer
at a sweep speed of 300 mm/sec and a sample gate of 1 mm. The
following echocardiographic parameters associated with PH were
measured: From the parasternal short axis view at the mid-papillary
muscle level, distance 1 (D1) was measured as the left ventricular
minor axis diameter perpendicular to the septum, and distance 2
(D2) was measured as the left ventricular minor axis diameter
parallel to the septum. The eccentricity index (EI) was defined as
the ratio between D1 and D2 (EI=D1/D2). The tricuspid annular plane
systolic excursion (TAPSE) was measured using M-mode across the
tricuspid valve annulus at the right ventricle (RV) by positioning
the echo cursor on the junction between the tricuspid valve plane
and the RV-free wall (Fw) using the apical four-chamber view. In
the short-axis view at the aorta level, the ratio between the main
pulmonary artery (MPA) diameter and the aortic diameter (Ao),
MPA/Ao, and the ratio between the acceleration time (AT) and
ejection time (ET), AT/ET, of the pulmonary artery (PA) flow
velocity were measured (25,27,30,37).
The RV Tei index was calculated as the sum of the isovolumetric
contraction time (ICT), and the isovolumetric relaxation time
(IRT), divided by the ET, (ICT + IRT)/ET, using the right
parasternal RV outflow view (13,14).
Peak transtricuspid early diastolic wave velocity (E wave) was
measured using the Doppler signals for the tricuspid inflow in the
apical four-chamber view. The peak tissue Doppler tricuspid annular
velocities at systole (Sm) and early diastole (Ea) in the apical
four-chamber view were measured by focusing the sample volume of
the tissue Doppler imaging on the Fw and the septal wall (Mv),
respectively, of the tricuspid valve annulus, and E wave/Ea was
calculated (25,38). Representative echocardiographic
images of these measurements are presented in Fig. 1.
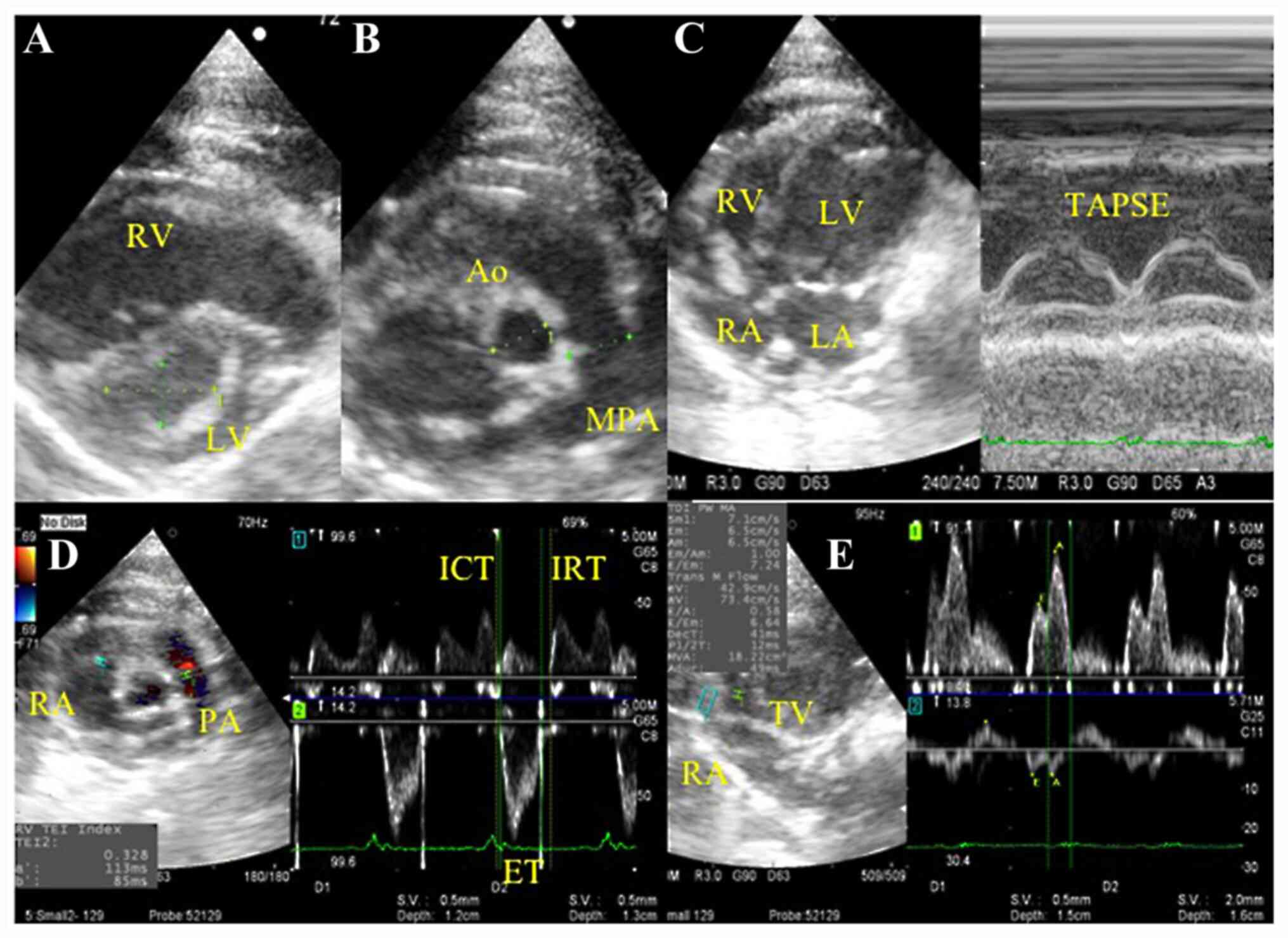 | Figure 1Representative echocardiogram images
of right heart functional evaluation. (A-D) Representative images
of the (A) eccentricity index from the parasternal short axis view,
(B) MPA/Ao in the short-axis view at the aorta level, (C) TAPSE in
the apical four-chamber view and (D) RV Tei index using the Dual
Doppler system in the right parasternal RV outflow view. (E) Peak
transtricuspid early diastolic wave velocity and tissue Doppler
tricuspid annular velocities at systole and at early diastole were
measured using the Dual Doppler system in the apical four-chamber
view. Ao, aortic diameter; ET, ejection time; ICT, isovolumetric
contraction time; IRT, isovolumetric relaxation time; LA, left
atrium; LV, left ventricle; MPA, main pulmonary artery; PA,
pulmonary artery; RA, right atrium; RV, right ventricle; TAPSE,
tricuspid annular plane systolic excursion by M-mode imaging; TV,
tricuspid valve. |
Hemodynamic measurements
Following the echocardiographic measurements, the
rats were reoriented into a supine position to directly measure PAP
under thoracotomy. The trachea of each rat was incised for tracheal
tube insertion. Following intubation, the respiration mode was
switched from spontaneous respiration to artificial respiration
using an artificial ventilator (Harvard Apparatus). A catheter was
inserted into the left carotid artery to monitor the invasive
arterial pressure to measure heart rates, systolic blood pressure,
mean blood pressure and diastolic blood pressure. The isoflurane
concentration was carefully adjusted to maintain a mean blood
pressure (MBP) >60 mmHg, according to a pressure transducer. To
measure the mean PAP (MPAP), the chest was opened at the fourth
intercostal space and a catheter was directly inserted into the PA.
The catheter was connected to a physiological pressure transducer
and amplifier system (Life Scope BSM-5192; Nihon Kohden Co., Ltd.)
to record pressure oscillations. PAP was recorded following the
calibration and stabilization of the cardiac rhythm after
anesthesia, as monitored using electrocardiogram leads and pulse
oximetry.
Histological analysis
Lung tissue was fixed overnight in 10% buffered
formalin at room temperature. Paraffin sections (5 µm) were
generated from each lobe, stained with hematoxylin & eosin. All
slide samples were washed with xylene for 5 min three times for
deparaffinization before being dripped into methanol three times
and washed in water for 1 min. The slides were then shaken and
stained with hematoxylin for 10 min at room temperature and eosin
for 6 min at room temperature. All sections were analyzed with a
light microscope to assess lung vascular pathology. Pulmonary
arteries of 50-200 µm in diameter were evaluated by measuring
arterial wall thickness at x400 magnification. For each rat, ≥10
randomly selected circular or oval-shaped blood vessels were
measured. The percentage medial wall thickness (MWT) of the
arteries was calculated using the following formula: MWT=[(external
diameter-internal diameter)/external diameter] x100. The internal
diameter was measured as the luminal diameter of the vessel, and
the external diameter was measured as the total diameter of the
vessel. The vessel area was also measured; wall area (WA)=[(total
vessel area-luminal area)/total vessel area] x100 (26,31,37,39).
Total vessel area and luminal area were measured using PCD software
version 1.7 (FLOVEL Co., Ltd.).
Big ET-1 ELISA assay
Big ET-1 is a pre-cleavage pro-peptide of ET-1,
which has a longer half-life compared with ET-1 and is therefore
less likely to be inactivated than ET-1(17). Blood samples (4 ml) from the rats
were collected from the carotid artery in tubes containing
aprotinin. Serum was obtained following centrifugation (1,200 x g;
10 min; 4˚C), and it was stored at -80˚C until required. At the
time of experimentation, frozen samples were thawed at room
temperature and the extraction and concentration of Big ET-1 was
performed using Sep Pak C18 cartridges (Waters Corporation). The
samples were eluted through the Sep Pak C18 cartridges using 2 ml
0.1% trifluoroacetic Acid + 60% acetonitrile (Wako Pure Chemical
Industries, Ltd.). Serum big ET-1 concentrations were measured
using a commercial rat big ET-1 ELISA kit (cat. no. 27168;
Immuno-Biological Laboratories Co., Ltd.), according to the
manufacturer's protocol. Because the primary antibody was
immobilized on the plate, samples and exogenous standards were
added to the plate to perform the primary reaction. After washing,
horseradish peroxidase-labeled secondary antibody was added to the
plate to perform the secondary reaction. After the reaction, excess
secondary antibody was washed away and
3,3',5'5'-tetramethylbenzidine was added to the wells. The
resulting coloration was proportional to the concentration of rat
big ET-1 in the sample, as measured using a microplate absorbance
reader (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data analysis was performed using Microsoft Excel
2016 (Microsoft Corporation), GraphPad Prism version 5.0a and
InStat version 3.05 software (GraphPad Software, Inc.). All data
are presented as mean ± SD. Normality of all data was analyzed
using a Bartlett's test. For normally distributed parameters,
differences between groups were analyzed using a one-way ANOVA
followed by post hoc analysis with Bonferroni correction. For
non-parametric parameters, differences between groups were
evaluated using a non-parametric Kruskal-Wallis test followed by
post hoc analysis with Dunn's multiple comparison test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Hemodynamic analysis of blood pressure
and PAP
No significant differences were observed in the
heart rates, systolic blood pressure, mean blood pressure,
diastolic blood pressure, between the sham, PH and MT groups
(Fig. 2A-C; Table I). Rats in the PH group developed
severe PH compared with rats in the sham group, indicating the
successful establishment of the PH model. Systolic PAP, mean PAP
and diastolic PAP values in the PH group were significantly higher
compared with the sham group, which were in turn reduced in the MT
group compared with the PH group. Therefore, metformin treatment
could reduce PAP to levels comparable with that in the sham group
(Fig. 2D-F).
 | Figure 2Blood pressure and pulmonary arterial
pressure measurements in vivo. Measurements of (A) SBP, (B) MBP,
(C) DBP, (D) SPAP, (E) MPAP and (F) DPAP in the sham group (n=9),
MT group (n=8) and PH group (n=10). Horizontal lines represent the
median for each group. *P<0.05. DBP, diastolic blood
pressure; DPAP, diastolic pulmonary artery pressure; MBP, mean
blood pressure; MPAP, mean pulmonary artery pressure; MT, metformin
treatment; PH, pulmonary hypertension; SBP, systolic blood
pressure; SPAP, systolic pulmonary artery pressure. |
 | Table IComparisons of the right heart
functional parameters. |
Table I
Comparisons of the right heart
functional parameters.
| Variables | Sham group | MT group | PH group |
|---|
| Heart rate
(beats/min) | 313.00±34.00 | 301.00±34.00 | 317.00±31.00 |
| Eccentricity
index | 1.01±0.03 | 1.04±0.09 | 1.19±0.22 |
| Main pulmonary
artery/aortic artery | 0.95±0.12 | 0.97±0.05 |
1.16±0.22a |
| Accelerator
time/ejection time | 0.43±0.05 |
0.34±0.06a |
0.19±0.05a,b |
| Right ventricle Tei
index | 0.36±0.03 | 0.35±0.05 |
0.47±0.07a,b |
| Peak transtricuspid
early diastolic wave velocity (m/sec) | 0.69±0.16 | 0.63±0.22 | 0.62±0.16 |
| Early systole free
wall (cm/sec) | 6.56±1.54 | 6.86±1.78 | 7.03±1.45 |
| Early systole
septal wall (cm/sec) | 6.62±1.23 | 5.53±1.13 | 5.52±1.12 |
| Systole
(cm/sec) | 5.65±0.89 | 5.51±1.22 | 5.71±1.38 |
| E wave/Ea free
wall | 10.69±1.32 | 9.41±3.43 | 8.86±1.89 |
| E wave/Ea septal
wall | 10.42±1.17 | 11.42±4.00 | 11.35±2.67 |
| Tricuspid annular
plane systolic excursion (mm) | 3.02±0.20 | 2.81±0.57 |
2.27±0.51a |
Histopathological analysis of
pulmonary arteries
Pulmonary vascular remodeling is central to the
pathology of PH, and the MWT and WA of PAs is an indicator of
pulmonary vascular remodeling (26,31,37,39).
Histopathological analysis of the lung tissue demonstrated that
rats in the PH group exhibited markedly thickened vascular walls
within the pulmonary arterioles and increased proliferation of lung
stromal cells (Fig. 3A and B). The pulmonary arteriole MWT
(53.11±8.25%) and WA (75.62±7.40) were significantly increased in
the PH group compared with the sham group (MWT, 20.26±2.67%; WA,
37.00±5.43) (Fig. 3C and D). Metformin treatment prevented
PH-induced PA thickening in the MT group, with the pulmonary
arteriolar MWT (27.33±3.40%) and WA (49.28±6.10) in the MT group
being significantly reduced compared with the PH group. These
results indicated that metformin treatment significantly inhibited
the pathological pulmonary vascular remodeling induced by PH.
Echocardiographic right heart
functional evaluation
Comparisons of the right heart functional parameters
between the groups are presented in Table I. No significant differences were
identified between groups in EI, E wave, Ea Fw, Ea Mv, Sm, E/Ea Fw
or E/Ea Mv values (Table I). The
AT/ET ratio of both the PH and MT groups were significantly reduced
compared with that in the sham group. Although the AT/ET ratio
improved in the MT group compared with the PH group, it did not
reach that of the sham group (Fig.
4A-C; Table I). There were
significant differences in the MPA/Ao and TAPSE values between the
sham and PH groups, but no significant differences were observed
for these variables between the MT and PH groups (Table I). The RV Tei index, which is
indicative of overall RV function (13,14),
presented significant differences between the sham and PH group,
and significantly improved in the MT group relative to the PH group
(Table I). This observation
suggests that metformin treatment could improve echocardiographic
parameters in patients with PH.
Serum big ET-1 levels are upregulated
in PH and decreased by metformin treatment
Big ET-1 concentrations were significantly increased
in the PH group (3.59±0.74 pg/ml) compared with the sham group
(2.70±0.64 pg/ml) (Fig. 5), but
were reduced upon metformin treatment (2.82±0.33 pg/ml), with the
MT group demonstrating significantly decreased serum levels of big
ET-1 compared with the PH group. There was no significant
difference reported between the MT group and the sham group
(Fig. 5). These data suggest that
blood big ET-1 levels were elevated in patients with P, which were
alleviated following metformin treatment.
Discussion
Although pulmonary vascular endothelial dysfunction
and vascular remodeling are central to the pathology of PH, the
pathology of PH remains relatively unknown (40,41).
The present study focused on the AMPK activator, metformin, as AMPK
is important for the maintenance of pulmonary vascular endothelial
function (22,42-44).
Utilizing an MCT-induced PH rat model to evaluate the therapeutic
effects of metformin in PH, a relationship between metformin and
serum ET-1, which is elevated in PH, was determined.
Although the mechanism by which MCT causes PH
remains unclear, pulmonary vascular remodeling, which progresses to
cause pulmonary arterial vasculitis, is one of the possible
mechanisms (45,46). In the present study,
histopathological analysis confirmed that MCT promoted pulmonary
blood vessel remodeling and induced PH. In addition to the MT group
exhibiting significant decreases in MWT and WA values relative to
the PH group, PAP was significantly reduced by metformin treatment;
the mechanism by which metformin reduces PAP may be associated with
its ability to inhibit vascular remodeling. In the context of PH,
metformin most likely maintains pulmonary vascular homeostasis by
improving vascular endothelial cell function, as well as
suppressing pulmonary vascular endothelial cell apoptosis and
vascular smooth muscle cell remodeling (22).
In addition, metformin treatment decreased serum big
ET-1 levels in PH. Big ET-1 is a vasoconstrictive and
vasoproliferative factor produced by endothelial cells, and it is
thought to potentiate pulmonary arterial vasoconstriction and
remodeling in the context of PH (14,17,47,48).
These results suggested that the therapeutic effects of metformin
in PH may be related to decreased peripheral big ET-1
concentrations. AMPK contributes to the maintenance of
intracellular energy homeostasis, and it has been demonstrated to
have antiapoptotic effects in endothelial cells and anti-remodeling
effects in vascular smooth muscle cells (20-22).
Tang et al (49) revealed
that increased AMPK activity reduced ET-1 levels, by suppressing
the NF-κB/NF-κB inhibitor (IκB)α axis. NF-κB forms part of a
protein complex that regulates the immune response and cellular
proliferation by controlling the expression of proinflammatory and
pro-survival genes (50). AMPK
suppresses NF-κB activity in a number of ways; thus, the
therapeutic effects of metformin in the context of PH may be
associated with the NF-κB/IκBα axis. The study by Tang et al
(49) suggested that dipeptidyl
peptidase 4 inhibitor reduced ET-1 expression in the vascular
endothelium by suppressing NF-κB/IκBα signaling through AMPK
activation in diabetic rats. Therefore, reduced expression levels
of big ET-1 in the serum of PH model rats following metformin
treatment may be due to suppressed NF-κB/IκBα signaling.
In the present study, AT/ET was considered to be the
most sensitive echocardiographic parameter for estimating the
therapeutic effects of metformin on PH. Numerous previous studies
have reported that AT/ET is decreased in patients with PH (25,27,30,37).
Similarly, the results obtained in this study demonstrated that
AT/ET was significantly reduced in the PH model rats compared with
the other groups. In addition, AT/ET reflected the therapeutic
effect of metformin between MT group and PH group. The RV Tei index
is an echocardiographic parameter that reflects the severity of PH
(51,52). The RV Tei index was significantly
higher in the PH group compared to the other groups; however,
because no significant difference was observed between the MT group
and the sham group, it indicated that the RV Tei index may not be
as sensitive as AT/ET in evaluating PH severity. Both AT/ET and RV
Tei index are considered to be more useful compared with TAPSE and
MPA/PA values for the evaluation of PH severity in the present
study. Although clear evidence remains to be lacking, AT/ET and RV
Tei index appear to be sensitive because they can capture changes
in blood flow with higher degrees of sensitivity in the present
study.
Nonetheless, there are limitations to this study and
the results should be interpreted with caution. Metformin was
administered prophylactically prior to MCT induction of PH;
therefore, the therapeutic effects of metformin following the onset
of PH remain unclear. Further studies regarding the anti-remodeling
effects of metformin are also necessary, and the detailed
mechanisms underlying these effects must be investigated at the
molecular level. Furthermore, big ET-1 concentrations and
expression of preproET-1 mRNA, which encodes the precursor protein
of big ET-1 in tissues, in pulmonary vascular endothelial tissue
should be examined to identify the origin of serum big ET-1, in
addition to measuring other biomarkers associated with PH.
Additionally, it remains unclear whether the dose of metformin used
in this study was within the physiological range permitted for
humans; as such, the dose of metformin should also be investigated
in further studies.
To the best of our knowledge, this is the first
study to report that metformin prevented the development of
MCT-induced PH by decreasing the serum ET-1 concentration. The
possibility of this finding being associated with the AMPK pathway
warrants further future study. Multiple previous studies have
reported that the homeostatic improvement of vascular endothelial
cells by AMPK was effective for the treatment of PH (20,22,43).
However, in addition to this mechanism, it can be hypothesized that
the increased expression of ET-1, produced in vascular endothelial
cells, is suppressed by improved homeostasis of vascular
endothelial cells following AMPK activation.
In conclusion, the present study demonstrated that
early-stage metformin treatment significantly attenuated the
development of MCT-induced PH, hemodynamically and
histopathologically, which may have been related to suppression of
serum big ET-1. These results suggested that metformin may be a
putative therapeutic strategy for PH, but further studies are
required to understand the detailed mechanisms underlying these
effects.
Acknowledgements
The authors would like to thank the Fujifilm Monolis
Co., Ltd. (Tokyo, Japan) for technical assistance with experiments
and providing the facilities.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TY designed the study and wrote the initial draft of
the manuscript. DM, KSh, RN and PK maintained the animals. KM
contributed to analysis and interpretation of data. KSu prepared
tissue specimens and provided advise for the histopathological
studies. SG, AU and RT contributed to data interpretation and
critically reviewed the manuscript. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
All experiments were conducted in accordance with
the Regulations on Animal Experiments of Tokyo University of
Agriculture and Technology (Tokyo, Japan) and with the Guide for
the Care and Use of Laboratory Animals. This study was approved by
the Animal Experimental subcommittee of Tokyo University of
Agriculture and Technology (permit no. 30-88).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky
EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM,
Kernis JT, et al: Survival in patients with primary pulmonary
hypertension: Results from a national prospective registry. Ann
Intern Med. 115:343–349. 1991.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gall H, Felix JF, Schneck FK, Milger K,
Sommer N, Voswinckel R, Franco OH, Hofman A, Schermuly RT,
Weissmann N, et al: The giessen pulmonary hypertension registry:
Survival in pulmonary hypertension subgroups. J Heart Lung
Transplant. 36:957–967. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Escribano-Subias P, Blanco I,
López-Meseguer M, Lopez-Guarch CJ, Roman A, Morales P,
Castillo-Palma MJ, Segovia J, Gómez-Sanchez MA and Barberà JA:
REHAP investigators: Survival in pulmonary hypertension in Spa in:
Insights from the Spanish registry. Eur Respir J. 40:596–603.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Galiè N, Humbert M, Vachiery JL, Gibbs S,
Lang I, Torbicki A, Simonneau G, Peacock A, Noordegraaf AV,
Beghetti M, et al: 2015 ESC/ERS guidelines for the diagnosis and
treatment of pulmonary hypertension: The joint task force for the
diagnosis and treatment of pulmonary hypertension of the european
society of cardiology (ESC) and the european respiratory society
(ERS): Endorsed by: Association for european paediatric and
congenital cardiology (AEPC), international society for heart and
lung transplantation (ISHLT). Eur Heart J. 37:67–119.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Barberà JA, Román A, Gómez-Sánchez MÁ,
Blanco I, Otero R, López-Reyes R, Otero I, Pérez-Peñate G, Sala E
and Escribano P: Guidelines on the diagnosis and treatment of
pulmonary hypertension: Summary of recommendations. Arch
Bronconeumol. 54:205–215. 2018.(In English, Spanish). PubMed/NCBI View Article : Google Scholar
|
|
6
|
Barst RJ, McGoon M, Torbicki A, Sitbon O,
Krowka MJ, Olschewski H and Gaine S: Diagnosis and differential
assessment of pulmonary arterial hypertension. J Am Coll Cardiol.
43:S40–S47. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lai YC, Potoka KC, Champion HC, Mora AL
and Gladwin MT: Pulmonary arterial hypertension: The clinical
syndrome. Circ Res. 115:115–130. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gupta H, Ghimire G and Naeije R: The value
of tools to assess pulmonary arterial hypertension. Eur Respir Rev.
20:222–235. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chester AH, Yacoub MH and Moncada S:
Nitric oxide and pulmonary arterial hypertension. Glob Cardiol Sci
Pract. 2017(14)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cracowski JL and Leuchte HH: The potential
of biomarkers in pulmonary arterial hypertension. Am J Cardiol. 110
(Suppl 6):S32–S38. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shao D, Park JE and Wort SJ: The role of
endothelin-1 in the pathogenesis of pulmonary arterial
hypertension. Pharmacol Res. 63:504–511. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang X, Xu Q, Li T, Rong Y, Hong W, Huang
Y and Guo X: Intratracheal administration of isosorbide dinitrate
improves pulmonary artery pressure and ventricular remodeling in a
rat model of heart failure following myocardial infarction. Exp
Ther Med. 14:1399–1408. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Montani D, Souza R, Binkert C, Fischli W,
Simonneau G, Clozel M and Humbert M: Endothelin-1/endothelin-3
ratio: A potential prognostic factor of pulmonary arterial
hypertension. Chest. 131:101–108. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Simon M, Battistini B, Kim YJ and Tsang J:
Plasma levels of endothelin-1, big endothelin-1 and thromboxane
following acute pulmonary air embolism. Respir Physiol Neurobiol.
138:97–106. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Satwiko MG, Ikeda K, Nakayama K, Yagi K,
Hocher B, Hirata K and Emoto N: Targeted activation of endothelin-1
exacerbates hypoxia-induced pulmonary hypertension. Biochem Biophys
Res Commun. 465:356–362. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Vizza CD, Letizia C, Badagliacca R, Poscia
R, Pezzuto B, Gambardella C, Nona A, Papa S, Marcon S, Mancone M,
et al: Relationship between baseline ET-1 plasma levels and outcome
in patients with idiopathic pulmonary hypertension treated with
bosentan. Int J Cardiol. 167:220–224. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fukumoto S, Hanazono K, Miyasho T, Endo Y,
Kadosawa T, Iwano H and Uchide T: Serum big endothelin-1 as a
clinical marker for cardiopulmonary and neoplastic diseases in
dogs. Life Sci. 118:329–332. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ibe JCF, Zhou Q, Chen T, Tang H, Yuan JXJ,
Raj JU and Zhou G: Adenosine monophosphate-activated protein kinase
is required for pulmonary artery smooth muscle cell survival and
the development of hypoxic pulmonary hypertension. Am J Respir Cell
Mol Biol. 49:609–618. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Evans AM, Hardie DG, Peers C and Mahmoud
A: Hypoxic pulmonary vasoconstriction: Mechanisms of
oxygen-sensing. Curr Opin Anaesthesiol. 24(13)2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu Y, Liu L, Zhang Y, Wang G, Han D, Ke R,
Li S, Feng W and Li M: Activation of AMPK inhibits pulmonary
arterial smooth muscle cells proliferation. Exp Lung Res.
40:251–258. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Agard C, Rolli-Derkinderen M,
Dumas-de-La-Roque E, Rio M, Sagan C, Savineau JP, Loirand G and
Pacaud P: Protective role of the antidiabetic drug metformin
against chronic experimental pulmonary hypertension. Br J
Pharmacol. 158:1285–1294. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Omura J, Satoh K, Kikuchi N, Satoh T,
Kurosawa R, Nogi M, Otsuki T, Kozu K, Numano K, Suzuki K, et al:
Protective roles of endothelial AMP-activated protein kinase
against hypoxia-induced pulmonary hypertension in mice. Circ Res.
119:197–209. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
National Researrch Council: Guide for the
Care and Use of Laboratory Animals. 8th edition. National Academies
Press. Washingthon DC, 2010.
|
|
24
|
Gomez-Arroyo JG, Farkas L, Alhussaini AA,
Farkas D, Kraskauskas D, Voelkel NF and Bogaard HJ: The
monocrotaline model of pulmonary hypertension in perspective. Am J
Physiol Lung Cell Mol Physiol. 302:L363–L369. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nakata TM, Tanaka R, Yoshiyuki R, Fukayama
T, Goya S and Fukushima R: Effects of single drug and combined
short-term administration of sildenafil, pimobendan, and nicorandil
on right ventricular function in rats with monocrotaline-induced
pulmonary hypertension. J Cardiovasc Pharmacol.
65(640)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bogdan S, Seferian A, Totoescu A,
Dumitrache-Rujinski S, Ceausu M, Coman C, Ardelean CM, Dorobantu M
and Bogdan M: Sildenafil reduces inflammation and prevents
pulmonary arterial remodeling of the monocrotaline-induced disease
in the Wistar rats. Maedica. 7(109)2012.PubMed/NCBI
|
|
27
|
Wang Y, Tian W, Xiu C, Yan M, Wang S and
Mei Y: Urantide improves the structure and function of right
ventricle as determined by echocardiography in
monocrotaline-induced pulmonary hypertension rat model. Clin
Rheumatol. 38:29–35. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tawa M, Furukawa T, Tongu H, Sugihara M,
Taguwa S, Yamanaka M, Yano Y, Matsumori H, Kitada R, Sawano T, et
al: Stimulation of nitric oxide-sensitive soluble guanylate cyclase
in monocrotaline-induced pulmonary hypertensive rats. Life Sci.
203:203–209. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Breitling S, Krauszman A, Parihar R,
Walther T, Friedberg MK and Kuebler WM: Dose-dependent, therapeutic
potential of angiotensin-(1-7) for the treatment of pulmonary
arterial hypertension. Pulm Circ. 5:649–657. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Pacagnelli FL, Sabela AKD, Mariano TB,
Ozaki GAT, Castoldi RC, Carmo EM, Carvalho RF, Tomasi C, Okoshi K
and Vanderlei LCM: Fractal dimension in quantifying
experimental-pulmonary-hypertension-induced cardiac dysfunction in
rats. Arq Bras Cardiol. 107:33–39. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Karasu-Minareci E, Ozbudak IH, Ozbilim G
and Sadan G: Acute effects of vardenafil on pulmonary artery
responsiveness in pulmonary hypertension. ScientificWorldJournal.
2012(718279)2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bae HK, Lee H, Kim KC and Hong YM: The
effect of sildenafil on right ventricular remodeling in a rat model
of monocrotaline-induced right ventricular failure. Korean J
Pediatr. 59:262–270. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tsukamoto A, Uchida K, Maesato S, Sato R,
Kanai E and Inomata T: Combining isoflurane anesthesia with
midazolam and butorphanol in rats. Exp Anim. 65:223–230.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Aimbire F, Penna SC, Rodrigues KC,
Lopes-Martins RAB and Serté JAA: Effect of hydroalcoholic extract
of zingiber officinalis rhizomes on LPS-induced rat airway
hyperreactivity and lung inflammation. Prostaglandins Leukot Essent
Fatty Acids. 77:129–138. 2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Albrecht M, Henke J, Tacke S, Markert M
and Guth B: Effects of isoflurane, ketamine-xylazine and a
combination of medetomidine, midazolam and fentanyl on
physiological variables continuously measured by telemetry in
wistar rats. BMC Vet Res. 198:10–23. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gades NM, Danneman PJ, Wixson SK and
Tolley EA: The magnitude and duration of the analgesic effect of
morphine, butorphanol, and buprenorphine in rats and mice. Contemp
Top Lab Anim Sci. 39:8–13. 2000.PubMed/NCBI
|
|
37
|
Lee JH, Park BK, Oh KS, Yi KY, Lim CJ, Seo
HW and Lee BH: A urotensin II receptor antagonist, KR36676,
decreases vascular remodeling and inflammation in experimental
pulmonary hypertension. Int Immunopharmacol. 40:196–202.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kimura K, Daimon M, Morita H, Kawata T,
Nakao T, Okano T, Lee SL, Takenaka K, Nagai R, Yatomi Y and Komuro
I: Evaluation of right ventricle by speckle tracking and
conventional echocardiography in rats with right ventricular heart
failure. Int Heart J. 56:349–353. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Itoh T, Nagaya N, Fujii T, Iwase T,
Nakanishi N, Hamada K, Kangawa K and Kimura H: A combination of
oral sildenafil and beraprost ameliorates pulmonary hypertension in
rats. Am J Respir Crit Care Med. 169:34–38. 2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hirose S, Hosoda Y, Furuya S, Otsuki T and
Ikeda E: Expression of vascular endothelial growth factor and its
receptors correlates closely with formation of the plexiform lesion
in human pulmonary hypertension. Pathol Int. 50:472–479.
2000.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tuder RM, Groves B, Badesch DB and Voelkel
NF: Exuberant endothelial cell growth and elements of inflammation
are present in plexiform lesions of pulmonary hypertension. Am J
Pathol. 144:275–285. 1994.PubMed/NCBI
|
|
42
|
Dean A, Nilsen M, Loughlin L, Salt IP and
MacLean MR: Metformin reverses development of pulmonary
hypertension via aromatase inhibition. Hypertension. 68:446–454.
2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhai C, Shi W, Feng W, Zhu Y, Wang J, Li
S, Yan X, Wang Q, Zhang Q, Chai L, et al: Activation of AMPK
prevents monocrotaline-induced pulmonary arterial hypertension by
suppression of NF-κB-mediated autophagy activation. Life Sci.
208:87–95. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hattori Y, Suzuki K, Hattori S and Kasai
K: Metformin inhibits cytokine-induced nuclear factor kappaB
activation via AMP-activated protein kinase activation in vascular
endothelial cells. Hypertension. 47:1183–1188. 2006.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lalich J and Merkow L: Pulmonary arteritis
produced in rats by feeding crotalaria spectabilis. Lab Invest.
10:744–750. 1961.PubMed/NCBI
|
|
46
|
Yamaguchi K, Kanai Y, Asano K, Takasugi T,
Tanaka T, Yasuoka M and Hosoda Y: Temporal alterations of
endothelial-vasodilator functions in lung injury induced by
monocrotaline. Respir Physiol. 107:47–58. 1997.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Rubens C, Ewert R, Halank M, Wensel R,
Orzechowski HD, Schultheiss HP and Hoeffken G: Big endothelin-1 and
endothelin-1 plasma levels are correlated with the severity of
primary pulmonary hypertension. Chest. 120:1562–1569.
2001.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Stangl K, Dschietzig T, Richter C, Laule
M, Stangl V, Tanis E, Baumann G and Felix SB: Pulmonary release and
coronary and peripheral consumption of big endothelin and
endothelin-1 in severe heart failure: Acute effects of vasodilator
therapy. Circulation. 102:1132–1138. 2000.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Tang ST, Su H, Zhang Q, Tang HQ, Wang CJ,
Zhou Q, Wei W, Zhu HQ and Wang Y: Sitagliptin inhibits endothelin-1
expression in the aortic endothelium of rats with
streptozotocin-induced diabetes by suppressing the nuclear
factor-κB/IκBα system through the activation of AMP-activated
protein kinase. Int J Mol Med. 37:1558–1566. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Barnes PJ and Karin M: Nuclear factor-κB:
A pivotal transcription factor in chronic inflammatory diseases. N
Engl J Med. 336:1066–1071. 1997.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Tei C, Dujardin KS, Hodge DO, Bailey KR,
McGoon MD, Tajik AJ and Seward SB: Doppler echocardiographic index
for assessment of global right ventricular function. J Am Soc
Echocardiogr. 9:838–847. 1996.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Seyfarth HJ, Pankau H, Hammerschmidt S,
Schauer J, Wirtz H and Winkler J: Bosentan improves exercise
tolerance and Tei index in patients with pulmonary hypertension and
prostanoid therapy. Chest. 128:709–713. 2005.PubMed/NCBI View Article : Google Scholar
|



















