Introduction
Retinal arterial occlusion affects mainly older
people, with a mean age of 60 years at presentation. Young patients
are affected under specific conditions. Central retinal arterial
occlusion presents abrupt, painless loss of vision. Visual acuity
is typically reduced to the level of counting fingers or hand
movements, unless there is a separate cilioretinal artery supplying
the macula (1-4).
Relative afferent pupillary defect (RAPD) is present.
There are several causes for central retinal artery
occlusion. Emboli are 75% cholesterolic, 15% thrombi and 10%
calcific (1,5,6).
Thrombosis develops at the site of an atherosclerotic plaque,
vasculitic occlusions are associated with inflammatory disorders
i.e. Giant cell arteritis, Behcet disease, Polyarteritis
nodosa, Wegener's granulomatosis, Systemic Lupus erythematosus,
Susac's disease or dermatomyositis, and infectious conditions also
cause an inflammatory response in the retina with possible
occlusive complications (7-12).
Other causes can be medical procedures, such as arterial
angiography, vitreoretinal surgery, retrobulbar injections or
cervical manipulation (13),
structural anomalies, such as pre-papillary arterial loops and
optic disk drusen (14) and
vasospasm induced by migraines or drug use (1,15).
However, the most common causes of central retinal artery occlusion
in young patients are coagulation disorders (1).
Retinal vein occlusion is the most frequent primary
vascular disorder of the retina. Central retinal vein occlusion
takes place at a mean age between 60 and 70 years. About 10% of the
patients are younger than 50 years (16). It is generally monocular, but 5 to
11% of patients will suffer from occlusion in the contralateral eye
within five years (17).
Symptoms include floaters, black spots or
metamorphopsia, as well as blurred vision, which may occur after
getting up in the morning and fade during the day. The visual
acuity might deteriorate over a couple of days, leading the patient
to visit an ophthalmology clinic only after 1-3 weeks have passed
(18). The patient usually presents
with a visual acuity of 0.1-0.5.
Risk factors for central retinal vein occlusion may
be cardiovascular, local (trauma, retinal vasculitis, glaucoma,
optic disk drusen), coagulation disorders and hyperviscosity
syndromes (16).
Thrombophilia refers to a diverse group of disorders
that predispose an individual to the development of thrombosis.
Congenital thrombophilic states are inherited disorders, which
result in disturbance of the coagulation system either through
increased levels of procoagulants, deficiencies of anticoagulants
or reduced fibrinolysis (1,19-21).
Acquired causes of thrombophilia include a diverse group of
conditions. In the antiphospholipid syndrome autoantibodies
(22) to phospholipids form immune
complexes resulting in thrombosis formation. Hyperhomocysteinemia
leads to the damage of the vascular endothelium promoting thrombus
formation (23,24). Other associations with retinal
arterial and venous occlusion include hyperviscosity states, such
as myeloproliferative disorders, and pregnancy (1,16).
Case report
A 32-year old pregnant female patient was admitted
in another clinic in 2010 for sudden decrease of visual acuity in
the right eye, with no apparent precipitating factors. The patient
had no relevant family history or ophthalmological afflictions, but
she suffered from a spontaneous abortion at three weeks, one year
prior to this event, followed by a normal pregnancy. At
presentation, her best corrected visual acuity was 20/30 (0.2
logMAR) for the right eye and 20/20 (0 logMAR) for the left eye
with no correction. The intraocular pressure by Goldmann
applanation tonometry (GAT) was 17 mmHg in the right eye and 18
mmHg in the left eye. After external examination, slit-lamp
examination of the anterior and posterior segment and paraclinical
investigations, she was diagnosed with central retinal artery
occlusion in the right eye and was recommended to undergo a
hematological examination. The perimetric exam revealed a central
scotoma in the right eye.
The following paraclinical examination (Table I) revealed mildly higher RDW and PDW
and lower platelet number (~60,000/mcl). However, it is known that
platelet values can decrease during pregnancy without pathological
significance. Later, the platelet count returned to pseudo normal
values (489,000/mcl), which fluctuated over time. Considering this,
she was recommended permanent treatment with low molecular weight
heparin, platelet antiaggregant, peripheral vasodilator,
neuroprotectors and screening for clotting disorders.
 | Table IParaclinical investigations. |
Table I
Paraclinical investigations.
| Hereditary
thrombophilia |
|---|
| Tests | Results |
|---|
| C Protein | Normal |
| S Protein | Slightly ↓ - 46.58%
(N: 54.7-123.7%) |
| Antithrombin | Normal |
| Activated protein C
resistance | Normal |
| Factor V
leiden | Normal |
| Prothrombin gene
nutation | Absent |
| Homocysteine | Slightly ↑ - 17
µmol/l (N: 12-15 µmol/l) |
| Coagulogram (APTT,
PT, fibrinogen, ESR) | Normal |
| Acquired
thrombophilia |
| Tests | Results |
| Anti-phospholipidic
antibodies | Negative |
| Lupus anticoagulant
(LAC) | Negative |
| Anti-cardiolipin
antibodies (ACL) | Negative |
| Beta 2
glicoprotein-1 antibodies | Negative |
| Autoimmune
disorders |
| Tests | Results |
| Antibodies: RNP/Sm,
Sm, SS-A, RO-52, SS-B, Scl-70, PM-Scl100, JO-1, CB, | Negative |
| PCNA, dSDNA, NUC,
RIB |
| HLAB27 | Negative |
| AMA-M2 | Class +:
Inconclusive |
| DFS70 | Class +++: Highly
positive |
Regarding the tests for hereditary thrombophilia,
Antithrombin (AT), Activated Protein C Resistance (APCR), Factor V
Leiden (FVL), Protein C were normal. Prothrombin gene mutation
(PGM) was absent and Protein S was only slightly lower. The
coagulogram (APTT, PT, Fibrinogen, ESR) was normal. However,
homocysteine levels were slightly risen. Antibodies associated with
acquired thrombophilia (Anti-phospholipidic antibodies, Lupic
anticoagulant, Anti-cardiolipin antibodies, Beta 2 glicoprotein-1
antibodies) were absent.
Most antigens present in autoimmune disorders were
negative (HLAB27, RNP/Sm, Sm, SS-A, RO-52, SS-B, Scl-70, PM-Scl100,
JO-1, CB, PCNA, dSDNA, NUC, RIB). AMA-M2 test result was uncertain
and DFS70, a type of anti-nuclear antigen, was strongly
positive.
Additional investigations (Table II) showed normal blood pressure,
normal cerebral MRI, normal dental exam. The screening for
Paroxysmal Nocturnal Hemoglobinuria by immunophenotyping (GPI
deficit, PNH clone) was negative. However, the screening for
mutations of genes that control homocysteine metabolism revealed
MTHFR C677T and MTHFR A1298C heterozygote mutations, associated
with a high risk of spontaneous abortions, congenital malformations
and thromboembolic evens (25-28).
The patient was diagnosed with thrombophilia.
 | Table IIAdditional paraclinical
investigations. |
Table II
Additional paraclinical
investigations.
| Additional
investigations |
|---|
| Investigation | Results |
|---|
| Blood pressure | Within normal
range |
| Cerebral MRI | Normal |
| Dental
examination | Normal |
| Paroxysmal
nocturnal haemoglobinuria (GPI deficit, PNH clone) | Negative |
| screening for
homocysteine metabolism genes | MTHFR C677T
heterozygote mutation |
| | MTHFR A1298C
heterozygote mutation |
| Screening for
myeloproliferative disorders |
| Investigation | Results |
| Blood smear | Inconclusive |
| Abdominal
echography | Normal spleen |
| FAL | Negative |
| c-MPL mutation
(W515L, W515K, SN505N) | Absent |
| JAK2 V617F gene
mutation | Present |
| Medullar
biopsy | Positive for
essential thrombocythemia |
The screening for myeloproliferative disorders
showed a normal abdominal echography, with a normal sized spleen,
absence of FAL and c-MPL mutation (W515L, W515K, SN505N), and
presence of JAK2 V617F gene mutation. This mutation is associated
with Polycythemia vera (PV), Idiopathic myelofibrosis (IMF) and
Essential thrombocythemia (ET).
PV is characterized by an increase in red cells,
white cells and platelets. The patients have a plethoric
appearance, pruritus and splenomegaly. Complications include
hemorrhage and thromboembolic events and they can progress to
myelofibrosis and acute leukemia (29). ET is characterized by an increased
platelet count. In most cases, it is clinically asymptomatic, but
it can manifest with thromboembolic events that may lead to disease
detection (29). IMF is defined by
splenomegaly, bone marrow fibrosis and a leukoerythroblastic blood
picture that includes anaemia, thrombocythemia or thrombocytopenia
and variable white cell counts. The disease usually progresses to
transfusion dependent anaemia, symptomatic splenomegaly and
transformation to acute leukemia (29).
Considering these aspects, essential thrombocythemia
was the most likely diagnosis. For confirmation, a medullar biopsy
was needed, which was positive.
Two years later, in 2012, the patient requested a
routine check-up in our clinic, and the visual acuity of the right
eye had decreased to 20/160 (0.9 logMAR). The optical coherence
tomography (OCT) of the right macula showed severe macular atrophy
(Fig. 1).
In 2018, 8 years after the arterial occlusion, the
patient was admitted again to our clinic for sudden decrease of
visual acuity in the left eye. At presentation, her best corrected
visual acuity was 20/160 (0.9 logMAR) for the right eye and 20/40
(0.3 logMAR) for the left eye without correction. The intraocular
pressure by GAT was 16 mmHg in the right eye and 13 mmHg in the
left eye.
External examination and slit-lamp examination of
the anterior segment revealed no abnormal findings. Relative
afferent pupillary defect was absent.
The fundus of each eye was examined after
pharmaceutical mydriasis with 0.5% tropicamide and 10%
phenylephrine hydrochloride ophthalmic solutions. The right eye had
a pallid papilla in the temporal half, spastic arteries, moderately
dilated veins and no foveolar reflex. The left eye had a
protruding, hyperemic papilla, with imprecise delimited margins,
turgescent veins, cotton wool spots and intraretinal haemorrhages
concentrated around the papilla (Fig.
2).
Perimetry was assessed by the Humphrey Visual Field
Analyzer, central 24-2 threshold program, with a size III white
stimulus. Reliability indices were very good in visual fields for
both eyes. It demonstrated centrocecal scotoma with inferonasal
extension for the right eye and superior arcuate scotoma for the
left eye (Fig. 3).
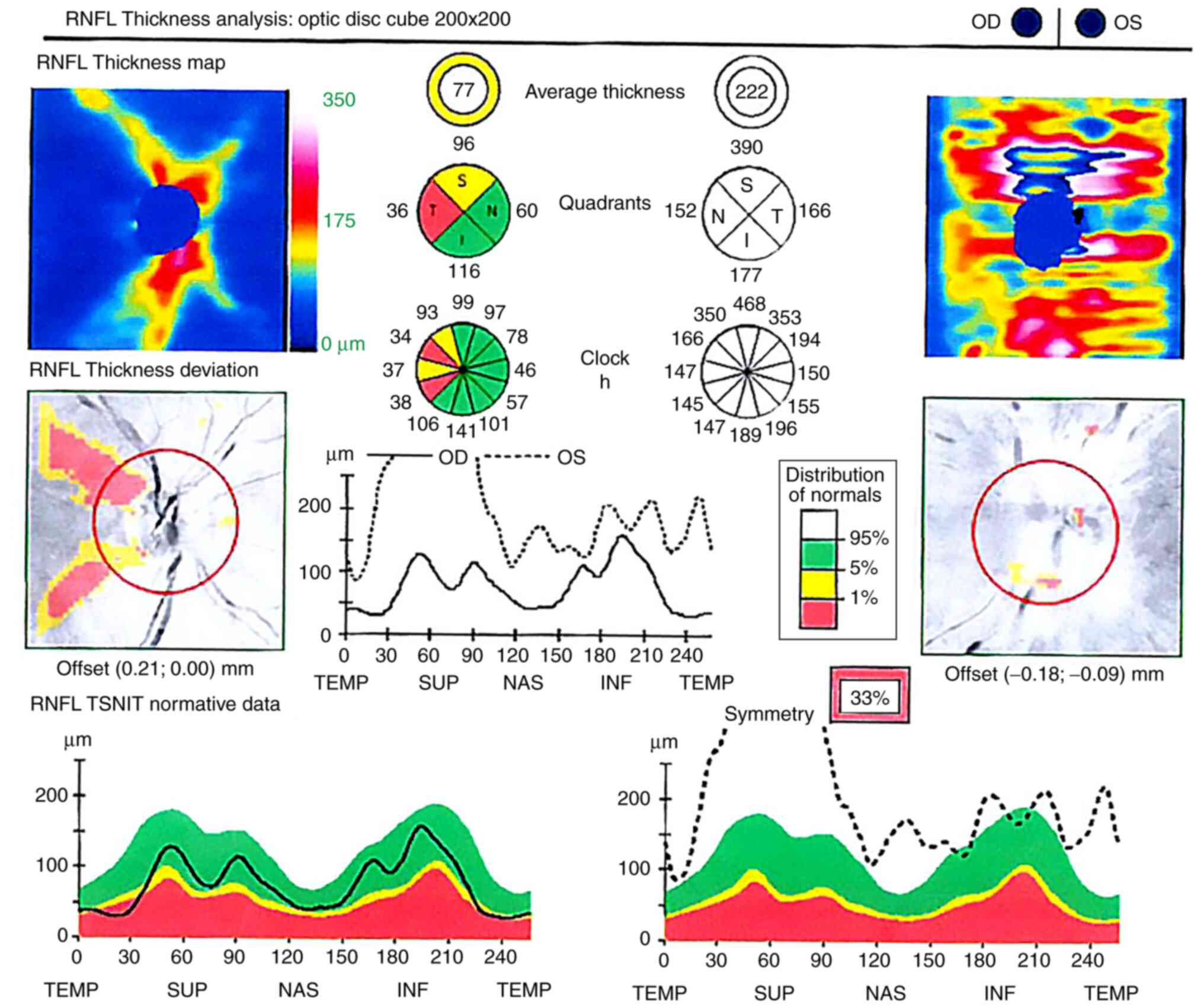
Optical coherence tomography (OCT) of the optic
nerve showed temporal atrophy of the retinal nerve fiber layer
(RNFL) in the right eye and thickening of the nerve fiber layer for
360˚ around the optic disc in the left eye, secondary to the
papillary edema (Fig. 4). The
macular cube analysis revealed severe atrophy of the right macula
and subretinal fluid in the interpapillo-macular region of the left
eye (Fig. 5).
Based on this clinical and paraclinical
investigations, we established the working diagnosis of
Papillophlebitis. The patient was further investigated in order to
establish the course of treatment. We recommended maintaining the
treatment with low molecular weight heparin, platelet
antiaggregant, peripheral vasodilator, neuroprotectors and regular
hematological check-ups.
The differential diagnosis (Table III) included causes of papillary
edema associated with cotton wool spots and retinal hemorrhages.
Diabetic retinopathy, hypertensive retinopathy and infections,
which can combine all three signs (papillary edema, hemorrhages and
exudates) were excluded considering the normal glucose levels,
blood pressure and inflammatory markers. Other causes of retinal
hemorrhage and exudates, like ocular ischemic syndrome, were also
excluded (1,16,18,30,31).
 | Table IIIDifferential diagnosis for
pathologies overlapping papillary edema, retinal hemorrhages and
cotton wool spots. |
Table III
Differential diagnosis for
pathologies overlapping papillary edema, retinal hemorrhages and
cotton wool spots.
| Papillary
edema | Retinal
hemorrhages | Cotton wool
spots |
|---|
| Central retinal
vein occlusion | Central retinal
vein occlusion | Central retinal
vein occlusion |
| Diabetic
retinopathy | Diabetic
retinopathy | Diabetic
retinopathy |
| Hypertensive
retinopathy | Hypertensive
retinopathy | Hypertensive
retinopathy |
| Infections | Infections | Infections |
| Inflammatory
diseases | Inflammatory
diseases | Purtscher
retinopathy |
| Trauma | Trauma | Radiotherapy |
| NA-AION | Ocular ischemic
syndrome | Ocular ischemic
syndrome |
| Congenital disk
anomalies | Valsalva
retinopathy | Siclemy |
| Leber optic
neuropathy | Retinal
macroaneurysm | Drug reaction
(Interferon) |
| Intracranial
hypertension | Terson
syndrome | Talc emboli |
| Expansive
processes | | Metastatic
cancer |
| Infiltrative
diseases | | |
Central retinal vein occlusion occurs in patients
over 50 years of age or under 50 with coagulation disorders
(16). The decrease in visual
acuity is acute, nonprogressive, painless and monocular. Papillary
edema is associated with macular edema, retinal hemorrhages in all
quadrants, tortuous, dilated veins and cotton wool spots with a
characteristic aspect of ‘blood and thunder fundus’.
Papillophlebitis is a type of central retinal vein
occlusion that occurs in otherwise healthy young people with
moderate visual acuity changes with no relative afferent pupillary
defect. Papillary edema is predominant and macular edema is rare.
Retinal haemorrhages and cotton wool spots are situated mainly
peripapillary (16).
Given the above exclusion criteria, the genetic
constellation and the fact that the patient presented with several
elements common for central retinal vein occlusion, the positive
diagnosis included: Thrombophilia with hyperhomocysteinemia,
essential thrombocythemia, old central retinal artery occlusion
(CRA) in the right eye and central retinal vein occlusion (CRV) in
the left eye.
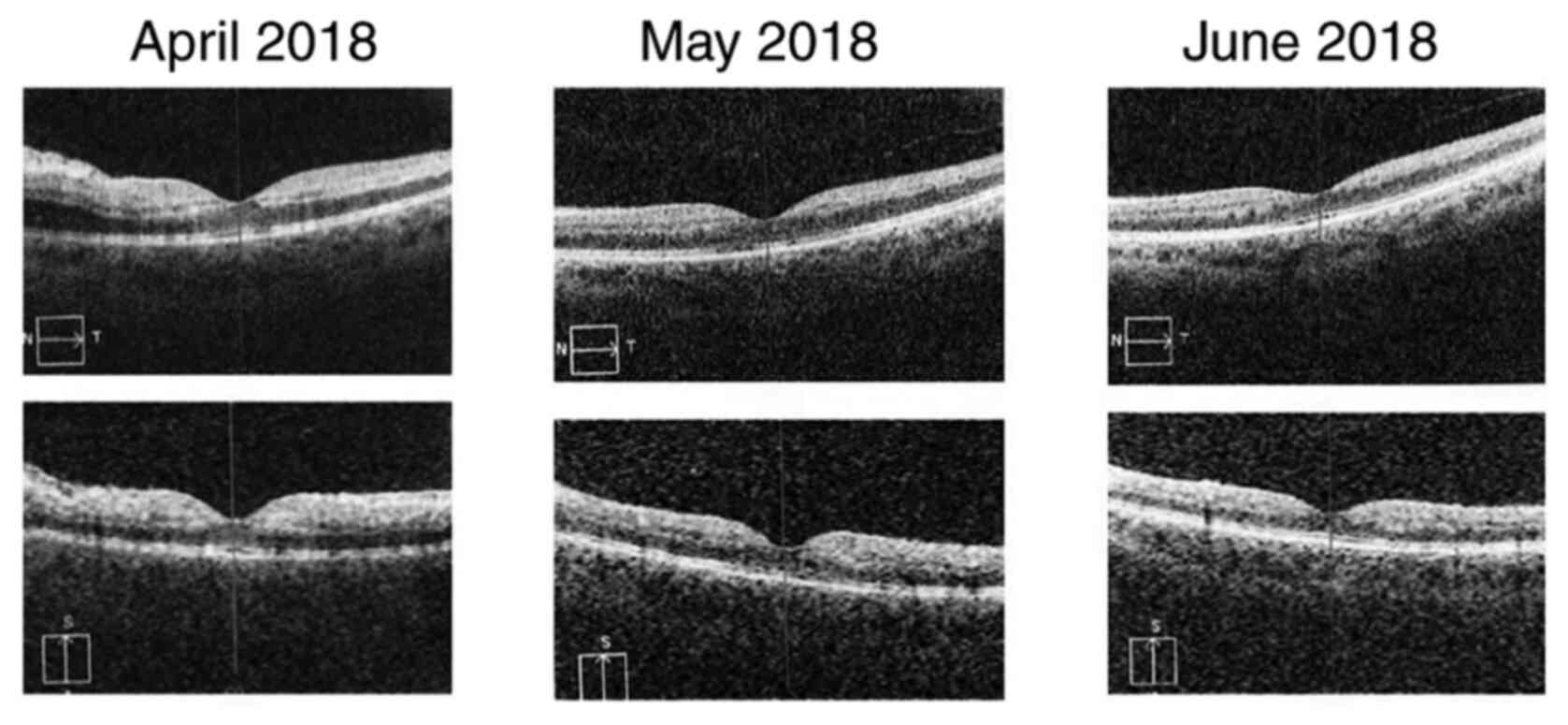
The patient was followed-up for 2 months. The right
eye presented no changes during the follow-up period. For the left
eye, best corrected central visual acuity increased from 20/40 (0.3
logMAR) (in April 2018) to 20/20 (0 logMAR) (in June 2018). The
aspect of the optic disc improved with the remission of the edema
(Fig. 6). The cotton wool spots
disappeared completely and only a few retinal hemorrhages remained
(Fig. 6, black arrows). The caliber
of the veins also showed an improvement.
Perimetry was assessed again after 2 months. During
the follow-up period there was an improvement of the visual field
in the left eye, with significant reduction of the superior arcuate
scotoma. The centrocecal scotoma in the right eye remained
unchanged (Fig. 7).
The evolution of the optical nerve OCT for the left
eye showed partial regression of the papillary edema after 3 weeks,
followed at 2 months by total resorption of the papillary edema
(Fig. 8). In the right eye, which
suffered the central retinal artery occlusion back in 2010, the
RNFL atrophy of the temporal quadrant and severe macular atrophy
persisted. The macula of the left eye regained its normal thickness
by subretinal fluid evanescence (Figs.
9 and 10). However, atrophy
gradually appeared in the ganglion cell layer, caused by the
previous nerve fiber layer inflammation, which hindered the
axoplasmic flow and led to the axonal atrophy (Fig. 11).
The best corrected central visual acuity improved
progressively in 2 months from 20/40 (0.3 logMAR) to 20/20 (0
logMAR). The visual field underwent positive changes with the
persistence of a small ring-shaped scotoma. The RNFL thickness
returned to normal values; however, ganglion cell atrophy was
identified by OCT imaging 2 months after the central retinal vein
occlusion.
Discussion
This case is particular because the young patient
had two retinal vascular occlusive episodes 8 years apart: The
central retinal artery occlusion when she was 32 years old and the
central retinal vein occlusion at 40. The venous occlusion occurred
despite being on chronic treatment with low molecular weight
heparin, platelet antiaggregant and peripheral vasodilator. She has
two genetic defects that predispose to thromboembolic events.
Methylenetetrahydrofolate Reductase (MTHFR)
Deficiency is the most common genetic cause of elevated levels of
homocysteine in the plasma. The MTHFR enzyme has a role in
processing amino acids, specifically, the conversion of
homocysteine to methionine. Genetic variations in the MTHFR
gene can lead to impaired function or inactivation of this enzyme,
which results in mildly elevated levels of homocysteine (25,32-34).
Up until recent times, it was believed that MTHFR deficiency led to
an increased risk of venous thrombosis, coronary heart disease, and
recurrent pregnancy loss, by causing elevated homocysteine levels
(25-28).
However, more recent studies have not found an association between
elevated homocysteine levels and the risk of venous thrombosis or
coronary heart disease (25,35).
The myeloproliferative disorders (MPD) are a group
of hematological conditions where there is a primary defect at the
level of the multi-potent hematopoietic stem cell leading to
increased production in one or more blood cell types. The main
disorders are polycythemia vera (PV), essential thrombocythemia
(ET) and idiopathic myelofibrosis (IMF). ET is characterized by an
increased platelet count. In most cases, it is clinically
asymptomatic, but it can manifest with thromboembolic events that
may lead to disease detection (29).
The JAK2 gene is a member of a family of
Janus kinases. It has a role as an upstream signaling molecule
directly linked to the erythropoietin receptor. Hematopoietic stem
cells from MPD patients are hypersensitive to a range of growth
factors and use JAK2 for signaling. The activity of JAK2 is
disrupted by the presence of the V617F mutation (29).
The MRC-PT-1 prospective study of ET compared JAK2
V617F mutation positive and negative patients (36). Those with the mutation had features
resembling PV, had a higher rate of transformation to PV, higher
hemoglobin, neutrophil counts, were more prone to venous
thrombosis, but showed lower serum erythropoietin levels and
ferritin levels.
A factor that might have had an influence on the
initial coagulation imbalance, which led to the arterial occlusion,
was the fact that the patient was pregnant. Several changes occur
to the coagulation system as pregnancy progresses, with the largest
changes being seen at term gestation (37-39).
While plasma volume increases up to 40%, red blood cell volume
increases by only 25%, which leads to a decrease in hemoglobin
concentration known as the physiological anemia of pregnancy
(40). Platelet counts usually
decrease, caused by hemodilution and consumption by the
uteroplacental unit. However, this decrease is rarely great enough
to influence bleeding (41,42).
Coagulation factor concentrations change
significantly throughout pregnancy. Factors II, V and protein C do
not change, factor IX is variable, factors VIII, IX, X, XII, VWF
and fibrinogen increase more than 100%, D-dimer up to 400% and
factor VII up to 1000%. The platelet count decreases up to 20% and
protein S and factor XIII can decrease up to 50% (36-46).
The total of all these changes leads to roughly double the
coagulation activity seen when compared with the non-pregnant
state, thus causing pregnancy to be a hypercoagulable state
(37).
DFS70, a type of anti-nuclear antigen was strongly
positive for this patient. Some studies described an immune
procoagulant state involving anti-DFS70 antibodies (47). However, this antigen, associated
with various diseases, like atopic dermatitis (48), alopecia areata (49) and Vogt-Harada syndrome (50), is positive in 6% of healthy people
(51,52).
Therefore, the hypercoagulable state caused by the
pregnancy acted as a precipitating factor on an organism that
already had two genetic mutations predisposing to vascular
occlusions. After this incident, more specific paraclinical
investigations were recommended, which led to the discovery of the
Methylenetetrahydrofolate Reductase and Janus Kinase 2 gene
mutations.
The visual short-term prognosis for the left eye is
good, with complete central visual acuity regain. The visual acuity
of the right eye with severe macular atrophy will most likely never
recover. The long-term prognosis for this case is, however,
uncertain. There is a risk of retinal or iris neovascularization as
a complication of the vascular occlusion. New retinal artery or
vein occlusions could occur and there is also a risk of thrombosis
in other areas, like cerebral, pulmonary or renal, due to the
general coagulability imbalance. Essential thrombocythemia also has
a small probability to progress towards myelofibrosis and acute
leukemia, which may be influenced by the treatment modalities used
(36).
The visual and systemic impact of chronic
hypercoagulability states is significant. Since they affect
patients at a relatively young age, the quality of life is reduced
at an active stage of life. Treatment is mandatory, but disease
control is not always acquired, and the long-term prognosis varies
according to etiology and the presence of additional risk
factors.
Acknowledgements
Professional editing, linguistic and technical
assistance performed by Irina Radu, Individual Service Provider,
certified translator in Medicine and Pharmacy (certificate
credentials: Series E no. 0048).
Funding
No funding was received.
Availability of data and materials
All data and materials supporting the results of the
present case are available in the published article.
Authors' contributions
HTS contributed to the conception and design of the
study, the acquisition, analysis and interpretation of data of the
study. He also contributed to the drafting of the work and its
critical revision for important intellectual content. BT
contributed to the acquisition, the analysis and interpretation of
data of the study, to the drafting of the work and its critical
revision for important intellectual content. SS and MMa contributed
to the conception and design of the study, the acquisition,
analysis and interpretation of data of the study, contributed to
the drafting of the work and its critical revision for important
intellectual content. MMu, FB, CR and DMD contributed to
the conception and design of the study, to the drafting of the work
and its critical revision for important intellectual content. ACT
contributed to the analysis and interpretation of data of the
study, to the drafting of the work and its critical revision for
important intellectual content. All authors read and approved the
final version of the manuscript and agreed to be accountable for
all aspects of the study in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
‘Prof. Dr. Agrippa Ionescu’ Emergency Clinical Hospital (approval
no., 34/11.06.2018; Bucharest, Romania).
Patient consent for publication
Written informed consent obtained from the patient
prior to publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Joussen AM, Gardner TW, Kirchhof B and
Ryan SJ (eds): Retinal vascular disease. Section III, Ch. 21,
Retinal Artery Occlusion, pp507-518, 2007.
|
|
2
|
Brown GC and Magargal LE: Central retinal
artery obstruction and visual acuity. Ophthalmology. 89:14–19.
1982.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Landa E, Rehany U and Rumelt S: Visual
functions following recovery from non-arteritic central retinal
artery occlusion. Ophthalmic Surg Lasers Imaging. 35:103–108.
2004.PubMed/NCBI
|
|
4
|
Stanca HT, Petrović Z and Munteanu M:
Transluminal Nd: YAG laser Embolysis - a reasonable method to
reperfuse occluded branch retinal arteries. Vojnosanit Pregl.
71:1072–1077. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Arruga J and Sanders MD: Ophthalmologic
findings in 70 patients with evidence of retinal embolism.
Ophthalmology. 89:1336–13347. 1982.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mitchell P, Wang JJ and Smith W: Risk
factors and significance of finding asymptomatic retinal emboli.
Clin Exp Ophthalmol. 28:13–17. 2000.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Stanca HT, Suvac E, Munteanu M, Jianu DC,
Motoc AGM, Roşca GC and Boruga O: Giant cell arteritis with
arteritic anterior ischemic optic neuropathy. Rom J Morphol
Embryol. 58:281–285. 2017.PubMed/NCBI
|
|
8
|
Braunstein RA and Gass JD: Branch artery
obstruction caused by acute toxoplasmosis. Arch Ophthalmol.
98:512–513. 1980.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cohen SM, Davis JL and Gass DM: Branch
retinal arterial occlusions in multifocal retinitis with optic
nerve oedema. Arch Ophthalmol. 113:1271–1276. 1995.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lightman DA and Brod RD: Branch retinal
artery occlusion associated with Lyme disease. Arch Ophthalmol.
109:1198–1199. 1991.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Solley WA, Martin DF, Newman NJ, King R,
Callanan DG, Zacchei T, Wallace T, Parks DJ, Bridges W and
Sternberg P Jr: Cat scratch disease: Posterior segment
manifestations. Ophthalmology. 106:1546–1553. 1999.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yokoi M and Kase M: Retinal vasculitis due
to secondary syphilis. Jpn J Ophthalmol. 48:65–67. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Klein ML, Jampol LM, Condon PI, Rice TA
and Serjeant GR: Central retinal artery occlusion without
retrobulbar hemorrhage after retrobulbar anesthesia. Am J
Ophthalmol. 93:573–577. 1982.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Newman NJ, Lessell S and Brandt EM:
Bilateral central retinal artery occlusions, disk drusen, and
migraine. Am J Ophthalmol. 107:236–240. 1989.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wallace RT, Brown GC, Benson W and
Sivalingham A: Sudden retinal manifestations of intranasal cocaine
and methamphetamine abuse. Am J Ophthalmol. 114:158–160.
1992.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Joussen AM, Gardner TW, Kirchhof B and
Ryan SJ (eds): Retinal vascular disease. Section III, Ch. 21,
Central Retinal Vein Occlusion, pp443-461, 2007.
|
|
17
|
Hayreh SS, Zimmerman MB and Podhajsky P:
Incidence of various types of retinal vein occlusion and their
recurrence and demographic characteristics. Am J Ophthalmol.
117:429–441. 1994.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fong A, Schatz H, McDonald HR, Burton TC,
Maberley AL, Joffe L, Zegarra H, Nadel AJ and Johnson RN: Central
retinal vein occlusion in young adults (papillophlebitis). Retina.
11:3–11. 1991.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Golub BM, Sibony PA and Coller BS: Protein
S deficiency associated with central retinal artery occlusion. Arch
Ophthalmol. 108(918)1990.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nelson ME, Talbot JF and Preston FE:
Recurrent multiple-branch retinal arteriolar occlusions in a
patient with protein C deficiency. Graefes Arch Clin Exp
Ophthalmol. 227:443–447. 1989.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Weger M, Renner W, Pinter O, Stanger O,
Temmel W, Fellner P, Schmut O and Haas A: Role of factor V Leiden
and prothrombin 20210A in patients with retinal artery occlusion.
Eye (Lond). 17:731–734. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cobo-Soriano R, Sanchez-Ramon S, Aparicio
MJ, Teijeiro MA, Vidal P, Suarez-Leoz M, Rodriguez-Mahou M,
Rodriguez-Huerta A, Fernández-Cruz E and Cortés C: Antiphospholipid
antibodies and retinal thrombosis in patients without risk factors:
A prospective case-control study. Am J Ophthalmol. 128:725–732.
1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cahill M, Karabatzaki M, Meleady R, Refsum
H, Ueland P, Shields D, Mooney D and Graham I: Raised plasma
homocysteine as a risk factor for retinal vascular occlusive
disease. Br J Ophthalmol. 84:154–157. 2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pianka P, Almog Y, Man O, Goldstein M,
Sela BA and Loewenstein A: Hyperhomocystinemia in patients with
nonarteritic anterior ischemic optic neuropathy, central retinal
artery occlusion, and central retinal vein occlusion.
Ophthalmology. 107:1588–1592. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dean L: Methylenetetrahydrofolate
Reductase Deficiency. Mar 8, 2012 [Updated 2016 Oct 27]. In: Pratt
VM, McLeod HL, Rubinstein WS, et al (eds). Medical Genetics
Summaries [Internet]. Bethesda (MD), National Center for
Biotechnology Information (US), 2012. Available from urihttps://www.ncbi.nlm.nih.gov/books/NBK66131/simplehttps://www.ncbi.nlm.nih.gov/books/NBK66131/.
Accessed on July 10, 2020.
|
|
26
|
Humphrey LL, Fu R, Rogers K, Freeman M and
Helfand M: Homocysteine level and coronary heart disease incidence:
A systematic review and Meta-analysis. Mayo Clin Proc.
83:1203–1212. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
den Heijer M, Rosendaal FR, Blom HJ,
Gerrits WB and Bos GM: Hyperhomocysteinemia and venous thrombosis:
A meta-analysis. Thromb Haemost. 80:874–877. 1998.PubMed/NCBI
|
|
28
|
Kupferminc MJ, Eldor A, Steinman N, Many
A, Bar-Am A, Jaffa A, Fait G and Lessing JB: Increased frequency of
genetic thrombophilia in women with complications of pregnancy. N
Engl J Med. 340:9–13. 1999.PubMed/NCBI View Article : Google Scholar
|
|
29
|
McLornan D, Percy M and McMullin MF: JAK2
V617F: A single mutation in the myeloproliferative group of
disorders. Ulster Med J. 75:112–119. 2006.PubMed/NCBI
|
|
30
|
Jackson TL and Moorfields Eye Hospital:
Moorfields Manual of Ophthalmology. Philadelphia, PA, Mosby
Elsevier, 2008.
|
|
31
|
Munteanu M, Rosca C and Stanca HT:
Sub-inner limiting membrane hemorrhage in a patient with Terson
syndrome. Int Ophthalmol. 39:461–464. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Holmes MV, Newcombe P, Hubacek JA, Sofat
R, Ricketts SL, Cooper J, Breteler MM, Bautista LE, Sharma P,
Whittaker JC, et al: Effect modification by population dietary
folate on the association between MTHFR genotype, homocysteine, and
stroke risk: A meta-analysis of genetic studies and randomised
trials. Lancet. 378:584–594. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shiran A, Remer E, Asmer I, Karkabi B,
Zittan E, Cassel A, Barak M, Rozenberg O, Karkabi K and Flugelman
MY: Association of vitamin B12 deficiency with homozygosity of the
TT MTHFR C677T genotype, hyperhomocysteinemia, and endothelial cell
dysfunction. Isr Med Assoc J. 17:288–292. 2015.PubMed/NCBI
|
|
34
|
van der Put NM, Gabreels F, Stevens EM,
Smeitink JA, Trijbels FJ, Eskes TK, van den Heuvel LP and Blom HJ:
A second common mutation in the methylenetetrahydrofolate reductase
gene: An additional risk factor for neural-tube defects? Am J Hum
Genet. 62:1044–1051. 1998.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Clarke R, Bennett DA, Parish S, Verhoef P,
Dötsch-Klerk M, Lathrop M, Xu P, Nordestgaard BG, Holm H, Hopewell
JC, et al: Homocysteine and coronary heart disease: Meta-analysis
of MTHFR case-control studies, avoiding publication bias. PLoS Med.
9(e1001177)2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Campbell PJ, Scott LM, Buck G, Wheatley K,
East CL, Marsden JT, Duffy A, Boyd EM, Bench AJ, Scott MA, et al:
United kingdom myeloproliferative disorders study group; Medical
research council adult leukaemia working party; Australasian
leukaemia and lymphoma group definition of subtypes of essential
thrombocythaemia and relation to polycythaemia vera based on JAK2
V617F mutation status: A prospective study. Lancet. 366:1945–1953.
2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Katz D and Beilin Y: Disorders of
coagulation in pregnancy. Br J Anaesth. 115 (Suppl 2):ii75–ii88.
2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Brenner B: Haemostatic changes in
pregnancy. Thromb Res. 114:409–414. 2004.PubMed/NCBI View Article : Google Scholar
|
|
39
|
James AH: Pregnancy and thrombotic risk.
Crit Care Med. 38(Suppl 2):S57–S63. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Abbassi-Ghanavati M, Greer LG and
Cunningham FG: Pregnancy and laboratory studies: A reference Table
for clinicians. Obstet Gynecol. 114:1326–1331. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cerneca F, Ricci G, Simeone R, Malisano M,
Alberico S and Guaschino S: Coagulation and fibrinolysis changes in
normal pregnancy. Increased levels of procoagulants and reduced
levels of inhibitors during pregnancy induce a hypercoagulable
state, combined with a reactive fibrinolysis. Eur J Obstet Gynecol
Reprod Biol. 73:31–36. 1997.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Prisco D, Ciuti G and Falciani M:
Hemostatic changes in normal pregnancy. Hematol Meet Reports
(Formerly Haematol Reports). 1:1–5. 2005.
|
|
43
|
O'Riordan MN and Higgins JR: Haemostasis
in normal and abnormal pregnancy. Best Pract Res Clin Obstet
Gynaecol. 17:385–396. 2003.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bremme K, Ostlund E, Almqvist I, Heinonen
K and Blombäck M: Enhanced thrombin generation and fibrinolytic
activity in normal pregnancy and the puerperium. Obstet Gynecol.
80:132–137. 1992.PubMed/NCBI
|
|
45
|
Szecsi PB, Jørgensen M, Klajnbard A,
Andersen MR, Colov NP and Stender S: Haemostatic reference
intervals in pregnancy. Thromb Haemost. 103:718–727.
2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Comp PC, Thurnau GR, Welsh J and Esmon CT:
Functional and immunologic protein S levels are decreased during
pregnancy. Blood. 68:881–885. 1986.PubMed/NCBI
|
|
47
|
Marlet J, Ankri A, Charuel JL,
Ghillani-Dalbin P, Perret A, Martin-Toutain I, Haroche J, Amoura Z,
Musset L and Miyara M: Thrombophilia associated with anti-DFS70
autoantibodies. PLoS One. 10(e0138671)2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ochs RL, Muro Y, Si Y, Ge H, Chan EK and
Tan EM: Autoantibodies to DFS 70 kd/transcription coactivator p75
in atopic dermatitis and other conditions. J Allergy Clin Immunol.
105:1211–1220. 2000.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Okamoto M, Ogawa Y, Watanabe A, Sugiura K,
Shimomura Y, Aoki N, Nagasaka T, Tomita Y and Muro Y:
Autoantibodies to DFS70/LEDGF are increased in alopecia areata
patients. J Autoimmun. 23:257–266. 2004.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Shinohara T, Singh DP and Chylack LT Jr:
Review: Age-related cataract: Immunity and lens epithelium-derived
growth factor (LEDGF). J Ocul Pharmacol Ther. 16:181–191.
2000.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yamada K, Senju S, Shinohara T, Nakatsura
T, Murata Y, Ishihara M, Nakamura S, Ohno S, Negi A and Nishimura
Y: Humoral immune response directed against LEDGF in patients with
VKH. Immunol Lett. 78:161–168. 2001.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Watanabe A, Kodera M, Sugiura K, Usuda T,
Tan EM Takasaki Y, Tomita Y and Muro Y: Anti-DFS70 antibodies in
597 healthy hospital workers. Arthritis Rheum. 50:892–900.
2004.PubMed/NCBI View Article : Google Scholar
|