Introduction
The skin serves as a barrier against extrinsic
factors and is continuously in contact with potentially harmful
elements, such as toxic substances, microbial organisms and solar
radiation. Exposure to ultraviolet (UV) radiation has detrimental
effects, which may manifest as sunburn, inflammation, tumorigenesis
and aging (1). The latter is also
referred to as photoaging and is characterized by connective tissue
degradation. Solar UV radiation, the primary cause of extrinsic
skin aging, is divided into three subgroups according to
wavelength: UVA, 320-400 nm; UVB, 280-320 nm; and UVC, 200-280 nm.
Of the two UV subgroups that can reach the human skin, UVA is
responsible for ~95% of total radiation exposure compared with UVB
(2). UVA-exposed skin begins to
exhibit photoaging symptoms, including loss of elasticity and
strength, inflammation and wrinkle formation. In addition to the
DNA damage, the damage caused by UV irradiation is primarily due to
rapid degradation of the extracellular matrix (ECM), which is the
result of an increase in the production of ECM-degrading enzymes,
namely matrix metalloproteinases (MMPs) (3). Among different types of MMPs that are
expressed in various parts of the body, collagenase MMP-1 and
gelatinase MMP-9 are predominantly found in skin cells. Degradation
of the main components of the ECM is primarily carried out by MMP-1
and MMP-9. UVA irradiation induces the expression and activation of
MMP-1 and MMP-9 in keratinocytes, subsequently increasing collagen
degradation and decreasing collagen production (4). Therefore, ameliorating UVA-induced
alterations in MMP expression and collagen production may serve to
prevent photoaging. Medicinal plants are widely recognized for
their various secondary metabolites with beneficial health effects.
Apart from their medicinal uses for the treatment of diseases and
complications, certain plants, such as Rosmarinus
officinalis, Thymus vulgaris and Smallanthus
sonchifolius, exert skin-protective effects, including
inhibition of UV-mediated MMP-1 activity (5). Similar MMP-1 inhibitory effects were
reported for natural plants of Asian origin, such as Typha
orientalis Nymphaea tetragona and Filipendula glaberrima
(6). Camellia japonica
(C. japonica) is a plant native to Asia that is widely
cultivated worldwide as a garden plant. Therefore, cultivars of
C. japonica are widely available and its flowers may be
easily found. Over the past decades, C. japonica has
attracted attention as a cosmeceutical ingredient due to its
beneficial moisturizing, antimicrobial, anti-inflammatory and
antioxidant properties (7).
Therefore, the aim of the present study was to investigate the
effect of Camellioside A (CMDA), a triterpenoid saponin from C.
japonica, against UVA-induced photoaging in HaCaT human
keratinocytes with respect to MMP-1 expression and collagen
production and degradation.
Materials and methods
Isolation and characterization of
CMDA
C. japonica flowers were hand-collected in
Namwon-eup (Jeju island, Republic of Korea) in January 2018.
Samples were identified by Dr Gwanpil Song (Jeju Biological
Resource Co.), and a voucher specimen (no. AP-0104) was deposited
at the Plant Archive of Amorepacific Research and Development
Center for future reference. The collected flowers were sun-dried
and ground into a fine powder using an electric mill. Powdered
samples were stored in a sealed container at 4˚C until further
use.
Dried C. japonica flowers (200 g) were
extracted three times with 80% ethanol (1 l) over 3 days.
Evaporation of the solvent under vacuum gave the crude extract (98
g). The crude extract was partitioned between ethyl acetate (2 l)
and H2O (1 l) mixture to give an ethyl acetate soluble
fraction (4.5 g) and aqueous phase, which was extracted with
1-butanol (1lx2) to give 1-butanol fraction (31 g). A part of the
1-butanol fraction (5.6 g) was subjected to reverse-phase silica
gel column to give eight fractions. Fractions 6, 7 and 8 contained
crude compound 1 (587 mg). Crude compound 1 fractions were purified
by preparative high-performance liquid chromatography (HPLC;
MeCN-H2O =35:65, v/v; both solvents were acidificed by
0.1% TFA, flow rate =15 ml/min, column temperature =30˚C) to give
pure compound 1 (243 mg). A Gilson HPLC system (Gilson, Inc.) was
used for preparative HPLC possessing a UV/Vis-155 detector, binary
pumps, and a GX-271 liquid handler. The HPLC column for preparative
HPLC was a Luna C18(2) column
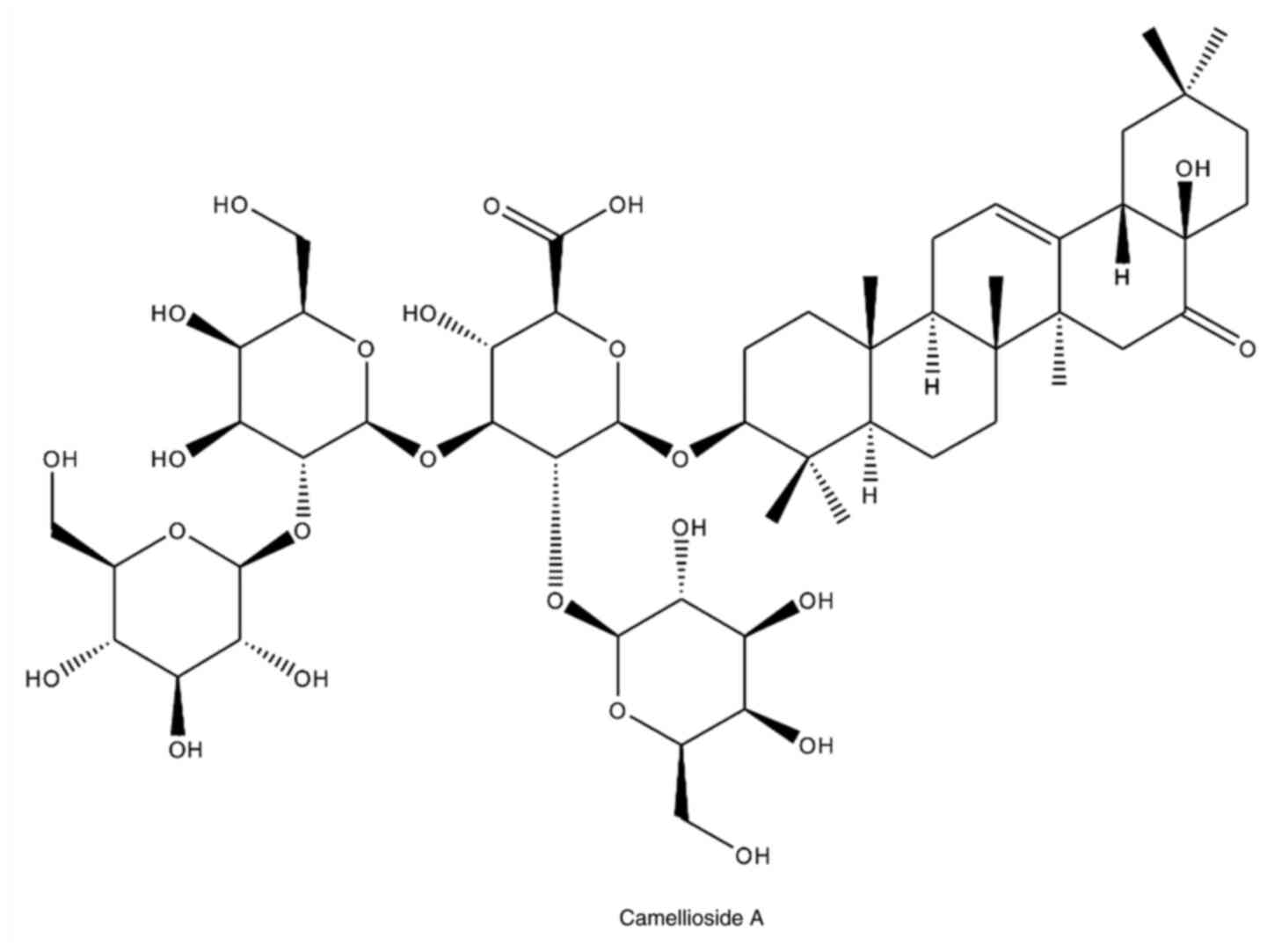
(21.2x250 mm I.D., 5 µm; Phenomenex). Compound 1 isolated from the
flower of C. japonica was identified as Camellioside A
(CMDA) by nuclear magnetic resonance spectroscopy (NMR) and mass
spectroscopic analyses (Fig. 1).
The spectroscopic data of CMDA matched published values (7).
HaCaT human keratinocyte culture and
maintenance
HaCaT cells (cat. no. 300493; CLS Cell Line Service
GmbH) were cultured in 6-well plates with DMEM (Sigma-Aldrich;
Merck KGaA) supplemented with 10% FBS (Sigma-Aldrich; Merck KGaA)
at 37˚C with 5% CO2.
Cell viability assay
The viability of HaCaT keratinocytes was analyzed by
conducting a colorimetric MTT assay. HaCaT cells (1x104
cells/well) were cultured in 96-well plates and incubated for 24 h
at 37˚C prior to treatment with different concentrations (1, 5 and
10 µM) of CMDA that were introduced in serum-free fresh medium.
After incubation for 24 h at 37˚C, the supernatant was removed and
100 µl MTT [1 mg/ml (m/v)] in PBS was added to the cells. Following
incubation for 4 h at 37˚C, 50 µl DMSO was added to each well to
stop the reaction and solubilize the formazan crystals. The optical
density of each well was measured at a wavelength of 540 nm using a
GENios® microplate reader (Tecan Group, Ltd.). Cell
viability was plotted as a relative percentage against the
untreated control group.
UVA irradiation
UVA irradiation was performed using a Bio-Sun UV
Irradiation System (Vilber Lourmat) fitted with a UVA source
designed for microplates. HaCaT cells grown in 6-well plates
(1.5x106 cells/well) were placed in the UVA irradiation
system and exposed to UVA (10 J/cm2). Cells were
irradiated in PBS without the plastic lid. When the irradiation
matched the desired programmed energy (10 J/cm2), the
UVA irradiation automatically stopped, and the cells were then
incubated in previously mentioned culture medium, with or without
CMDA treatment, until analysis.
MMP-1 and pro-collagen Iα1 ELISA
The production of MMP-1 and type Iα1 pro-collagen
was investigated by performing ELISA. HaCaT keratinocytes were
pre-incubated in 6-well plates (1.5x106 cells/well) for
24 h at 37˚C and washed with PBS before UVA (10 J/cm2)
exposure. After UVA irradiation, the cells were treated with or
without different concentrations of CMDA for 24 h at 37˚C. The
contents of MMP-1 and type Iα1 pro-collagen in the cell culture
media was assessed using an ELISA kit (Human Total MMP-1 DuoSet
ELISA, cat no. DY901B; Human Pro-Collagen I alpha 1 DuoSet ELISA,
cat. no. DY6220; both from R&D Systems, Inc.) according to the
manufacturer's protocol.
Reverse
transcription-semi-quantitative PCR analysis
HaCaT keratinocytes were grown to confluence in
6-well plates (1.5x106 cells/well) and the control group
was subjected to UVA irradiation (10 J/cm2) only.
Following UVA irradiation, cells were treated with CMDA for 24 h at
37˚C. Total RNA was extracted from HaCaT keratinocytes using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA (2 µg) was reverse-transcribed into cDNA using
CellScript All-in-One cDNA synthesis Master Mix (CellSafe Co.,
Ltd.) following manufacturer's protocol with T100 thermal cycler
(Bio-Rad Laboratories, Inc.). The following temperature protocol
was used for reverse transcription: 42˚C for 60 min and 72˚C for 5
min. Subsequently, qPCR was performed using the following primers:
MMP-1 forward, 5'-GGAGCCAGCTCCCTCTATTT-3' and reverse,
5'-GGCTACATGGGAACAGCCTA-3'; type I pro-collagen forward,
5'-AGAAGGAAATGGCTGCAGAA-3' and reverse, 5'-GCTCGGCTTCCAGTATTGAG-3';
and β-actin forward, 5'-CCACAGCTGAGAGGGAAATC-3' and reverse,
5'-AAGGAAGGCTGGAAAAGAGC-3'. Amplification of cDNA was performed
using the Thermal Cycler Dice Real-Time System TP800 (Takara Bio,
Inc.) using Luna® Universal qPCR Mix (New England
Biolabs, Inc.) according to manufacturer's protocol. The following
thermocycling conditions were used for qPCR: 30 cycles at 95˚C for
45 sec, 60˚C for 1 min and 72˚C for 45 sec. The final PCR products
were separated by electrophoresis for 30 min at 100 V on a 1.5%
agarose gel. Following staining with 1 mg/ml ethidium bromide, gels
were imaged under a UV light using a CAS-400SM Davinch-Chemi
Imager™ (Davinch-K).
Western blotting
HaCaT cells (1.5x106 cells/well) cultured
in 6-well plates were treated with or without CMDA for 24 h at 37˚C
after UVA (10 J/cm2) irradiation. Protein levels in
cells were investigated using standard western blotting techniques.
Briefly, cell lysates were prepared by vigorous pipetting of each
well with 1 ml RIPA buffer (Sigma-Aldrich; Merck KGaA) at 4˚C. The
nuclear fraction extraction was carried out using a NE-PERTM
Nuclear Extraction kit (cat. no. 78835; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Total protein
was quantified using a bicinchoninic acid protein assay (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Proteins (20 µg) were separated via 12% SDS-PAGE at 100 V and
transferred onto PVDF membranes (Amersham; Cytiva) using a wet
system run at 100 V for 1 h at 4˚C. The membranes were then
incubated for 1 h at room temperature in 5% skimmed milk for
blocking. Following blocking, the membranes were washed with 1X
TBST (0.1% Tween-20) and incubated with primary antibodies [1:1,000
in primary antibody dilution buffer containing 1X TBST with 5%
bovine serum albumin (Sigma-Aldrich; Merck KGaA)] overnight at 4˚C.
The following primary antibodies were used: MMP-1 (cat. no.
sc-6837; Santa Cruz Biotechnology, Inc.), MMP-9 (cat. no. 393857;
Cell Signaling Technology, Inc.), type I pro-collagen (cat. no.
sc-8782; Santa Cruz Biotechnology, Inc.), p38 (cat. no. 8690; Cell
Signaling Technology, Inc.), phosphorylated (p)-p38 (cat. no. 4511;
Cell Signaling Technology, Inc.), JNK (cat. no. LF-PA0047; Thermo
Fisher Scientific, Inc.), p-JNK (cat. no. sc-293136; Santa Cruz
Biotechnology, Inc.), ERK (cat. no. 4695; Cell Signaling
Technology, Inc.), p-ERK (cat. no. 4370; Cell Signaling Technology,
Inc.), c-Jun (cat. no. sc-74543; Santa Cruz Biotechnology, Inc.),
p-c-Jun (cat. no. sc-822; Santa Cruz Biotechnology, Inc.), c-Fos
(cat. no. sc-7202; Santa Cruz Biotechnology, Inc.), p-c-Fos (cat.
no. 5348s; Cell Signaling Technology, Inc.), β-actin (cat. no.
sc-47778; Santa Cruz Biotechnology, Inc.) and lamin B1 (cat. no.
sc-374015; Santa Cruz Biotechnology, Inc.). Subsequently, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies (1:1,000) for 1 h at room temperature. The
following source specific secondary antibodies were used:
anti-mouse (cat. no. 7076; Cell Signaling Technology, Inc.),
anti-rabbit (cat. no. 7074; Cell Signaling Technology, Inc.) and
anti-goat (cat. no. sc-2354; Santa Cruz Biotechnology, Inc.).
Protein bands were visualized using an ECL Western blot detection
kit (Amersham; Cytiva). Protein bands were imaged with CAS-400SM
Davinch-Chemi imager™ (Davinch-K).
Statistical analysis
All experiments were performed in triplicate. Data
are presented as the mean ± standard deviation. Comparisons among
multiple groups were analyzed using one-way ANOVA followed by
Tukey's post hoc test. Statistical analyses were conducted using
SAS software (version 9.1; SAS Institute, Inc.).
Results
CMDA inhibits UVA-induced expression
of MMP-1 and collagen
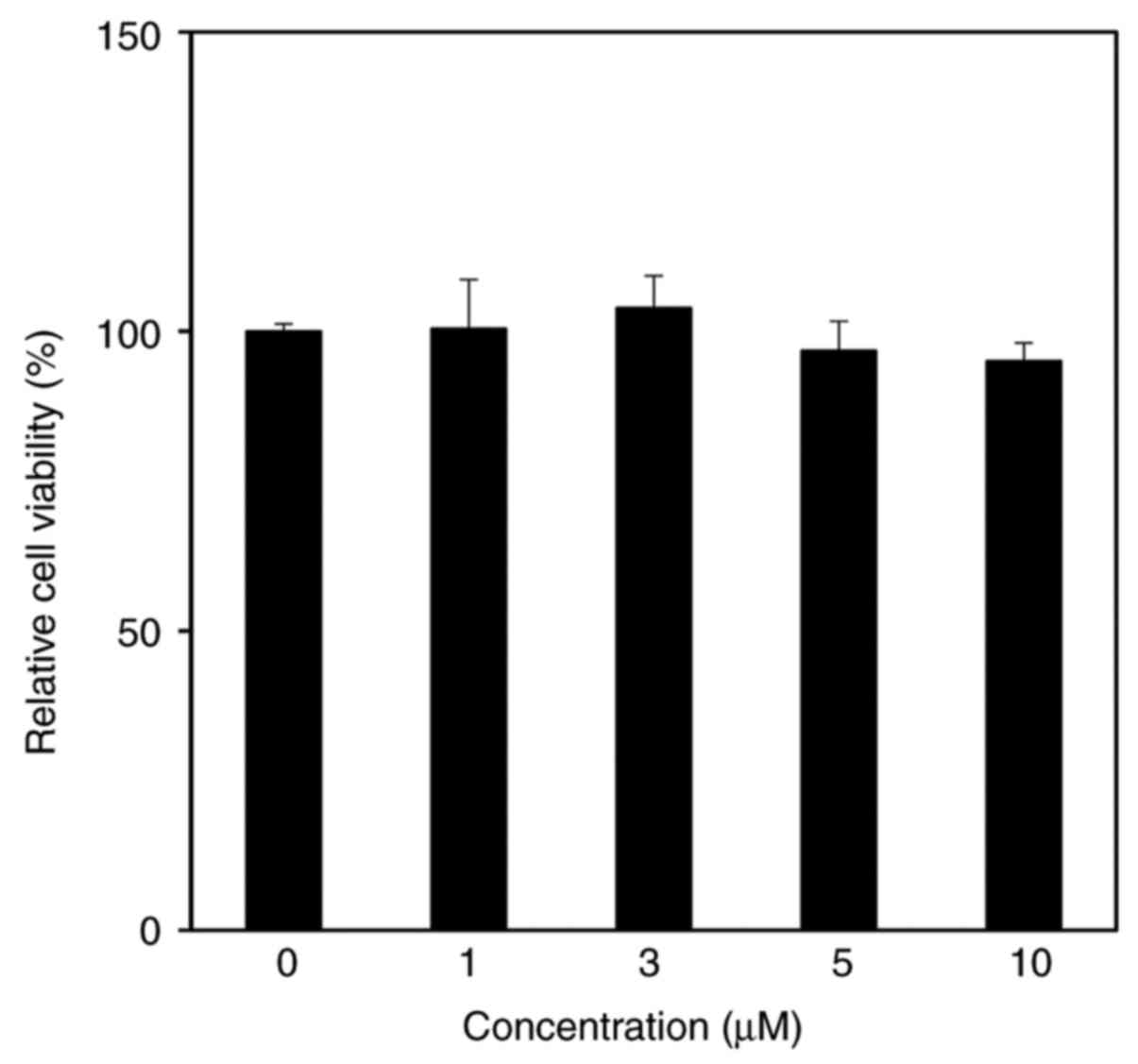
The cytotoxicity of CMDA in HaCaT keratinocytes was
assessed by performing an MTT assay. The results suggested that
CMDA treatment up to 10 µM did not exert any toxic effects on HaCaT
keratinocytes (Fig. 2).
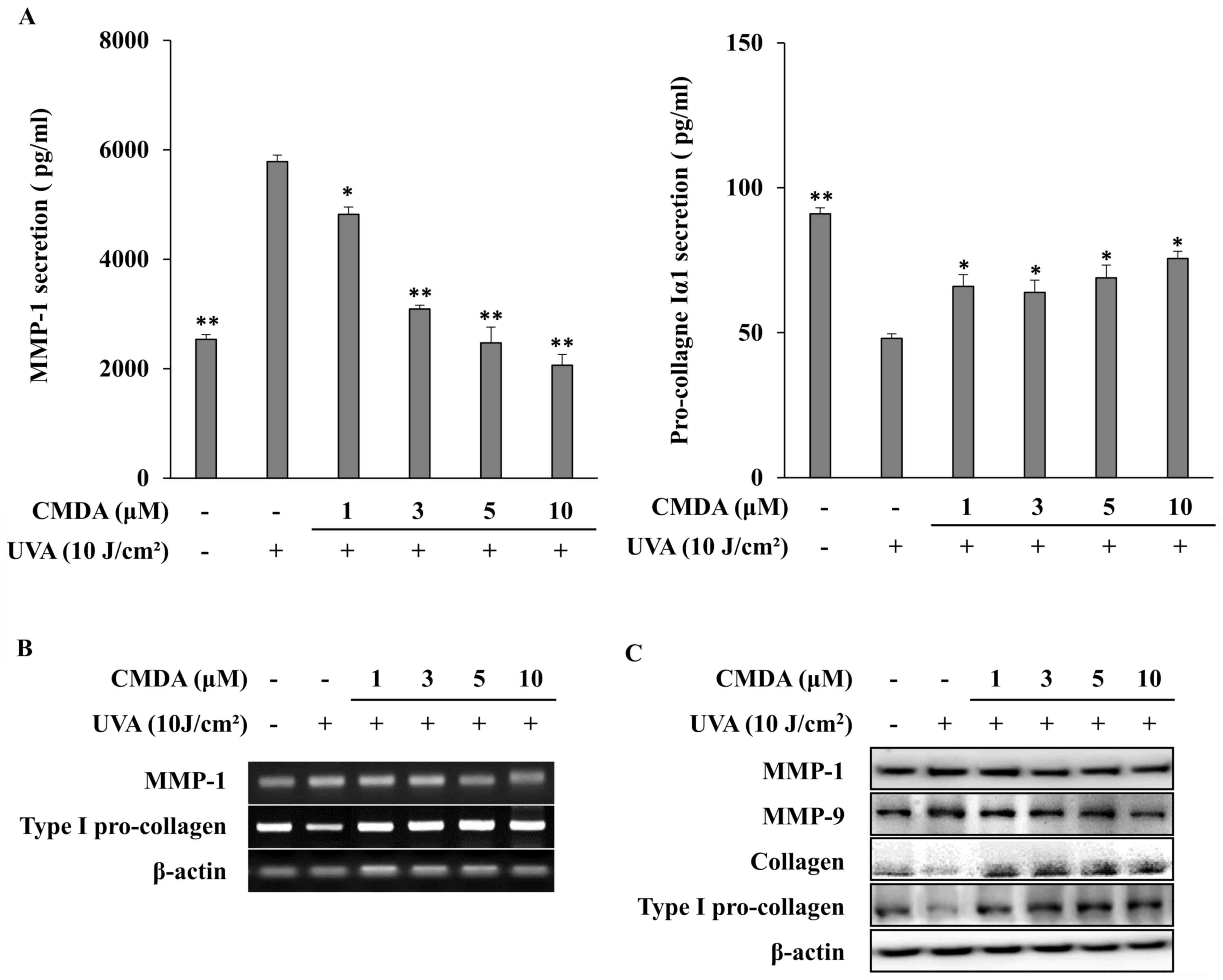
Subsequently, to verify the effect of CMDA on UVA-induced changes
in MMP-1 and collagen production, cellular MMP-1 and pro-collagen
Iα1 release were investigated using ELISA. Exposure to UVA (10
J/cm2) increased MMP-1 release to 5,782.7 pg/ml from
2,536.3 pg/ml (non-irradiated control), whereas pro-collagen Iα1
release was decreased to 48.1 pg/ml from 91.0 pg/ml (Fig. 3A). CMDA treatment of HaCaT
keratinocytes following UVA-irradiation dose-dependently inhibited
MMP-1 release. At 10 µM, CMDA-treated keratinocyte culture medium
contained 2,063.0 pg/ml MMP-1, which was a 64.3% decrease compared
with the UVA treatment-only group. Parallel effects were observed
for pro-collagen Iα1 release; however, dose dependency was not
observed, as 1, 3 and 5 µM CMDA increased pro-collagen Iα1 levels
to 65.9, 63.9 and 68.9 pg/ml, respectively, whereas keratinocytes
treated with 10 µM CMDA produced 75.6 pg/ml pro-collagen Iα1, which
was a 57.2% increase compared with the UVA treatment-only
group.
The results obtained from mRNA and protein
expression analyses were in agreement with the ELISA results. CMDA
treatment dose-dependently inhibited the mRNA (Fig. 3B) and protein (Fig. 3C) expression of MMP-1 in
UVA-irradiated HaCaT keratinocytes. Type I pro-collagen mRNA
expression was also upregulated following CMDA treatment. The
protein levels of collagen and type I pro-collagen were similarly
stimulated by CMDA treatment, which indicated that CMDA-treated
keratinocytes exhibited an increase in their ability to produce
collagen, which was inhibited by UVA irradiation. In addition, CMDA
treatment also suppressed UVA-induced MMP-9 levels in
keratinocytes.
CMDA suppresses UVA-stimulated
activation of the mitogen-activated protein kinase (MAPK)/activator
protein 1 (AP-1) signaling pathway
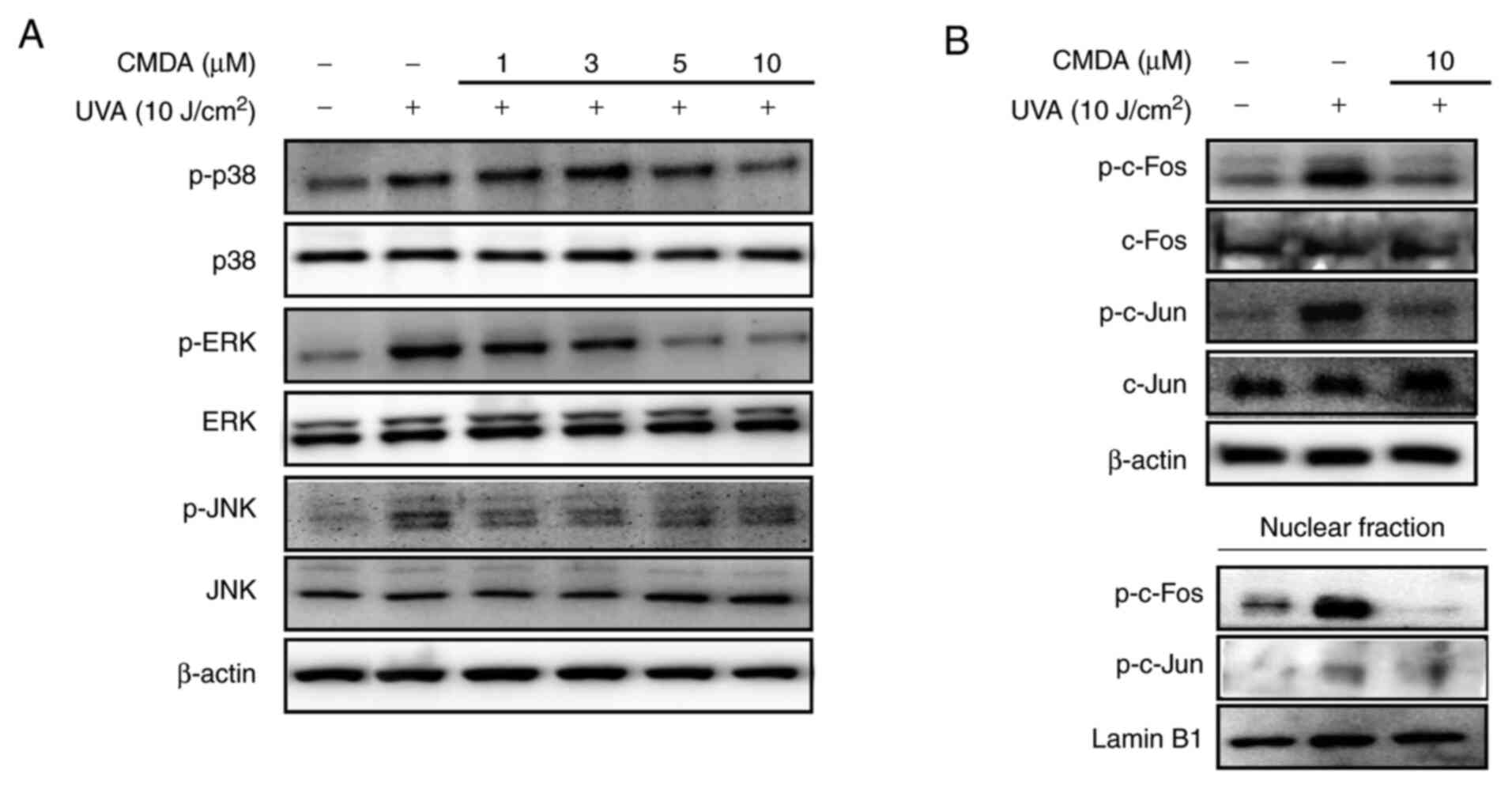
MAPK/AP-1 signaling regulation was investigated in
order to determine the mechanism underlying CMDA-mediated MMP-1
suppression. UV irradiation stimulates the activation of p38, ERK
and JNK MAPKs, leading to the phosphorylation of the c-Fos and
c-Jun proteins. The cascade facilitates the formation of AP-1
transcription factor and its nuclear translocation (6). Keratinocytes irradiated by UVA
displayed increased levels of phosphorylated p38, ERK and JNK MAPKs
(Fig. 4A). Treatment with CMDA (1,
3, 5 and 10 µM) dose-dependently suppressed UVA-induced
phosphorylation of MAPKs, whereas the total protein levels of MAPKs
were not altered. Subsequently, the phosphorylation levels of MAPK
downstream proteins for MMP-1 transcriptional expression (c-Fos and
c-Jun) were investigated. UVA irradiation significantly increased
phosphorylated c-Fos and c-Jun levels in total cell lysates and
nuclear fractions (Fig. 4B), which
was parallel to an increase in MAPK activation. The UVA-stimulated
increase of AP-1-forming proteins was notably suppressed following
treatment with 10 µM CMDA. These results suggested that CMDA
suppresses UVA-mediated MMP-1 production via suppression of AP-1
regulated transcriptional activity.
Discussion
C. japonica is a flowering plant endemic to East
Asia with known cosmeceutical benefits. It was previously
demonstrated that C. japonica oil promotes skin barrier
function and type I pro-collagen production (8). Based on these properties, several
bioactive compounds have been isolated from C. japonica, and
their bioactivities have been reported (7,9). CMDA
is one such compound, an oleanane triterpenoid saponin. In addition
to the MMP-1 inhibitory and pro-collagen production stimulatory
effects of C. japonica oil (9), the present study was conducted to
investigate the possible effects of CMDA against UVA-induced skin
aging using MMP-1 and type I pro-collagen production as
markers.
UV irradiation disrupts the collagen formation of
ECM, which is one of the leading causes of extrinsic skin aging,
also referred to as photoaging (2).
It was previously demonstrated that solar UVA radiation caused
elevated MMP-1 production along with diminished collagen
production, which in turn resulted in excessive degradation of skin
collagen. Increased degradation of the collagen framework of the
skin results in skin abnormalities, such as wrinkles (4). In the present study, HaCaT
keratinocytes released increased amounts of MMP-1 and significantly
decreased amounts of type Iα1 pro-collagen following UVA
irradiation, whereas CMDA treatment attenuated MMP-1 release and
increased type Iα1 pro-collagen release. The results suggested that
CMDA may serve a role in reversing UVA-induced damage of the skin
ECM.
Expression of the MMP-1 gene is transcriptionally
regulated by the AP-1 transcription factor, which is a
heterodimerized form of phosphorylated c-Fos and c-Jun proteins
(10). UVA irradiation contributes
to MMP-1 expression via activation of MAPK signaling pathways,
which are responsible for c-Fos and c-Jun heterodimerization and
subsequent nuclear translocation. Translocation of AP-1 initiates
MMP-1 expression (8). The results
of the present study further indicated that UVA irradiation caused
increased phosphorylation of the p38, ERK and JNK MAPKs, which were
responsible for the phosphorylation of the c-Fos and c-Jun
proteins. In addition, the nuclear fractions of UVA-irradiated
HaCaT keratinocytes exhibited significantly higher levels of
phosphorylated c-Fos and c-Jun compared with the non-irradiated
control group. The presence of CMDA in the culture medium after UVA
irradiation exerted suppressive effects on the activation of MAPK
signaling, resulting in decreased levels of phosphorylated p38,
MAPK and ERK. This effect of CMDA was also observed in both total
cell lysate and nuclear fraction levels of phosphorylated c-Fos and
c-Jun. Overall, the results of the present study indicated that
CMDA suppressed AP-1 transcription factor formation, thereby
inhibiting UVA-induced MMP-1 production.
In conclusion, the present study suggested that CMDA
may serve as a potential anti-photoaging agent by exerting
protective effects against UVA-induced collagen degradation in
keratinocytes. The results also indicated that CMDA exerted its
effects via suppression of AP-1-regulated MMP-1 expression.
Therefore, CMDA and its source, C. japonica extract, may be
useful in the cosmeceutical industry for the development of
products that protect against skin aging. However, further studies
are required to elucidate the underlying mechanism and to verify
the results of the present study in vivo.
Acknowledgements
Not applicable.
Funding
The present study was performed within the program
of the AMOREPACIFIC Open Research (grant no. SRT-01-R19E80012)
supported by a grant from AMOREPACIFIC.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FK, JHO and CSK conceived the study, designed the
experiments and supplied the necessary materials. FK, JHO and HRK
performed the experiments and collected the data. JK conducted the
isolation and chemical elucidation analysis. FK interpreted and
analyzed the data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dupont E, Gomez J and Bilodeau D: Beyond
UV radiation: A skin under challenge. Int J Cosmet Sci. 35:224–232.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Widel M, Krzywon A, Gajda K, Skonieczna M
and Rzeszowska-Wolny J: Induction of bystander effects by UVA, UVB,
and UVC radiation in human fibroblasts and the implication of
reactive oxygen species. Free Radic Biol Med. 68:278–287.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Battie C, Jitsukawa S, Bernerd F, Del Bino
S, Marionnet C and Verschoore M: New insights in photoaging, UVA
induced damage and skin types. Exp Dermatol. 23 (Suppl 1):S7–S12.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dong KK, Damaghi N, Picart SD, Markova NG,
Obayashi K, Okano Y, Masaki H, Grether-Beck S, Krutmann J, Smiles
KA and Yarosh DB: UV-induced DNA damage initiates release of MMP-1
in human skin. Exp Dermatol. 17:1037–1044. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Duque L, Bravo K and Osorio E: A holistic
anti-aging approach applied in selected cultivated medicinal
plants: A view of photoprotection of the skin by different
mechanisms. Ind Crops Prod. 97:431–439. 2017.
|
|
6
|
Kim YH, Kim KS, Han CS, Yang HC, Park SH,
Ko KI, Lee SH, Kim KH, Lee NH, Kim JM and Son KH: Inhibitory
effects of natural plants of Jeju Island on elastase and MMP-1
expression. Int J Cosmetic Sci. 29:487–488. 2007.PubMed/NCBI
|
|
7
|
Nakamura S, Moriura T, Park S, Fujimoto K,
Matsumoto T, Ohta T, Matsuda H and Yoshikawa M: Melanogenesis
inhibitory and fibroblast proliferation accelerating effects of
noroleanane- and oleanane-type triterpene oligoglycosides from the
flower buds of Camellia japonica. J Nat Prod. 75:1425–1430.
2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Panich U, Chaiprasongsuk A, Lohakul J,
Soontrapa K and Sampattavanich S: Photoprotective role of Nrf2 in
UVA-mediated MMP-1 via MAPK/AP-1 signaling in keratinocyte HaCaT
cells and mouse skin: The photoprotective effects of sulforaphane.
Free Radic Biol Med. 100(S44)2016.
|
|
9
|
Kim M, Son D, Shin S, Park D, Byun S and
Jung E: Protective effects of Camellia japonica flower
extract against urban air pollutants. BMC Complement Altern Med.
19(30)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang X and Bi Z: UVB-irradiated human
keratinocytes and interleukin-1alpha indirectly increase MAP
kinase/AP-1 activation and MMP-1 production in UVA-irradiated
dermal fibroblasts. Chin Med J (Engl). 119:827–831. 2006.PubMed/NCBI
|