Introduction
Asthma is a common chronic inflammatory airway
disease that is accompanied by respiratory symptoms, including
difficulty breathing, chest tightness, wheezing and airway
hyperresponsiveness (1). Globally,
>300 million patients suffer from asthma and the prevalence of
this disease is increasing (2-4).
Montelukast is a selective leukotriene receptor-1 antagonist
(LTRAs) and is widely used for the treatment of asthma (5-7).
The Global Initiative for Asthma guidelines recommend LTRAs as a
second-line alternative medication to inhaled corticosteroids (ICS)
(8,9). In children, montelukast is preferred
due to the advantages of its pharmaceutical form compared with ICS
(7). However, certain
post-marketing studies have indicated that chronic montelukast
treatment may be linked to neuropsychiatric disorders, including
aggressive behavior, sleep disorders and depression (5-9).
In 2008, due to the increase of these reports, the Food and Drug
Administration of the US (FDA) issued a warning and indicated the
enhanced risk of neuropsychiatric events associated with the use of
antileukotriene agents (10).
Previous clinical studies have reported an increase in suicidal
tendency, depression, hallucinations and nightmares following the
administration of montelukast (5,6). In
2009, the prescribing information of Singulair®
(montelukast sodium) was revised and the warnings and precautions
section was edited to include neuropsychiatric events (11,12).
However, despite the results of important case reports and the
warning by the FDA, no sufficient data was found to demonstrate an
association between montelukast and depression (13,14).
Furthermore, to the best of our knowledge, no experimental studies
have been conducted to investigate the association between
depression-like behavior and montelukast treatment. Furthermore,
asthma is considered to be a risk factor for depression (15-17).
The association between asthma and depression has been examined by
numerous studies and the results have demonstrated a high
comorbidity between the two diseases (18-21).
Depressive disorders have been reported to have almost 2-fold
higher prevalence in young asthmatic patients compared with young
non-asthmatics (22). The current
study aimed to investigate the possible association between chronic
montelukast treatment and depression-like behavior, and the effect
of asthma on depression-like behavior in mice.
Materials and methods
Animals
A total of 130 Swiss mice (male, 65; female, 65;
age, 8-12 weeks; weight, 20-25 g) were purchased from Kobay D.H.L.
A.S. and were used in all experiments. Equal numbers of male and
female mice were used in each experimental group. The mice were
housed in a room at constant humidity and temperature (22±1˚C) with
12 h light/dark cycles and provided with food and water ad
libitum. All experiments were carried out according to the EU
Directive 2010/63/EU (23) for
animal experiments and approved by the Animal Experimentations
Local Ethics Board of Hacettepe University, Ankara, Turkey
(approval no. 2018-3/2; updated by committee in the meeting
commencing in 2020 for in vivo analysis of airway function,
approval no. 2020-3/12).
Drug administration and experimental
design
The mice were divided into four groups for the
experimental asthma and forced swim test (FST) as follows: i)
Control group (n=24 for 1st, n=18 for 2nd and n=12 for 3rd FST);
ii) ovalbumin (OVA; n=24 for 1st, n=18 for 2nd and n=12 for 3rd
FST); iii) montelukast-treated controls (n=18 for 2nd and n=12 for
3rd FST); and iv) montelukast-treated OVA (n=18 for 2nd and n=12
for 3rd FST). Montelukast treatment was started after inflammation
was occurred with the first challenge. In the first FST, mice were
administrated montelukast for only 1 day, as the primary aim of the
current study was to investigate the chronic effects of
montelukast. The first FST was not performed in montelukast-treated
groups. (Fig. 1). Following each
FST, 6 mice were euthanized for histopathological analysis. In the
treatment groups (montelukast-treated OVA and montelukast-treated
control groups), montelukast (Abdi Ibrahim Pharmaceuticals; 20
mg/kg/day) was administrated orally through the drinking water
every day at the same hour, starting on the first day after the
first OVA challenge and montelukast treatment was continued for 20
days for 2nd challenge and 40 days for 3rd challenge (Fig. 1). Additional groups were used for
the open field test (n=10/group) and the in vivo assessment
of airway function (n=8/group). Open field tests were performed
using three other groups of mice: i) The OVA group; ii) the
montelukast-treated (20 days) control group; and iii) the control
group. In vivo assessment of airway function was performed
in another set of OVA and control groups.
Experimental asthma
An experimental asthma model was established using
OVA (Sigma-Aldrich; Merck KGaA) in the OVA and montelukast-treated
OVA groups (24). The OVA-induced
asthma model was modified to extend the duration of asthma to two
months. The mice were sensitized on days 1 and 8 by an
intraperitoneal injection of 10 µg OVA dissolved in 200 µl PBS
(Sigma-Aldrich; Merck KGaA) containing 1 mg aluminum hydroxide
(Sigma-Aldrich; Merck KGaA). Airway inflammation was induced by
intranasal OVA-challenges (0.25 µg OVA in 25 µl PBS) on days 15, 16
and 17 (1st challenge), 34, 35 and 36 (2nd challenge) and 53, 54
and 55 (3rd challenge) to induce and maintain chronic airway
inflammation. The control groups were sensitized with OVA (0.25 µg
OVA in 25 µl PBS) and challenged with intranasal PBS (25 µl PBS;
Fig. 1). A stratified design was
used for the experiments and, therefore, equal numbers of animals
from each group were used on the same days for all experiments.
In vivo assessment of airway
function
Airway function was assessed in the OVA and control
groups by evaluating the changes in airway resistance using the
Buxco Finepoint Resistance and Compliance System (Buxco
Electronics, Inc.). Testing was performed one day following the 1st
OVA challenge. Mice were anesthetized with 100 mg/kg ketamine/10
mg/kg xylazine intraperitoneally, tracheally cannulated and placed
into individual plethysmography chambers (Buxco Electronics, Inc.).
The lungs of the mice were mechanically ventilated at a respiratory
rate of 150 breaths/min with a tidal volume of 200 µl and a
positive end expiratory pressure of 2 cmH2O. The basal
airway resistance was recorded for 2 min and PBS was used for
control responses. Methacholine (1.5-48 mg/ml) was administered
intratracheally to measure the dose-dependent increase in
respiratory resistance using an ultrasonic nebulizer (Buxco
Electronics, Inc.). The maximum value measured following each dose
for respiratory resistance was used as the measure of response.
Airway resistance (cmH2O.s/ml) results were normalized
to values measured following PBS nebulization.
Forced swim test
A modified version of Porsolt's FST was performed to
assess the effect of montelukast treatment on depression-like
behavior and repeated following each challenge (25,26).
Briefly, glass cylinders (height, 19 cm; diameter, 12 cm) were
filled with 25±1˚C water to a 15 cm depth. The mice were placed
into the cylinders for 8 min and the immobility time of the last 6
min was determined. Immobility, in which the mice remained immobile
or made only small limb movements necessary to float, were regarded
as depression-like behavior. Following FST, 6 mice from each group
were euthanized by cervical dislocation after each FST. Blood
samples were collected by cardiac puncture for the determination of
plasma montelukast concentration. The lungs were isolated and fixed
in 10% formaldehyde solution for histopathological analysis
(Fig. 1).
Histopathological analysis
Isolated lungs, following each FST were fixed in 10%
formaldehyde at room temperature for ≥48 h. Slides (thickness, 0.4
µm) were prepared from paraffin embedded sections and were stained
with hematoxylin and eosin at room temperature for 1 h with an
automated slide stainer (Thermo Shandon Varistain Gemini™; Thermo
Fisher Scientific, Inc.). Histopathological analysis was performed
for the OVA, control and montelukast-treated OVA groups to confirm
the inflammation in the lungs following OVA and to examine whether
there is a reduction in the inflammation following montelukast
treatment. A total of 6 mice from each group were analyzed.
Histopathologically, interalveolar (interstitial) thickening,
peribronchial and perivascular inflammation were evaluated and each
of these parameters were graded semiquantitatively in a blinded
manner. The inflammation parameters were individually scored from
0-4 (0, no inflammation; 1, mild inflammation; 2, moderate
inflammation; 3, severe inflammation; 4, very severe inflammation)
by evaluating the extent of inflammatory cells, tissue injury and
interalveolar (interstitial) thickening (Leica Microsystems GmbH)
Final scores were defined as the sum of peribronchial (0-4) and
perivascular (0-4) inflammation.
Determination of plasma montelukast
concentration
At the end of the experimental protocol, montelukast
concentrations were confirmed in mice that had been treated with
montelukast 40 days orally through drinking water. Blood samples
were collected in heparinized tubes and centrifuged at 3.305 x g
for 15 min to obtain plasma. The proteins in the plasma samples
(100 µl) were crushed with 100 µl of acetonitrile (Sigma-Aldrich;
Merck KGaA). The amount of montelukast in the plasma samples was
quantified by liquid chromatography-electrospray ionization-mass
spectrometry (LCMS-8030; Shimadzu Corporation). The mass
spectrometric detection of montelukast was undertaken in the
positive electrospray ionization and multiple reaction-monitoring
mode using 586.0-422.10 m/z transitions using 19 eV of collision
energy. The nebulizer gas flow and drying gas flow were 3 ml/min
and 15 ml/min, respectively. The heat blocking temperature,
desolvation line temperature and collision-induced dissociation gas
pressure were 400˚C, 250˚C and 15 kPa, respectively.
Chromatographic separation was achieved on the C18
column (Hypersil ODS-4; GL Sciences; 50x2.1 mm; 3 µm) using a
mobile phase consisting of acetonitrile containing 0.1% formic acid
and water with 0.1% formic acid (70:30; v/v) at a flow rate of 0.3
ml/min.
Open field tests
Open field tests were used to assess any changes in
the locomotor activity of the mice following the administration of
OVA or montelukast. The test was performed according to previous
studies (27,28). Briefly, black locomotor activity
cages (40x40x40 cm) equipped with an infrared sensor system
(Activity Meter; Commat, Ltd.) were used. The mice were
individually placed in the center of the cages and tracked for 5
min. The locomotor activities of the mice were assessed based on
the total distance travelled in the cage as measured by the
analyzing system. The test was performed in the OVA group 1 day
after the completion of one OVA-challenge, a separate group that
had been administered only montelukast (20 mg/kg/day) for 20 days
and the control group.
Statistical analysis
Data are expressed as mean ± standard error of the
mean. Statistical analysis was performed using unpaired Student's
t-test for in vivo assessment of airway function and one-way
ANOVA followed by Tukey's post hoc test or the Kruskal Wallis test
followed by Dunn's test for other experiments. Data were analyzed
using GraphPad Prism software (version 5.0; GraphPad Software
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Development and establishment of
experimental asthma model
In the current study, the relationship between
airway inflammation, chronic montelukast treatment and
depression-like behavior was investigated. The induction of
experimental asthma was confirmed by histopathological changes in
lung segments and development of airway hyperresponsiveness to
methacholine challenge. In order to maintain the allergic airway
inflammation throughout the experimental protocol, the
OVA-challenges were performed in triplicate as described in
Fig. 1. This protocol allowed for
the investigation of the effect of chronic allergic inflammation
and chronic montelukast treatment on depression-like behavior. The
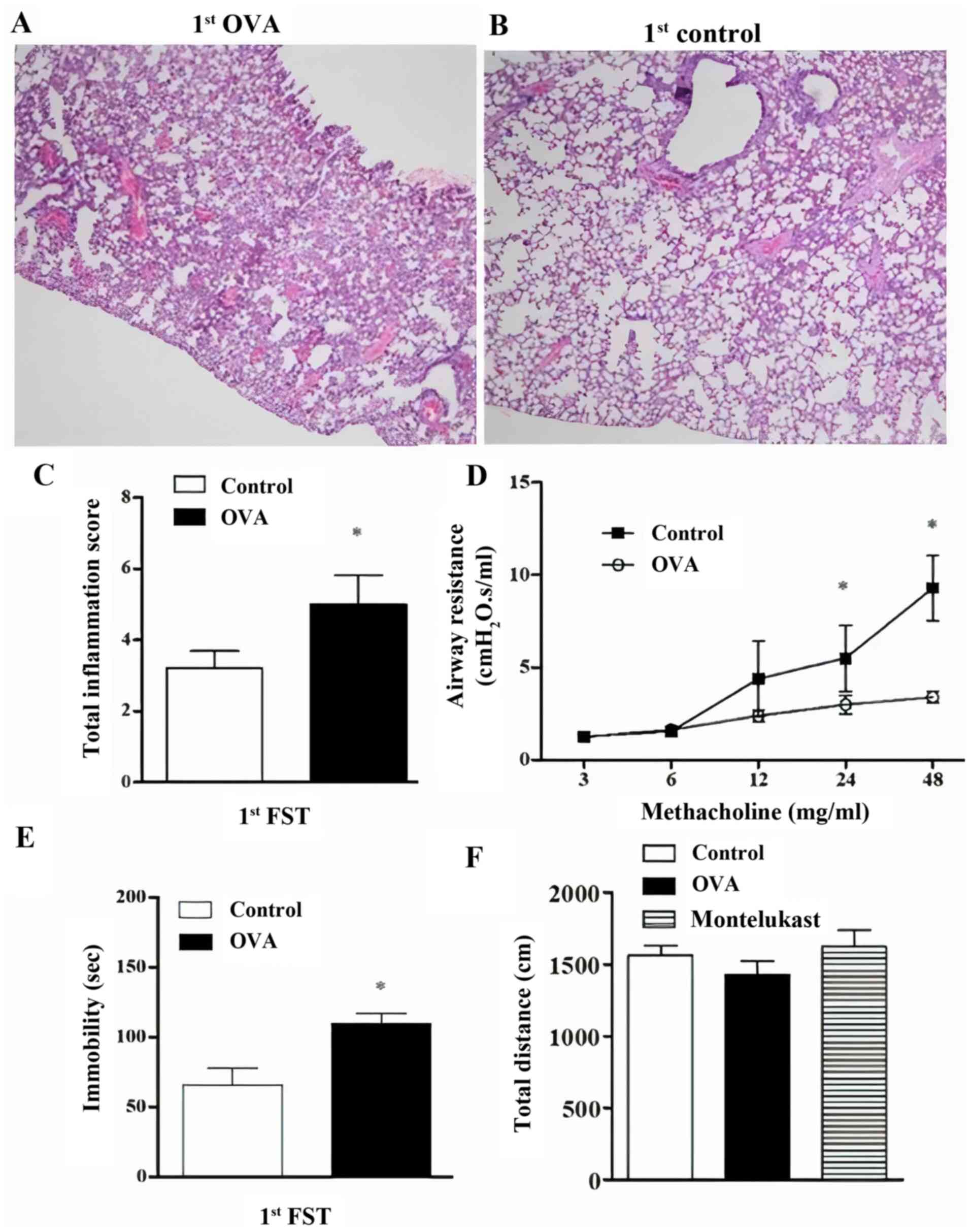
occurrence of inflammation was presented as histopathological
images (Fig. 2A and B). Total inflammation scores (Fig. 2C) and airway resistance (Fig. 2D) were significantly increased
following the first OVA challenge in the OVA group compared with
controls. Immobility time was significantly higher in the OVA group
compared with controls (Fig. 2E).
Furthermore, open field tests were performed to eliminate the
possible effects of OVA and montelukast treatment on the locomotor
activities of the mice. Total distances travelled in the cages in
the OVA group were not significantly different compared with
controls (Fig. 2F). Similarly, the
montelukast-treated OVA group (20 days of treatment) did not
exhibit a significant difference in total distances travelled,
which was indicated the locomotor activity. Additionally,
montelukast concentrations at a steady state were determined to be
226.40±34.59 ng/ml in mice administrated montelukast for >40
days (data not shown).
Effect of experimental asthma and
montelukast treatment in FST
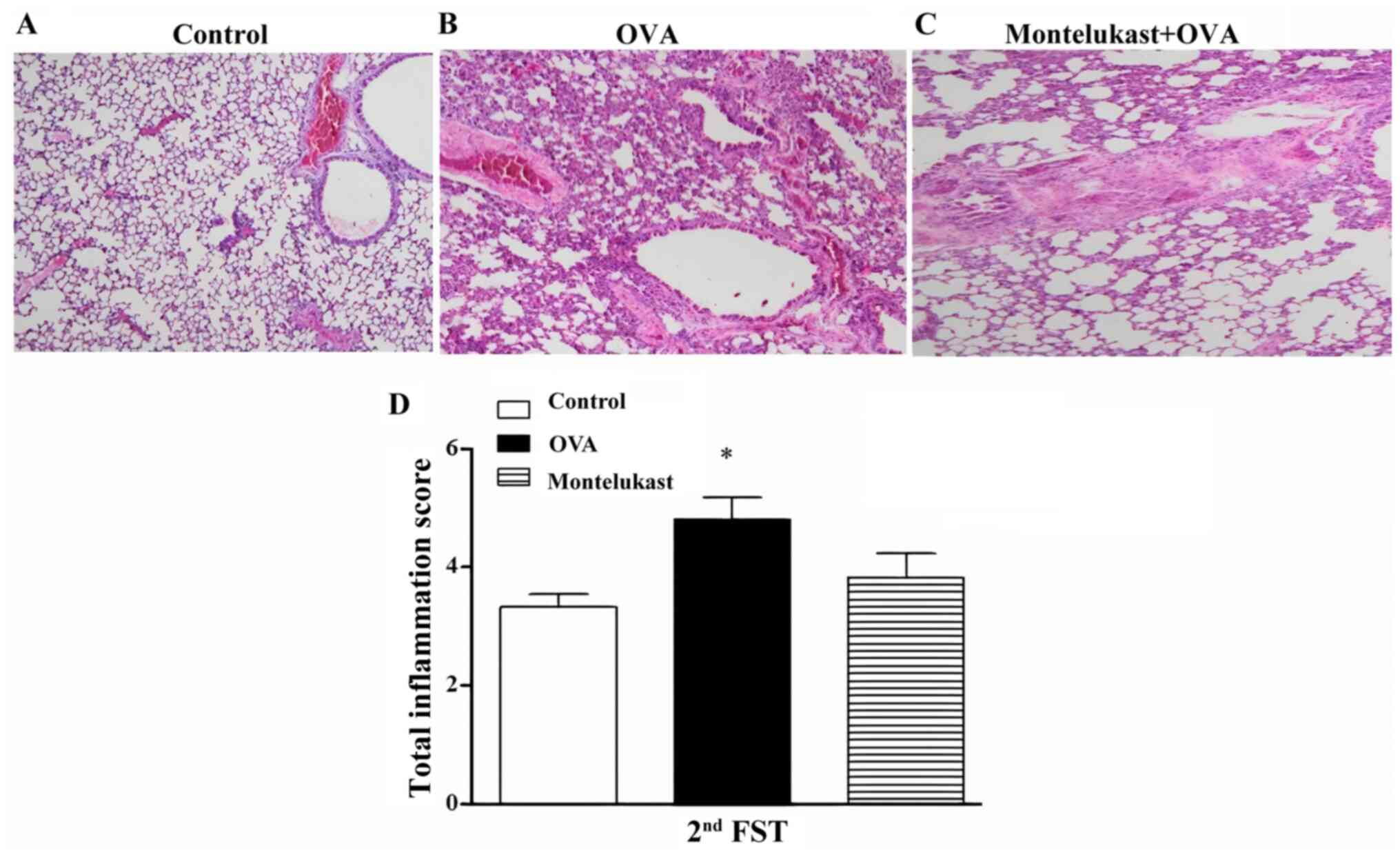
Airway inflammation observed in the OVA group
compared with control group was slightly reduced in the
montelukast-treated OVA group (20 days of treatment) following the
2nd FST (Fig. 3A-C). The total
inflammation score of the OVA group was statistically increased
compared with the control group (Fig.
3D). However, the decrease in the total inflammation score of
the montelukast-treated OVA group was not statistically significant
compared with OVA group. Furthermore, there was no statistical
differences between the montelukast-treated OVA and control
groups.
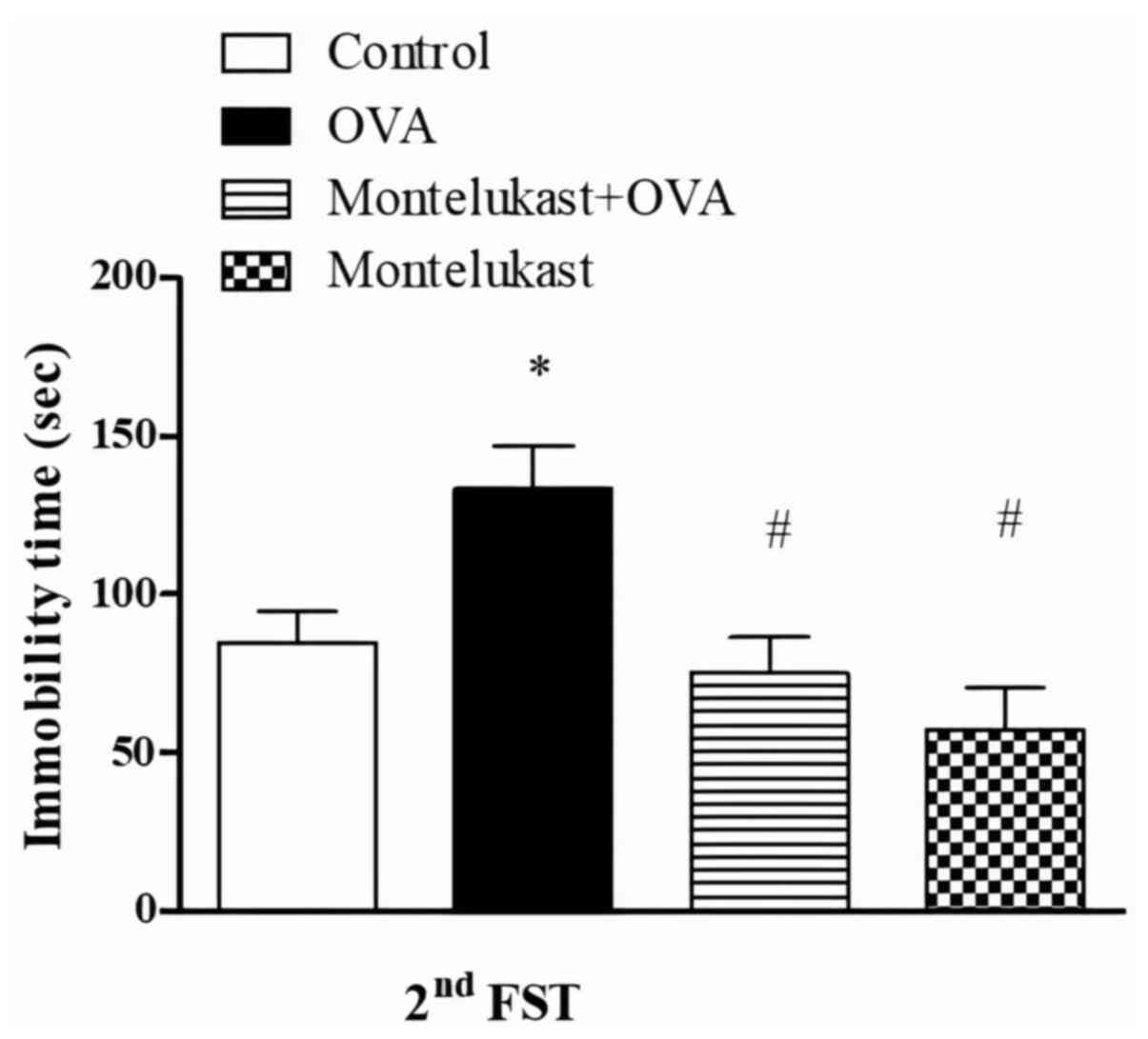
FST was performed following each OVA challenge phase
to examine the short- and long-term effects of asthma on
depression-like behavior. Immobility times were significantly
increased in the OVA group compared with the control group
following the second challenge (Fig.
4). Furthermore, immobility times were significantly decreased
in the montelukast-treated OVA group compared with the OVA group.
Immobility times in the montelukast-treated control group were not
significantly different between control and montelukast-treated OVA
groups. Furthermore, the immobility times of montelukast-treated
control group were significantly lower compared with the OVA
group.
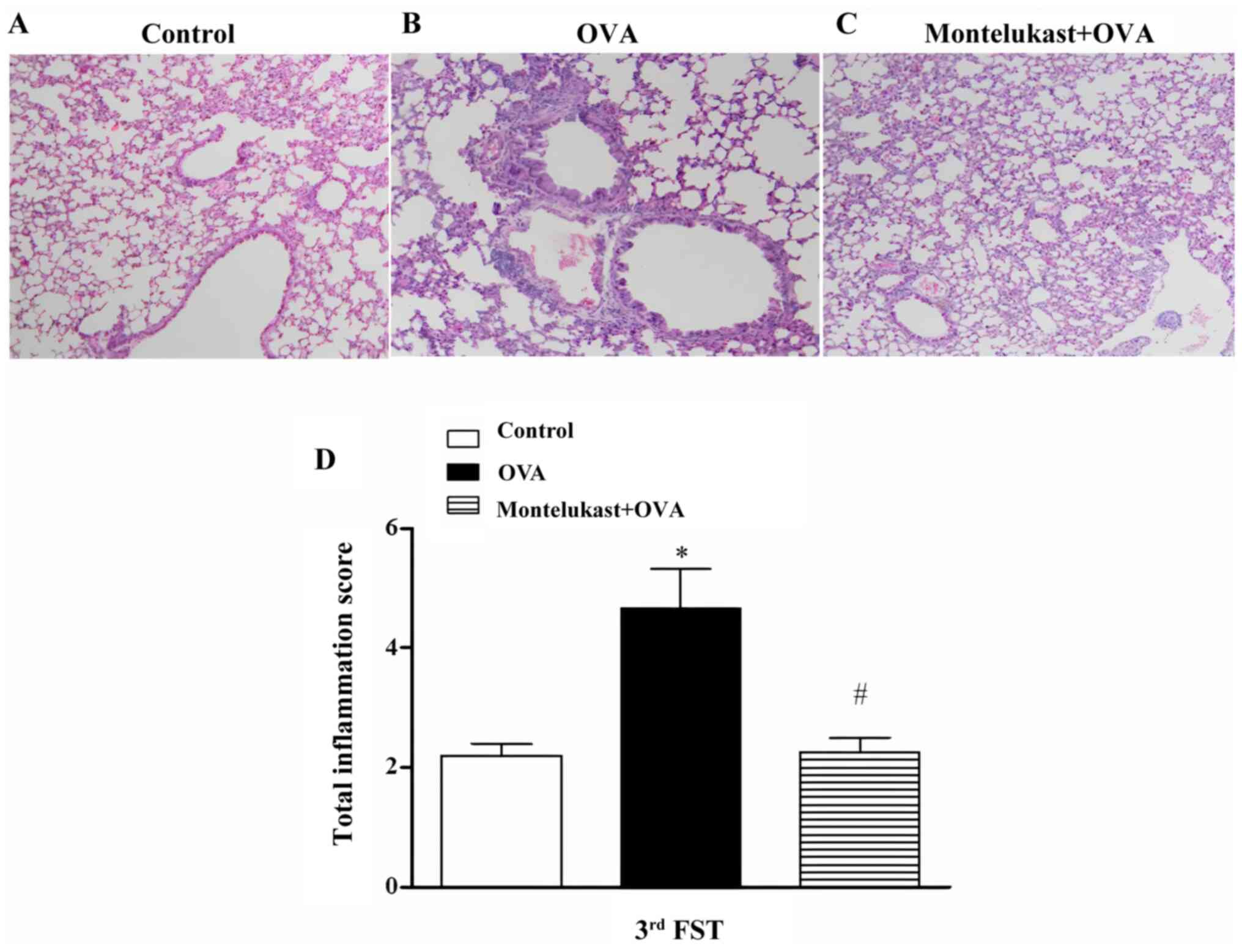
Following the third challenge, inflammation in
OVA-treated lungs was reduced followed by ongoing montelukast
treatment compared with the control group (5A-C) and total
inflammation scores of the montelukast-treated OVA group were
significantly decreased compared with the OVA group (Fig. 5D). Following the third challenge,
chronic montelukast application to the OVA-treated group (40 days
of treatment) significantly decreased immobility times compared
with the OVA group (Fig. 6).
Immobility times of the montelukast-treated control group were not
statistically different compared with controls; however, they were
significantly decreased compared with the OVA group (Fig. 6). Furthermore, there were no
significant differences between the immobility times of three time
points in the OVA-treated groups (data not shown).
Discussion
Montelukast is an imperative medication that is
widely used and is recommended in the guidelines for the treatment
of asthma (6,29). In the present study, the
depression-like behavioral effects of chronic montelukast
administration were investigated in a murine model of experimental
asthma. In accordance with the clinical use of montelukast, an
OVA-induced asthma model was used to assess both the possible
depression-like effects of montelukast treatment and asthma itself.
The OVA model is well-established and is the most commonly used
experimental asthma model (30),
causing airway hyperreactivity, inflammatory cell infiltration and
parenchymal inflammation (24).
The current study confirmed the development of
experimental asthma with OVA by pathological assessments and OVA
was demonstrated to result in a statistically significant increase
in lung inflammation following the first challenge. Furthermore,
the increase of airway resistance in response to methacholine
challenge in the OVA-treated mice indicated that allergic asthma
was successfully induced. These results confirmed changes in the
lungs may have led to impaired respiratory function, which in turn,
may have reduced the mobility of the mice. Furthermore, open field
tests prior to FST were performed to eliminate any possible changes
in motor activity that may affect the mice and cause false negative
or positive results in FST. However, there were no significant
difference in the total distance travelled by the mice between the
OVA, montelukast-treated OVA and control groups. This indicated
that differences observed in FST were not caused by motor
disability, confirming the reliability of this test.
Furthermore, the standard OVA protocol was modified
to maintain the persistence of the disease and repeated challenges
were applied to the mice at the indicated intervals. The modified
model allowed for the evaluation of the relationship between
ongoing respiratory tract inflammation, chronic montelukast
treatment and depression-like behavior at different time points
over two months. Montelukast was administrated to the mice via
drinking water during this period to avoid the stress of daily
gavage. The plasma montelukast concentrations of mice were verified
at the end of the current study and concentrations were at
therapeutic concentrations consistent with the plasma
concentrations of humans and rats (31,32).
The association between asthma and depression
following each OVA-challenge point was investigated. For all three
time points, the immobility of the OVA mice was significantly
increased compared with controls. However, immobility times did not
differ between time points in the OVA group. This result is
consistent with the pathological analysis and indicated that the
repeated OVA challenges did not aggravate the disease; instead, it
maintained inflammation. Furthermore, the role of chronic
montelukast treatment in depression was assessed with FST performed
following the second and third OVA challenges. Montelukast
treatment was found to ameliorate lung inflammation as confirmed by
histopathological analysis. These results indicated that
montelukast dose used in the present study was within the
therapeutic range. Inflammation was reduced by 20 days of
montelukast treatment and was completely reversed by the end of 40
days of treatment. Additionally, montelukast treatment reduced the
immobility times of mice following both FST compared with the OVA
group. This indicated that chronic montelukast treatment in asthma
does not cause depression-like behavior. Furthermore, the reduced
immobility times indicated that montelukast decreased
asthma-induced depression by suppressing airway inflammation.
Asthma has been demonstrated to be closely associated with
depression (33-36).
Since montelukast is most commonly used in the treatment of
allergic asthma, the side effect of depression-like behavior caused
by montelukast has been debated in terms of whether it is
associated with the drug or the disease itself (9,33-36).
Therefore, the effect of montelukast on the control mice was
evaluated to test whether the drug caused depression-like behavior
under physiological conditions. Montelukast application to the
control mice did not cause any change in the immobility times
following the second and third FST compared with controls at the
same time points.
During FST, an increase in immobility time indicated
behavioral despair and depression-like behavior (26,37,38).
FST is one of the oldest screening tests as it has been used for
numerous years and is one of the most accepted models for the
investigations of antidepressant activity (30,39,40).
Various drugs used in the treatment of depression were first
assessed using this test (30,40).
However, this test has certain disadvantages. For instance, FST is
weak at reflecting clinical situations. However, it is the most
preferred pharmacological test for assessing antidepressant
activity and will be beneficial in supporting future results using
other methods, including the elevated plus maze or sucrose
tolerance tests, which asses depressive-like behavior using
different mechanisms (41).
In the current study, depression-like behavior was
increased in asthmatic mice; however, there was no indicated
association between montelukast treatment and depression. The
association between allergic asthma and depression has been
reported by several studies (41,42).
Numerous patients with chronic allergic asthma have a history of
depression and patients with asthma are more susceptible to suicide
(43,44). Allergic asthma dependent-chronic
stress may lead to depression or these two chronic diseases may
coexist as they are affected by similar inflammatory pathways
(21,45). Increases in the levels of
inflammatory mediators, including cytokines and interleukins have
been demonstrated in depression and allergic asthma (21,45).
Uz et al (46) indicated
that in arachidonate 5-lipoxygenase knock-out animals,
depression-like behavior declined due to a decrease in
leukotrienes. Furthermore, previous studies have reported that the
immobility time of mice and rats was increased by OVA-induced
allergic asthma (47,48); these results are consistent with the
data of the current study. Pharmacokinetic studies have reported
that the distribution of montelukast across the blood brain barrier
is limited (12) and that the
cysteinyl leukotriene receptor-1 is weakly expressed in brain
tissue (49,50). It has also been indicated that when
montelukast treatment is not sufficient for allergic asthma
therapy, mediators, including pro-inflammatory cytokines, increase
due to unresolved asthma and cause sleep disorders, depression and
suicidal behavior (15,51).
Neuropsychiatric side effects were not reported in
clinical trials and were not included in the prescribing
information when Singulair® was first authorized.
However, with the widespread use of montelukast, several
post-marketing studies indicated the drug caused depression,
hallucinations, sleep disorders and suicidal behavior (5). These side effects were reported
following the beginning of treatment and were revealed to disappear
following the cessation of the drug (52,53).
Some studies have not indicated an association between depression
and montelukast treatment (54,55).
Furthermore, a previous study indicated that there was no
difference in the side effect profiles of montelukast-treated and
placebo groups (56). Clinical
studies have several limitations in the assessment of behavioral
adverse effects of drugs. In particular, the selection of patients
for clinical studies serves a key role in determining the
reliability of the research and it is challenging to exclude those
who are susceptible to psychiatric diseases (57). Furthermore, diagnosis of depression
primarily depends on the self-reports of patients, which can lead
to abundant variation among study populations (57). Thus, contradictory results are
expected in clinical studies on adverse behavioral effects
(57).
The present experimental study had certain
limitations, such as only using FST for assessing depression-like
behavior. Although FST is a commonly used test for the evaluation
of depressed activity, additional behavioral tests, including those
for anxiety and hedonia, can be performed to confirm the side
effects of montelukast.
In conclusion, montelukast is an efficient drug and
is frequently the preferred treatment for asthma (9). Further experimental studies should be
conducted to increase the reliability of clinical data, to achieve
a better understanding of the mechanisms of neuropsychiatric side
effects and to provide information for the safer use of
montelukast.
Acknowledgements
The authors would like to thank Associate Professor
Mikael Adner (Experimental Asthma and Allergy Research and The
Centre for Allergy Research, Karolinska Institutet, Solna, Sweden)
for his valuable contribution in developing the chronic OVA-induced
murine asthma model and Dr Yasemin Karaman-Kutluay (Department of
Pharmacy, Hacettepe University Faculty of Pharmacy, Ankara, Turkey)
for her help in the in vivo assessment of airway
function.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BCT, TEB and GT conceived and designed the current
study. BT and TEB conducted and GT performed the experiments. EN
performed and evaluated analytical measurements. SO evaluated
pathological data. GT analyzed data and wrote the original draft of
the manuscript. BCT and TEB reviewed and edited the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by the Animal
Experimentations Local Ethics Board of Hacettepe University,
Ankara, Turkey (approval nos. 2018/3-2 and 2020/3-13).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Harris K, Kneale D, Lasserson TJ, McDonald
VM, Grigg J and Thomas J: School-based self-management
interventions for asthma in children and adolescents: A mixed
methods systematic review. Cochrane Database Syst Rev.
1(CD011651)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pawankar R, Canonica GW, Holgate ST and
Lockey RF: Allergic diseases and asthma: A major global health
concern. Curr Opin Allergy Clin Immunol. 12:39–41. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Woo LN, Guo WY, Wang X, Young A, Salehi S,
Hin A, Zhang Y, Scott JA and Chow CW: A 4-week model of house dust
mite (HDM) induced allergic airways inflammation with airway
remodeling. Sci Rep. 8(6925)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Barnes PJ: New therapies for asthma: Is
there any progress? Trends Pharmacol Sci. 31:335–343.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Calapai G, Casciaro M, Miroddi M, Calapai
F, Navarra M and Gangemi S: Montelukast-induced adverse drug
reactions: A review of case reports in the literature.
Pharmacology. 94:60–70. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Haarman MG, van Hunsel F and de Vries TW:
Adverse drug reactions of montelukast in children and adults.
Pharmacol Res Perspect. 5(e00341)2017.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Aldea Perona A, Garcia-Saiz M and Sanz
Alvarez E: Psychiatric disorders and montelukast in children: A
disproportionality analysis of the VigiBase((R)). Drug Saf.
39:69–78. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Benard B, Bastien V, Vinet B, Yang R,
Krajinovic M and Ducharme FM: Neuropsychiatric adverse drug
reactions in children initiated on montelukast in real-life
practice. Eur Respir J. 50(1700148)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
urihttps://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdfsimplehttps://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdf.
|
|
10
|
FDA: Early Communication About an Ongoing
Safety Review of Montelukast (Singulair). 3/23/2008 04/18/2013
8/7/2018]; Available from: urihttps://wayback.archive-it.org/7993/20170112033551/http:/www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm070618.htmsimplehttps://wayback.archive-it.org/7993/20170112033551/http:/www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm070618.htm.
|
|
11
|
FDA: Updated Information on Leukotriene
Inhibitors: Montelukast (marketed as Singulair), Zafirlukast
(marketed as Accolate), and Zileuton (marketed as Zyflo and Zyflo
CR). 8/28/2009 07/08/2015; urihttps://wayback.archive-it.org/7993/20170111080414/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm165489.htmsimplehttps://wayback.archive-it.org/7993/20170111080414/http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm165489.htm.
|
|
12
|
FDA. SINGULAIR® (Montelukast
sodium) Tablets, Chewable Tablets, And Oral Granules 8/19/2009;
Available from: urihttps://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020829s051_020830s052_021409s028lbl.pdfsimplehttps://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020829s051_020830s052_021409s028lbl.pdf.
|
|
13
|
Manalai P, Woo JM and Postolache TT:
Suicidality and montelukast. Expert Opin Drug Saf. 8:273–282.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Schumock GT, Stayner LT, Valuck RJ, Joo
MJ, Gibbons RD and Lee TA: Risk of suicide attempt in asthmatic
children and young adults prescribed leukotriene-modifying agents:
A nested case-control study. J Allergy Clin Immunol. 130:368–375.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Postolache TT, Komarow H and Tonelli LH:
Allergy: A risk factor for suicide? Curr Treat Options Neurol.
10:363–376. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hurwitz EL and Morgenstern H:
Cross-sectional associations of asthma, hay fever, and other
allergies with major depression and low-back pain among adults aged
20-39 years in the United States. Am J Epidemiol. 150:1107–1116.
1999.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Addolorato G, Ancona C, Capristo E,
Graziosetto R, Di Rienzo L, Maurizi M and Gasbarrini G: State and
trait anxiety in women affected by allergic and vasomotor rhinitis.
J Psychosom Res. 46:283–289. 1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Trojan TD, Khan DA, Defina LF, Akpotaire
O, Goodwin RD and Brown ES: Asthma and depression: The cooper
center longitudinal study. Ann Allergy Asthma Immunol. 112:432–436.
2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Oraka E, King ME and Callahan DB: Asthma
and serious psychological distress: Prevalence and risk factors
among US adults, 2001-2007. Chest. 137:609–616. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wong KO, Rowe BH, Douwes J and
Senthilselvan A: Asthma and wheezing are associated with depression
and anxiety in adults: An analysis from 54 countries. Pulm Med.
2013(929028)2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jiang M, Qin P and Yang X: Comorbidity
between depression and asthma via immune-inflammatory pathways: A
meta-analysis. J Affect Disord. 166:22–29. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Katon W, Lozano P, Russo J, McCauley E,
Richardson L and Bush T: The prevalence of DSM-IV anxiety and
depressive disorders in youth with asthma compared with controls. J
Adolesc Health. 41:455–463. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
urihttp://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:En:PDFsimpleeurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:En:PDF.
|
|
24
|
Swedin L, Ellis R, Kemi C, Ryrfeldt A,
Inman M, Dahlén SE and Adner M: Comparison of aerosol and
intranasal challenge in a mouse model of allergic airway
inflammation and hyperresponsiveness. Int Arch Allergy Immunol.
153:249–258. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Porsolt RD, Bertin A and Jalfre M:
Behavioral despair in mice: A primary screening test for
antidepressants. Arch Int Pharmacodyn Ther. 229:327–336.
1977.PubMed/NCBI
|
|
26
|
Porsolt RD, Le Pichon M and Jalfre M:
Depression: A new animal model sensitive to antidepressant
treatments. Nature. 266:730–732. 1977.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Painsipp E, Wultsch T, Edelsbrunner ME,
Tasan RO, Singewald N, Herzog H and Holzer P: Reduced anxiety-like
and depression-related behavior in neuropeptide Y Y4 receptor
knockout mice. Genes Brain Behav. 7:532–542. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Brunner SM, Farzi A, Locker F, Holub BS,
Drexel M, Reichmann F, Lang AA, Mayr JA, Vilches JJ, Navarro X, et
al: GAL3 receptor KO mice exhibit an anxiety-like phenotype. Proc
Natl Acad Sci USA. 111:7138–7143. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
British Thoracic Society; Scottish
Intercollegiate Guidelines Network. British guideline on the
management of asthma. Thorax. 69 (Suppl 1):1–192. 2014.PubMed/NCBI
|
|
30
|
Aun MV, Bonamichi-Santos R, Arantes-Costa
FM, Kalil J and Giavina-Bianchi P: Animal models of asthma: Utility
and limitations. J Asthma Allergy. 10:293–301. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhao JJ, Rogers JD, Holland SD, Larson P,
Amin RD, Haesen R, Freeman A, Seiberling M, Merz M and Cheng H:
Pharmacokinetics and bioavailability of montelukast sodium
(MK-0476) in healthy young and elderly volunteers. Biopharm Drug
Dispos. 18:769–777. 1997.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Marschallinger J, Krampert M,
Couillard-Despres S, Heuchel R, Bogdahn U and Aigner L:
Age-dependent and differential effects of Smad7DEx1 on neural
progenitor cell proliferation and on neurogenesis. Exp Gerontol.
57:149–154. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Brunner WM, Schreiner PJ, Sood A and
Jacobs DR Jr: Depression and risk of incident asthma in adults. The
CARDIA study. Am J Respir Crit Care Med. 189:1044–1051.
2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
de Miguel Diez J, Hernández Barrera V,
Puente Maestu L, Carrasco Garrido P, Gómez García T and Jiménez
García R: Psychiatric comorbidity in asthma patients. Associated
factors. J Asthma. 48:253–258. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Di Marco F, Santus P and Centanni S:
Anxiety and depression in asthma. Curr Opin Pulm Med. 17:39–44.
2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gerald JK and Moreno FA: Asthma and
depression: It's complicated. J Allergy Clin Immunol Pract.
4:74–75. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Petit-Demouliere B, Chenu F and Bourin M:
Forced swimming test in mice: A review of antidepressant activity.
Psychopharmacology (Berl). 177:245–255. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kara NZ, Stukalin Y and Einat H:
Revisiting the validity of the mouse forced swim test: Systematic
review and meta-analysis of the effects of prototypic
antidepressants. Neurosci Biobehav Rev. 84:1–11. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Slattery DA and Cryan JF: Using the rat
forced swim test to assess antidepressant-like activity in rodents.
Nat Protoc. 7:1009–1014. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Slattery DA and Cryan JF: The ups and
downs of modelling mood disorders in rodents. ILAR J. 55:297–309.
2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Van Lieshout RJ, Bienenstock J and
MacQueen GM: A review of candidate pathways underlying the
association between asthma and major depressive disorder. Psychosom
Med. 71:187–195. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gao YH, Zhao HS, Zhang FR, Gao Y, Shen P,
Chen RC and Zhang GJ: The relationship between depression and
asthma: A meta-analysis of prospective studies. PLoS One.
10(e0132424)2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Postolache TT, Langenberg P, Zimmerman SA,
Lapidus M, Komarow H, McDonald JS, Furst N, Dzhanashvili N,
Scrandis D, Bai J, et al: Changes in severity of allergy and
anxiety symptoms are positively correlated in patients with
recurrent mood disorders who are exposed to seasonal peaks of
aeroallergens. Int J Child Health Hum Dev. 1:313–322.
2008.PubMed/NCBI
|
|
44
|
Goodwin RD and Eaton WW: Asthma, suicidal
ideation, and suicide attempts: Findings from the Baltimore
epidemiologic catchment area follow-up. Am J Public Health.
95:717–722. 2005.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Loerbroks A, Gadinger MC, Bosch JA,
Stürmer T and Amelang M: Work-related stress, inability to relax
after work and risk of adult asthma: A population-based cohort
study. Allergy. 65:1298–1305. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Uz T, Dwivedi Y, Pandey GN, Roberts RC,
Conley RR, Manev R and Manev H: 5-Lipoxygenase in the prefrontal
cortex of suicide victims. Open Neuropsychopharmacol J. 1:1–5.
2008.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zuo HX, Li JQ, Han B, Ke CJ, Liu XD, Zhang
YC, Li L and Yang X: Di-(n-butyl)-phthalate-induced oxidative
stress and depression-like behavior in mice with or without
ovalbumin immunization. Biomed Environ Sci. 27:268–280.
2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Neamati A, Chaman F, Hosseini M and
Boskabady MH: The effects of Valeriana officinalis L.
hydro-alcoholic extract on depression like behavior in ovalbumin
sensitized rats. J Pharm Bioallied Sci. 6:97–103. 2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lynch KR, O'Neill GP, Liu Q, Im DS, Sawyer
N, Metters KM, Coulombe N, Abramovitz M, Figueroa DJ, Zeng Z, et
al: Characterization of the human cysteinyl leukotriene CysLT1
receptor. Nature. 399:789–793. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
50
|
Jones TR and Rodger IW: Role of
leukotrienes and leukotriene receptor antagonists in asthma. Pulm
Pharmacol Ther. 12:107–110. 1999.PubMed/NCBI View Article : Google Scholar
|
|
51
|
de Vries TW and van Hunsel F: Adverse drug
reactions of systemic antihistamines in children in the
netherlands. Arch Dis Child. 101:968–970. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Callero-Viera A, Infante S,
Fuentes-Aparicio V, Zapatero L and Alonso-Lebrero E:
Neuropsychiatric reactions to montelukast. J Investig Allergol Clin
Immunol. 22:452–453. 2012.PubMed/NCBI
|
|
53
|
Alkhuja S, Gazizov N and Alexander ME:
Sleeptalking! Sleepwalking! Side effects of montelukast. Case Rep
Pulmonol. 2013(813786)2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ali MM, O'Brien CE, Cleves MA and Martin
BC: Exploring the possible association between montelukast and
neuropsychiatric events among children with asthma: A matched
nested case-control study. Pharmacoepidemiol Drug Saf. 24:435–445.
2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Holbrook JT and Harik-Khan R: Montelukast
and emotional well-being as a marker for depression: Results from 3
randomized, double-masked clinical trials. J Allergy Clin Immunol.
122:828–829. 2008.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Philip G, Hustad C, Noonan G, Malice MP,
Ezekowitz A, Reiss TF and Knorr B: Reports of suicidality in
clinical trials of montelukast. J Allergy Clin Immunol. 124:691–696
e6. 2009.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Philip G, Hustad CM, Malice MP, Noonan G,
Ezekowitz A, Reiss TF and Knorr B: Analysis of behavior-related
adverse experiences in clinical trials of montelukast. J Allergy
Clin Immunol. 124:699–706 e8. 2009.PubMed/NCBI View Article : Google Scholar
|