Introduction
Chronic liver disease and its associated
complications are responsible for >2 million deaths per year
worldwide (1-3).
Viral and alcoholic liver disease, drug induced liver disease
(DILD), primary biliary cirrhosis (PBC) and autoimmune hepatitis
(AIH) are among the most common types of liver diseases observed in
clinical practice (4,5). However, these diseases lack unique
clinical characteristics, making their diagnoses difficult to
distinguish. Generally, the causes of viral liver disease,
alcoholic liver disease and DILD are clearly defined. However, PBC
and AIH are autoimmune diseases that have no known clear causes.
Certain autoimmune antibodies, including anti-mitochondrial
antibodies (AMAs) and anti-nuclear antibodies (ANAs), have been
reported to be important for the diagnosis of autoimmune diseases
(6). A disrupted balance between
pathogen recognition and the innate and adaptive immune systems is
a common cause of liver disease progression (7).
Immunoglobulin G (IgG) is a major antibody isotype
in the blood that protects the body against pathogenic infection.
The IgG family is comprised of IgG1, IgG2, IgG3 and IgG4(8). Differences in the structural
composition of the IgG subclasses determines their function, which
include antigen binding, immune complex formation, complement
activation, triggering of effector cells, half-life and placental
transport (9). IgG1 is the most
abundant subclass in the serum, whilst IgG3 has the shortest
half-life (7 days) (10). In
addition, responses to different antigens will lead to marked
skewing towards specific IgG antibody subclasses (11). Although deficiencies in selective
subclasses are not usually harmful, they may lead to enhanced
susceptibility towards specific classes of pathogens. However, this
condition is rarely observed (12).
IgG subclasses contribute to the immunopathogenesis of liver
diseases by regulating immunoglobulin Fcγ receptor (FcgR) and
complement interactions (13).
Radioimmunodiffusion or ELISA assays can be used to measure the
levels of specific serum IgG subclasses but are not particularly
accurate or convenient (14).
Immunonephelometric assays are therefore the preferred method for
this application (15).
IgG4-related disease (IgG4-RD) is a
fibroinflammatory, immune system-mediated systemic disease that was
reported for the first time in 2014 in Japan (16). IgG4-RD is described as a novel
clinical entity of unknown origin that involves multiple organs
(17). The current diagnostic
approach for IgG4-RD includes serum IgG4 levels >135 mg/dl
(18). Other diagnostic criteria
include IgG4-related tests, imaging and typical histopathological
examination (19). However,
accurate diagnosis of IgG4-RD is challenging, since IgG4-RD can be
easily misdiagnosed as autoimmune pancreatitis, inflammatory
pseudotumour and Küttner's tumor (20). IgG4-related hepatobiliary diseases
include IgG4-related sclerosing cholangitis and IgG4-related
hepatopathy (21).
Previous studies have focused on the relationship
between the IgG subclasses and diseases that affect the body's
immunity, including the significant increase of IgG1 and IgG3 in
patients with rheumatoid arthritis (22), IgG subclass determination in renal
disease progression (23) and
distinguishing patients with human immunodeficiency virus
infections who exhibit varying degrees of infection (24). However, few prior studies have
previously analyzed serum IgG subclass levels fully in clinically
common liver diseases and limited data are available regarding IgG4
levels in larger patient cohorts for diseases other than IgG4-RD
(25-27).
Both liver diseases and IgG4-RD exhibit changes in IgG4 levels
(28). Therefore, to further
understanding, provide an early diagnosis strategy of different
liver diseases and evaluation of IgG4 levels in patients with
non-IgG4-RD, the present study analyzed serum IgG subclass levels
in patients with five common liver diseases.
Materials and methods
Patients
Clinical information from 32 patients with viral
liver disease, 26 with alcoholic liver disease, 39 with DILD, 28
with PBC, 29 with AIH and 30 healthy controls (HCs) was recorded at
the Clinical Laboratory Center, Beijing YouAn Hospital, Capital
Medical University (Beijing, China) between May 2018 and March
2019. Patients with other types of liver or autoimmune diseases,
including acute and chronic fatty liver disease, metabolic liver
diseases, rheumatal-immune diseases and systemic lupus
erythematosus, were excluded from the present study. The patients
recruited into the present study only had diseases affecting the
liver. All patients with viral liver disease met the guidelines of
Prevention and Treatment for Chronic hepatitis B (29) and C (30). Patients with alcoholic liver disease
met the 2010 American Association for the Study of Liver Diseases
diagnosis (AALSD) (31). Those with
DILD met the diagnostic criteria of the Roussel Uclaf Causal
Relationship Assessment Method (RUCAM), with a RUCAM causality
scale of >6 points (32).
Patients with PBC met the 2018 AALSD PBC diagnosis guidelines
(33). Patients with AIH met the
2015 Chinese Consensus on the Diagnosis and Management of
Autoimmune Hepatitis criteria (34). The laboratory test results,
including alanine transaminase (ALT), aspartate aminotransferase
(AST), γ-glutamyl transferase (GGT), alkaline phosphatase (ALP),
bilirubin and albumin of HCs, were found to lie within the normal
range. All baseline information of the patients recruited into the
present study, including age, sex distribution and laboratory
parameters, are listed in Table I.
The present study was approved by the Ethics Committee of Beijing
YouAn Hospital, Capital Medical University (Beijing, China).
 | Table IBaseline characteristics of
participants in the five liver disease cohorts. |
Table I
Baseline characteristics of
participants in the five liver disease cohorts.
| Variables | HC (n=30) | V-LD (n=32) | LD (n=26) | DILD (n=39) | PBC (n=28) | AIH (n=29) | Statistics | P-value |
|---|
| Age, years | 53.8±8.8 | 50.2±15.4 | 57.0±10.7 | 50.1±16.1 | 56.9±12.3 | 54.3±14.2 | F=1.58 | 0.167 |
| Female/male | 13/17 | 13/19 | 1/25a | 23/16 | 25/3a | 23/6 |
χ2=51.58 | <0.001 |
| Laboratory
parameters (normal range) | | | | | | | | |
|
ALT (9-50
U/l) | 23.5 (16.8,
29.2) | 40.6 (17.9,
74.4) | 29.4 (16.2,
105.5) | 66.9 (32.7,
206.1)b | 42.8 (16.8,
74.1) | 40.1 (17.4,
136.5) | H=21.85 | 0.001 |
|
AST
(15-40-U/l) | 23.5 (20.8,
27.2) | 60.6 (30.6,
99.0)b | 58.8 (37.9,
109.9)b | 96.8 (42.1,
168.1)b | 62.9 (28.1,
133.4)b | 82.1 (28.0,
144.0)b | H=45.7 | <0.001 |
|
GGT (10-60
U/l) | 17.0 (12.0,
22.0) | 74.1 (38.7,
164.2)a | 105.1 (38.0,
723.2)b | 147.6 (93.9,
359.8)b | 131.5 (35.5,
258.9)b | 99.2 (46.6,
206.6)b | H=36.90 | <0.001 |
|
ALP (45-125
U/l) | 51.0 (41.0,
59.5) | 113.0 (83.0,
182.0)b | 167.0 (95.2,
315.3)b | 146.0 (109.0,
214.5)b | 215.7 (117.0,
424.0)b | 120.6 (78.6,
181.8)b | H=42.15 | <0.001 |
|
Bilirubin
(5-21 µmol/l) | 18.3 (14.0,
20.0) | 28.5 (14.8,
96.6) | 122.3 (22.3,
209.7)a | 42.5 (25.3,
221.4)a | 33.8 (17.1,
118.6) | 31.2 (17.3,
80.4) | H=17.74 | 0.003 |
|
Albumin
(40-55 g/l) | 44.8±2.3 |
32.9±6.5b |
32.9±4.8b |
35.7±5.0a |
33.7±5.7b |
33.5±6.2b | F=10.63 | <0.001 |
IgG and other indices measured
Baseline blood samples were collected from all
participants and all samples were processed at the Clinical
Laboratory Center of Beijing YouAn hospital, Capital Medical
University. Serum IgG subclass levels were measured using
immunonephelometric assays using molecular biology kits (N Latex
IgG1, cat. no. OQXI; N Latex IgG2, cat. no. OQXK; N Latex IgG3,
cat. no. OPAV; N Latex IgG4, cat. no. OPAU and N Supplementary
Reagent/Precipitation; all Siemens Healthineers) in Siemens BNII
automatic protein analyzer (Siemens Healthineers) according to the
manufacturer's protocols. IgG levels were determined regardless of
disease stage. The normal ranges of adult IgG subclasses were
defined as follows: i) IgG1, 4.05-10.11 g/l; ii) IgG2, 1.69-7.86
g/l; iii) IgG3, 0.11-0.85 g/l; and iv) IgG4, 0.03-2.01 g/l (normal
ranges derived from IgG subclass kit instructions). Serum IgG
levels were defined as the sum of IgG1, IgG2, IgG3 and IgG4.
IgM (cat. no. OSAT) and IgA (cat. no. OSAR) levels
were also determined by electrochemiluminescence using a Modular
E170 analyzer (Roche Diagnostics). Anti-mitochondrial antibody
(AMA), anti-nuclear antibody (ANA) and anti-smooth muscle antibody
(ASMA) levels were measured using an indirect immunofluorescence
assay (cat. no. FA1510-1; EuroImmun AG; PerkinElmer, Inc.). AMA
type 2 (AMA-M2) levels were measured using ELISA (cat. no.
EA1590-9601-8G; EuroImmun AG; PerkinElmer, Inc.). Autoantibodies
titers ≥1:100 were considered positive and an absorbance value of
AMA-M2 ≥25 RU/ml was also considered positive.
The model for end-stage liver disease (MELD) score
used in the present study was obtained from the United Network for
Organ Sharing (UNOS) for the prioritization of transplant organs,
which was calculated using the following formula (35): MELD=3.8x{Ln [serum bilirubin
(mg/dl)]} + 11.2x{Ln [international normalized ratio (INR)]} +
9.6x{Ln [serum creatinine (mg/dl)]} + 6.4x (constant for liver
disease etiology). Ln was the natural logarithm with base e. If
disease etiology was biliary or alcoholic, constant for liver
disease etiology was considered to be 0 whereas other etiologies,
such as viral hepatitis, were set to 1. A MELD score >6 was
considered to be abnormal.
Statistical analysis
Normally distributed numerical variables were
assessed using Q-Q plot graphical methods and were presented as the
mean ± SD. Data were instead presented as the median (interquartile
range, 25th and 75th percentile) when numerical variables were not
normally distributed. Categorical variables were presented as
numbers and percentages. One-way ANOVA and Kruskal-Wallis tests
were performed to compare IgG subclass levels. Multiple comparison
between the groups was performed using Scheffe's post hoc test.
Statistical differences between two groups were analyzed using
Student's t-test or Mann-Whitney test. χ2 test was
applied to analyze the percentages of elevated IgG subclasses.
Additionally, Pearson's correlation coefficient was used to
evaluate the relationship between the levels of two subclasses of
antibodies. P<0.05 was considered to indicate a statistically
significant difference. SPSS version 22.0 (IBM Corp.) and GraphPad
Prism version 7.0 for Windows (GraphPad Software, Inc.) were used
for data analysis. All experiments were repeated three times and
there were three replicates for each experiment.
Results
Basic characteristics of study
participants
Blood samples from patients with five types of liver
disease were analyzed. The viral liver disease cohort included a
total of 32 patients (13 females and 19 males; age, 18-75 years)
who were infected with the hepatitis virus, mainly hepatitis B and
hepatitis C virus. In the DILD cohort, there were a total of 39
patients (23 females and 16 males; age, 12-83 years) who had liver
damage caused by adverse drug reactions. These drug reactions
included those caused by herbals and antimicrobials such as
amoxicillin-clavulanate, nitrofurantoin and
sulfamethoxazole-trimethoprim, which of which can cause liver
diseases (36,37). Additionally, 26 patients, 28
patients and 29 patients were respectively included into the
alcoholic liver disease cohort (1 female and 25 males; age, 36-77
years), the PBC cohort (25 females and 3 males; age, 20-78 years)
and the AIH cohort (23 females and 6 males; age, 16-76 years) and
30 patients comprised the HC cohort (13 females and 17 males; age,
28-75 years).
The baseline characteristics of the participants in
the present study are presented in Table I. Among the five cohorts, there were
significant differences in sex, with more males exhibiting
alcoholic liver disease and more females exhibiting PBC compared
with those in the HC group. Laboratory parameters also
significantly differed among the six cohorts. All patients with
liver diseases exhibited higher AST, GGT and ALP levels but lower
albumin levels compared those in the HC group. In the DILD group,
patients exhibited higher ALT and bilirubin levels compared with
those in the HC group. Data were presented as the median
(interquartile range, 25th and 75th percentile). Bilirubin levels
in the alcoholic liver disease group were demonstrated to be ~7X
higher compared with those in the HC group.
Serum IgG subclass levels among
patients with different liver diseases
Serum concentrations of IgG subclasses are presented
in Table II and Fig. 1. Levels of each IgG subclass and
ratio of the IgG subclasses to total IgG significantly differed
among the six cohorts in the present study. However, this was not
observed for IgG2. IgG1 levels in patients with AIH and the
IgG1/IgG ratio in patients with viral liver disease were
significantly higher compared with those in the HC group.
Additionally, higher levels of IgG3 and IgG3/IgG, and lower levels
of IgG4 and IgG4/IgG were detected in patients with PBC compared
with those in the HC group. No notable in the distribution
characteristics of IgG subclasses could be observed in the
alcoholic liver disease and DILD cohorts in the present study. IgG1
levels were also significantly correlated with those of serum
albumin in patients with AIH (r=0.488), PBC (r=0.709), DILD
(r=0.578), alcoholic liver disease (r=0.644) and viral liver
disease (r=0.534). The results also revealed that ALT, AST, GGT,
ALP and bilirubin levels were not significantly associated with the
levels of any of the IgG subclasses.
 | Table IISerum IgG subclass levels. |
Table II
Serum IgG subclass levels.
| A, IgG subclass
measurements |
|---|
| IgG subclass | HCs (n=30) | V-LD (n=32) | A-LD (n=26) | DILD (n=39) | PBC (n=28) | AIH (n=29) | Statistic | P-value |
|---|
| IgG1, g/l | 7.49±1.53 |
12.62±4.52a | 10.57±3.93 | 10.45±5.16 |
12.96±6.20a |
15.64±7.25b | F=8.826 | <0.001 |
| IgG2, g/l | 4.65 (3.63,
5.46) | 4.12 (2.53,
5.66) | 5.08 (3.49,
7.91) | 3.89 (3.00,
5.23) | 4.46 (3.01,
7.53) | 5.22 (3.72,
7.93) | H=10.581 | 0.060 |
| IgG3, g/l | 0.26 (0.15,
0.51) | 0.35 (0.20,
0.56) | 0.34 (0.23,
0.65) | 0.30 (0.20,
0.55) | 1.11 (0.60,
2.49)b | 0.43 (0.28,
0.73) | H=34.486 | <0.001 |
| IgG4, g/l | 0.51 (0.26,
0.68) | 0.67 (0.20,
0.93) | 0.41 (0.23,
0.84) | 0.46 (0.25,
0.77) | 0.22 (0.05,
0.59)a | 0.56 (0.20,
1.53) | H=10.824 | 0.028 |
| IgG, g/l | 13.14±2.88 |
18.25±6.29a |
18.17±6.36a | 15.72±5.62 |
21.11±10.21a |
23.07±7.92b | F=8.325 | <0.001 |
| B, Ratios of each
IgG subclass with total IgG |
| IgG subclass | HCs (n=30) | V-LD (n=32) | A-LD (n=26) | DILD (n=39) | PBC (n=28) | AIH (n=29) | Statistic | P-value |
| IgG1/IgG, % | 58.01±9.47 |
69.69±9.64a | 59.26±12.86 | 64.82±10.89 | 63.23±10.81 | 66.49±12.99 | F=4.712 | <0.001 |
| IgG2/IgG, % | 34.75±9.44 |
24.15±8.09a | 32.16±9.57 | 28.50±10.20 |
26.10±8.39a |
26.03±9.87a | F=5.658 | <0.001 |
| IgG3/IgG, % | 1.72 (2.89,
3.72) | 2.15 (1.14,
3.56) | 1.95 (1.60,
3.35) | 2.27 (1.28,
3.04) | 5.84 (3.55,
13.77)b | 2.22 (0.94,
3.46) | H=31.794 | <0.001 |
| IgG4/IgG, % | 3.82 (2.06,
5.94) | 3.29 (1.73,
5.16) | 2.50 (1.21,
6.90) | 3.10 (2.06,
4.80) | 1.40 (0.50,
2.20)b | 3.10 (1.00,
5.61) | H=21.888 | 0.001 |
IgG subclass levels in each patient
cohort
The percentage of patients with PBC exhibiting
elevated IgG3 levels was four times higher compared with that in
the other groups (Fig. 2). The
frequency of elevated IgG1 in patients with AIH was the highest of
all cohorts, whilst the percentage of elevated IgG1 in patients
with DILD was the lowest compared with that in the five disease
groups. Compared with other IgG subclasses, the percentages of IgG1
and IgG4 elevations were the highest and lowest, respectively. In
addition, the percentage of IgG1 elevation was markedly higher in
all five cohorts compared with the other IgG subclasses.
Frequency of elevated IgG subclasses
in each group and the merged cohort
Overall, 68.2% (105/154) participants in the merged
cohort (five cohorts combined) had ≥ one type of elevated IgG
subclass (Table III). The most
frequently observed increase was one type of increased IgG
subclass, whilst four types of elevated IgG subclasses was the
least common. In 25.3% (39/154) patients, ≥ two IgG subclasses were
simultaneously increased.
 | Table IIIFrequency of elevated IgG subclasses
in each group and all five cohorts combined. |
Table III
Frequency of elevated IgG subclasses
in each group and all five cohorts combined.
| Patient group | One type of
increased IgG subclassa (n, %) | Two types of
increased IgG subclassesb (n, %) | Three types of
increased IgG subclassesc (n, %) | IgG1, IgG2, IgG3
and IgG4 increasedd
(n, %) | Only IgG4 increased
(n, %) |
|---|
| V-LD (n=32) | 17 (53.1) | 3 (9.4) | 2 (6.2) | 0 (0) | 0 (0) |
| A-LD (n=26) | 12 (46.2) | 3 (11.5) | 2 (7.7) | 1 (3.8) | 3 (11.5) |
| DILD (n=39) | 11 (28.2) | 6 (15.4) | 0 (0) | 0 (0) | 2 (5.1) |
| PBC (n=28) | 8 (28.6) | 9 (32.1) | 4 (14.3) | 1 (3.6) | 1 (3.6) |
| AIH (n=29) | 18 (62.1) | 5 (17.2) | 2 (6.9) | 1 (3.4) | 6 (20.7) |
| All five cohorts
combined (n=154) | 66 (42.8) | 26 (16.9) | 10 (6.5) | 3 (1.9) | 12 (7.8) |
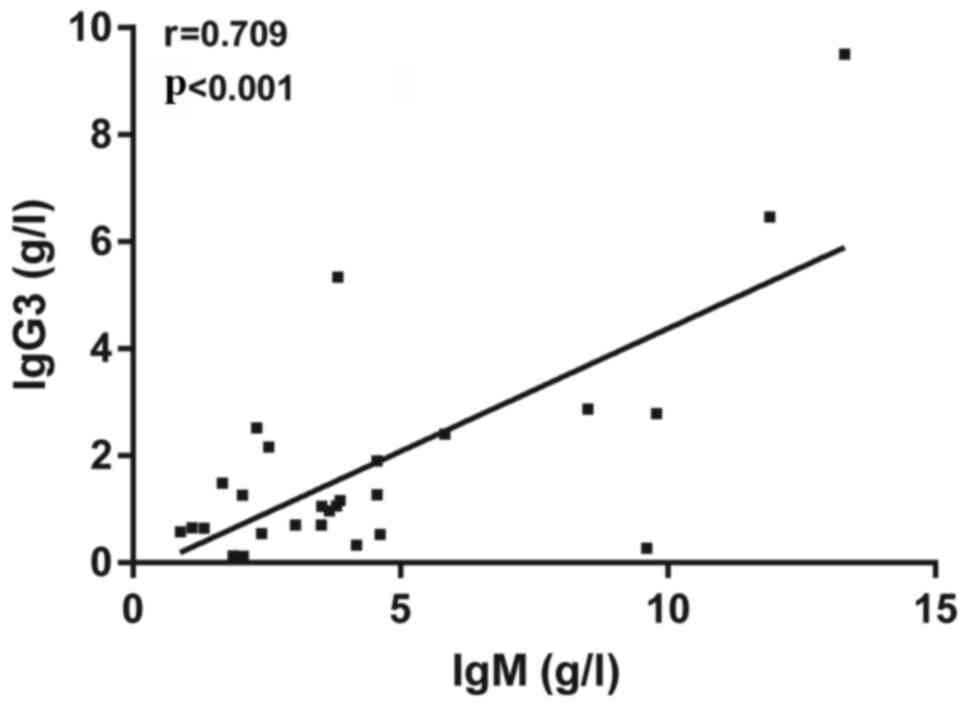
Relationship between IgG3 and IgM
concentrations in patients with PBC
IgA levels in patients with PBC were found to be
3.65 (1.78, 5.82) g/l and 3.06 (1.53, 6.30) g/l in patients with
AIH. Data were presented as the median (interquartile range, 25 and
75th percentile). IgA did not significantly differ between patients
with PBC and AIH. IgM levels in patients with PBC were 3.60 (2.12,
4.60) g/l and 1.80 (1.0, 3.0) g/l in patients with with AIH. IgM
levels in patients with PBC were significantly higher compared with
that in patients with AIH. Pearson's correlation coefficient
analysis was subsequently used to evaluate the relationship between
IgG3 and IgM concentrations in patients with PBC. The results
revealed that IgG3 levels were significantly correlated with those
of serum IgM in patients with PBC (Fig.
3). These results suggested that IgG3 and IgM may serve
synergistic effects in patients with PBC.
Relationship between concentrations of
IgG3 and AMA-M2 in patients with PBC
PBC is an autoimmune disease of the liver
characterized by the presence of AMA in 90-95% of patients
(38). Serum AMA, particularly that
of the AMA-M2 subtype, is regarded to be one of the most specific
and acceptable diagnostic indicators of PBC (39). In the present study, IgG3 levels
were not found to be significantly associated with AMA-M2 titers in
patients with PBC (Fig. 4). A total
of 94.1% patients with PBC demonstrated medium to high AMA-M2
titers (AMA-M2 ≥200 RU/ml) when IgG3 was elevated. When elevated
IgG3 was 1-2X higher than the reference interval, AMA-M2 titers
were also higher compared with two groups of 1X and >2X
reference interval of IgG3 (Fig.
4). The positive rate of the ANA autoantibody in patients with
PBC was 80.8% (21/26) and 89.3% (25/28) in patients with AIH
(Table IV), due to two patients
with PBC and one patient with AIH lacking ANA test data. ANA titers
did not significantly differ between patients with PBC and AIH.
ASMA could not be detected in patients in the PBC group whereas the
positive rate of ASMA in patients with AIH was 32.1% (9/28)
(Table IV). The incidence of ASMA
positivity in patients with AIH were significantly higher compared
with those in the PBC cohort (χ2=10.03; P=0.002).
 | Table IVThe titer of ANA and ASMA between PBC
and AIH patients. |
Table IV
The titer of ANA and ASMA between PBC
and AIH patients.
| | ANA titer | ASMA titer | Positive rates (%,
n) |
|---|
| Group | Negative | 1:100 | 1:320 | 1:1,000 | Negative | 1:100 | 1:320 | 1:1,000 | ANA | ASMA |
|---|
| PBC
(n=26)a | 5 | 2 | 1 | 18 | 26 | 0 | 0 | 0 | 80.8% (21/26) | 0% (0/26) |
| AIH
(n=28)b | 3 | 3 | 6 | 16 | 19 | 3 | 4 | 2 | 89.3% (25/28) | 32.1% (9/28) |
Association between IgG subclass
concentration and liver damage in patients with AIH
MELD scores are used to calculate the degree of
liver damage, such that the higher the MELD score, the greater the
liver damage (40). Patients with
AIH demonstrated higher MELD scores (13.28±6.10) compared with
those in the PBC cohort (12.63±5.27), but statistical significance
was not observed. In patients with AIH, IgG1 levels did not
significantly associate with the MELD scores. However, within the
same cohort, MELD scores were the highest in the cohort exhibiting
IgG1 levels > two times higher than the reference intervals
(Fig. 5). IgG1 levels and MELD
scores may therefore share a synergistic relationship.
Frequency of elevated serum IgG4
levels in patients
Serum IgG4 levels >135 mg/dl are considered to be
diagnostic criteria for IgG4-RD (18). The number and frequency of patients
with serum IgG4 levels >135 mg/dl were therefore calculated
among the five cohorts. Among the 154 patients, only 17 (11.0%) had
serum IgG4 levels >135 mg/dl (Table
V). In addition, no significant difference in the frequency of
elevated serum IgG4 levels was identified between patients with
viral liver disease and patients without non-viral liver disease
(Table V).
 | Table VFrequency of elevated serum IgG4
levels in patients. |
Table V
Frequency of elevated serum IgG4
levels in patients.
| Groups | Cases | N (IgG4 >135
mg/dl, %) |
P-valuea |
|---|
| V-LD | 32 | 3 (9.4) | - |
| A-LD | 26 | 4 (15.4) | 0.222 |
| DILD | 39 | 3 (7.7) | 0.809 |
| PBC | 28 | 1 (3.6) | 0.165 |
| AIH | 29 | 6 (20.7) | 0.079 |
Discussion
Liver diseases can be caused by a variety of
factors, including genetic predisposition, infection, autoimmunity
or metabolism, which renders diagnosis challenging (4). In the present retrospective study,
serum IgG subclass levels were analyzed in patients with five
common liver diseases to further their understanding and the
development of efficient novel early diagnosis strategies. The
results revealed that certain serum IgG subclass levels were
selectively increased or decreased depending of the type of
disease.
Previous studies have demonstrated that serum IgG3
levels are elevated patients with PBC (41,42).
However, little is known about IgG subclasses in patients with AIH.
The results of the present study suggested that there were higher
levels of IgG3 and IgG3/IgG but lower levels of IgG4 and IgG4/IgG
in patients with PBC compared with the HC group, further validating
the results of aforementioned studies. Additionally, compared with
those in the HC group, IgG1 levels were significantly higher in
patients with AIH. PBC and AIH are autoimmune diseases. The results
of the present study indicated that IgG subclasses may serve a role
in the differential diagnosis of patients with PBC and AIH. The
four subclasses of IgG differ in their constant regions,
particularly in their hinges and CH2 domains. IgG1 has the highest
FcγR binding affinity, followed by IgG3, IgG2 and IgG4 in that
order (43). When antigens enter
the body, their chemical compositions stimulate an immune reaction,
resulting in differential patterns of class switching. For
different antigens, such as adhesion protein desmoglein 3,
interleukin (IL)-4 and IL-10, class switching tends to occur from
IgG1 or IgG3 to IgG4 (44,45).
A previous study revealed that alcoholic liver
disease was associated with low serum concentrations of IgG and
reduced IgG following alcohol administration in mice (46). However, the results of the present
study demonstrated that IgG levels were elevated, which was not
consistent with observations from this previous study. This may be
due to different effects exerted by alcohol in murine and human
systems. Alonso et al (46)
previously injected alcohol into mice and measured IgG
concentrations after 4 weeks and found that alcohol administration
appeared to decrease IgG subclass concentrations. Patients with
alcoholic liver disease frequently have a long history of drinking
(47). It therefore may take some
time for the IgG subclasses to be produced in patients with
alcoholic liver disease. Additionally, Alonso's research objects
were mice, the physiological functions of which were different from
those of humans. As a result, further research is required to
assess these affects in humans.
Previous studies have demonstrated that when
compared with HCs, serum IgG1 and IgG3 levels were higher in
virus-infected individuals (48).
In the present study, IgG1/IgG levels were significantly increased
in viral liver disease group when compared with HCs. IgG3 was also
higher compared with that of HCs, but without significance. IgG
serves a pivotal role in viral neutralization (49). Antigens can trigger B-cells
directly, leading to a plethora of secondary signals that regulates
cell differentiation and subsequent antibody production (50,51).
The induction of specific IgG1 and IgG3 antibodies is therefore
more likely to achieve the clearance of HBV and HCV (49).
The results of the present study revealed that the
expression of IgG3 in patients with PBC was significantly
correlated with the levels of serum IgM. Furthermore, 94.1%
patients with PBC demonstrated medium to high AMA-M2 titers (AMA-M2
≥200 RU/ml) when IgG3 was elevated. Serum IgM and AMA-M2 levels are
two of the most important and specific diagnostic indicators of PBC
(52). IgG3, IgM and AMA-M2 may
have synergistic effects in PBC, which may be involved in the
pathogenesis of PBC. In addition, since AMA-M2 is primarily of the
IgG-AMA subclass (52) and that
currently available commercial kits are customized for IgG-AMA
detection, measurement of AMA and IgG3 levels may be more accurate
than radioimmunodiffusion in diagnosing PBC.
The MELD score was designed to calculate the degree
of liver damage, where higher MELD score implies greater damage to
the liver (40). In the present
study, it was demonstrated that IgG1 levels and MELD scores may
have a synergistic relationship in patients with AIH. Different
antigens can stimulate an IgG1 response (51). Therefore, excessive IgG1 levels are
more likely to harm the liver and cause autoimmune liver disease
(53,54).
IgG4-RD is a systemic disease involving a number of
organs, including the pancreas, lacrimal glands, lungs, liver and
kidney (55). Two types of liver
disease involvement have been reported in relation to IgG4-RD:
IgG4-related AIH and IgG4-hepatopathy (55). Certain common liver diseases have
been reported to share similarities with IgG4-RD. IgG4-related AIH
is clinicopathologically similar to that of AIH, except for the
elevated serum IgG4 levels and heavy infiltration of IgG4-positive
plasma cells in the liver tissue (56). In addition, IgG4-related AIH can be
hard to diagnose as well-known IgG4-RD(s) (55). Yamamoto et al (28) revealed that except for IgG4-RD,
increased IgG4 levels were observed several clinical cases, such as
Churg-Strauss syndrome, multicentric Castleman's disease,
eosinophilic disorders, and some patients with rheumatoid
arthritis, systemic sclerosis, chronic hepatitis, and liver
cirrhosis. Additionally, Ebbo et al (57) reported that 13.6% patients whose
serum IgG4 levels were > the cutoff value of 135 mg/dl were
diagnosed with IgG4-RD. In the present study, only 11% patients
exhibited serum IgG4 levels >135 mg/dl, which was lower compared
with that of previous studies. The results suggested that serum
IgG4 levels in the five patient cohorts of the present study were
different from those with IgG4-RD in the previous studies
aforementioned. These data may prove to be useful for differential
diagnosis between IgG4-RD and five liver disease cohorts, although
they share similarities of elevated serum IgG4 levels in pathogenic
processes. Additionally, the present study suggested that IgG4 and
other IgG subclasses may be used to distinguish liver diseases from
IgG4-RD.
The present study has several limitations. Firstly,
since this was an observational study, the effect of IgG4 on the
clinical manifestation of patients was not determined. Therefore, a
follow-up process should be performed in future studies. Secondly,
different laboratories could have slightly different definitions
for elevated IgG4. Finally, the number of samples in the present
study was relatively small and the present study lacked patients at
different stages of liver disease.
In conclusion, different liver diseases were found
to be associated with different serum IgG subclass distributions.
The present study suggested that IgG subclasses may serve as
biomarkers for the early diagnosis of liver diseases. However,
further studies on serum IgG subclass distributions may further
elucidate the immunopathogenesis of liver disease.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Beijing
Natural Science Foundation (grant nos. 7191004 and 7202069), the
Beijing Municipal Science & Technology Commission (grant no.
Z171100001017078), the Beijing municipal administration of
hospitals (grant nos. DFL20181701 and ZYLX201711) and the Beijing
Key Laboratory (grant no. BZ0373). The Capital health research and
development of special (grant no. Capital development
2020-2-1153).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ drafted the manuscript. WZ and FJ collected the
data. JS, YW, YJ, QG and JL performed the experiments. WZ and YZ
performed the statistical analysis and participated in the study
design. WZ and YZ participated in the acquisition, analysis or
interpretation of the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Beijing YouAn Hospital, Capital Medical University
(Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Asrani SK, Devarbhavi H, Eaton J and
Kamath PS: Burden of liver diseases in the world. J Hepatol.
70:151–171. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rowe IA: Lessons from epidemiology: The
burden of liver disease. Dig Dis. 35:304–309. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
GBD 2013 DALYs and HALE Collaborators.
Murray CJ, Barber RM, Foreman KJ, Abbasoglu Ozgoren A, Abd-Allah F,
Abera SF, Aboyans V, Abraham JP, Abubakar I, et al: Global,
regional, and national disability-adjusted life years (DALYs) for
306 diseases and injuries and healthy life expectancy (HALE) for
188 countries, 1990-2013: Quantifying the epidemiological
transition. Lancet. 386:2145–2191. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang FS, Fan JG, Zhang Z, Gao B and Wang
HY: The global burden of liver disease: The major impact of China.
Hepatology. 60:2099–2108. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Carbone M and Neuberger JM: Autoimmune
liver disease, autoimmunity and liver transplantation. J Hepatol.
60:210–223. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kapsogeorgou EK and Tzioufas AG:
Autoantibodies in autoimmune diseases: Clinical and critical
evaluation. Isr Med Assoc J. 18:519–524. 2016.PubMed/NCBI
|
|
7
|
Dusseaux M, Masse-Ranson G, Darche S,
Ahodantin J, Li Y, Fiquet O, Beaumont E, Moreau P, Riviere L,
Neuveut C, et al: Viral load affects the immune response to HBV in
mice with humanized immune system and liver. Gastroenterology.
153:1647–1661.e9. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Berry AA, Gottlieb ER, Kouriba B, Diarra
I, Thera MA, Dutta S, Coulibaly D, Ouattara A, Niangaly A, Kone AK,
et al: Immunoglobulin G subclass and antibody avidity responses in
Malian children immunized with Plasmodium falciparum apical
membrane antigen 1 vaccine candidate FMP2.1/AS02A. Malar
J. 18(13)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vidarsson G, Dekkers G and Rispens T: IgG
subclasses and allotypes: From structure to effector functions.
Front Immunol. 5(520)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Valenzuela NM and Schaub S: The Biology of
IgG subclasses and their clinical relevance to transplantation.
Transplantation. 102 (Suppl 1):S7–S13. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jansen A, Mandić AD, Bennek E, Frehn L,
Verdier J, Tebrügge I, Lutz H, Streetz K, Trautwein C and Sellge G:
Anti-food and anti-microbial IgG subclass antibodies in
inflammatory bowel disease. Scand J Gastroenterol. 51:1453–1461.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Khokar A and Gupta S: Clinical and
immunological features of 78 adult patients with primary selective
IgG subclass deficiencies. Arch Immunol Ther Exp (Warsz).
67:325–334. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shen H, Zhang M, Kaita K, Minuk GY, Rempel
J and Gong Y: Expression of Fc fragment receptors of immunoglobulin
G (FcγRs) in rat hepatic stellate cells. Dig Dis Sci. 50:181–187.
2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pressler T, Mansa B, Pedersen SS, Espersen
F, Høiby N and Koch C: Methodologic problems in establishing normal
values for IgG subclass concentrations in a pediatric population;
comparison of radial immunodiffusion and ELISA methods. Allergy.
49:772–777. 1994.PubMed/NCBI View Article : Google Scholar
|
|
15
|
van der Gugten G, DeMarco ML, Chen LYC,
Chin A, Carruthers M, Holmes DT and Mattman A: Resolution of
spurious immunonephelometric IgG subclass measurement discrepancies
by LC-MS/MS. Clin Chem. 64:735–742. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Brito-Zeron P, Ramos-Casals M, Bosch X and
Stone JH: The clinical spectrum of IgG4-related disease. Autoimmun
Rev. 13:1203–1210. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Okazaki K and Umehara H: Current concept
of IgG4-related disease. Curr Top Microbiol Immunol. 401:1–17.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Khosroshahi A, Wallace ZS, Crowe JL,
Akamizu T, Azumi A, Carruthers MN, Chari ST, Della-Torre E,
Frulloni L, Goto H, et al: International consensus guidance
statement on the management and treatment of IgG4-related disease.
Arthritis Rheumatol. 67:1688–1699. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Brito-Zerón P, Bosch X, Ramos-Casals M and
Stone JH: IgG4-related disease: Advances in the diagnosis and
treatment. Best Pract Res Clin Rheumatol. 30:261–278.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kamisawa T, Zen Y, Pillai S and Stone JH:
IgG4-related disease. Lancet. 385:1460–1471. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Culver EL and Chapman RW: IgG4-related
hepatobiliary disease: An overview. Nat Rev Gastroenterol Hepatol.
13:601–612. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Westra J, van Assen S, Wilting KR, Land J,
Horst G, de Haan A and Bijl M: Rituximab impairs immunoglobulin
(Ig)M and IgG (subclass) responses after influenza vaccination in
rheumatoid arthritis patients. Clin Exp Immunol. 178:40–47.
2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Huang CC, Lehman A, Albawardi A, Satoskar
A, Brodsky S, Nadasdy G, Hebert L, Rovin B and Nadasdy T: IgG
subclass staining in renal biopsies with membranous
glomerulonephritis indicates subclass switch during disease
progression. Mod Pathol. 26:799–805. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sadanand S, Das J, Chung AW, Schoen MK,
Lane S, Suscovich TJ, Streeck H, Smith DM, Little SJ, Lauffenburger
DA, et al: Temporal variation in HIV-specific IgG subclass
antibodies during acute infection differentiates spontaneous
controllers from chronic progressors. AIDS. 32:443–450.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang YL, Wang ZF and Chen N: Expression
of serum IgG4 in patients with different diseases. Beijing Da Xue
Xue Bao Yi Xue Ban. 49:961–964. 2017.PubMed/NCBI(In Chinese).
|
|
26
|
Zuo Y, Evangelista F, Culton D, Guilabert
A, Lin L, Li N, Diaz L and Liu Z: IgG4 autoantibodies are
inhibitory in the autoimmune disease bullous pemphigoid. J
Autoimmun. 73:111–119. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu Y and Li J: Preferentially
immunoglobulin (IgG) subclasses production in primary Sjogren's
syndrome patients. Clin Chem Lab Med. 50:345–349. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yamamoto M, Tabeya T, Naishiro Y, Yajima
H, Ishigami K, Shimizu Y, Obara M, Suzuki C, Yamashita K, Yamamoto
H, et al: Value of serum IgG4 in the diagnosis of IgG4-related
disease and in differentiation from rheumatic diseases and other
diseases. Mod Rheumatol. 22:419–425. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chinese Society of Hepatology, Chinese
Medical Association; Chinese Society of Infectious Diseases,
Chinese Medical Association. Hou JL and Lai W: The guideline of
prevention and treatment for chronic hepatitis B: A 2015 update.
Zhonghua Gan Zang Bing Za Zhi. 23:888–905. 2015.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
30
|
Chinese Society of Hepatology, Chinese
Medical Association; Wei L; Chinese Society of Infectious Diseases,
Chinese Medical Association and Hou JL. The guideline of prevention
and treatment for hepatitis C: A 2015 update. Zhonghua Gan Zang
Bing Za Zhi. 23:906–923. 2015.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
31
|
O'Shea RS, Dasarathy S and McCullough AJ:
Alcoholic liver disease. Am J Gastroenterol. 105:14–32.
2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Danan G and Teschke R: Roussel Uclaf
causality assessment method for drug-induced liver injury: Present
and future. Front Pharmacol. 10(853)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lindor KD, Bowlus CL, Boyer J, Levy C and
Mayo M: Primary biliary cholangitis: 2018 practice guidance from
the american association for the study of liver diseases.
Hepatology. 69:394–419. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chinese Society of Hepatology, Chinese
Society of Gastroenterology & Chinese Society of Infectious
Diseases. Chinese consensus on the diagnosis and management of
autoimmune hepatitis (2015). J Dig Dis. 18:247–264. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kim S, Zerillo J, Tabrizian P, Wax D, Lin
HM, Evans A, Florman S and DeMaria S Jr: Postoperative meld-lactate
and isolated lactate values as outcome predictors following
orthotopic liver transplantation. Shock. 48:36–42. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fisher K, Vuppalanchi R and Saxena R:
Drug-induced liver injury. Arch Pathol Lab Med. 139:876–887.
2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kleiner DE, Chalasani NP, Lee WM, Fontana
RJ, Bonkovsky HL, Watkins PB, Hayashi PH, Davern TJ, Navarro V,
Reddy R, et al: Hepatic histological findings in suspected
drug-induced liver injury: Systematic evaluation and clinical
associations. Hepatology. 59:661–670. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
de Liso F, Matinato C, Ronchi M and
Maiavacca R: The diagnostic accuracy of biomarkers for diagnosis of
primary biliary cholangitis (PBC) in anti-mitochondrial antibody
(AMA)-negative PBC patients: A review of literature. Clin Chem Lab
Med. 56:25–31. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Huang YQ: Recent advances in the diagnosis
and treatment of primary biliary cholangitis. World J Hepatol.
8:1419–1441. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lebray P and Varnous S: Combined heart and
liver transplantation: State of knowledge and outlooks. Clin Res
Hepatol Gastroenterol. 43:123–130. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rigopoulou EI, Davies ET, Bogdanos DP,
Liaskos C, Mytilinaiou M, Koukoulis GK, Dalekos GN and Vergani D:
Antimitochondrial antibodies of immunoglobulin G3 subclass are
associated with a more severe disease course in primary biliary
cirrhosis. Liver Int. 27:1226–1231. 2007.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhang H, Li P, Wu D, Xu D, Hou Y, Wang Q,
Li M, Li Y, Zeng X, Zhang F and Shi Q: Serum IgG subclasses in
autoimmune diseases. Medicine (Baltimore). 94(e387)2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yu J, Song Y and Tian W: How to select IgG
subclasses in developing anti-tumor therapeutic antibodies. J
Hematol Oncol. 13(45)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ellebrecht CT, Mukherjee EM, Zheng Q, Choi
EJ, Reddy SG, Mao X and Payne AS: Autoreactive IgG and IgA B cells
evolve through distinct subclass switch pathways in the autoimmune
disease pemphigus vulgaris. Cell Rep. 24:2370–2380. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Nualnoi T, Kirosingh A, Basallo K, Hau D,
Gates-Hollingsworth MA, Thorkildson P, Crump RB, Reed DE, Pandit S
and AuCoin DP: Immunoglobulin G subclass switching impacts
sensitivity of an immunoassay targeting Francisella tularensis
lipopolysaccharide. PLoS One. 13(e0195308)2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Alonso M, Gomez-Rial J, Gude F, Vidal C
and Gonzalez-Quintela A: Influence of experimental alcohol
administration on serum immunoglobulin levels: Contrasting effects
on IgE and other immunoglobulin classes. Int J Immunopathol
Pharmacol. 25:645–655. 2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Mathurin P and Bataller R: Trends in the
management and burden of alcoholic liver disease. J Hepatol. 62 (1
Suppl):S38–S46. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Walker MR, Eltahla AA, Mina MM, Li H,
Lloyd AR and Bull RA: Envelope-specific IgG3 and IgG1 responses are
associated with clearance of acute hepatitis C virus infection.
Viruses. 12(75)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Jin J, Xu H, Wu R, Gao N, Wu N, Li S and
Niu J: Identification of key genes and pathways associated with
different immune statuses of hepatitis B virus infection. J Cell
Mol Med. 23:7474–7489. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Pone EJ, Zhang J, Mai T, White CA, Li G,
Sakakura JK, Patel PJ, Al-Qahtani A, Zan H, Xu Z and Casali P:
BCR-signalling synergizes with TLR-signalling for induction of AID
and immunoglobulin class-switching through the non-canonical
NF-kappaB pathway. Nat Commun. 3(767)2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Giuntini S, Granoff DM, Beernink PT, Ihle
O, Bratlie D and Michaelsen TE: Human IgG1, IgG3, and IgG3
hinge-truncated mutants show different protection capabilities
against meningococci depending on the target antigen and epitope
specificity. Clin Vaccine Immunol. 23:698–706. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Tang L, Zhong R, He X, Wang W, Liu J, Zhu
Y, Li Y and Hou J: Evidence for the association between
IgG-antimitochondrial antibody and biochemical response to
ursodeoxycholic acid treatment in primary biliary cholangitis. J
Gastroenterol Hepatol. 32:659–666. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Behairy OG, Behiry EG, El Defrawy MS and
El Adly AN: Diagnostic value of soluble programmed cell death
protein-1 in type-1 autoimmune hepatitis in Egyptian children.
Scand J Clin Lab Invest. 80:59–65. 2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Than NN, Ching DK, Hodson J, McDowell P,
Mann J, Gupta R, Salazar E, Ngu JH and Oo YH: Difference in
clinical presentation, immunology profile and treatment response of
type 1 autoimmune hepatitis between United Kingdom and Singapore
patients. Hepatol Int. 10:673–679. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Nakanuma Y, Ishizu Y, Zen Y, Harada K and
Umemura T: Histopathology of IgG4-related autoimmune hepatitis and
IgG4-related hepatopathy in IgG4-related disease. Semin Liver Dis.
36:229–241. 2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Lee HE and Zhang L: Immunoglobulin
G4-related hepatobiliary disease. Semin Diagn Pathol. 36:423–433.
2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ebbo M, Grados A, Bernit E, Vély F,
Boucraut J, Harlé JR, Daniel L and Schleinitz N: Pathologies
associated with serum IgG4 elevation. Int J Rheumatol.
2012(602809)2012.PubMed/NCBI View Article : Google Scholar
|