Introduction
Hepatocellular carcinoma (HCC) is an aggressive
cancer and is the third leading cause of cancer related mortality
worldwide (1). A number of patients
with HCC are not considered candidates for surgery due to the late
stage diagnosis (2) and HCC is
highly resistant to chemo therapy (3). Therefore, novel drugs and treatment
targets are urgently needed to develop therapies for HCC.
Peroxisome proliferator-activated receptor γ (PPARγ)
is a class of ligand activated nuclear transcription factor. Upon
activation by its ligand, PPARγ can inhibit tumor cell
proliferation and metastasis, as well as promote apoptosis
(4,5). For example, activated PPARγ promotes
the expression of the pro-metastatic genes MMP9, MMP13 to regulate
cell metastasis; overexpression of PPARγ also promotes expression
of caspase-3, caspase-7 and other caspases (6,7).
Previous studies have also indicated that PPARγ transcriptionally
inhibits NF-κB signaling in HCC (8,9).
Cyclooxygenase-2 (Cox-2), a prostaglandin synthetase, is a
rate-limiting enzyme that is highly expressed in various types of
cancer, including HCC, and exerts anticancer effects (10). The promoter region of Cox-2 contains
several known sequences, including a binding site for NF-κB
(11).
Plantamajoside (PMS) is a major component of
Plantago asiatica L, which has several pharmacological
properties, including anti-proliferative, anti-inflammatory and
anti-tumor effects (12,13). Previous studies have reported that
PMS suppresses the growth and metastasis of breast cancer and
squamous cell carcinoma (14,15).
Furthermore, it has been reported that the biological effects of
PMS are mediated through the regulation of MMP9 and 2; NF-κB;
PI3K/Akt; and MAPK signaling (13,15-17).
To the best of our knowledge, the present study is
the first study that has demonstrated that PMS inhibits the
biological functions of HCC cells and as such, may be employed as a
novel therapeutic agent for human HCC.
Materials and methods
Reagents
PMS was purchased from Shifeng Biological Technology
Co., Ltd., and dissolved in a solution of ethanol and double
distilled water at a ratio of 1:1. Sorafenib (10 mM) was purchased
from Selleck Chemicals to be used as positive control and was
digested in a solution containing DMSO (63 mg/ml, warmed to 25˚C).
T0070907 was purchased from Selleck Chemicals. It has been
previously reported that T0070907 is a selective ligand for PPARγ,
functioning as an antagonist (18).
Cell culture and treatment
Huh7 cells, PLC/PRF 5 and THLE-2 cells (The Cell
Bank of Type Culture Collection of Chinese Academy of Sciences)
were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) at 37˚C
in an atmosphere containing 5% CO2. Cells were treated
with PMS at a concentration of 25, 50 or 100 µg/ml according to a
previous study (14), or were
treated with sorafenib at 5 or 20 µM. T0070907 was used as a
pre-treatment to inhibit PPARγ.
Cell viability assay
To investigate the effects of PMS on cell growth,
MTT assays were used. Huh7 cells were seeded in 96-well plates at a
density of 1x104 cells/well for 24 h. After cell
attachment, the cells were treated with sorafenib or PMS at 25, 50
or 100 µg/ml, or were not treated at 37˚C. The Optical density (OD)
value at 490 nm was detected at 12, 24, 36 and 48 h.
Wound healing assay
Cells (1x106 cells/well) were seeded in
6-well plates and incubated for 48 h until ~100% confluent. Cells
were washed with serum-free medium and pre-treated with mitomycin C
(10 µg/ml at 37˚C for 30 min). Subsequently, the incubation medium
was replaced with serum-free DMEM. A scratch was created in the
cell layer using a 200 µl pipette tip, followed by incubation with
medium (non-treated control), sorafenib (positive control) or PMS
at 25, 50 or 100 µg/ml for 48 h. Cell migration was examined under
a light microscope (magnification, x100; Olympus Corporation). The
wound healing distance was calculated using the following equation:
(Initial width at 0 h-final width at 48)/0 h width. The relative
wound healing distance was obtained by normalizing to the control
group.
Transwell assay
Cells were seeded at a density of 1x104
cells/well in the upper chamber of a transwell plate (8 µm
pore-size filter; Merck KGaA) and received no treatment (control),
were treated with sorafenib (positive control) or were treated with
PMS at 25, 50 or 100 µg/ml at 37˚C for 48 h. Following addition of
100 µl FBS free medium and treatment with mitomycin C at 37˚C for
30 min, the upper chambers were placed in a 24-well plate. The
lower chambers were filled with 500 µl medium supplemented with 10%
FBS for 24 h. The cells that had migrated to the lower surface of
the filter were stained with 0.1% crystal violet solution. Images
were captured using a light microscope (magnification, x100;
Olympus Corporation) and processed by IPP 6.0 software (Media
Cybernetics, Inc.).
Cell apoptosis and Cell cycle
Cells (4x104 cells/well) were cultured
with sorafenib and PMS at 25, 50 and 100 µg/ml for 48 h prior to
analysis. The cells were subsequently fixed with 70% pre-cooled
ethanol for 12-14 h, washed with PBS and resuspended in PBS
containing RNase and PI/Triton X-100 (20 µg/0.1% Triton X-100) for
15 min at 37˚C, then the cell cycle was analyzed (1x104
cells/sample) using the BD AccuriC6 flow cytometer (BD
Biosciences).
Cells were cultured with sorafenib and PMS at 25, 50
and 100 µg/ml for 48 h prior to analysis, stained with the Annexin
V-FITC Apoptosis Detection kit (Beyotime Institute of
Biotechnology) according to manufacturer's instructions and cell
apoptosis was detected using the BD AccuriC6 flow cytometer (BD
Biosciences). Data on the cell cycle distribution and cell
apoptosis were further analyzed using FlowJo software (version 10;
FlowJo LLC).
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from Huh7 cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's instructions. RNA concentrations were
evaluated using a spectrophotometer (Beckman Coulter, Inc.). cDNA
was synthesized using a Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions manual. The temperature protocol for RT
was as follows: 37˚C for 15 min, followed by 85˚C for 5 sec and
holding at 4˚C. cDNA was amplified by PCR using SYBR (Invitrogen;
Thermo Fisher Scientific, Inc.), and the specific primers used were
as follows: GAPDH (reference gene) forward,
5'-GAACGGGAAGCTCACTGG-3' and reverse, 5'-GCCTGCTTCACCACCTTCT-3;
NF-κB forward, 5'-AAGCACGAATGACAGAGGC-3' and reverse,
5'-CTTGGCGGATTAGCTCTTTT-3'; Cox-2 forward,
5'-TGTGCAACACTTGAGTGGCT-3' and reverse, 5'-ACTTTCTGTACTGCGGGTGG-3';
and PPAR-γ forward, 5'-GCAGGAGCAGAGCAAAGAG-3' and reverse,
5'-GAGGAGAGTTACTTGGTCGTTC-3'. The thermocycling conditions were as
follows: 95˚C for 2 min, followed by 40 cycles of 95˚C for 20 sec
and 65˚C for 40 sec. Expression levels of target genes were
normalized to the endogenous control GAPDH using the
2-∆∆Cq method (19).
Western blotting
Cells (1x106 cells/well) were seeded into
6-well plates and received no treatment (control) or were treated
with sorafenib (positive control) and PMS at 25, 50 or 100 µg/ml
for 48 h. Cells were lysed using lysis buffer (Shanghai Yanjin
Biological Technology Co., Ltd.) and the protein concentration was
detected using spectrophotometry. Equal masses of protein samples
(30 µg extract loaded per lane) were subjected to 12% SDS-PAGE
transferred to PVDF membranes (Invitrogen; Thermo Fisher
Scientific, Inc.). After blocking with 5% non-fat milk at room
temperature for 2 h, samples were incubated with the indicated
primary antibody, namely; NF-κB (p65; cat. no. MW 65; 1:1,000; Cell
Signaling Technologiy, Inc.), Cox-2 (cat. no. MW 74; 1:1,000; Cell
Signaling Technology, Inc.), PPAR-γ (cat. no. MW 55; 1:1,000; Cell
Signaling Technologies, Inc.), MMP2 (cat. no. MW 72; 1:1,000; Cell
Signaling Technology, Inc.), MMP9 (cat. no. MW 92; 1:1,000; Cell
Signaling Technology, Inc.), cyclin D1 (cat. no. MW 36; 1:1,000;
Cell Signaling Technology, Inc.), cleaved-caspase-3 (cat. no. MW
35, 1:1,000; Cell Signaling Technology, Inc.), caspase-3 (cat. no.
MW 17; 1:1,000; Cell Signaling Technology, Inc.), cytochrome C
(Cyt-C; cat. no. MW 14; 1:1,000; Cell Signaling Technology, Inc.),
GAPDH (cat. no. MW 37; loading control, 1:1,000; Cell Signaling
Technology, Inc.). Subsequently, the target proteins were evaluated
by binding with horseradish peroxidase-conjugated anti-rabbit (cat.
no. ab205718; 1:10,000) or an anti-mouse (cat. no. ab205719;
1:10,000; both Abcam) secondary antibodies and an ECL kit (Wuhan
Servicebio Technology Co., Ltd.). Gray scale scanning was used to
analyze the protein bands.
Statistical analysis
Graphpad PRISM 6.0 (GraphPad Software, Inc.) was
used to analyze the data. Numerical results are presented as the
mean ± SD from three independent experiments. Differences between
groups were compared using one-way ANOVAs and two-way ANOVAs,
followed by Tukey's post-hoc multiple comparisons tests. A P-value
<0.05 was considered to indicate statistically significant
differences.
Results
PMS inhibits the viability of HCC
cells
To investigate the anti-HCC effect, Huh7 and
PLC/PRF5 cells were treated with PMS at various doses and MTT
assays were performed to determine cell viability. Sorafenib, as a
positive control, significantly reduced the viability of Huh7 and
PLC/PRF5 cells at all measured time-points. PMS, at 100 µg/ml, also
exhibited a similar efficacy to sorafenib in Huh7 and PLC/PRF5. PMS
at 50 µg/ml also showed a significant reduction in the cell
viability at 48 h in Huh7 and PLC/PRF5 cells compared with the
untreated control, but the treatment in PLC/PRF5 did not show the
same level of significance as that in Huh7 cells. In addition, PMS
at 25 µg/ml did not exhibit a significant impact compared with the
untreated control in both HCC cells, only having a significant
impact on the PCL/PRF5 cells at 48 h (Fig. 1). PMS at 100 µg/ml promoted the
expression of mRNA and proteins of PPARγ in the Huh7 and PLC/PRF5
cell line compared with the control group (Fig. 2). The cell viability of Huh-7 and
PLC/PRF5 cells, which was reduced by Sorafenib and 100 µg/ml PMS,
was reversed by the PPARγ inhibitor, T0070907 when compared with
PMS 100 µg/ml (Fig. 3). PPARγ may
be a potential mechanism by which PMS exerted its anti-tumor
effects.
HCC cell migration is inhibited by
PMS
As shown in Fig. 4,
wound healing assays showed a significant decrease in the cell
migration in the sorafenib and PMS 100 µg/ml groups compared with
the untreated cells, with no significant difference being found
between these two treatments. PMS 50 µg/ml caused a significant
reduction in the cell migration in both cells. The low dose group
(PMS 25 µg/ml) did not exhibit a notable change compared with the
untreated control group. To further investigate whether PPARγ
participated in the effects of PMS, transwell assays were performed
and it was demonstrated that 50 and 100 µg/ml PMS markedly
inhibited cell migration compared with the untreated control group,
and that treatment with the PPARγ inhibitor, T0070907, reversed the
effects of PMS on cell migration compared with 100 µg/ml PMS
(Fig. 5).
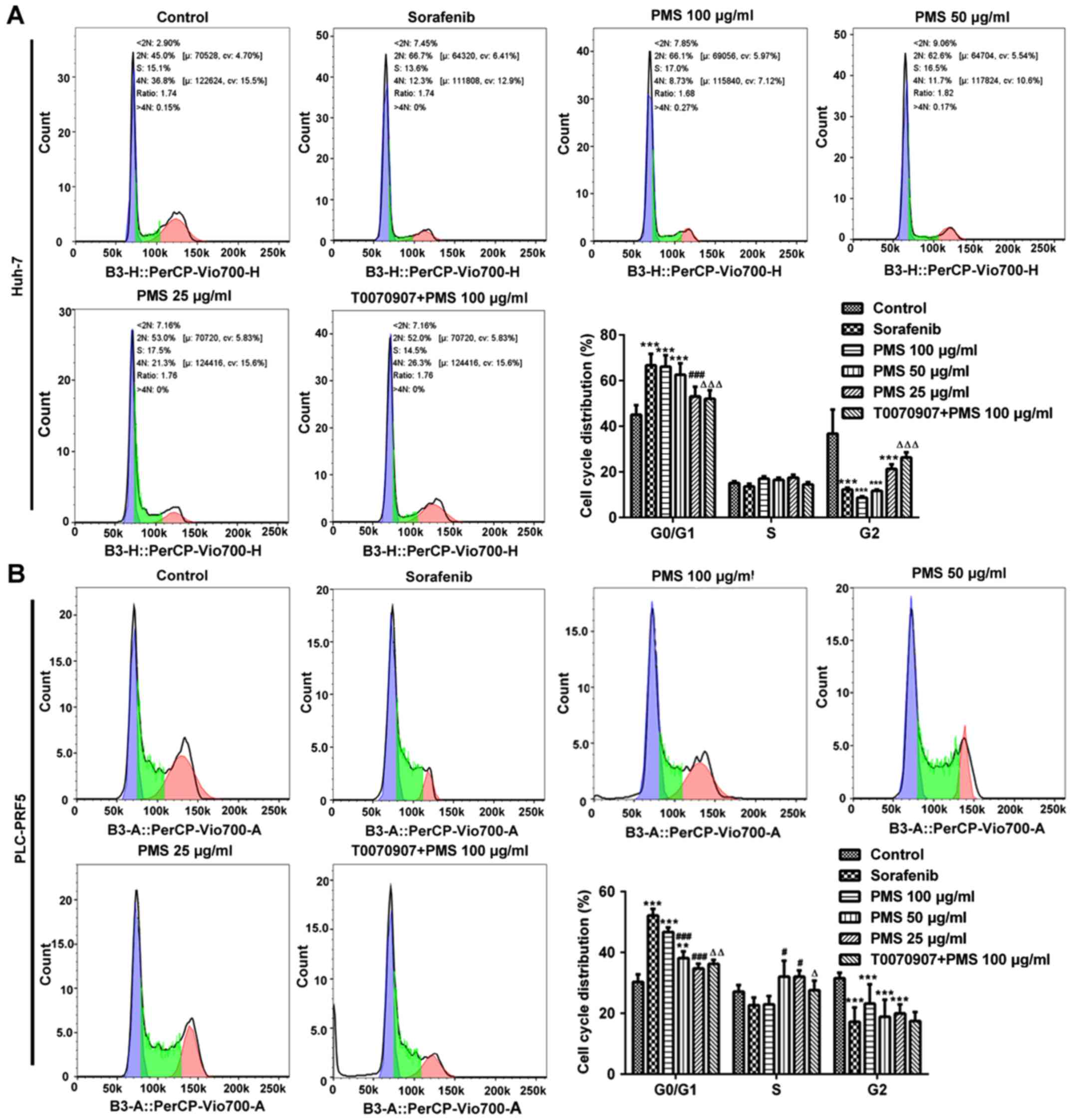
PMS induces cell cycle arrest at the
G0/G1 phase and promotes apoptosis in HCC cells
To further investigate the effects of PMS on cell
proliferation, the cell cycle was analyzed in Huh-7 and PLC/PRF5
cells. It was found that there were significantly more cells in the
G0/G1 phase in the sorafenib, PMS 100 µg/ml and PMS 50 µg/ml
treated groups compared with the untreated control group. The
effect of PMS on the cell cycle appeared to be directly associated
with the dose administered. Cell cycle arrest at the G0/G1 phase
was reversed by treatment with T0070907 (Fig. 6).
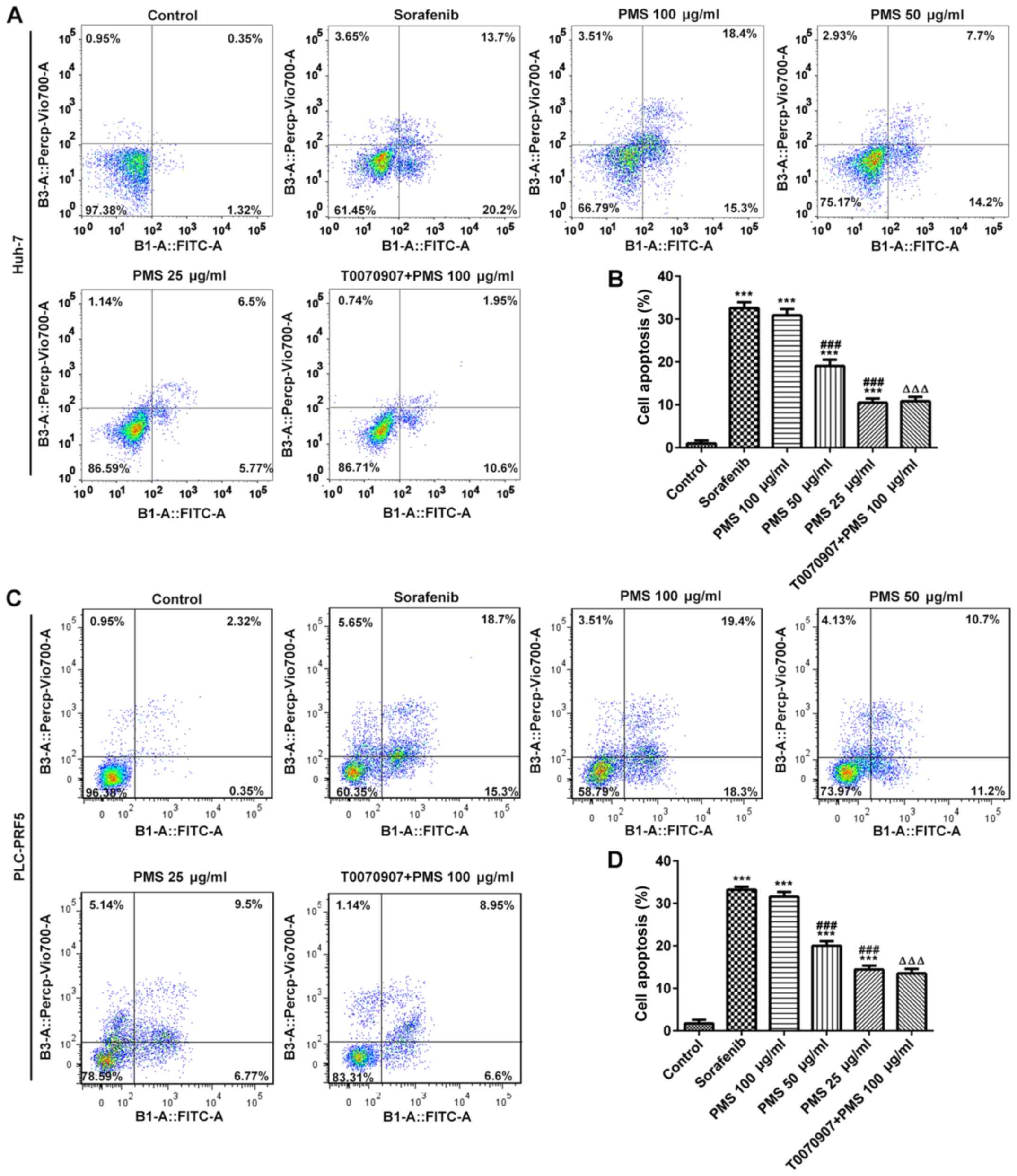
In addition, cell apoptosis in Huh-7 and PLC/PRF5
cells was significantly enhanced by PMS and sorafenib treatment.
PMS at a high dose (100 µg/ml) exerted effects comparable to those
of sorafenib and the efficacy of PMS was directly associated with
the dose administered, that is, the higher the dose of PMS, the
stronger the promotive effect on apoptosis. The raised apoptosis
observed following PMS 100 µg/ml treatment to both cell lines was
significantly reversed by treatment with a PPARγ inhibitor
(T0070907) in both cell lines, although this did not reach levels
observed in the untreated cells (Fig.
7).
Further mechanisms of PMS on cell
migration, proliferation, the cell cycle and cell apoptosis
To further confirm that the effects of PMS on cell
migration, proliferation, the cell cycle and cell apoptosis are
PPARγ-dependent, the expression levels of biomarkers involved in
the above processes including MMP2, MMP9, Cyclin D1, cleaved
caspase-3/caspase-3 and Cyt-C were detected (Fig. 8). MMPs play a role in the
degradation of the extracellular matrix, leading to metastasis
(20). Consistently, the expression
levels of MMP2 and MMP9 were significantly decreased in the
sorafenib and PMS 100 µg/ml treated groups, compared with the
untreated control. The PPARγ inhibitor, T0070907 reversed the
inhibitory effect of PMS. Cyclin D1, a major cell cycle regulators
of G1 phase progression (21), was
expressed at significantly lower levels in the sorafenib, PMS 100
µg/ml and PMS 50 µg/ml treated groups, than in the control. Again,
the effects of PMS on the expression levels of cyclin D1 were
significantly reversed by T0070907. In regards to apoptosis related
proteins, caspase-3 and Cyt-C are involved in apoptosis activation
(22). The expression levels of
cleaved-caspase-3, a compound caused by the activation of caspase-3
activation (22), were
significantly increased in the sorafenib and PMS 100 and 50 µg/ml
treated cells compared with the control. The expression levels of
Cyt-C were also increased in the sorafenib treated group, as well
as in the PMS 100 and 50 µg/ml treated groups, compared with the
control. Furthermore, the expression levels of both
cleaved-caspase-3 and Cyt-C were significantly reversed by T0070907
treatment compared with the 100 µg/ml treated group, although this
did not reach the levels of the control group.
 | Figure 8The role of PMS on cell
proliferation, migration and apoptosis. The expression levels of
proteins involved in migration (MMP2 and MMP9), apoptosis
(caspase-3 and Cyt-C) and cell cycle (cyclin D1) were detected
using (A) western blotting and (B) gray scan analysis from the
Huh-7 can PLC/PRF5 cells following treatment with sorafenib, PMS
and T0070907 + PMS. Data are presented as the mean ± SD.
*P<0.05, **P<0.01,
***P<0.001 vs. the control group;
#P<0.05, ##P<0.01,
###P<0.001 vs. sorafenib; ΔP<0.05,
ΔΔP<0.01 vs. PMS 100 µg/ml. T0070907 was used for
PPARγ inhibition. Cyt-C, cytochrome C; MMP, matrix
metalloproteinase; PMS, plantamajoside; PPARγ, peroxisome
proliferator-activated receptor γ. |
PMS exerts its biological effects
through the PPARγ/NF-κB/Cox-2 signaling pathway
Compared with the normal liver cell line THLE-2, the
expression levels of PPARγ was significantly decreased, whereas
that of Cox-2 and NF-κB was significantly increased in both
untreated Huh7 and PLC/PRF5 cells. Western blotting analysis
results revealed that the expression levels of NF-κB and Cox-2 were
significantly decreased following treatment with sorafenib and high
dose PMS (100 µg/ml), with no significant difference between the
two groups. The intermediate (50 µg/ml) and low (25 µg/ml) PMS dose
groups also exhibited a decrease in the expression of these
proteins, but to a lesser degree compared with the high dose group,
compared with the control group. PPARγ expression was increased in
the sorafenib and PMS groups, with the effects of PMS appearing to
be concentration-dependent. Inhibition of PPARγ significantly
upregulated the expression of Cox-2 and NF-κB, compared with the
PMS 100 µg/ml group (Fig. 9).
Discussion
Herbal medicine is becoming increasingly attractive
as a potential cancer therapy (23). PMS is extracted from Plantago
major L which has been reported to exert inhibitory effects on
certain types of cancer, such as breast cancer and esophageal
squamous cell carcinoma (13,14).
However, the effect of PMS on HCC and the underlying mechanism of
action remain unclear. It was hypothesized that PMS may be
beneficial for the treatment of HCC.
To investigate this hypothesis, the biological
effects of PMS on HCC cells were investigated. A high dose of PMS
was found to significantly inhibit cell proliferation and
migration, as well as to promote apoptosis. Sorafenib is used to
prevent tumor relapses and metastasis in patients with HCC who
undergo tumor resection (24).
Sorafenib, a kinase inhibitor, is the only systemic treatment that
is currently used to provide clinical improvements in patients with
advanced HCC (25). As sorafenib is
a useful treatment for advanced HCC and in most cases, liver cancer
is discovered at a later stage, sorafenib was used as a control due
to its proven efficacy (23). The
present study herein demonstrated that a high dose of PMS exerted
similar effects with the sorafenib positive control when inhibiting
HCC, indicating the potential role that PMS played on HCC cells
in vitro.
It has previously been shown that PMS regulates cell
migration, proliferation and apoptosis, and promotes the expression
of PPARγ (13,14). To examine whether PPARγ was involved
in these biological effects in HCC cells, HCC cells were pretreated
with the PPARγ inhibitor, T0070907, before PMS treatment. PPARγ has
been reported to inhibit MMP2 and MMP9, to exert an anti-tumor
metastasis effect in mice with HCC (26,27).
Activation of PPARγ by its ligands are attributed to the
suppression of proliferation and induction of apoptosis (28). The present results revealed that
PPARγ mediated the effects of PMS on cell migration, proliferation
and apoptosis. Moreover, inhibition of PPARγ also blocked the
expression levels of caspase-3 and Cyt-C, which were promoted by
PMS. Cyt-C is a marker of the mitochondrial respiration chain
(29), therefore, PMS may promote
mitochondrial apoptosis in a PPARγ-mediated manner. Cyclin D1 is a
regulator of the progression from G1 to S, as reflected by the
accumulation in G1(30). The
present stdy showed that PMS inhibits cell proliferation and this
effect is linked with the downregulation of cyclin D1 expression.
In addition, T0070907 reversed the effects of PMS on cell
proliferation.
In the present study, it was also observed that
Cox-2 expression was significantly decreased following treatment
with PMS, while PPARγ expression was significantly increased, and
the two may have mutual constraints. PPARγ is a member of the
ligand regulated nuclear receptor superfamily, which exerts diverse
biological effects, such as promoting tumor cell growth,
angiogenesis and invasion (31-33).
PPARγ ligands can interact with Cox-2 and affect shared pathways.
Previous studies have suggested that Cox-2 expression can be
inhibited with PPARγ activators in human cervical cancer (34,35).
The dysregulation of the NF-κB signaling pathway has recently been
confirmed to be involved in the biological response to various
types of cancer (36). NF-κB not
only promotes tumor cell survival and protects cells against
apoptotic stimuli, but may also promote proliferation and
metastasis of tumor cells (37).
The human Cox-2 promotor includes several transcription
factor-binding sites, such as for NF-κB and NF-IL6(34). It has been previously demonstrated
that increased PPARγ expression inhibits the expression of Cox-2
and its promoter activity (38). In
the present study, the expression of PPARγ was significantly
promoted by PMS, whereas the expression of Cox-2 and NF-κB was
downregulated in Huh7 and PLC/PRF5 cells. Taken together, these
findings indicated that PMS may have acted as an activator of the
PPARγ/NF-κB/Cox-2 signaling pathway in HCC cells.
In conclusion, the present study evaluated the
effects of PMS on HCC cell metastasis, apoptosis, cell cycle
distribution and proliferation. PMS was found to inhibit the
proliferation and migration and promote the apoptosis of Huh7 and
PLC/PRF5 cells. PMS also triggered G0/G1 phase arrest in Huh7 and
PLC/PRF cells. The PPARγ/NF-κB/Cox-2 axis may be the mechanism
underlying its regulatory biological effects. These results
indicated that PMS may be a promising agent for the treatment of
HCC. However, more HCC cell lines as well as in vivo
investigations should be explored in further research to confirm
the findings of the present study.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation for Young Scientists of China (grant nos.
81703940 and 815503267).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and SL conceived and designed the current study.
SL, XJ, GY and YL acquired the data. ZL, LM, JW and HW analyzed the
data. SL and YL drafted the manuscript and revised it for
critically important intellectual content. All authors read and
approved the final manuscript. YL and SL confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jiang BG, Wang N, Huang J, Yang Y, Sun LL,
Pan ZY and Zhou WP: Tumor SOCS3 methylation status predicts the
treatment response to TACE and prognosis in HCC patients.
Oncotarget. 8:28621–28627. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Guo XL, Li D, Hu F, Song JR, Zhang SS,
Deng WJ, Sun K, Zhao QD, Xie XQ, Song YJ, et al: Targeting
autophagy potentiates chemotherapy-induced apoptosis and
proliferation inhibition in hepatocarcinoma cells. Cancer Lett.
320:171–179. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tontonoz P and Spiegelman BM: Fat and
beyond: The diverse biology of PPARgamma. Ann Rev Biochemistry.
77:289–312. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Heudobler D, Rechenmacher M, Lüke F,
Vogelhuber M, Pukrop T, Herr W, Ghibelli L, Gerner C and Reichle A:
Peroxisome proliferator-activated receptors (PPAR)γ agonists as
master modulators of tumor tissue. Int J Mol Sci.
19(3540)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shen B, Chu ES, Zhao G, Man K, Wu CW,
Cheng JT, Li G, Nie Y, Lo CM, Teoh N, et al: PPARgamma inhibits
hepatocellular carcinoma metastases in vitro and in mice. Br J
Cancer. 106:1486–1494. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yu J, Shen B, Chu ES, Teoh N, Cheung KF,
Wu CW, Wang S, Lam CN, Feng H, Zhao J, et al: Inhibitory role of
peroxisome proliferator-activated receptor gamma in
hepatocarcinogenesis in mice and in vitro. Hepatology.
51:2008–2019. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nojima H, Kuboki S, Shinoda K, Shimizu H,
Ohtsuka M, Kato A, Yoshitomi H, Furukawa K, Takayashiki T and
Miyazaki M: Activation of peroxisome proliferator-activated
receptor-gamma inhibits tumor growth by negatively regulating
nuclear factor-κB activation in patients with hepatocellular
carcinoma. J Hepatobiliary Pancreat Sci. 23:574–584.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Zhang N, Chu ES, Zhang J, Li X, Liang Q,
Chen J, Chen M, Teoh N, Farrell G, Sung JJ and Yu J: Peroxisome
proliferator activated receptor alpha inhibits hepatocarcinogenesis
through mediating NF-kappaB signaling pathway. Oncotarget.
5:8330–8340. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kern MA, Schoneweiss MM, Sahi D, Bahlo M,
Haugg AM, Kasper HU, Dienes HP, Käferstein H, Breuhahn K and
Schirmacher P: Cyclooxygenase-2 inhibitors suppress the growth of
human hepatocellular carcinoma implants in nude mice.
Carcinogenesis. 25:1193–1199. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tsatsanis C, Androulidaki A, Venihaki M
and Margioris AN: Signalling networks regulating cyclooxygenase-2.
Int J Biochem Cell Biol. 38:1654–1661. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wu H, Zhao G, Jiang K, Chen X, Zhu Z, Qiu
C, Li C and Deng G: Plantamajoside ameliorates
lipopolysaccharide-induced acute lung injury via suppressing NF-κB
and MAPK activation. Int Immunopharmacol. 35:315–322.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pei S, Yang X, Wang H, Zhang H, Zhou B,
Zhang D and Lin D: Plantamajoside, a potential anti-tumor herbal
medicine inhibits breast cancer growth and pulmonary metastasis by
decreasing the activity of matrix metalloproteinase-9 and -2. BMC
Cancer. 15(965)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li X, Chen D, Li M, Gao X, Shi G and Zhao
H: Plantamajoside inhibits lipopolysaccharide-induced
epithelial-mesenchymal transition through suppressing the
NF-κB/IL-6 signaling in esophageal squamous cell carcinoma cells.
Biomed Pharmacother. 102:1045–1051. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Han AR, Nam MH and Lee KW: Plantamajoside
inhibits UVB and advanced glycation end products-induced MMP-1
expression by suppressing the MAPK and NF-κB pathways in HaCaT
cells. Photochem Photobiol. 92:708–719. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ma C and Ma W: Plantamajoside inhibits
lipopolysaccharide-induced MUC5AC expression and inflammation
through suppressing the PI3K/Akt and NF-κB signaling pathways in
human airway epithelial cells. Inflammation. 41:795–802.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Son WR, Nam MH, Hong CO, Kim Y and Lee KW:
Plantamajoside from Plantago asiatica modulates human umbilical
vein endothelial cell dysfunction by glyceraldehyde-induced AGEs
via MAPK/NF-κB. BMC Complement Altern Med. 17(66)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee G, Elwood F, McNally J, Weiszmann J,
Lindstrom M, Amaral K, Nakamura M, Miao S, Cao P, Learned RM, et
al: T0070907, a selective ligand for peroxisome
proliferator-activated receptor gamma, functions as an antagonist
of biochemical and cellular activities. J Biol Chem.
277:19649–19657. 2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yao P, Li Y, Shen W, Xu X, Zhu W, Yang X,
Cao J and Xing C: ANKHD1 silencing suppresses the proliferation,
migration and invasion of CRC cells by inhibiting YAP1-induced
activation of EMT. Am J Cancer Res. 8:2311–2324. 2018.PubMed/NCBI
|
|
21
|
Tchakarska G and Sola B: The double
dealing of cyclin D1. Cell Cycle. 19:163–178. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kalpage HA, Bazylianska V, Recanati MA,
Fite A, Liu J, Wan J, Mantena N, Malek MH, Podgorski I, Heath EI,
et al: Tissue-specific regulation of cytochrome c by
post-translational modifications: Respiration, the mitochondrial
membrane potential, ROS, and apoptosis. FASEB J. 33:1540–1553.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sarris J, Panossian A, Schweitzer I,
Stough C and Scholey A: Herbal medicine for depression, anxiety and
insomnia: A review of psychopharmacology and clinical evidence. Eur
Neuropsychopharmacol. 21:841–860. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Feng YX, Wang T, Deng YZ, Yang P, Li JJ,
Guan DX, Yao F, Zhu YQ, Qin Y, Wang H, et al: Sorafenib suppresses
postsurgical recurrence and metastasis of hepatocellular carcinoma
in an orthotopic mouse model. Hepatology. 53:483–492.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Han M, Gao H, Ju P, Gao MQ, Yuan YP, Chen
XH, Liu KL, Han YT and Han ZW: Hispidulin inhibits hepatocellular
carcinoma growth and metastasis through AMPK and ERK signaling
mediated activation of PPARγ. Biomed Pharmacother. 103:272–283.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu YI, Liu Z, Chen Y, Xu K and Dong J:
PPARγ activation reduces ischemia/reperfusion-induced metastasis in
a murine model of hepatocellular carcinoma. Exp Ther Med.
11:387–396. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Takahashi N, Okumura T, Motomura W,
Fujimoto Y, Kawabata I and Kohgo Y: Activation of PPARγ inhibits
cell growth and induces apoptosis in human gastric cancer cells.
FEBS Lett. 455:135–139. 1999.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bock FJ and Tait SWG: Mitochondria as
multifaceted regulators of cell death. Nature reviews. Mol Cell
Biol. 21:85–100. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bendris N, Lemmers B and Blanchard JM:
Cell cycle, cytoskeleton dynamics and beyond: The many functions of
cyclins and CDK inhibitors. Cell Cycle. 14:1786–1798.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Niu Z, Shi Q, Zhang W, Shu Y, Yang N, Chen
B, Wang Q, Zhao X, Chen J, Cheng N, et al: Caspase-1 cleaves PPARγ
for potentiating the pro-tumor action of TAMs. Nat Commun.
8(766)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhao T, Du H, Blum JS and Yan C: Critical
role of PPARγ in myeloid-derived suppressor cell-stimulated cancer
cell proliferation and metastasis. Oncotarget. 7:1529–1543.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cheng J, Miao B, Hu KQ, Fu X and Wang XD:
Apo-10'-lycopenoic acid inhibits cancer cell migration and
angiogenesis and induces peroxisome proliferator-activated receptor
γ. J Nutr Biochem. 56:26–34. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ikawa H, Kameda H, Kamitani H, Baek SJ,
Nixon JB, Hsi LC and Eling TE: Effect of PPAR activators on
cytokine-stimulated cyclooxygenase-2 expression in human colorectal
carcinoma cells. Exp Cell Res. 267:73–80. 2001.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Han S, Inoue H, Flowers LC and Sidell N:
Control of COX-2 gene expression through peroxisome
proliferator-activated receptor gamma in human cervical cancer
cells. Clin Cancer Res. 9:4627–4635. 2003.PubMed/NCBI
|
|
36
|
Cildir G, Low KC and Tergaonkar V:
Noncanonical NF-κB signaling in health and disease. Trends Mol Med.
22:414–429. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Xiong S, Wang Y, Li H and Zhang X: Low
dose of bisphenol a activates NF-κB/IL-6 signals to increase
malignancy of neuroblastoma cells. Cell Mol Neurobiol.
37:1095–1103. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bren-Mattison Y, Meyer AM, Van Putten V,
Li H, Kuhn K, Stearman R, Weiser-Evans M, Winn RA, Heasley LE and
Nemenoff RA: Antitumorigenic effects of peroxisome
proliferator-activated receptor-gamma in non-small-cell lung cancer
cells are mediated by suppression of cyclooxygenase-2 via
inhibition of nuclear factor-kappaB. Mol Pharmacol. 73:709–717.
2008.PubMed/NCBI View Article : Google Scholar
|