Introduction
Osteoarthritis (OA) is one of the most common
arthritic diseases (1) and a major
cause of chronic pain and dysfunction in patients. OA is a chronic
degenerative disease that is characterized by joint disease,
cartilage degeneration and erosion, fibrosis, and osteophyte
formation. In the worst cases, OA leads to pain and disability and
severely affects the quality of life of patients (2-4).
However, the origins and causes of OA have not been fully
elucidated to date.
Chondrocytes are the only type of cell present in
articular cartilage, where they are responsible for maintaining the
normal synthesis and renewal of the cartilage matrix (4). A previous study associated
pro-inflammatory cytokines [e.g., interleukin (IL)-1β] with OA
pathogenesis and revealed that it plays a pivotal role in OA
(3). The activation of
inflammation-related signaling pathways was revealed to be
initiated by IL-1β. Then, IL-1β stimulated the expression of matrix
metalloproteinases (MMPs) and nitric oxide (NO), which resulted in
the destruction of the cartilage matrix (5). MMP-3 and MMP-13 have been recognized
as the two most important collagenases that are involved in the
degradation of the cartilage matrix; these are also crucial factors
in OA (6,7). It has been revealed that the
expression levels of MMP-3 and MMP-13 are increased in synovial
fluid from patients with OA (8).
The overexpression of metalloproteinases promotes degradation of
type II collagen (Col II) (4).
Col II is the main component of the extracellular
matrix (ECM) (4). The fibrillar Col
II is the major collagen of articular cartilage, and includes both
procollagen with both amino (N)-terminal (PIINP and PIIANP) and
carboxy (C)-terminal propeptide (PIICP) (9). The PIICP levels have been correlated
with the progression of OA in the knee (10). When Col II breaks down and produces
fragments, such as collagenase, type II collagen cleavage
neoepitope (C2C) and collagen type-II C-telopeptide fragments
(CTX-II) are generated (11,12).
C2C is a useful biomarker that reflects the cleavage of Col II in
OA and the levels of CTX-II have been demonstrated to be associated
with the total damage of osteophytes (13).
Numerous studies have revealed that IL-1β can
activate the nuclear factor-κB (NF-κB) pathway (14-16).
NF-κB has been identified as an inducible transcription factor
(17) and the NF-κB pathway has
been reported to regulate the balance in the catabolism that
controls numerous inflammation-related genes (18). When NF-κB has been stimulated by
IL-1β, inactive NF-κB becomes active and translocates from the
cytoplasm to the nucleus; moreover, the phosphorylation of p65 is
translated into the nucleus and the expression of downstream genes
is increased (14,19). Therefore, OA is associated with
inflammation, and reducing activation of the NF-κB pathway may be a
therapeutic strategy for the treatment of OA.
As a class of molecules with high sensitivity,
biomarkers associated with OA have been extensively studied and
have broad application prospects (20). However, in previous studies,
chondrocytes induced with 1 ng/ml IL-1α or 10 ng/ml IL-1β to
simulate an in vitro OA model mostly focused on a single
marker at a certain time-point (21,22),
while not studying the change trend of these markers in OA, and
thus, not reflecting OA progression. In the present study, the
variation trend of biomarkers was observed at four different
time-points. Currently, advanced OA can only be alleviated through
analgesia or joint replacement, both of which are expensive
(23). Therefore, clarifying the
pathogenesis as well as the early diagnosis of OA is important for
the prevention and treatment of OA. The present study explored the
optimal conditions for IL-1β-induced OA in a rat articular
chondrocyte cell model. Additionally, the expression of MMPs and
subsequent degradation of type II collagen in the course of OA were
analyzed. The presented variation trend of the concentration of
molecular markers provides a theoretical basis for the diagnosis
and screening of OA molecular markers.
Materials and methods
Chondrocyte isolation and culture
Chondrocytes were isolated from male Sprague-Dawley
(SD) rats aged 14-24 days, weight 30-50 g. SD rats were bought from
thr Animal Experimental Base of Heilongjiang University of
Traditional Chinese Medicine. All the animals were kept separately
in the animal room. The room was well ventilated, clean and
comfortable: The temperature was 21±3˚C, the humidity was 50±15%,
the light time was 12 h per day, the food and water supply was
sufficient, and the bedding material was changed regularly. The use
of rats in the present study was approved by the Laboratory Animal
Welfare and Ethics Committee of Northeast Agricultural University
(Harbin, China). Chondrocytes from different rats were pooled.
Animals were euthanized by placing in a clean, fluoroscopically
closed box and CO2 was perfused at a rate of 10-30% of
the replacement volume/min into the box. Animal sacrifice was
ascertained when the animal was motionless, not breathing, and the
pupils became dilated; then CO2 was turned off. The
animals were observed for an additional 2 min to confirm sacrifice.
In a sterile environment, chondrocytes were isolated from articular
cartilage and digested with 0.25% trypsin (Gibco; Thermo Fisher
Scientific, Inc.) for 30 min at 37˚C. Subsequently, DMEM/F12
containing 10% FBS (Corning, Inc.) and penicillin-streptomycin
(Corning, Inc.) containing 0.2% collagenase type II (Biofroxx) were
mixed for 4 h at 37˚C in a shaker to isolate chondrocytes. The cell
suspension was centrifuged (400 x g; 7 min) to harvest primary
chondrocytes at room temperature. Primary chondrocytes were
cultured in DMEM/F12 at 37˚C with 5% CO2 in growth
medium. The medium was changed at 24-h intervals. When the cells
reached 80% confluence, they were passaged, and the chondrocytes of
passage 2 were used for all subsequent experiments.
Cell model
Passage 2 cells were washed three times with
serum-starved medium (0.5% FBS) and incubated with DMEM/F12 for 12
h at 37˚C. Chondrocytes were treated with IL-1β (10 ng/ml)
(PeproTech, Inc.) for 0, 12, 24 and 48 h at 37˚C. OA is a
pathologic process with varying degrees of severity (24). Chondrocytes treated with IL-1β at
different time-points were used to simulate an in vitro
model of OA. The variation trend of biomarkers at different
time-points was observed with the development of OA (14).
Toluidine blue staining
Chondrocytes were washed three times in
phosphate-buffered saline (PBS) and fixed with 10% buffered
formalin for 30 h at 4˚C. After washing for 5 min with distilled
water, chondrocytes were gently washed for 15 min under running
water, and then evenly dyed with 1% toluidine blue dye (Gibco;
Thermo Fisher Scientific, Inc.) for 2 h at room temperature
Finally, the samples were washed with distilled water and examined
under a laser confocal optical microscope.
ELISA
According to the manufacturer's instructions, the
concentrations of PIICP (cat. no. 88-1052), C2C (cat. no.
EHJ-96082r), and CTX-II (cat. no. EHJ-96093r) were measured by
ELISA kits (Xiamen Huijia Biological Technology Co., Ltd.). The
expression of NO was measured by NO assay kit (cat. no. A013-2;
NanJing JianCheng Bioengineering Institute). All assays were
repeated three times. All kits are were operated according to the
manufacturers' instructions.
Western blot analysis
The protein concentrations were determined using the
bicinchoninic acid assay. Cells were rinsed with ice-cold PBS and
harvested in lysis buffer (Beyotime Institute of Biotechnology) to
obtain total cellular protein, nuclear, or cytoplasmic fractions.
Equal quantities (30 µg/lane) of proteins were subjected to 8-12%
SDS-PAGE (Beyotime Institute of Biotechnology) and transferred to
nitrocellulose filter (NC) membranes (Pall Corporation). Then, the
membranes were blocked in PBS containing 20% Tween-20 (PBST;
Beyotime Biotechnology, Shanghai, China) plus 5% non-fat dry milk
for 1 h at room temperature, and were incubated with primary
antibodies overnight at 4˚C. The membranes were washed three times
in Tris-buffered saline (TBS) with 20% Tween-20. The blots were
then incubated with the secondary antibody conjugated with
horseradish peroxidase for 1 h at room temperature. Finally, the
western blots were developed using ECL reagent (Beyotime Institute
of Biotechnology) through a western blotting detection system.
Primary antibodies included mouse anti-GAPDH
(1:3,000, cat. no. TA-08; ZSGB-BIO; OriGene Technologies, Inc.),
mouse anti-MMP-13 (1:1,000; cat. no. NB100-91878V; Novus
Biologicals, Inc.), rabbit anti-MMP-3 (1:1,000; cat. no. 69926),
NF-κB p65 (1:1,000; cat. no. 8242), phospho-NF-κB p65 (1:1,000,
cat. no. 3033) (all from Cell Signalling Technology, Inc.) and
polyclonal antibody rabbit anti-iNOS (1:500; cat. no.
NB300-605AF405; Novus Biologicals, Inc.). Secondary antibodies
included goat anti-rabbit-IgG and goat anti-mouse-IgG (1:1,000;
cat. no. ZB2305/ZB-2301, ZSGB-BIO; OriGene Technologies, Inc.).
Statistical analysis
All experimental data was analysed using SPSS
software (version 19.0 for Windows; SPSS, Inc.). Values are
expressed as the mean ± standard deviation (SD). Statistical
comparisons between multiple groups were conducted by one-way
analysis of variance (ANOVA), followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Results of chondrocytes
When the chondrocytes cover 80% of the culture
bottle, primary cells can be sub-cultured. After more than three
generations, the cells undergo a process known as
‘dedifferentiation’ (25). In the
present study, undifferentiated second-generation chondrocytes were
used and the cell morphology is shown in Fig. 1.
Results of toluidine blue
staining
After dyeing, proteoglycans and the nucleus
separated in chondrocytes, where proteoglycans were blue-purple and
the nucleus was mazarine. Fig. 2
clearly revealed that the cultured cells were chondrocytes.
Effect of the expression levels of
MMP-3 and MMP-13 in IL-1β-induced chondrocytes
The expression levels of MMP-3 and MMP-13 are
presented in Fig. 3. The expression
levels of MMP-3 increased in a time-dependent manner in
IL-1β-induced chondrocytes at 12, 24 and 48 h. MMP-13 expression
levels were significantly increased at 12, 24 and 48 h, and were
highest at 24 h.
Effect of iNOS and NO expression in
IL-1β-induced chondrocytes
Western blot results for the expression level of
iNOS are presented in Fig. 4. After
IL-1β stimulation, the iNOS expression levels at 12, 24 and 48 h
were significantly increased compared to those at 0 h, and reached
the maximum value at 24 h (Fig.
4A). As revealed in Fig. 4B,
the NO levels differed in culture supernatants at 12, 24, and 48 h
compared with the 0-h time-point. In addition, the level of NO was
significantly increased at 12, 24 and 48 h, and reached the maximum
value at 24 h.
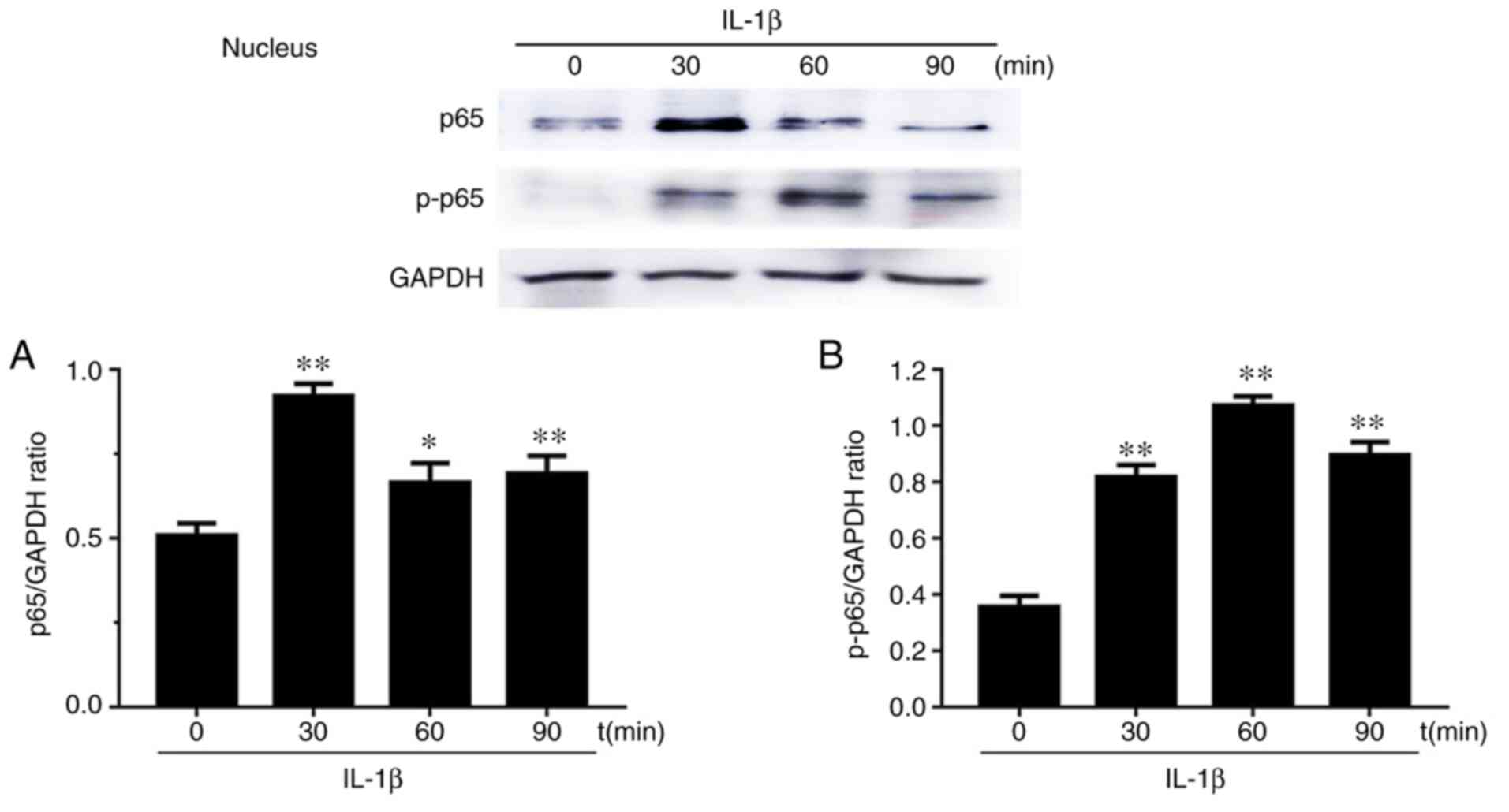
Effects of p65 on the nucleus
translocation in IL-1β-induced chondrocytes
Western blot results of the expression levels of p65
and phosphorylated (p)-p65 after chondrocyte stimulation with IL-1β
at 0, 30, 60 and 90 min in both the cytoplasm and nucleus are
presented in Figs. 5 and 6. Firstly, cytoplasmic extracts exhibited
a significantly reduced level of p65 upon IL-1β stimulation.
However, the cytoplasmic extracts exhibited the opposite result for
p-p65. Secondly, the levels of p65 and p-p65 in chondrocyte nuclei
after IL-1β stimulation for 30, 60 and 90 min were increased
compared to the group without stimulation (0 min). p65 levels first
increased at 30 min and then decreased at 60 and 90 min. p-p65
levels increased and reached the maximum value at 60 min.
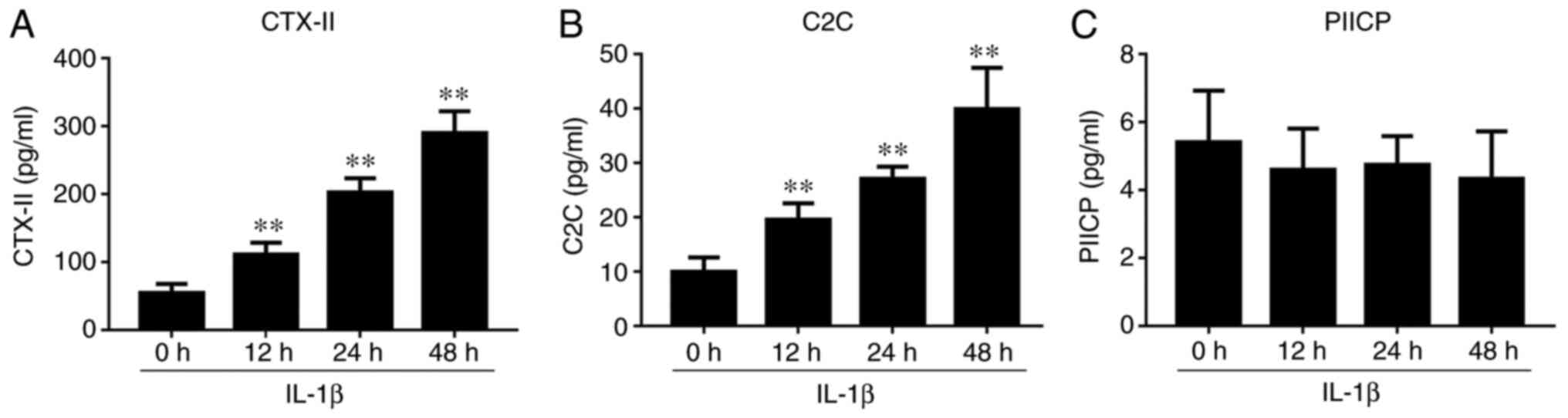
IL-1β-induced chondrocyte effect on
the levels of CTX-II, C2C and PIICP
ELISA assays revealed that the levels of CTX-II and
C2C were significantly increased in IL-1β-induced culture
supernatants, and reached a maximum value at 48 h (Fig. 7). However, no significant change was
observed in the levels of PIICP after IL-1β treatment.
Discussion
Biomarkers are a class of highly sensitive
molecules, and have been extensively studied in OA (9). It is important to study the mechanisms
and molecular biomarkers of OA cartilage. Articular cartilage can
be damaged by abnormal wear or pathological processes. At the early
stage of OA, the ECM changes and the cartilage surface remains
intact (25). This change leads to
the expression of markers such as Runx2, ColX, and MMP-13(26). As OA further develops, the
composition and structure of articular cartilage changes, the
proteoglycan and collagen networks break down, the cartilage
degrades, and the integrity of the cartilage is lost (27,28).
The chondrocytes undergo apoptosis and eventually, the articular
cartilage disappears (29). Part of
the cartilage forms osteophytes (30). IL-1β (10 ng/ml) (14) has been revealed as an important
pro-inflammatory compound that plays key roles in numerous
inflammatory responses (31). The
development of OA is accompanied by an inflammatory response
(32). Therefore, IL-1β, as a
pro-inflammatory agent, initiates the activation of inflammation
and induces OA. It can be confirmed that articular cartilage is
destroyed in OA development (12).
In a study by De Visser et al articular cartilage damage was
induced on femoral condyles and it was revealed that Fib3-3 was
upregulated and may be a biomarker and related to joint
degenerative changes (33). In
addition, it was revealed by observing different biomarkers in an
in vitro OA rat model induced with IL-1α (1 ng/ml) that
membrane-free stem cell components (MFSCC) had an effect on
cartilage regeneration (34). In
these experiments, biomarkers were assessed at specific time-points
and did not exhibit trends. In the present study model,
rat-specific chondrocytes were used, and different time-points were
treated with IL-1β, which could reflect the human OA pathological
process to some extent. OA progression is more intuitively
reflected by observing biomarker changes at different
time-points.
The conducted present experiment only diagnosed OA
biomarkers at the cellular level. In follow-up research, we will
verify this effect in vivo, which will provide a theoretical
basis for the early diagnosis and treatment of OA. The present
study revealed that the expression levels of MMP-3, MMP-13, and
iNOS and the production of NO were upregulated in IL-1β-induced
chondrocytes (35,36). In the progression of OA, MMPs
(including MMP-3 and MMP-13) are important regulators. MMP-3, as a
matrix lysin, can decrease the expression of collagen and
proteoglycans and activate other collagenases (37). MMP-13 is a collagenase that
regulates matrix degradation and binds to type II collagen in the
ECM (7,38). In addition, proteolytic enzymes and
MMPs could be stimulated by IL-1β, which are important factors for
cartilage destruction (13). The
results of a previous study on type II collagen degradation in
chondrocytes were consistent with those of the present study
(4). Furthermore, the NF-κB
signaling pathway activated by IL-1β, and western blot results
revealed that p65 and p-p65 levels increased, which also confirmed
the inflammatory response of chondrocytes (39). NF-κB is a transcription factor that
plays a vital role in the response to cellular stress as a
transcriptional regulator (40).
Inflammatory cytokines, such as IL-1β, activate NF-κB to regulate
the facilitation of OA. Thus, NF-κB is an important molecule that
controls both the normal development and the pathological
destruction of cartilage (41).
NF-κB is a heterodimeric, and p65 is one of its subunits (42). In the present study, IL-1β
stimulated the NF-κB signaling pathway; then, p65 was
phosphorylated in the cytoplasm and transferred to the nucleus.
Therefore, the level of p65 significantly decreased in cytoplasmic
extracts. However, the level of p-p65 exhibited the opposite
result. In the chondrocyte nucleus, the level of p65 increased
first and then decreased, while the level of p-p65 levels
increased.
Western blot analyses revealed that the expression
of iNOS increased in chondrocytes, and also, the concentration of
NO increased in the chondrocyte culture supernatant (43,44).
This suggests that activation of the NF-κB pathway leads to the
activation of related transcription factors, further leading to an
increase in the synthesis of iNOS and NO. However, the increase of
NO concentration stimulated the degradation of the cartilage
matrix. In summary, in OA, the NF-κB pathway is activated, which
induces a cascade response downstream of the pathway, which
promotes cartilage damage. In the present study, the in
vitro OA model was induced by treating chondrocytes with IL-1β
for different periods of time to observe biomarker changes.
According to the results, not only was the pathogenesis and early
diagnosis of OA clarified, but also the screening of drugs used for
the treatment of OA. The goal of the present study was to provide a
summary and guide for the application of in vitro biomarkers
for the development of drugs for OA. The present study provided
insight into the treatment and relief of OA pathological
processes.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Key R&D
Program of China (project no. 2017YFD0502200) and the Applied
Technology Research and Development Plan of Heilongjiang (grant no.
GX18B023).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XM and LG designed the study. XM, ZZ and RL
performed the cell experiments. XM, MS and XJ collected and
analyzed data. XM, ZZ, YM, LG and ZW interpreted the data. XM wrote
and edited the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The use of rats in the present study was approved by
the Laboratory Animal Welfare and Ethics Committee of Northeast
Agricultural University (Harbin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
French HP, Galvin R, Horgan F and Kenny
RA: Prevalence and burden of osteoarthritis amongst older people in
Ireland: Findings from The Irish LongituDinal study on ageing
(TILDA). Osteoarthritis Cartilage. 23(A188)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Abramson SB and Attur M: Developments in
the scientific understanding of osteoarthritis. Arthritis Res Ther.
11(227)2009.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Scanzello CR and Goldring SR: The role of
synovitis in osteoarthritis pathogenesis. Bone. 51:249–257.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Momin M, Guoqing L, Yang W and Li C:
Protective effects of gemigliptin against type II collagen
degradation in human chondrocytes. Biomed Pharmacother.
104:590–954. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Haseeb A and Haqqi TM: Immunopathogenesis
of osteoarthritis. Clin Immunol. 146:185–196. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Muto T, Kokubu T, Mifune Y, Inui A, Harada
Y, Yoshifumi Takase F, Kuroda R and Kurosaka M: Temporary
inductions of matrix metalloprotease-3 (MMP-3) expression and cell
apoptosis are associated with tendon degeneration or rupture after
corticosteroid injection. J Orthop Res. 32:1297–1304.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mitchell PG, Magna HA, Reeves LM,
Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF and Hambor
JE: Cloning, expression, and type II collagenolytic activity of
matrix metalloproteinase-13 from human osteoarthritic cartilage. J
Clin Invest. 97:761–768. 1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ying X, Chen X, Cheng S, Shen Y, Peng L
and Xu HZ: Piperine inhibits IL-β induced expression of
inflammatory mediators in human osteoarthritis chondrocyte. Int
Immunopharmacol. 17:293–299. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Patra D and Sandell L: Evolving biomarkers
in osteoarthritis. J Knee Surg. 24:241–249. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sugiyama S, Itokazu M, Suzuki Y and
Shimizu K: Procollagen II C propeptide level in the synovial fluid
as a predictor of radiographic progression in early knee
osteoarthritis. Ann Rheum Dis. 62:27–32. 2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dejica VM, Mort JS, Laverty S, Antoniou J,
Zukor DJ, Tanzer M and Poole AR: Increased type II collagen
cleavage by cathepsin K and collagenase activities with aging and
osteoarthritis in human articular cartilage. Arthritis Res Ther.
14(R113)2012.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Boeth H, Macmahon A, Poole AR, Buttgereit
F, Önnerfjord P, Lorenzo P, Klint C, Pramhed A and Duda GN:
Differences in biomarkers of cartilage matrix turnover and their
changes over 2 years in adolescent and adult volleyball athletes. J
Exp Orthop. 4(7)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Madzuki IN, Lau SF, Che Ahmad Tantowi NA,
Mohd Ishak NI and Mohamed S: Labisia pumila prevented
osteoarthritis cartilage degeneration by attenuating joint
inflammation and collagen breakdown in postmenopausal rat model.
Infammopharmacology. 26:1207–1217. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li Y, Wang J, Song X, Bai H, Ma T, Zhang
Z, Li X, Jiang R, Wang G, Fan X, et al: Effects of baicalein on
IL-1β-induced inflammation and apoptosis in rat articular
chondrocytes. Oncotarget 8:. 8:90781–90795. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu B, Zhou Y, Chen X and Peng D:
IL-1β-mediated NF-κB signaling augments the osteosarcoma cell
growth through modulating miR-376c/TGFA axis. Die Pharmazie.
72:419–424. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu H, Zhang M, Li W, Zhu S and Zhang D:
Stachydrine attenuates IL-1β-induced inflammatory response in
osteoarthritis chondrocytes through the NF-κB signaling pathway.
Chem Biol Interact. 326(109136)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liacini A, Sylvester J, Li WQ and
Zafarullah M: Inhibition of interleukin-1-stimulated MAP kinases,
activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B)
transcription factors down-regulates matrix metalloproteinase gene
expression in articular chondrocytes. Matrix Biol. 21:251–162.
2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zheng W, Chen C, Zhang C, Cai L and Chen
H: The protective effect of phloretin in osteoarthritis: An in
vitro and in vivo study. Food Funct. 9:263–278. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Perkins ND: The diverse and complex roles
of NF-κB subunits in cancer. Nat Rev Cancer. 12:121–132.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Mobasheri A, Bay-Jensen AC, van Spil WE,
Larkin J and Levesque MC: Osteoarthritis year in review 2016:
Biomarkers (biochemical markers). Osteoarthritis Cartilage.
25:199–208. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang L, Ma S, Su H and Cheng J:
Isoliquiritigenin inhibits IL-1β-induced production of matrix
metalloproteinase in articular chondrocytes. Mol Ther Methods Clin
Dev. 9:153–159. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Huang X, Pan Q, Mao Z, Zhang R, Ma X, Xi Y
and You H: Sinapic acid inhibits the IL-1β-induced inflammation via
MAPK downregulation in rat chondrocytes. Inflammation. 41:562–568.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hayashi D, Roemer FW and Guermazi A:
Imaging for osteoarthritis. Ann Phys Rehabil Med. 59:161–169.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mobasheri A and Batt M: An update on the
pathophysiology of osteoarthritis. Ann Phys Rehabil Med.
59:333–339. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Charlier E, Deroyer C, Ciregia F, Malaise
O, Neuville S, Plener Z, Malaise M and de Seny D: Chondrocyte
dedifferentiation and osteoarthritis (OA). Biochem Pharmacol.
165:49–65. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li H, Wang D, Yuan Y and Min J: New
insights on the MMP-13 regulatory network in the pathogenesis of
early osteoarthritis. Arthritis Res Ther. 19(248)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wu W, Billinghurst RC, Pidoux I, Antoniou
J, Zukor D, Tanzer M and Poole AR: Sites of collagenase cleavage
and denaturation of type II collagen in aging and osteoarthritic
articular cartilage and their relationship to the distribution of
matrix metalloproteinase 1 and matrix metalloproteinase 13.
Arthritis Rheum. 46:2087–2094. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hollander AP, Pidoux I, Reiner A, Rorabeck
C, Bourne R and Poole AR: Damage to type II collagen in aging and
osteoarthritis starts at the articular surface, originates around
chondrocytes, and extends into the cartilage with progressive
degeneration. J Clin Invest. 96:2859–2869. 1995.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mehana EE, Khafaga AF and El-Blehi SS: The
role of matrix metalloproteinases in osteoarthritis pathogenesis:
An updated review. Life Sci. 234(116786)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xia B, Di Chen Zhang J, Hu S, Jin H and
Tong P: Osteoarthritis pathogenesis: A review of molecular
mechanisms. Calcif Tissue Int. 95:495–505. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhou K, Hu L, Liao W, Yin D and Rui F:
Coptisine prevented IL-β-induced expression of inflammatory
mediators in chondrocytes. Inflammation. 39:1558–1565.
2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Berenbaum F: Osteoarthritis as an
inflammatory disease (osteoarthritis is not osteoarthrosis!).
Osteoarthritis Cartilage. 21:16–21. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
de Visser HM, Sanchez C, Mastbergen SC,
Lafeber FPJG, Henrotin YE and Weinans H: Fib3-3 as a biomarker for
osteoarthritis in a rat model with metabolic dysregulation.
Cartilage. 10:329–334. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lee HJ, Lee SM, Moon YG, Jung YS, Lee JH,
Venkatarame Gowda Saralamma V, Kim YS, Pak JE, Lee HJ, Kim GS and
Heo JD: Membrane-free stem cell components inhibit
interleukin-1α-stimulated inflammation and cartilage degradation in
vitro and in vivo: A rat model of osteoarthritis. Int J Mol Sci.
20(4869)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vilá S: Inflammation in osteoarthritis. P
R Health Sci J. 36:123–129. 2017.PubMed/NCBI
|
|
36
|
Goldring MB, Otero M, Plumb DA, Dragomir
C, Favero M, El Hachem K, Hashimoto K, Roach HI, Olivotto E, Borzì
RM and Marcu KB: Roles of inflammatory and anabolic cytokines in
cartilage metabolism: Signals and multiple effectors converge upon
MMP-13 regulation in osteoarthritis. Eur Cell Mater. 21:202–220.
2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zeng JZ, Ma LF, Meng H, Yu HM, Zhang YK
and Guo A: Effect of Ginkgo biloba extract on matrix
metalloproteinase-3 expression in a rat model of chondrocyte
injury. Genet Mol Res. 14:18280–18286. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Piecha D, Weik J, Kheil H, Becher G,
Timmermann A, Jaworski A, Burger M and Hofmann MW: Novel selective
MMP-13 inhibitors reduce collagen degradation in bovine articular
and human osteoarthritis cartilage explants. Inflamm Res.
59:379–389. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Rigoglou S and Papavassiliou AG: The NF-κB
signalling pathway in osteoarthritis. Int J Biochem Cell Biol.
45:2580–2584. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jimi E, Fei H and Nakatomi C: NF-κB
signaling regulates physiological and pathological chondrogenesis.
Int J Mol Sci. 20(6275)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
De Luca F: Role of nuclear factor kappa B
(NF-κB) in growth plate chondrogenesis. Pediatr Endocrinol Rev.
13:720–730. 2016.PubMed/NCBI
|
|
42
|
Robinson SM and Mann DA: Role of nuclear
factor kappaB in liver health and disease. Clin Sci (Lond).
118:691–705. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Dai W, Liang Z, Liu H, Zhao G and Ju C:
Lunasin abrogates the expression of matrix metalloproteinases and
reduction of type II collagen. Artif Cells Nanomed Biotechnol.
47:3259–3264. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Moqbel SAA, He Y, Xu L, Ma C, Ran J, Xu K
and Wu L: Rat chondrocyte inflammation and osteoarthritis are
ameliorated by madecassoside. Oxid Med Cell Longev.
2020(7540197)2020.PubMed/NCBI View Article : Google Scholar
|