Introduction
Xiaochaihu decoction is a traditional Chinese
medicine that is prepared from seven herbs (Bupleuri Radix,
Pinelliae Tuber, Scutellariae Radix, Zizyphi Fructus, Ginseng
Radix, Glycyrrhizae Radix, Zingiberis Rhizoma) (1), which has attracted increasing
attention as an alternative treatment and supplement. Xiaochaihu
decoction has been widely applied for the treatment of 15 types of
262 diseases, including diseases of the digestive, respiratory and
nervous system (2). To facilitate
the use of Xiaochaihu decoction in the clinical setting,
researchers have developed several dosage forms of Xiaochaihu
decoction, including granules, capsules, tablets and effervescent
tablets, which have been recorded in the 2015 edition of Chinese
Pharmacopoeia (Part I) (3). With
continuous experimental and clinical exploration, Xiaochaihu
decoction has been co-administered with various synthesised drugs
such as rabeprazole, isosorbide mononitrate, and entecavir for
digestive, cardiovascular, cerebrovascular and immune diseases,
respectively (4-6).
The concomitant administration of two or more drugs
may lead to drug-drug interactions (DDIs), which are considered a
common cause of adverse drug reactions (ADRs) (7). Previous studies have reported that
co-administration of Xiaochaihu decoction with tolbutamide
(8), carbamazepine (9) and cyclosporine A (CsA) (10) affects the in vivo efficacy of
the latter drugs. The interaction between Xiaochaihu decoction and
other concomitant drugs is a problem worthy of attention for basic
and clinical researchers.
Despite many reasons for DDIs, metabolic
interactions account for ~40% of them. Metabolic interactions are
often caused by enzymes involved in drug metabolism, mainly hepatic
enzymes in the cytochrome P450 (CYP) family. CYPs are mixed
functional oxidases involved in the metabolism of endogenous and
exogenous substances (11,12), and they can significantly affect
drug metabolism (13). Many drugs
act as substrates, inducers or inhibitors of CYPs. When a drug
induces or inhibits CYPs, it causes changes in the physiological
environment of another drug, consequently altering their efficacy
and even leading to toxicity (14).
Therefore, changes in the content or activity of CYPs by one or
more concomitant drugs are one of the main causes of DDIs in the
clinical setting. It is important to study the effects of drugs on
CYPs for understanding the interactions between drugs used in
combination.
With the continuous development as well as
improvements in the knowledge and understanding of traditional
Chinese medicine, Xiaochaihu decoction and other formulations would
be increasingly used in combination with synthesised drugs.
Xiaochaihu decoction in combination with other drugs may alter the
metabolism of the concomitant drug and lead to changes in the blood
levels of the drugs. It may further decrease the efficacy of drugs
or increase the likelihood of ADRs. To effectively obtain the
benefits and avoid the disadvantages of combination therapy to the
utmost, it is necessary to identify and confirm the mechanisms
underlying these interactions at the earliest. At present, there is
not sufficient experimental information on the effects of
Xiaochaihu decoction on CYPs. Therefore, further research is needed
to confirm the existing conclusions. Modern pharmacological
toxicology studies have shown that many drugs affect the gene
transcription and protein expression of CYPs (14,15).
Therefore, rats were selected to investigate the effects of
Xiaochaihu decoction on gene transcription and protein expression
of different subtypes of CYPs. The findings will help better
elucidate the effects of combining Xiaochaihu decoction with other
drugs that are metabolised by CYPs.
Materials and methods
Medicinal materials and reagents
The crude components of Xiaochaihu decoction,
including Bupleuri Radix, Pinelliae Tuber, Scutellariae Radix,
Zizyphi Fructus, Ginseng Radix, Glycyrrhizae Radix, and Zingiberis
Rhizoma, were all purchased from the Department of Pharmacy in the
Affiliated Hospital of Zunyi Medical University (Guizhou, China)
and identified as quality Chinese herb medicines by Professor
Jianwen Yang in Department of Pharmacognosy, Zunyi Medical
University. Rifampicin was purchased from Chengdu ALFA
Biotechnology Co., Ltd. Baicalin was purchased from Guizhou Dida
Technology Co., Ltd. All other reagents were obtained from local
reagent companies.
Preparation of Xiaochaihu
decoction
Xiaochaihu decoction was prepared according to the
proportion of Xiaochaihu granules in the Pharmacopoeia of the
People's Republic of China 2015 edition (3) and the optimal extraction scheme of
Xiaochaihu decoction reported by Cai et al (16). Briefly, 125 g Bupleuri Radix, 45 g
Pinelliae Tuber, 45 g Scutellariae Radix, 45 g Zizyphi Fructus, 45
g ginger, 45 g Codonopsis Pilosula and 45 g Glycyrrhizae Radix were
mixed with an 8-fold volume of distilled water evenly. After the
herbs were moistened thoroughly, the mixture was boiled at 100˚C
for 40 min and filtered through a gauze to obtain the filtrate.
Subsequently, the dregs were boiled with an 8-fold volume of
distilled water at 100˚C for 40 min and filtered again.
Subsequently, the filtrates were mixed together and concentrated to
a brown sticky extract (1 g/ml) in a rotary evaporator to obtain
the decoction for experiments. The decoctions were stored at
4˚C.
Rifampicin solution
Rifampicin powder was suspended in distilled water
and mixed in a vortex mixer to obtain rifampicin at a concentration
of 5 mg/ml prior to each administration.
Animals
Eighty specific pathogen-free (SPF) male SD rats
(220±20 g) for the experiments were provided by Liaoning Changsheng
Biotechnology Co., Ltd. [Animal license number of the rats was
SCXK: (Liaoning) 2015-0001]. All the rats were housed in
environmentally controlled conditions (temperature, 20-24˚C; and
relative humidity, 40-60%) with a 12-h light/dark cycle. Rats were
acclimated to the environment for 7 days before the experiments.
All animal experiments were strictly carried out in accordance with
the NIH guidelines for the Care and Use of Laboratory Animals (NIH
publications no. 80-23; revised 1996). The study protocol was
approved by the Animal Experimentation Ethics Committee of Zunyi
Medical University, China (approval no. ZMUER2014-2-069).
Experiment grouping
Eighty normal SPF SD male rats were randomly divided
according to their body weight into two groups corresponding to the
duration of administration (3 and 6 days). Each group of rats was
further divided into blank control (2 ml 5% sodium carboxymethyl
cellulose solution), positive control of CYP inducer (50 mg/kg/day
rifampicin), and experimental groups (Xiaochaihu decoction: Low
dose, 1.7 g/kg/day; medium dose, 3.4 g/kg/day; and high dose, 6.8
g/kg/day), with eight rats in each group. These drug doses given to
animals were selected primarily in reference to previous studies
(17,18) and pre-experimental results.
Intragastric administration and sample
extraction
The corresponding treatments were administered to
the rats in each group every morning. After the last dose, the rats
were fasted for 24 h and euthanised by cervical dislocation.
Animals were anesthetized via intraperitoneal injection with
pentobarbital sodium (60 mg/kg) prior to cervical dislocation. The
livers of the rats were excised, frozen in liquid nitrogen, and
stored in a refrigerator at -80˚C.
Quality control of Xiaochaihu
decoction by HPLC
Quality control of the Xiaochaihu decoction was
carried out by using high pressure liquid chromatography (HPLC) to
determine baicalin content in Xiaochaihu decoction. HPLC method was
adapted from previously established method (19). Briefly, by using an Agilent-1260
HPLC system (Agilent Technologies, Inc.) with a photodiode array
detector, the analysis was achieved through a TSK gel ODS C18
(250x4.6 mm; 5 µm; Tosoh) column with mobile phase of
methanol-water phosphoric acid (65:35:0.7) at a flow rate of 1.0
ml/min. The detection wavelength was set as 280 nm, the column
temperature as 30˚C, and the injection volume as 5 µl.
Total RNA isolation and reverse
transcription-quantitative (RT-q)PCR assays
Total RNA was isolated from rat liver tissue using
RNAiso Plus (Berkeley), and the concentration was quantified by
spectrophotometer (A260/A280). The RNA was reverse transcribed into
complementary DNA (cDNA) using a FlexCycler and the RevertAid First
Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions. Target gene
expression was analysed via qPCR system (Bio-Rad Laboratories,
Inc.) with SYBR Green PCR kit (Berkeley). The reactions for each of
the target mRNAs consisted of 25-µl volumes including 12.5 µl TB
Green Premix Ex Taq II (Takara Bio, Inc.), 1 µl specific forward
primer, 1 µl specific reverse primer, 2 µl cDNA and 8.5 µl sterile
water. A standard PCR amplification procedure was performed as
follow: 95˚C for 30 sec, followed by 40 cycles of 95˚C for 5 sec
and 60˚C for 30 sec. Analysis of each specimen for each target was
repeated three times. Gene transcription was analyzed by the
2-ΔΔCq method (20)
using β-actin expression as a reference. The forward and reverse
primer sequences used in this study are shown in Table I.
 | Table IPrimer sequences used for reverse
transcription-quantitative PCR reactions. |
Table I
Primer sequences used for reverse
transcription-quantitative PCR reactions.
| Gene | Forward (5' to
3') | Reverse (5' to
3') |
|---|
| CYP1A2 |
aggtcaaccatgatgagaagcagtg |
aggaggatggctaagaagaggaagac |
| CYP1B1 |
gagagttggtggcagtgttggtg |
ctcggcatcgtcgtggttgtac |
| CYP2D6 |
gtgctgccttcgctgaccatag |
tccagattcctcctcaagagtgtcc |
| CYP3A1 |
cgttcaccagtggaagactcaagg |
acttctttcacagggacaggtttgc |
| β-actin |
cacccgcgagtacaaccttc |
cccatacccaccatcacacc |
Western blot analysis
The protein levels of Cyp1a2, 1b1, 2d6
and 3a1 in rat livers were evaluated using western blotting
assay. Briefly, total protein was extracted from rat liver tissue
using RIPA lysis buffer (Beijing Solarbio Science & Technology
Co., Ltd.) containing with 1% proteinase inhibitor
phenylmethylsulfonyl fluoride (PMSF). After centrifugation, the
supernatant of the lysates was obtained. The protein concentration
was quantified using a bicinchoninic acid (BCA) assay kit (Beijing
Solarbio Science & Technology Co., Ltd.). The protein samples
(50 µg) were added to per lane, and separated on 10% sodium dodecyl
sulphate-polyacrylamide gels by electrophoresis and then
transferred onto polyvinylidene difluoride (PVDF) membranes. The
membranes were blocked with 5% non-fat milk at room temperature for
2 h. The primary antibodies GAPDH (1:5,000, cat. no. BL006B;
Biosharp life sciences Co., Ltd), CYP1A2 antibody (1:4,000, cat.
no. bs-2589R; Biosynthesis Biotechnology Co., Ltd.), CYP1B1
antibody (1:4,000; cat. no. bs-12926R; Biosynthesis Biotechnology
Co., Ltd.), CYP3A1 antibody (1:4,000, cat. no. bs-20586R;
Biosynthesis Biotechnology Co., Ltd.) and CYP2D6 (1:4,000, cat. no.
bs-1725R; Biosynthesis Biotechnology Co., Ltd.) were incubated at
4˚C overnight. Goat anti-rabbit IgG secondary antibody (1:5,000;
cat. no. BL003A; Biosharp Life Sciences) was added to the membranes
and incubated for 1 h at room temperature. The protein bands were
visualized using the ECL prime detection reagent (cat. no.
KGP1121-KGP1123; Nanjing KeyGen Biotech Co., Ltd.) and using a
ChemiDoc MP imaging system (Bio-Rad Laboratories, Inc.). Image Lab
version 6.0 gel imaging system software (Bio-Rad Laboratories,
Inc.) was used to analyze the gray value of the target strip.
Statistical analysis
SPSS20.0 (IBM Corp.) was used for statistical
analysis of all data; and the Kolmogorov-Smirnov test was used as a
distribution normality test. The comparison between groups was
evaluated using one-way analysis of variance (ANOVA). When variance
was equal, the last significant difference method was used for
multiple comparisons between groups. When assessed variance was not
equal, the Dunnett's T3 method was used for multiple comparisons
between groups. Results are presented as mean ± SE. P<0.05 was
considered to indicate a statistically significant difference.
Results
Quality control of Xiaochaihu
decoction
The HPLC chromatograms of reference substance and
baicalin in Xiaochaihu decoction are presented in Fig. 1, and the retention time of baicalin
in reference substance and in Xiaochaihu decoction were 4.87 and
4.85 min, respectively. As shown in Table II, the average content of baicalin
in Xiaochaihu decoction was 3.26 mg/ml, which was consistent with
previous results (3.39 mg/ml) (19), and the decoction could be used for
follow-up research.
 | Table IIContent of baicalin in Xiaochaihu
decoction (n=3). |
Table II
Content of baicalin in Xiaochaihu
decoction (n=3).
| Batch no. | Content, mg/ml | Average content,
mg/ml |
|---|
| 1 | 3.21 | |
| 2 | 3.27 | 3.26 |
| 3 | 3.30 | |
Effects of Xiaochaihu decoction on the
mRNA and protein expression of Cyp1a2 in the liver
Fig. 2 shows the
relative expression of Cyp1a2 mRNA in the liver tissue of
each group. Compared with that in the control, Cyp1a2 mRNA
was downregulated in the rifampicin group after 3 days of
administration (P<0.05) but slightly upregulated after 6 days of
administration. The results showed that rifampicin had no
significant effects on the expression of Cyp1a2 when
administered for a relatively longer time. Liver-specific
Cyp1a2 mRNA and protein expression levels were significantly
downregulated in the medium-dose treatment groups after 3 days
compared with those in the control group (P<0.01). In the
high-dose treatment group, there was a slight but insignificant
increase in Cyp1a2 mRNA and protein expression after 3 days
(P>0.05). After 6 days, Cyp1a2 was upregulated in the
medium-dose group and upregulated slightly in other groups
(P>0.05). These results showed that short-term administration of
Xiaochaihu decoction can slightly inhibit Cyp1a2 expression
in rats; however, this inhibitory effect disappeared with prolonged
administration.
Effects of Xiaochaihu decoction on
mRNA and protein expression of Cyp3a1 in the liver
Fig. 3 shows the
relative expression of Cyp3a1 mRNA in the livers of each
group. Cyp3a1 mRNA and protein levels in the liver were
increased in the rifampicin group after 6 days of administration
compared with those in the control. There was a significant
increase in Cyp3a1 mRNA expression in the medium-dose group
compared with that in the low-dose group. Furthermore, there was a
significant increase in Cyp3a1 mRNA expression in the
high-dose group compared with that in the medium-dose group.
Cyp3a1 protein levels were significantly higher in the
high-dose group than in the low- and medium-dose groups. Thus,
Xiaochaihu decoction was shown to induce Cyp3a1 mRNA and
protein expression in a dose-dependent manner.
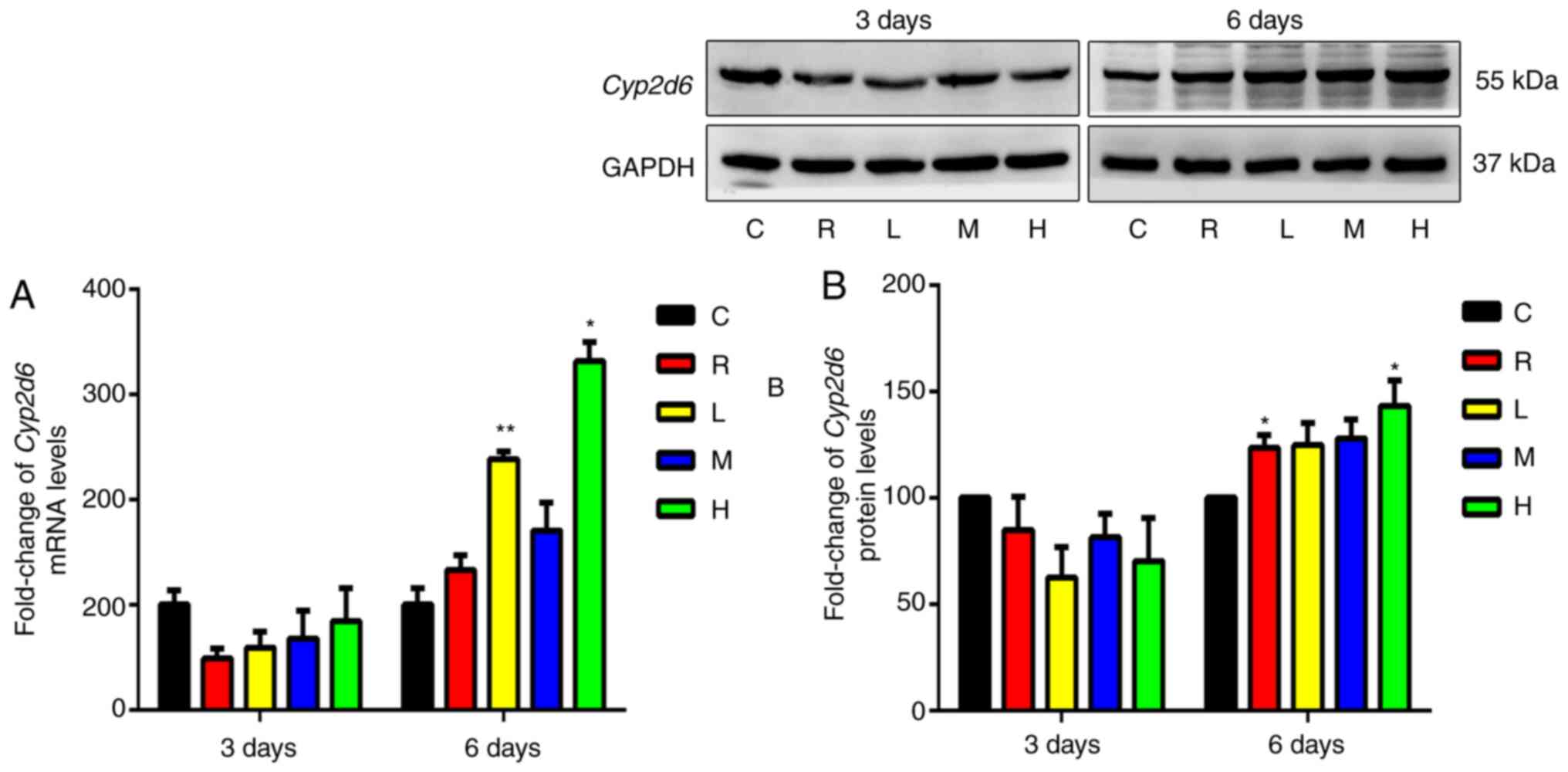
Effects of Xiaochaihu decoction on
mRNA and protein levels of Cyp2d6 in the liver
As shown in Fig. 4,
the differences in Cyp2d6 mRNA or protein expression between
the rifampicin or each Xiaochaihu group and the control after 3
days of administration were unchanged. After 6 days, Cyp2d6
mRNA and protein levels were mildly upregulated in the livers of
the rifampicin group. Cyp2d6 mRNA was significantly
upregulated in the livers of the low- and high-dose groups
(P<0.05). Cyp2d6 protein levels were significantly
upregulated in the livers of the high-dose group (P<0.05).
Therefore, Xiaochaihu decoction induced Cyp2d6 mRNA and
protein expression after administration for 6 days.
Effects of Xiaochaihu decoction on
mRNA and protein expression of Cyp1b1 in the liver
As demonstrated by Fig.
5, the differences in Cyp1b1 mRNA or protein levels
between the rifampicin for each Xiaochaihu group and the control
were not significant (P>0.05). Xiaochaihu decoction may not
affect the mRNA and protein expression of Cyp1b1.
Discussion
Although several modern formulations of Xiaochaihu,
including granules and tablets, have been developed from Xiaochaihu
decoction, and are widely used in clinical practice, the
concentrated decoction is still administered in many cases. To
better mimic the clinical use of drugs and consider the reference
value of the research results for different forms of dosage,
concentrated Xiaochaihu decoction was selected in the present
study.
The main reasons for selecting the drug doses given
to animals were selected primarily in reference to previous studies
(17,18); and pre-experimental results found
that low-dose Xiaochaihu decoction has slightly stronger induction
effect on Cyp1A2 and Cyp3A1 gene expression compared
with medium and high dose at the doses of 13.6, 6.8 and 3.4
g/kg/day. It was assumed that a lower dose of Xiaochaihu decoction
may also have a certain induction effect on some CYPs, and the
doses of Xiaochaihu decoction given to animals were decided as low
dose of 1.7, medium dose of 3.4 and high dose 6.8 g/kg/day for
subsequent experiments.
Scutellariae Radix is a main herb in the
prescription of Xiaochaihu decoction. As a traditional Chinese
herbal medicine, Scutellariae Radix exhibits important effects in
the treatment of various diseases, including emesis, hepatitis and
high blood pressure. Baicalin, the main bioactive ingredient in
Scutellariae Radix, exerts antidepressant (21), anti-inflammatory and antioxidant
effects (22). Therefore, baicalin
content is determined for quality control of many traditional
Chinese medicines containing Scutellariae Radix (23). In the Pharmacopoeia of the People's
Republic of China 2015 edition, baicalin content was specified as a
quality control parameter of Xiaochaihu granules (3). A quality control method for Xiaochaihu
decoctions in clinical use and experimental research was
established in the present study by referring to the quality
control methods used for Xiaochaihu granules in the Pharmacopoeia
of the People's Republic of China 2015 edition, and improving the
relevant chromatographic conditions (19). Xiaochaihu decoction prepared using
this method was uniform and quality was controllable.
Rifampicin is a broad-spectrum, classical,
non-specific inducer of hepatic enzymes, including CYP3A, 1A2, 2B6,
2C9, 2C19 and 2D6, and rifampicin has the greatest effects on the
expression of CYP3A in the liver (24). According to the US Food and Drug
Administration, rifampicin can be used as a strong index inducer of
CYP2B6 (moderate inducer), 2C8 (moderate inducer), 2C9 (moderate
inducer), 2C19, and 3A when evaluating drug interactions (25). For the consideration of animal
welfare (research on animals should involve the fewest number of
animals) and simplicity in interpretation of results, only
rifampicin was used as a positive drug for the different types of
CYPs in the experiments. This experiment design can be found in
some similar studies, in which only rifampicin or phenobarbital was
used as a positive control for different CYPs (26,27).
However, it is reasonable that using a positive control for
inhibition of hepatic enzymes may better reveal the different
effects of Xiaochaihu decoction on different CYPs. The lack of a
positive control for CYP inhibition is a limitation to the present
study.
The present results revealed that rifampicin induced
the gene and protein expression of Cyp3a1 after 6 days, and
the difference was significant. This is consistent with the
findings of a previous study in which rifampicin increased CYP3A4
expression (CYP3A4 in human was equivalent to CYP3A1 in rats)
(28). After 3 days of rifampicin
administration, the expression of Cyp1a2 gene was inhibited;
however, rifampicin had little effect on protein expression. The
uncoordinated regulation of Cyp1a2 gene and protein
expression in rats by rifampicin may be due to the regulation of
protein expression at the transcriptional and translational levels.
After 6 days of administration, rifampicin had little effect on the
expression of Cyp2d6 gene and protein. These results were
consistent with the findings by Rae et al (29) who showed that rifampicin had little
effect on CYP2D6 gene expression in primary human hepatocytes. A
previous study also found that rifampicin does not affect the gene
and protein expression of Cyp1b1 in rats (29). The present results indicated that
rifampicin had varying degrees of effects on the genes and proteins
of different subtypes of CYPs in rats, and that induction effects
varied with the duration of administration.
Effect of Xiaochaihu decoction on the gene
and protein expression of Cyp1a2 in rats
CYP1A2 accounts for ~13% of the total CYP enzyme
content in the liver, making it the third most abundant CYP in the
liver (28,30). CYP1A2 gene and its human counterpart
show a high homology of 80%. Additionally, amino acid homology
between CYP1A2 enzymes in humans and rats is 70% (31). CYP1A2 mediates 10% of clinical drug
metabolism (32), including in
vivo elimination of propranolol, clomipramine, phenacetin,
mexiletine, propanol, β-fluoroamine, verapamil and nifedipine
(13,33,34).
As shown in Fig. 2, short-term
administration of Xiaochaihu decoction inhibited the gene and
protein expression of Cyp1a2 in rats. This was consistent
with the result of the study by Saruwatari et al (35), in which Xiaochaihu decoction was
found to have a slight inhibitory effect on human CYP1A2 activity
using the probe drug method. It has also been reported that
astragalus, baicalein and baicalin have an inhibitory effect on
CYP1A2 activity (34-39).
The inhibitory effect of Xiaochaihu decoction on the gene and
protein expression of Cyp1a2 weakened with prolonged
administration. It may be speculated that some components in
Xiaochaihu decoction can slowly induce the expression of
Cyp1a2 gene and protein. Liquorice (a component in
Xiaochaihu decoction) can significantly increase the activity of
CYP1A2 (39-41).
The results showed that Xiaochaihu decoction had different effects
on Cyp1a2 gene and protein expression at different durations
of continuous administration. However, the inhibitory effects of
short-term administration of Xiaochaihu decoction need to be
considered when co-administered with the metabolic substrates of
CYP1A2, such as clomipramine, phenacetin, and theophylline, since
this inhibitory effect may increase the blood concentration of
concomitant drugs, as well as the risks of poisoning and other
ADRs.
Effect of Xiaochaihu decoction on the gene
and protein expression of Cyp3a1 in rats
CYP3A4 is one of the most important CYPs and
accounts for 60% of the total weight of CYP enzymes in human liver.
It participates in the metabolism of 50% of all drugs (13), including macrolide antibiotics,
imidazoles (antifungal medication), antivirals, rifamycins,
selective serotonin reuptake inhibitors, calcium antagonists,
β-hydroxy-β-methylglutaryl-CoA reductase inhibitors and
benzodiazepines. Cyp3a1 in rats is equivalent to CYP3A4 in
human. In the present study, short-term administration of low,
medium and high doses of Xiaochaihu decoction showed slight
inhibitory effects of varying degrees on the gene and protein
expression of Cyp3a1. Zhou et al (10) reported that a patient who had kidney
transplantation in the previous year experienced CsA poisoning when
Xiaochaihu granules were ingested concomitantly. Xiaochaihu
granules were assumed to inhibit the gene and protein expression of
CYP3A4, thus interfering with the metabolism of CsA and increasing
its blood concentration. Xiaochaihu decoction may inhibit the
expression of Cyp3a1 gene and protein because of the
inhibitory effects of some of its components on Cyp3a1. It
has been reported previously that both baicalein and astragalus
membranaceus extracts have inhibitory effects on CYP3A4 activity
(37,38).
After 6 days of continuous administration, low and
medium doses of Xiaochaihu decoction induced Cyp3a1 gene
expression, whereas the medium dose induced Cyp3a1 protein
expression. The long-term use of Xiaochaihu decoction was found to
induce Cyp3a1 gene and protein expression. This finding was
consistent with the study by Nose et al (42), who showed the induction of
Cyp3a1 gene by Xiaochaihu decoction in female SD rats. A
possible reason for this induction is that Xiaochaihu decoction
contains components that can induce the expression of Cyp3a1
gene and protein. As previously reported, saponins from Bupleurum
can induce Cyp3a1 in mice. A high-dose Bupleurum injection
(0.72 ml/kg) can induce the protein expression of CYP3A4 in mouse
liver (43), and liquorice can
significantly increase the enzymatic activity of CYP3A1/2 (37,41,44).
Therefore, when Xiaochaihu decoction is combined with the metabolic
substrates of CYP3A1, such as macrolide antibiotics and imidazoles,
the induction effects of Xiaochaihu decoction on CYP3A1 gene and
protein expression should be considered.
Effect of Xiaochaihu decoction on the gene
and protein expression of Cyp2d6 in rats
Although CYP2D6 only accounts for 2-4% of the total
CYP content in the liver, it is involved in 20-25% of drug
metabolism (45,46). It can metabolise more than 30 drugs,
such as dextromethorphan, statin, propranolol, codeine, selective
serotonin reuptake inhibitors, antiarrhythmic drugs and
antipsychotic drugs (47). In the
present study, after 6 days of continuous administration, high-dose
Xiaochaihu decoction induced Cyp2d6 gene and protein
expression. It is suggested that Xiaochaihu decoction may slowly
and dose dependently induce Cyp2d6 expression in rats
through its ingredients. Consistent with this finding, a previous
study reported that Xiaochaihu decoction can induce the expression
of Cyp2d6 (48). Thus, when
Xiaochaihu decoction is combined with the metabolic substrates of
CYP2D6, such as isoquinoline, dextromethorphan, propranolol,
statin, metoprolol and codeine, the induction of CYP2D6 expression
should be considered.
Effect of Xiaochaihu decoction on the gene
and protein expression of Cyp1b1 in rats
CYP1B1 accounts for 3% of drug metabolism and is
involved in the metabolic activation of 11% of precancerous
carcinogens, such as polycyclic aromatic hydrocarbons (32). It can affect the tumour sensitivity
of several chemotherapeutic drugs, such as paclitaxel, docetaxel
and cyclophosphamide (49). The
results of the present study showed that Xiaochaihu decoction does
not affect the expression of Cyp1b1 gene and protein in
normal rats. Thus, the effects of Xiaochaihu decoction on metabolic
enzymes may be not be relevant for drugs metabolised by CYP1B1.
Conclusions
The study revealed that Xiaochaihu decoction can
affect the expression of Cyp1a2, Cyp2d6 and Cyp3a1 in
rats in a dose- and/or time-dependent manner. Moreover, it was
found that there may be potential drug interactions when Xiaochaihu
decoction is concomitantly administered with substrates of
Cyp1a2, Cyp2d6 and Cyp3a1 (CYP1A2, CYP2D6 and CYP3A4
in humans).
Acknowledgements
The authors thank Ms Xue Lan in Zunyi Medical
University for Carrying out the quality control of the Xiaochaihu
decoction.
Funding
Funding: This study was supported by the Science and Technology
Foundation of Guizhou of China (grant no. Qian Kehe J word [2013]
2323), National Natural Science Foundation of China (grant no.
31460246), 2011 Collaborative Inovation Center of Guizhou
Traditional Chinese Medicine and Ethnic medicine (grant no.
Qianjiaokeyanfa [2012] 311), the Education Department of Guizhou
Province of China (grant nos. GNYL[2017]006 and YLXKJS-YS-05).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FT, YT and HL conceived and designed the
experiments; YT, HL, WW performed the experiments; HL, FT, CY
analyzed the data; and FT, HL and YT prepared the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal
Experimentation Ethics Committee of Zunyi Medical University of
China (approval no. ZMUER2014-2-069).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nishimura N, Uemura T, Iwamoto K and Naora
K: Change in tolbutamide permeability in rat jejunum and Caco-2
cells by Sho-saiko-to (Xiao Chai Hu Tang), a Chinese traditional
medicine. J Pharm Pharmacol. 62:651–657. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li JX, Cao Y, Zhang YW and Yang L:
Analysis of disease spectrum of Xiaochaihu decoction. J China Pres
Drug. 16:102–103. 2018.(In Chinese).
|
|
3
|
Chinese Pharmacopoeia Commission:
Pharmacopoeia of the People's Republic of China: One. Beijing:
China Medical Science Press, 2015: 574-576.
|
|
4
|
Liu CG and Du DH: Effects of Xiaochaihu
decoction combined with Lansoprazole on reflux esophagitis. Contemp
Med. 21:157–158. 2015.
|
|
5
|
Li WJ: Evaluation of the efficacy of
Xiaochaihu decoction in the treatment of angina pectoris. Res
Integr Tradit Chin West Med. 8:41–42. 2016.(In Chinese).
|
|
6
|
Lin JB, Zhang YG and Lin PJ: To analyze
the clinical effect of Xiaochaihu decoction combined with entecavir
on chronic hepatitis B. Guide China Med. 15:204–205. 2016.(In
Chinese).
|
|
7
|
Palleria C, Di Paolo A, Giofrè C, Caglioti
C, Leuzzi G, Siniscalchi A, De Sarro G and Gallelli L:
Pharmacokinetic drug-drug interaction and their implication in
clinical management. J Res Med Sci. 18:601–610. 2018.
|
|
8
|
Nishimura N, Uemura T, Iwamoto K and Naora
K: Effects of Sho-saiko-to on the pharmacokinetics and
pharmacodynamics of tolbutamide in rats. J Pharm Pharmacol.
50:231–236. 1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ohnishi N, Okada K, Yoshioka M, Kuroda K,
Nagasawa K, Takara K and Yokoyama T: Studies on interactions
between traditional herbal and western medicines. V. Effects of
Sho-saiko-to (Xiao-Cai-Hu-Tang) on the pharmacokinetics of
carbamazepine in rats. Biol Pharm Bull. 25:1461–1466.
2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhou Y, Chu XM, Ling SS, Cai WM and Wang
QW: Effects of oral administration of Xiaochaih granule on whole
blood cyclosporin A concentration in kidney transplantation
patients. Chin J Hosp Pharm. 19:713–714. 1999.(In Chinese).
|
|
11
|
Khojasteh SC, Driscoll JP, Jackson KD,
Miller GP, Mitra K, Rietjens IMCM and Zhang D: Novel advances in
cytochrome p450 research. Drug Discov Today. 16:793–799.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pillai VC, Strom SC, Caritis SN and
Venkataramanan R: A sensitive and specific Cyp cocktail assay for
the simultaneous assessment of human cytochrome p450 activities in
primary cultures of human hepatocytes using LC-MS/MS. J Pharm
Biomed Anal. 74:126–132. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zanger UM and Schwab M: Cytochrome p450
enzymes in drug metabolism: Regulation of gene expression, enzyme
activities, and impact of genetic variation. Pharmacol Ther.
138:103–141. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zanger UM, Turpeinen M, Klein K and Schwab
M: Functional pharmacogenetics/genomics of human cytochromes p450
involved in drug biotransformation. Anal Bioanal Chem.
392:1093–1108. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xiu LL, Yu X, Liu DN, Wang SR, Liu HY,
Chen SH and Chen F: Effects of different species of sargassum and
radix Glycyrrhizae in Haizao Yuhu decoction on cytochrome P450 in
goiter rats. China J Trad Chin Med Pharm. 32:2020–2025. 2017.(In
Chinese).
|
|
16
|
Cai H, Zhuang YS, Liu X and Cai BC:
Orthogonal optimization of extraction technology of Xiaochaihu
decoction. J Chin Med Mater. 35:2036–2039. 2012.(In Chinese).
|
|
17
|
Li J, Xie M and Gan Y: Effect of
Xiaochaihu decoction and different herbal formulation of component
on inhibiting H22 liver cancer in mice and enhancing immune
function. Zhongguo Zhong Yao Za Zhi. 33:1039–1044. 2008.PubMed/NCBI(In Chinese).
|
|
18
|
Wu FR, Ning LJ and Zhou R: Protective
effect of Xiaochaihu decoction chemical hepatic fibrosis in mice.
Chin J Clin Pharm Ther. 25:481–488. 2020.(In Chinese).
|
|
19
|
Lan X, Tang FS, Chen ZG, Zhang Y, Wang W
and Zhou XM: Determination of baicalin in Xiaochaihu decoction by
HPLC. J Zunyi Med Univ. 38:435–438. 2015.(In Chinese).
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhao F, Tao W, Shang Z, Zhang W, Ruan J,
Zhang C, Zhou L, Aiello H, Lai H and Qu R: Facilitating granule
cell survival and maturation in dentate gyrus with Baicalin for
antidepressant therapeutics. Front Pharmacol.
11(556845)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yang F, Feng C, Yao Y, Qin A, Shao H and
Qian K: Antiviral effect of baicalin on Marek's disease virus in
CEF cells. BMC Vet Res. 16(371)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li F, Wang WQ and You PJ: Advances in
extraction technology of flavonoids from radix astragali. Mod Chin
Med. 12:5–9. 2010.(In Chinese).
|
|
24
|
Niemi M, Backman JT, Fromm MF, Neuvonen PJ
and Kivistö KT: Pharmacokinetic interactions with rifampicin:
Clinical relevance. Clin Pharmacokinet. 42:819–850. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Huang SM, Temple R, Throckmorton DC and
Lesko LJ: Drug interaction studies: Study design, data analysis,
and implications for dosing and labeling. Clin Pharmacol Ther.
81:298–304. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mo CK, Xie YR, Chen YL, Huang M and He F:
The effect of Ginkgolide A on the expression of CYP450mRNA by
application of human primary hepatocyte. Pharm Today. 29:79–82.
2019.(In Chinese).
|
|
27
|
Li Q, Zhen YQ, Cao WL, Gao L, Wang YC,
Zhang XJ and Niu LY: Effects of saikosaponins and total glucosides
of paeony on CYP450 enzyme activity and liver function of rat liver
microsomes. Chin Tradit Pat Med. 4:3025–3028. 2019.(In
Chinese).
|
|
28
|
He M, Huang RB and Liu Z: Effects of
rifampicin on cytochrome P450 superfamily and related drugs. China
Pharm. 17:1664–1666. 2006.(In Chinese).
|
|
29
|
Rae JM, Johnson MD, Lippman ME and
Flockhart DA: Rifampin is a selective, pleiotropic inducer of drug
metabolism genes in human hepatocytes: Studies with cDNA and
oligonucleotide expression arrays. J Pharmacol Exp Ther.
299:849–857. 2001.PubMed/NCBI
|
|
30
|
Takeda K, Watanabe J, Inoue K and Kanamura
S: Rifampicin suppresses hepatic cyp2e1 expression and minimizes
DNA injury caused by carbon tetrachloride in perivenular
hepatocytes of mice. Alcohol Clin Exp Res. 24 (Suppl 4):87S–92S.
2000.PubMed/NCBI
|
|
31
|
Yue J, Peng RX, Yang J, Kong R and Liu J:
CYP2E1 mediated isoniazid-induced hepatotoxicity in rats. Acta
Pharmacol Sin. 25:699–704. 2004.PubMed/NCBI
|
|
32
|
Wu S, Yang HL, Li H and Sun ZX:
Prohibitive effect of processed polygonum multiflorum on CYP1A2
activities and mRNA expressions of rats. World Chin Med.
11:475–478-482. 2012.(In Chinese).
|
|
33
|
Pragyan P, Kesharwani SS, Nandekar PP,
Rathod V and Sangamwar AT: Predicting drug metabolism by CYP1A1,
CYP1A2, and CYP1B1: Insights from MetaSite, molecular docking and
quantum chemical calculations. Mol Divers. 18:865–878.
2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhou SF, Wang B, Yang LP and Liu JP:
Structure, function, regulation and polymorphism and the clinical
significance of human cytochrome P4501A2. Drug Metab Rev.
42:268–354. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Saruwatari J, Nakagawa K, Shindo J, Nachi
S, Echizen H and Ishizaki T: The in-vivo effects of sho-saiko-to, a
traditional Chinese herbal medicine, on two cytochrome P450 enzymes
(1A2 and 3A) and xanthine oxidase in man. J Pharm Pharmacol.
55:1553–1559. 2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wei LY, Zhang YJ, Wei BH, Ye J, Yang XY
and Sun GX: Effects of Coptis chinensis Franch, Scutellaria
baicalensis Georgi and their compatibility on five CYP450
subenzymes in rat liver microsomes. China J Chin Mater Med.
38:1426–1429. 2013.PubMed/NCBI(In Chinese).
|
|
37
|
Yao YC, Ni J, Han J, Feng LJ and Yan L:
Effects of Scutellaria extract on cytochrome P450 enzymes. Chin J
Exp Tradit Med Formul. 18:202–205. 2012.(In Chinese).
|
|
38
|
Tian S, He GR, Gao M, Sun JL, Wang FB and
Gui GH: Drug interaction potential of oral baicalein on metabolic
enzymes in rats. Chin J New Drug. 21:2230–2234+2301. 2012.(In
Chinese).
|
|
39
|
Huang K, Liu ZH and He Q: Inhibition of
Baicalin on activity of cytochrome P450 enzyme in rat and human
liver microsomes. Chin J Exp Tradit Med Formul. 22:20–23. 2016.(In
Chinese).
|
|
40
|
He YJ, Shi SY, Jin KT and Gao Y: The
modulation effect of Glycyrrhiza in combination with Euphorbia
pekinensis, Euphorbia kansui, and Daphne genkwa on the enzyme
activity of CYP1A2 in rat livers. Chin Remed Clin. 7:278–280.
2016.(In Chinese).
|
|
41
|
Xiao CR, Wang YG, Dai FG, Ma ZC, Tan HL
and Gao Y: The effect of aconitum coadministration with lilac
daphne on cytochrome P450 in rat liver. Chin J Exper Tradit Med
Formul. 12:48–50. 2006.(In Chinese).
|
|
42
|
Nose M, Tamura M, Ryu N, Mizukami H and
Ogihara Y: Sho-saiko-to and Saiko-keisi-to, the traditional Chinese
and Japanese herbal medicines, altered hepatic drug-metabolizing
enzymes in mice and rats when administered orally for a long time.
J Pharm Pharmacol. 55:1419–1426. 2003.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang YH, Qi JF and Lin M: Effect of total
saikosaponins on the intestinal first-pass effect and liver
Cyp3a,Cyp2el. Chin J Clin Pharm Ther. 16:740–748. 2011.(In
Chinese).
|
|
44
|
Cheng Y, Huang Y, Tian Y, Xu L, Liu GQ and
Zhang ZJ: Assessment of the effects of Radix Bupleuri and
vinegar-baked Radix Bupleuri on cytochrome 450 activity by a
six-drug cocktail approach. Chin J Nat Med. 11:302–308.
2013.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
45
|
Ingelman-Sundberg M: Genetic polymorphisms
of cytochrome P450 2D6 (CYP2D6): Clinical consequences,
evolutionary aspects and functional diversity. Pharmacogenomics J.
5:6–13. 2005.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kawakami M, Takenoshita-Nakaya S, Takeba
Y, Nishimura Y, Oda M, Watanabe M, Ohta Y, Kobayashi S, Ohtsubo T,
Kobayashi S, et al: Evaluation of CYP2D6 protein expression and
activity in the small intestine to determine its metabolic
capability in the Japanese population. Biol Pharm Bull.
40:1344–1351. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhou SF, Di YM, Chan E, Du YM, Chow VD,
Xue CC, Lai X, Wang JC, Li CG, Tian M and Duan W: Clinical
pharmacogenetics and potential application in personalized
medicine. Curr Drug Metab. 9:738–784. 2008.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Dai GF, Luo R, Wang YG, Xia CY, Tan HL,
Xiao CR, Zhao YH and Gao Y: Compatible effects of euphorbia and
glycyrrhiza on CYP2E1 expression and activity in rat liver. J Third
Mil Med Univ. 27:742–744. 2009.(In Chinese).
|
|
49
|
Liu MC, Meng QQ, Cui JH and Li JS: Recent
research progress on the CYP1B1 inhibitors. Prog Mod Biomed.
17:4190–4196. 2017.(In Chinese).
|