Introduction
The supplementation of drinking water with fluoride
is an established method to prevent dental caries (1). According to the Dean index, the
optimal concentration of fluoride in drinking water is 1 ppm
(2). Nevertheless, in In many areas
of the world, the natural fluoride levels in drinking water exceed
the recommended concentration (1).
In some areas, the natural fluoride levels in drinking water are
extremely high, such as in Colorado (11.2 mg/l), Oklahoma (12.0
mg/l), New Mexico (13.0 mg/l) and Idaho (15.9 mg/l) (3). With the development of dentistry,
fluoride has also been added to dental materials, including
toothpaste, varnish, foam and dental resin, to prevent the
occurrence of caries. All these fluoride additions increase the
morbidity of dental fluorosis (3),
which is currently a worldwide problem. A small ‘pea-sized’ amount
of toothpaste would contain 0.36-0.72 mg fluoride, which would
increase the risk of dental fluorosis in children (4). In America, the prevalence of dental
fluorosis in children aged 15-17 years increased from 22.6% in the
1986-1987 to 40.6% in the 1999-2004, which was provided by the
National Health and Nutrition Examination Survey (5). Although there are multiple causes of
dental fluorosis, the precise mechanism remains controversial
(6,7). The regulation of intracellular and
extracellular pH is an important mechanism that may lead to dental
fluorosis. According to the acid hypothesis (8), fluoride ions are unable to enter the
cell, whereas hydrogen fluoride (HF) can readily cross the cell
membrane under acidic conditions (9). An increased fluoride concentration in
the cytoplasm may induce oxidative stress by reducing the activity
of antioxidant enzymes, thereby affecting a variety of structures
and processes of normal cells due to reactive oxygen species
accumulation (8,10-12).
Furthermore, fluoride in the cytoplasm may induce endoplasmic
reticulum (ER) stress, including the phosphorylation of the
eukaryotic initiation factor 2 (eIF2), which may result in a
decrease in the overall protein production, including secretion of
the protease kallikrein 4 (KLK4) (8,13-15).
However, studies on fluoride transport have primarily used
fibroblasts and epithelial cells from the rat stomach and urinary
bladder, hamster cheek pouch and renal tubules of several species,
and very little research has been carried out in ameloblasts
(9,16). Sharma et al (13) reported that a low pH environment in
the maturation stage of ameloblasts facilitated the uptake of
fluoride. This hypothesis was tested by measuring changes in
stress-related genes and proteins. Fluorescent probes are a more
intuitive method to observe the accumulation of fluoride in
ameloblasts (17). However,
limitations remain in the majority of the reported fluorescent
fluoride probes in terms of determination and bioimaging (17-20).
For example, pyrene-based dye lies within the ultraviolet-visible
light range, increasing the difficulties in targeting fluoride in
living animals (17,18). Although the probes with the
lipophilic TBDPS moiety have high selectivity, their performance in
apparent optical signal changes to the naked-eye remains poor
(17,19,20).
To improve on these methods, a highly selective ratiometric visual
and red-emitting fluorescent dual-channel, probe 1, was developed.
The probe contains the hydrophilic compound
2-dicyanomethylene-3-cyano-4,5,5-trimethyl-2,5-dihydrofuran (TCF)
and the lipophilic tert-butyldiphenylsilyl (TBDPS); therefore, it
can enter cells easily (21,22).
Additionally, probe 1 possesses a latent internal charge transfer
structure, which makes it detect signals to the naked-eye (21,22).
Through cleavage of the Si-O bond, fluoride transforms probe 1 into
a red-emitting fluorescent form. Therefore, probe 1 reflects the
distribution of the fluoride ion may be used to determine fluoride
concentration (17,21,22).
The epithelial transport process of fluoride relies
on ion channels. To the best of our knowledge, no specialized
fluoride channels have been identified. It is generally accepted
that fluoride ions pass through the membrane via chloride channels
(23). There are differences in the
permeability and conductivity of fluoride between different
chloride channels (23). Simchowitz
(24). found that human neutrophils
were selective for haloid ions, and the order of affinity for these
ions was
Cl->Br->F->I-,
which were reverse-transported in a 1:1 ratio to extracellular
Cl-. Studies on the epithelium of the respiratory tract
have revealed that its chloride channels are highly selective for
anions and that their ionic permeability to halogens is in the
order of
I->Br-≥Cl->F-
(25,26). Additionally, Anderson et al
(27) demonstrated that the order
of selectivity of cystic fibrosis transcription factor for anions
was
Br->Cl->I->F-.
In mammals, several classes of chloride channels have been found,
including voltage-gated chloride channels (ClCs), the cystic
fibrosis transmembrane conductance regulator (CFTR),
calcium-activated chloride channels (CLCAs) and chloride
intracellular channels (CLICs) (28). There are nine ClCs, namely ClC-1 to
ClC-7, ClC-Ka, and ClC-Kb. ClCs-3-7 function as the main
Cl-/H+-exchangers, and facilitate vesicular
acidification by shunting currents of proton pumps and by
increasing vesicular Cl- concentration (29,30).
ClCs control the electrical excitability of muscles or neurons,
extra- and intracellular ion homeostasis and transepithelial
transport (30). Hou et al
(31) found that ClCs-1-7 were
expressed in tooth germs. ClC-5 has been reported in the dental
lamina, inner enamel epithelium, stratum intermedium, outer enamel
epithelium, odontoblasts and ameloblasts (32). CLCN7 mRNA has been reported in
ameloblasts at the maturation-stage (33). CFTR plays an important role in
transportation of bicarbonate into the enamel space to buffer
protons in ameloblasts and is located on the apical plasma membrane
during the maturation stage of ameloblasts (34). Lacruz et al (35) reported that the mRNA level of CFTR
in rat ameloblasts was higher at the early-mid and mid-late
maturation stages than at the secretory stage. Furthermore,
previous studies have indicated that CFTR is a critical factor in
the regulation of pH during the maturation of ameloblasts and is
essential for enamel mineralization (34,36,37).
Duan et al (38)
demonstrated that CFTR inhibition and treatment with CFTR siRNA may
increase intracellular pH. In our previous study, ClC-5 and ClC-7,
which are Cl-/H+-exchangers related to tooth
development and pH regulation were the focus (30).
As a member of the transforming growth factor-β
(TGF-β) superfamily, TGF-β1 regulates cellular biological
processes, such as proliferation, differentiation, apoptosis and
extracellular matrix (ECM) protein production (39). Furthermore, it plays an important
role in tooth development (32,40).
Duan et al (32) indicated
that ClC-5 regulates dentine development through the TGF-β1
pathway. Previous studies have revealed that fluoride affects
enamel protein content via TGF-β1-mediated KLK4 inhibition
(41,42). TGF-β1 may also impair the expression
and function of chloride ion channels in other epithelial cells,
including alveolar, bronchial, vas deferens and nasal polyp
epithelial cells (43-46).
However, whether the expression and function of chloride ion
channels in ameloblasts is regulated by TGF-β1 remains to be
elucidated.
In the present study the correlation between TGF-β1
and the intracellular fluoride concentration at a high
extracellular fluoride concentration was investigated. Furthermore,
the effects of exogenous TGF-β1 on ClC-5 and ClC-7 under a high
extracellular fluoride concentration were studied.
Materials and methods
Cell culture
The ameloblast-like cell line (LS8) was a gift from
Prof. Malcolm L. Snead (University of Southern California, USA),
and was cultured in DMEM (Corning, Inc.) supplemented with 10%
fetal bovine serum (FBS; Biological Industries) and antibiotics
(100 U/ml of penicillin and 100 µg/ml of streptomycin; Gibco;
Thermo Fisher Scientific, Inc.). The cells were incubated at 37˚C
in a 5% CO2 incubator and the medium was replaced with
fresh medium every 2 days. The experiments were performed in
triplicate.
Reverse transcription-quantitative PCR
(RT-qPCR)
The following groups of ameloblast-like LS8 cells
were established: Control; high fluoride, treated with a high
fluoride concentration (2.0 mM F-;Sigma-Aldrich; Merck
KGaA); TGF-β1, treated with 10 ng/ml TGF-β1 (Peprotech Inc.); and
FT, treated with 2.0 mM F- + 10 ng/ml TGF-β1. Cells were
plated into six-well plates at a density of 1x106
cells/ml and treated with different conditions as aforementioned
for 24 h. Total RNA of different groups were extracted using RNAiso
(Takara Bio, Inc.) according to the manufacturer's instructions.
cDNA was synthesized using the PrimeScript™ RT reagent kit with
gDNA Eraser using the temperature protocols of 37˚C for 15 min and
85˚C for 5 sec (Takara, Bio. Inc.). Mouse-specific primers for
GAPDH (47), TGF-β1, ClC-5 and
ClC-7, were used in conjunction with SYBR Premix Ex Taq™ II (Takara
Bio., Inc.) for RNA measurement. All qPCR reactions were conducted
under the following conditions: Hot start for 30 sec at 95˚C,
followed by 40 cycles of 5 sec at 95˚C and 30 sec at 60˚C. The
primer sequences used are presented in Table I. In the next step, the target mRNA
expression levels were determined using the LightCycler 480
Real-Time PCR System (Roche Diagnostics). The relative expression
of target genes was calculated using the 2-ΔΔCq method
(48). Each group was set with
three repeated wells and their mean values were calculated.
 | Table IPrimer sequences for reverse
transcription-quantitative PCR. |
Table I
Primer sequences for reverse
transcription-quantitative PCR.
| Gene | Forward sequence
(5'-3') | Reverse sequence
5'-3' |
|---|
| TGF-β1 |
CTTCAATACGTCAGACATTCGGG |
GTAACGCCAGGAATTGTTGCTA |
| ClC-5 |
GAGGAGCCAATCCCTGGTGTA |
TTGGTAATCTCTCGGTGCCTA |
| ClC-7 |
CGCCAGTCTCATTCTGCACT |
GAGGATCGACTTCCGGGTC |
| GAPDH |
TGACCTCAACTACATGGTCTACA |
CTTCCCATTCTCGGCCTTG |
Western blot analysis
Total protein was extracted from 60 mm dishes
(Corning, Inc.) containing the ameloblast-like LS8 cells with the
seeding density of 2x106 cells/ml and treated as
described in the previous section. The protein concentration of
each sample was determined with a bicinchoninic acid assay (Beijing
Solar Bio Science & Technology Co., Ltd.). To separate TGF-β1,
ClC-5 and ClC-7, equal amounts of proteins (40 µg) were loaded onto
10% SDS-PAGE gels and then the proteins were transferred onto PVDF
membranes. Membranes were blocked in Tris-buffered saline Tween
(hereafter called TBST) with 1% Tween and 5% non-fat dry milk for 1
h at room temperature and the membranes were labelled with
antibodies against TGF-β1, ClC-5 and ClC-7 at 4˚C overnight. The
probed membranes were washed with TBST three times and incubated
with appropriate secondary antibodies for 1 h at room temperature.
Protein bands were visualised using the enhanced chemiluminescence
(ECL, Thermo Fisher Scientific, Inc.) detection system Amersham
Imager 600 (GE Healthcare; Cytiva). The primary antibodies used
were all rabbit-derived and were anti-GAPDH at a ratio of 1:500
(cat. no. BA2913; Wuhan Boster Biological Technology Co., Ltd.),
anti-TGF-β1 at a ratio of 1:1,000 (cat. no. ab92486; Abcam),
anti-ClC-5 at a ratio of 1:1,000 (cat. no. ab188503; Abcam),
anti-ClC-7 at a ratio of 1:1,000 (cat. no. ab136016; Abcam).
Secondary antibodies used were horseradish
peroxidase-labelled-goat-derived anti-rabbit at a ratio of 1:5,000
(cat. no. BA1054; Wuhan Boster Biological Technology Co., Ltd.) and
horseradish peroxidase-labelled rabbit-derived anti-goat at a ratio
of 1:5,000 (cat. no. BA1060; Wuhan Boster Biological Technology
Co., Ltd.).
Intracellular fluoride detection
through confocal laser scanning microscopy
LS8 cells were seeded into 35 mm glass bottom dishes
(cat. no. P35G-1.5-14-C; MatTek Corporation) at a density of
4x105 cells/dish. After adhesion, cells were treated for
24 h, as described for RT-qPCR, before 10 µl the probe 1 was added
to each dish and the dishes maintained at room temperature for 30
min (49). The emitted fluorescence
intensity was determined using a confocal laser scanning microscope
(model no. LSM 780; Zeiss AG) at 543 nm. The mean fluorescence
intensities were calculated by ImageJ 1.52v. Probe 1 was provided
by Professor Baocun Zhu (Jinan University, Shandong, China).
Statistical analysis
All values are presented as the mean ± the standard
deviation (SD). Western blot and RT-qPCR results were analysed by
one-way ANOVA. Multiple comparisons between groups were performed
using the Tukey's test. P<0.05 was considered statistically
significant and P<0.01 values was considered highly
statistically significant.
Results
Fluoride treatment reduces TGF-β1
expression in LS8 cells
Fig. 1A indicates
the relative value (compared with the internal reference GAPDH) of
TGF-β1 mRNA expression. In the samples from the high fluoride
group, endogenous TGF-β1 mRNA expression levels were significantly
lower than those of the control group. Fig. 1B indicates the relative value
(compared with the internal reference GAPDH) of TGF-β1 protein
expression. Consistent with the RT-qPCR results, endogenous TGF-β1
protein expression in the high fluoride group was significantly
lower than that of the control group.
Fluoride increases the expression
levels of ClC-5 and ClC-7 in LS8 cells
Fig. 2A indicates
the relative values (compared with the internal reference GAPDH) of
ClC-5 and ClC-7 mRNA expression levels. In the high fluoride group,
ClC-5 and ClC-7 mRNA expression levels were significantly higher
than those of the control group. Fig.
2B indicates the relative values (compared with the internal
reference GAPDH) of ClC-5 and ClC-7 protein expression. ClC-5
protein expression in the high fluoride group was significantly
higher than that in the control group. ClC-7 protein expression in
the high fluoride group was higher than that in the control
group.
Exogenous TGF-β1 reduces the
expression of ClC-5 and ClC-7 in LS8 cells
Fig. 3A illustrates
the relative values (compared with the internal reference GAPDH) of
ClC-5 and ClC-7 mRNA expression levels. In the high fluoride group
the ClC-5 and ClC-7 mRNA expression levels were significantly
higher than those of the control group. In the exogenous TGF-β1
group, ClC-5 and ClC-7 mRNA expression levels were significantly
lower than those of the control group. In the FT group, ClC-5 and
ClC-7 mRNA expression levels were significantly lower than those of
the high fluoride group. Fig. 3B
illustrates the relative values (compared with the internal
reference GAPDH) of ClC-5 and ClC-7 protein expression. In the high
fluoride group, ClC-5 protein expression was higher than that of
the control group. In the exogenous TGF-β1 group, ClC-5 protein
expression was significantly lower than that of the control group.
In the FT group, ClC-5 protein expression was lower than that of
the high fluoride group. In those in the high fluoride group, ClC-7
protein expression levels were significantly higher than that of
the control group. In the exogenous TGF-β1 group, ClC-7 protein
expression levels were lower than those of the control group. In
the FT group, ClC-7 protein expression levels were lower than those
of the high fluoride group.
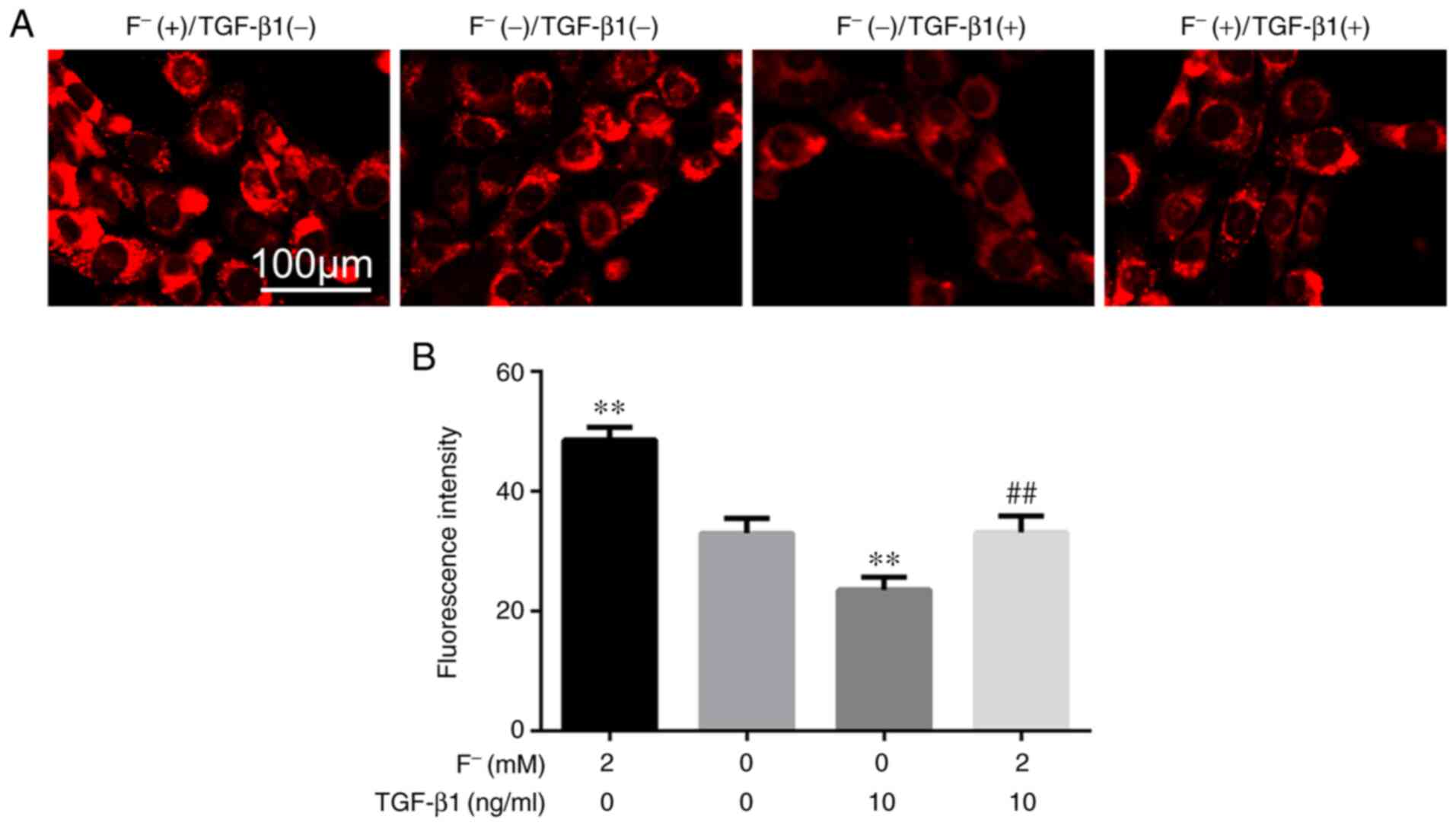
Exogenous TGF-β1 decreased the
fluorescence intensity of LS8 cells
Fig. 4 demonstrates
the fluorescence intensity of LS8 cells exposed to different
treatments. The fluorescence intensity of the high fluoride group
was significantly higher than that of the control group. The
fluorescence intensity of the exogenous TGF-β1 group was
significantly lower than that of the control group. In the FT
group, the fluorescence intensity was significantly lower than that
of the high fluoride group.
Discussion
The results of the present study indicated that a
high extracellular fluoride concentration had two effects on
ameloblasts, an increase in intracellular fluoride concentration
and an increase in ClC-5 and ClC-7 expression. Using probe 1, the
red fluorescence intensity of intracellular fluoride in the high
fluoride group was indicated to be stronger than that in the
control group. Fluoride cannot enter ameloblasts directly and must
be converted to HF beforehand (8).
Protons are released during the process of hydroxyapatite (HA)
deposition. Depending on the phosphate precursor, the precipitation
of HA releases 8-14 moles of hydrogen ions per mole of HA, which
acidifies the enamel matrix (8,13,50).
Additionally, a number of in vitro and in vivo
studies have proved that fluoride can accelerate crystal formation
and induce hypermineralized lines in secretory enamel (51,52).
Crystal growth produces a large number of protons, which can
acidify the microenvironment (53).
The low pH of the microenvironment promotes the conversion of
fluoride to HF. According to the Henderson-Hasselbalch equation,
more than 25-fold the amount of HF is produced at pH 6.0, compared
with that at pH 7.4 (8,13). Owing to the pH gradient, HF may
diffuse easily into the cytoplasm from the enamel matrix and revert
to fluoride ions in the neutral cytoplasm; consequently, it cannot
easily diffuse out of the cell (8,9,13,16).
Increased fluoride concentrations in the cytoplasm may induce
oxidative stress and endoplasmic reticulum stress, both of which
increase the occurrence of dental fluorosis (8,10-12,14,15).
In the present study, probe 1 was employed to
determine the intracellular fluoride concentration. Probe 1 is a
highly selective ratiometric visual and red-emitting fluorescent
dual-channel probe, which can detect fluoride in aqueous solution
and living systems (49). In
comparison to conventional technology, a ratiometric probe could
eliminate most or all ambiguities by self-calibration at two
wavelength intensities, which may avoid interference due to
instrumental stability, sample environments and probe distribution
(17,21,49,54).
Additionally, a ratiometric probe may display a large wavelength
shift (158 nm) for practical use in ratiometric determination. The
158 nm shift caused by fluoride changes the solution containing
probe 1 from yellow to blue (49).
Thus probe 1 may serve as a visual probe for fluoride because it
can be used to determine fluoride level changes by the naked-eye
(49). Additionally, probe 1 could
detect fluoride levels on the fluorescence spectrum, due to
considerable fluorescence enhancement at 612 nm caused by the
addition of fluoride (49).
Furthermore, the probe 1 is a rapid analytical method for the
detection of fluoride. Previous research suggested that the
fluorescence intensity at 612 nm increased with reaction time and
then leveled off at a reaction time >~25 min (49), which made probe 1 use easier. On the
basis of this finding 30 min was chosen as the time point to assess
the fluorescence intensity of fluoride using probe 1 in the present
study.
The second effect of fluoride on ameloblasts an
increase in ClC-5 and ClC-7 expression. As voltage-gated chloride
channels, ClC-5 and ClC-7 are responsible for exchanging
Cl- and H+ to maintain cell acidification.
High expression levels of ClC-5 promote the influx of fluoride into
the cytoplasm. ClC-7 localizes in vesicles of the
endocytotic-lysosomal pathway in different cell types (30). It has been indicated that during the
maturation stage of ameloblasts, the highest levels of ClC-7 are
localized in ameloblast vesicles (33,55).
Thus, the high expression of ClC-7 promotes the influx of fluoride
into the endosome/lysosome. This may induce toxicity in
ameloblasts, thereby leading to dental fluorosis (8,13).
CFTR stimulates the transport activity of Slc26a members, which
leads to bicarbonate efflux (37,56,57).
Additionally, CFTR is permeable to bicarbonate (37,58,59).
Although the results of previous studies have indicated that CFTR
is more permeable to Cl- than to bicarbonate, studies
have revealed that CFTR may be responsible for more than 50% of the
total bicarbonate efflux in pancreatic duct cells (37,53,58).
The low pH caused by HA deposition may lead to an increase in
expression of the electrolyte transporter responsible for the
efflux of bicarbonate (60,61). The released bicarbonate may
neutralize protons to produce a microenvironment that favors
crystal nucleation. However, in the present study, no significant
changes in CFTR were identified. This may be due to the use of
different cell types or low expression of CFTR in LS8 cells.
Further research is needed to better understand the mechanism
underlying CFTR expression. Duan et al (38) found that excess fluoride inhibited
endocytic activity of ameloblasts through the CFTR chloride channel
or other chloride channels. CFTR siRNA and CFTR-specific channel
inhibitor were employed to disturb the function of CFTR, which
could examine the effects on the transport activity in presence of
fluoride (38). Thus, future work
will assess the effects of ClCs inhibitors or silencing with siRNAs
on the expression and transport activity of ameloblasts.
TGF-β1 is distributed in most cells and is activated
through the release of latency-associated protein 1 and latent
TGF-β-binding protein 1 (62-64).
Active TGF-β1 may activate and affect organic homeostasis by
binding to the transforming growth factor-β receptor 1 (TGF-βR1)
through the TGF-β1-Smad signal pathway. Previous studies have shown
that fluoride may repress the expression of TGF-β1 (13,41,65,66)
and the present study confirmed this result. In the present study,
fluoride significantly reduced the expression levels of TGF-β1 in
ameloblasts at the gene and protein levels. TGF-β1 may also impair
the expression and function of chloride ion channels in other
epithelial cells, including alveolar, bronchial, vas deferens and
nasal polyp epithelial cells (43-46).
To investigate the relationship between TGF-β1 and chloride ion
channels, exogenous TGF-β1 was added to the culture medium. The
present results suggested a largely negative correlation between
them. Exogenous TGF-β1 significantly reduced the expression levels
of ClC-5 and ClC-7 in comparison with a control. When TGF-β1 and
high fluoride levels were added to the medium at the same time, the
increase in the expression levels of ClC-5 and ClC-7 caused by
fluoride was markedly reduced compared with high fluoride treatment
alone. These results suggested that the addition of exogenous
TGF-β1 may compensate for the changes in ClC-5 and ClC-7 caused by
the decrease of endogenous TGF-β1 induced by fluoride. With the
inhibition of ClC-5 and ClC-7 expression, the red fluorescence
intensity of intracellular fluoride was weaker compared with that
of the control group.
Both the decreased pH and the highly expressed ClC-5
and ClC-7 levels caused by the high fluoride concentration
accelerated the accumulation of fluoride in ameloblasts. If the
buffering capacity of ameloblasts is overwhelmed by an excessive
amount of fluoride, hypomineralization occurs and may cause dental
fluorosis. The present study may provide a new perspective for the
prevention of dental fluorosis through TGF-β-mediated reduction of
fluoride accumulation. The present study assessed the effect of
exogenous TGF-β1 on preventing the inflow of fluoride to
ameloblasts through regulation of ClC-5 and ClC-7 for 24 h. A
longer time course will be utilized in future studies.
Additionally, further studies are required to investigate the
precise mechanism by which the ions are transported. Studies will
also be conducted to confirm this hypothesis in vivo.
In conclusion, with the use of probe 1, it was
demonstrated that exogenous TGF-β1 may prevent the accumulation of
intracellular fluoride through the regulation of ClC-5 and ClC-7 in
ameloblasts under high extracellular fluoride concentrations.
Acknowledgements
The authors would like to thank Professor Malcolm L.
Snead (University of Southern California, USA) and Professor Baocun
Zhu (Jinan University, Shandong, China) for kindly donating LS8
cells and probe 1 respectively.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DZ and XD contributed to the conception and design
of the present study. MJ, XH and JS conducted all the experiments.
MJ and XD drafted the manuscript and revised it critically for
important intellectual content. MJ, XH and JS interpreted and
analyzed the data. MJ and XH were responsible for assessing the
authenticity of all the raw data. All authors read and approve the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Truman BI, Gooch BF, Sulemana I, Gift HC,
Horowitz AM, Evans CA, Griffin SO and Carande-Kulis VG: Task Force
on Community Preventive Services. Reviews of evidence on
interventions to prevent dental caries, oral and pharyngeal
cancers, and sports-related craniofacial injuries. Am J Prev Med.
23 (Suppl 1):S21–S54. 2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dean T, Jay P, Arnold F and Elvove E:
Domestic water and dental caries. II. A study of 2832 white
children, aged 12-14 years, of 8 suburban communities, including
Lactobacillus acidophilus studies of 1761 children. Public Health
Reports. 56:761–792. 1941.
|
|
3
|
Denbesten P and Li W: Chronic fluoride
toxicity: Dental fluorosis. Monogr Oral Sci. 22:81–96.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Institute of Medicine: Dietary reference
intakes for calcium, phosphorus, magnesium, Vitamin D, and
Fluoride. National Academies Press, Washington, DC, 1997.
|
|
5
|
Beltrán-Aguilar ED, Barker LK, Canto MT,
Dye BA, Gooch BF, Griffin SO, Hyman J, Jaramillo F, Kingman A,
Nowjack-Raymer R, et al: Surveillance for dental caries, dental
sealants, tooth retention, edentulism, and enamel fluorosis-United
States, 1988-1994 and 1999-2002. MMWR Surveill Summ. 54:1–43.
2005.PubMed/NCBI
|
|
6
|
Bawden JW, Crenshaw MA, Wright JT and
LeGeros RZ: Consideration of possible biologic mechanisms of
fluorosis. J Dent Res. 74:1349–1352. 1995.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Robinson C, Connell S, Kirkham J, Brookes
SJ, Shore RC and Smith AM: The effect of fluoride on the developing
tooth. Caries Res. 38:268–276. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sierant ML and Bartlett JD: Stress
response pathways in ameloblasts: Implications for amelogenesis and
dental fluorosis. Cells. 1:631–645. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kawase T and Suzuki A: Studies on the
transmembrane migration of fluoride and its effects on
proliferation of L-929 fibroblasts (L cells) in vitro. Arch Oral
Biol. 34:103–107. 1989.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mittal M and Flora SJ: Effects of
individual and combined exposure to sodium arsenite and sodium
fluoride on tissue oxidative stress, arsenic and fluoride levels in
male mice. Chem Biol Interact. 162:128–139. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jin XQ, Xu H, Shi HY, Zhang JM and Zhang
HQ: Fluoride-induced oxidative stress of osteoblasts and protective
effects of baicalein against fluoride toxicity. Biol Trace Elem
Res. 116:81–89. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Varol E, Icli A, Aksoy F, Bas HA, Sutcu R,
Ersoy IH, Varol S and Ozaydin M: Evaluation of total oxidative
status and total antioxidant capacity in patients with endemic
fluorosis. Toxicol Ind Health. 29:175–180. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sharma R, Tsuchiya M, Skobe Z, Tannous BA
and Bartlett JD: The acid test of fluoride: How pH modulates
toxicity. PLoS One. 5(e10895)2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sharma R, Tsuchiya M and Bartlett JD:
Fluoride induces endoplasmic reticulum stress and inhibits protein
synthesis and secretion. Environ Health Perspect. 116:1142–1146.
2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kubota K, Lee DH, Tsuchiya M, Young CS,
Everett ET, Martinez-Mier EA, Snead ML, Nguyen L, Urano F and
Bartlett JD: Fluoride induces endoplasmic reticulum stress in
ameloblasts responsible for dental enamel formation. J Biol Chem.
280:23194–23202. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
He H, Ganapathy V, Isales CM and Whitford
GM: pH-dependent fluoride transport in intestinal brush border
membrane vesicles. Biochim Biophys Acta. 1372:244–254.
1998.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jiao Y, Zhu B, Chen J and Duan X:
Fluorescent sensing of fluoride in cellular system. Theranostics.
5:173–187. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gai L, Chen H, Zhou B, Lu H, Lai G, Li Z
and Shen Z: Ratiometric fluorescence chemodosimeters for fluoride
anion based on pyrene excimer/monomer transformation. Chem Commun
(Camb). 48:10721–10723. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kim SY, Park J, Koh M, Park SB and Hong J:
Fluorescent probe for detection of fluoride in water and bioimaging
in A549 human lung carcinoma cells. Chem Commun (Camb).
21:4735–4737. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Ke B, Chen W, Ni N, Cheng Y, Dai C, Dinh H
and Wang B: A fluorescent probe for rapid aqueous fluoride
detection and cell imaging. Chem Commun (Camb). 49:2494–2496.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li Y, Zhang X, Zhu B, Yan J and Xu W: A
highly selective colorimetric and ‘off-on-off’ fluorescent probe
for fluoride ions. Anal Sci. 26:1077–1080. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhu B, Yuan F, Li R, Li Y, Wei Q, Ma Z, Du
B and Zhang X: A highly selective colorimetric and ratiometric
fluorescent chemodosimeter for imaging fluoride ions in living
cells. Chem Commun (Camb). 47:7098–7100. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dawson DC, Smith SS and Mansoura MK: CFTR:
Mechanism of anion conduction. Physiol Rev. 79 (Suppl 1):S47–S75.
1999.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Simchowitz L: Interactions of bromide,
iodide, and fluoride with the pathways of chloride transport and
diffusion in human neutrophils. J Gen Physiol. 91:835–860.
1988.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ran S, Fuller CM, Arrate MP, Latorre R and
Benos DJ: Functional reconstitution of a chloride channel protein
from bovine trachea. J Biol Chem. 267:20630–20637. 1992.PubMed/NCBI
|
|
26
|
Duszyk M, Liu D, Kamosinska B, French AS
and Man SF: Characterization and regulation of a chloride channel
from bovine tracheal epithelium. J Physiol. 489:81–93.
1995.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Anderson M, Gregory RJ, Thompson S, Souza
DW, Paul S, Mulligan RC, Smith AE and Welsh MJ: Demonstration that
CFTR is a chloride channel by alteration of its anion selectivity.
Science. 253:202–205. 1991.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jentsch TJ, Stein V, Weinreich F and
Zdebik AA: Molecular structure and physiological function of
chloride channels. Physiol Rev. 82:503–568. 2002.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Stauber T, Weinert S and Jentsch TJ: Cell
biology and physiology of CLC chloride channels and transporters.
Compr Physiol. 2:1701–1744. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Duan X: Ion channels, channelopathies, and
tooth formation. J Dent Res. 93:117–125. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hou J, Situ Z and Duan X: ClC chloride
channels in tooth germ and odontoblast-like MDPC-23 cells. Arch
Oral Biol. 53:874–878. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Duan X, Mao Y, Yang T, Wen X, Wang H, Hou
J, Xue Y and Zhang R: ClC-5 regulates dentin development through
TGF-beta1 pathway. Arch Oral Biol. 54:1118–1124. 2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lacruz RS, Brookes SJ, Wen X, Jimenez JM,
Vikman S, Hu P, White SN, Lyngstadaas SP, Okamoto CT, Smith CE and
Paine ML: Adaptor protein complex 2-mediated, clathrin-dependent
endocytosis, and related gene activities, are a prominent feature
during maturation stage amelogenesis. J Bone Miner Res. 28:672–687.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wright JT, Kiefer CL, Hall KI and Grubb
BR: Abnormal enamel development in a cystic fibrosis transgenic
mouse model. J Dent Res. 75:966–973. 1996.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lacruz RS, Smith CE, Moffatt P, Chang EH,
Bromage TG, Bringas P Jr, Nanci A, Baniwal SK, Zabner J, Welsh MJ,
et al: Requirements for ion and solute transport, and pH regulation
during enamel maturation. J Cell Physiol. 227:1776–1785.
2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sui W, Boyd C and Wright JT: Altered pH
regulation during enamel development in the cystic fibrosis mouse
incisor. J Dent Res. 82:388–392. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bronckers A, Kalogeraki L, Jorna HJ, Wilke
M, Bervoets TJ, Lyaruu DM, Zandieh-Doulabi B, Denbesten P and de
Jonge H: The cystic fibrosis transmembrane conductance regulator
(CFTR) is expressed in maturation stage ameloblasts, odontoblasts
and bone cells. Bone. 46:1188–1196. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Duan X, Mao Y, Wen X, Yang T and Xue Y:
Excess fluoride interferes with chloride-channel-dependent
endocytosis in ameloblasts. J Dent Res. 90:175–180. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Maeda H, Wada N, Tomokiyo A, Monnouchi S
and Akamine A: Prospective potency of TGF-β1 on maintenance and
regeneration of periodontal tissue. Int Rev Cell Mol Biol.
304:283–367. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhao H, Oka K, Bringas P, Kaartinen V and
Chai Y: TGF-beta type I receptor Alk5 regulates tooth initiation
and mandible patterning in a type II receptor-independent manner.
Dev Biol. 320:19–29. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Suzuki M, Shin M, Simmer JP and Bartlett
JD: Fluoride affects enamel protein content via TGF-β1-mediated
KLK4 inhibition. J Dent Res. 93:1022–1027. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Den Besten PK: Mechanism and timing of
fluoride effects on developing enamel. J Public Health Dent.
59:247–251. 1999.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Roux J, Carles M, Koh H, Goolaerts A,
Ganter MT, Chesebro BB, Howard M, Houseman BT, Finkbeiner W, Shokat
KM, et al: Transforming growth factor beta1 inhibits cystic
fibrosis transmembrane conductance regulator-dependent
cAMP-stimulated alveolar epithelial fluid transport via a
phosphatidylinositol 3-kinase-dependent mechanism. J Biol Chem.
285:4278–4290. 2010.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Snodgrass SM, Cihil KM, Cornuet PK,
Myerburg MM and Swiatecka-Urban A: Tgf-β1 inhibits Cftr biogenesis
and prevents functional rescue of ΔF508-Cftr in primary
differentiated human bronchial epithelial cells. PLoS One.
8(e63167)2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Yi S, Pierucci-Alves F and Schultz BD:
Transforming growth factor-β1 impairs CFTR-mediated anion secretion
across cultured porcine vas deferens epithelial monolayer via the
p38 MAPK pathway. Am J Physiol Cell Physiol. 305:C867–C876.
2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Pruliere-Escabasse V, Fanen P, Dazy AC,
Lechapt-Zalcman E, Rideau D, Edelman A, Escudier E and Coste A:
TGF-beta 1 downregulates CFTR expression and function in nasal
polyps of non-CF patients. Am J Physiol Lung Cell Mol Physiol.
288:L77–L83. 2005.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Faibish D, Suzuki M and Bartlett JD:
Appropriate real-time PCR reference genes for fluoride treatment
studies performed in vitro or in vivo. Arch Oral Biol. 62:33–42.
2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhu B, Kan H, Liu J, Liu H, Wei Q and Du
B: A highly selective ratiometric visual and red-emitting
fluorescent dual-channel probe for imaging fluoride anions in
living cells. Biosens Bioelectron. 52:298–303. 2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Smith CE, Chong DL, Bartlett JD and
Margolis HC: Mineral acquisition rates in developing enamel on
maxillary and mandibular incisors of rats and mice: Implications to
extracellular acid loading as apatite crystals mature. J Bone Miner
Res. 20:240–249. 2005.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lyaruu DM, Medina JF, Sarvide S, Bervoets
TJ, Everts V, Denbesten P, Smith CE and Bronckers AL: Barrier
formation: Potential molecular mechanism of enamel fluorosis. J
Dent Res. 93:96–102. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Bronckers AL, Lyaruu DM, Jansen ID, Medina
JF, Kellokumpu S, Hoeben KA, Gawenis LR, Oude-Elferink RP and
Everts V: Localization and function of the anion exchanger Ae2 in
developing teeth and orofacial bone in rodents. J Exp Zool B Mol
Dev Evol. 312B:375–387. 2009.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ji M, Xiao L, Xu L, Huang S and Zhang D:
How pH is regulated during amelogenesis in dental fluorosis. Exp
Ther Med. 16:3759–3765. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Fan J, Hu M, Zhan P and Peng X: Energy
transfer cassettes based on organic fluorophores: Construction and
applications in ratiometric sensing. Chem Soc Rev. 42:29–43.
2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Leisle L, Ludwig CF, Wagner FA, Jentsch TJ
and Stauber T: ClC-7 is a slowly voltage-gated
2Cl(-)/1H(+)-exchanger and requires Ostm1 for transport activity.
EMBO J. 30:2140–2152. 2011.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH,
Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ and Muallem S:
Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell
Biol. 6:343–350. 2004.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Mount DB and Romero MF: The SLC26 gene
family of multifunctional anion exchangers. Pflugers Arch.
447:710–721. 2004.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ishiguro H, Steward MC, Naruse S, Ko SB,
Goto H, Case RM, Kondo T and Yamamoto A: CFTR functions as a
bicarbonate channel in pancreatic duct cells. J Gen Physiol.
133:315–326. 2009.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Shcheynikov N, Kim KH, Kim KM, Dorwart MR,
Ko SB, Goto H, Naruse S, Thomas PJ and Muallem S: Dynamic control
of cystic fibrosis transmembrane conductance regulator
Cl(-)/HCO3(-) selectivity by external Cl(-). J Biol Chem.
279:21857–21865. 2004.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Paine ML, Snead ML, Wang HJ, Abuladze N,
Pushkin A, Liu W, Kao LY, Wall SM, Kim YH and Kurtz I: Role of
NBCe1 and AE2 in secretory ameloblasts. J Dent Res. 87:391–395.
2008.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Zheng L, Zhang Y, He P, Kim J, Schneider
R, Bronckers AL, Lyaruu DM and DenBesten PK: NBCe1 in mouse and
human ameloblasts may be indirectly regulated by fluoride. J Dent
Res. 90:782–787. 2011.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Lawrence DA, Pircher R and Jullien P:
Conversion of a high molecular weight latent beta-TGF from chicken
embryo fibroblasts into a low molecular weight active beta-TGF
under acidic conditions. Biochem Biophys Res Commun. 133:1026–1034.
1985.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Munger JS, Harpel JG, Gleizes PE, Mazzieri
R, Nunes I and Rifkin DB: Latent transforming growth factor-beta:
Structural features and mechanisms of activation. Kidney Int.
51:1376–1382. 1997.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Oklu R and Hesketh R: The latent
transforming growth factor beta binding protein (LTBP) family.
Biochem J 352 Pt. 3:601–610. 2000.PubMed/NCBI
|
|
65
|
Suzuki M, Everett ET, Whitford GM and
Bartlett JD: 4-phenylbutyrate mitigates fluoride-induced
cytotoxicity in ALC cells. Front Physiol. 8(302)2017.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Zhang X, Zhang Y, Xi S, Cheng G and Guo X:
The effect of different fluoride concentrations on the expression
of transforming growth factor-beta1 in ameloblast of rat incisor.
Hua Xi Kou Qiang Yi Xue Za Zhi. 30:434–438. 2012.PubMed/NCBI(In Chinese).
|