Introduction
Systemic lupus erythematosus (SLE) is a systemic
autoimmune disease that may injure the kidneys, joints, blood
vessels, lungs, heart and skin (1).
Lupus nephritis (LN), inflammation of the kidney due to lupus, is
one of the most severe complications seen in patients with SLE.
Furthermore, 50-60% of patients with SLE exhibit renal symptoms
within 10 years of the onset of disease (2). LN is characterized by progressive
renal function decline, microscopic hematuria and proteinuria
(3).
The pathogenesis of LN is associated with multiple
factors, including sex, steroids and excess salt intake, which lead
to the malfunction of immune self-tolerance and the development of
autoimmunity (4,5). Evidence from both human and
experimental models supports the hypothesis that cytokine
dysregulation contributes to kidney diseases, including LN
(6,7). Among these cytokines, IL-12 is an
important mediator of immunity that has been implicated in the
pathogenesis of SLE. IL-12 is secreted by innate immune cells upon
microbial stimulation (8,9). Studies have demonstrated that IL-12 is
a pro-inflammatory cytokine that can promote T helper 1 (Th1) and T
follicular helper cell differentiation. Studies have also reported
that IL-12 is essential for cytotoxic T cell activation and
function (8,10). Additionally, studies have
demonstrated that IL-12 could be an effective therapeutic target
for psoriasis, Crohn's disease and rheumatoid arthritis (11,12).
Vom Berg et al (13) found
that inhibition of IL-12/IL-23 signaling reduced Alzheimer's
disease-like pathology and cognitive decline. An increase in IL-12
concentration has been observed in patients with SLE compared with
healthy controls, and this is positively associated with the SLE
disease activity index (SLEDAI), a clinical assessment of lupus
disease activity in the preceding 10 days (14). However, Huang et al (15) suggested that IL-12 expression is
reduced in patients with SLE in comparison with controls.
Therefore, the precise role of IL-12 in the pathogenesis of SLE
remains to be elucidated.
To understand the role of IL-12 in the pathogenesis
of LN, the serum levels of IL-12 in patients with SLE and mice were
determined. The lupus model mice were treated with recombinant
IL-12 or an anti-IL-12 antibody. It was revealed that serum levels
of IL-12 were markedly increased in patients with SLE. Exogenous
IL-12 exacerbated LN-like symptoms in lupus model mice.
Materials and methods
Patients and healthy controls
A total of 30 patients with SLE and 30 healthy
volunteers were recruited at Taizhou Hospital Affiliated to Nanjing
University of Chinese Medicine (Taizhou, China) between January
2018 and December 2019 for the present study. All patients were
diagnosed with SLE according to the criteria set out by the
American College of Rheumatology, which were revised in 1997, and
all underwent a renal biopsy prior to the study, in order to reveal
cases of LN according to the International Society of
Nephrology/Renal Pathology Society 2003 classification (16,17).
The inclusion criteria were as follows: i) Patients aged 18 years;
and ii) patients with complete documented data. The exclusion
criteria were as follows: i) Infections, malignancies and other
inflammatory diseases; and ii) lack of key information that
rendered evaluation impossible. The demographic and clinical
characteristics of the patients with SLE are shown in Table I. The patients with SLE were divided
into two groups according to their SLEDAI score (17): Inactive patients, <5; active
patients, ≥5. Complement 3 and 4 (C3 and C4) was determined by
biochemical analyzer (Hitachi, Ltd.). The anti-anti-nuclear
antibody (ANA) antibody ELISA kit was from Zeus Scientific Inc.
(cat. no. 2Z29001G). Patient serum was prepared with 1:21 dilution,
following which 100 µl sample was added to each well coated with
inactivated antigen and incubated for 60 min at 20-25˚C. After
washing, the horseradish peroxidase (HRP)-conjugated goat
anti-human IgG was added for 30 min at 20-25˚C. After washing,
3,3',5,5'-Tetramethylbenzidine substrate solution (TMB) was added
for 30 min at 20-25˚C. Finally, the stop solution (1 M
H2SO4, 0.7 MHCl) was added and absorbance was
measured using a microplate reader at a wavelength of 450 nm.
 | Table ICharacteristics of patients with
SLE. |
Table I
Characteristics of patients with
SLE.
| Characteristic | Patients with
SLE | Healthy
control | P-value |
|---|
| N | 30 | 30 | N/A |
| Age, years | 29.13±7.24 | 31.34±5.41 | >0.05 |
| Sex,
male/female | 3/27 | 5/25 | >0.05 |
| Disease duration,
months, mean ± SD (range) | 37.04±16.04
(1-234) | ND | N/A |
| SLEDAI | 8.71±3.12 | ND | N/A |
| Scr (mg/dl) | 1.69±0.22 | 1.36±0.19 | <0.05 |
| Proteinuria
(g/day) | 3.44±0.53 | ND | N/A |
| Serum albumin
(g/l) | 29.84±1.52 | 43.34±2.11 | <0.01 |
|
Anti-dsDNA+ (%) | 76.52 | ND | N/A |
|
Anti-ANA+ (%) | 86.44 | ND | N/A |
| C3(g/l) | 0.57±0.17 | 0.91±0.22 | <0.01 |
| C4(g/l) | 0.17±0.06 | 0.49±0.12 | <0.01 |
| Mean blood pressure
(mmHg) | 97.05±11.04 | 86.06±16.08 | >0.05 |
| Hb (g/dl) | 8.91±2.32 | 15.33±1.74 | <0.01 |
| Urine RBC count
(104/ml) mean ± SD (range) | 349.03±105.02
(2-2300) | ND | N/A |
The anti-double-stranded DNA (ds-DNA) ELISA kit was
from Zeus Scientific Inc. (cat. no. 2Z2881G). The serum samples
from each patient was prepared in a 1:21 dilution before 100 µl
diluted serum was added each well coated with inactivated antigen
and incubated for 30 min at 20-25˚C. After washing, the
HRP-conjugated goat anti-human IgG was added for 30 min at 20-25˚C.
After washing, TMB was added for 30 min at 20-25˚C. Finally, the
stop solution (1 M H2SO4, 0.7 MHCl) was
added. A microplate reader was used to read absorbance in each well
at a wavelength of 450 nm. The calibrator within this ELISA kit has
been assigned with both a correction factor for the generation of
index values and a calibrator value for the generation of unit
values. According to the interpretation of results of the anti-ANA
and anti-ds-DNA ELISA kits, the serum in each patient was defined
as negative (anti-ANA-, anti-ds-DNA-) or
positive (anti-ANA+, anti-ds-DNA+). The
anti-ANA+ (%) in Table I
was referring to patients with anti-ANA+ among all
patients. The Anti-dsDNA+ (%) in Table I was referring to patients with
Anti-dsDNA+ among all patients. Written informed consent
was obtained from each participant. The present study was approved
by the Ethics Committee of Taizhou Hospital Affiliated to Nanjing
University of Chinese Medicine (Taizhou, China).
Mice
In total, 36 female MRL/MpJ-Faslpr and
six female C57BL/6 mice were obtained from the Laboratory Animal
Center, Academy of Military Medical Sciences (Beijing, China).
MRL/MpJ-Faslpr mice develop an autoimmune disease
resembling SLE, including an increase in anti-double-stranded DNA
(anti-dsDNA) antibodies in the blood, and develop severe nephritis
(18). All the mice used in this
study were housed at room temperature (20-24˚C) and 45-60% humidity
in a 12-h light/dark cycle. Mice had access to diet and water ad
libitum. Animal experiments were performed in accordance with
the guidelines and approved by the Committee of Experimental Animal
Administration of Taizhou Hospital Affiliated to Nanjing University
of Chinese Medicine (Taizhou, China).
Recombinant IL-12 or anti-IL-12
antibody treatment of MRL/MpJ-Faslpr mice
Six female 16-week-old MRL/MpJ-Faslpr
mice (35-40 g) were injected intraperitoneally with murine
recombinant IL-12 (100 µg/kg body weight; cat. no. 419-ML-050/CF;
R&D Systems, Inc.) for 7 consecutive days. Six 16-week-old
MRL/MpJ-Faslpr mice of the same age (35-40 g)that
received an equal volume of PBS served as controls. For anti-IL-12
treatment, six female 16-week-old MRL/MpJ-Faslpr mice
were injected intraperitoneally with the anti-IL-12 antibody (5
µg/g body weight, cat. no. MAB419-100; R&D Systems, Inc.) once.
Six female mice of the same age received an equal volume of rat
IgG2a isotype antibody (cat. no. MAB005R, R&D Systems, Inc.)
served as controls.
ELISA for IL-12
Whole blood was collected from patients with SLE,
healthy controls and mice. The blood was immediately centrifuged at
500 x g for 5 min at 4˚C for isolation of serum. Subsequently, the
serum was stored at -80˚C. All serum samples were brought to room
temperature before the assay. Human (cat. no. D1200) and mouse
(cat. no. M1270) IL-12 ELISA kits were purchased from R&D
Systems, Inc. All experiments were performed according to the
manufacturer's protocols.
Anti-dsDNA antibody
Anti-dsDNA antibodies in serum from mice were
detected as previously described (19). Briefly, 96-well microtiter plates
were coated with 50 µg/ml calf thymus dsDNA (Sigma-Aldrich; Merck
KGaA) overnight at 4˚C. After blocking with 1%BSA (Sigma-Aldrich;
Merck KGaA) in PBS at room temperature for 1 h, 100-fold diluted
serum (100 µl) was added, followed by incubation at room
temperature for 2 h. Subsequently, goat anti-mouse antibody
conjugated to horseradish peroxidase (1:2,000; cat. no. SA00001-1;
Proteintech Group, Inc.) was added, followed by incubation at room
temperature for 1 h. Next, 3,3',5,5'-Tetramethylbenzidine substrate
solution (cat. no. B019-1-1; Nanjing Jiancheng Bioengineering
Institute)was added, and plates were incubated at room temperature
for 30 min. Finally, stop solution (1 M
H2SO4) was added and the absorbance in each
well was monitored at 450 nmin a microplate reader (ELX808; BioTek
China).
Serum creatinine level and proteinuria
analysis
The creatinine levels in the serum of mice were
measured using quantitative enzyme colorimetric kits (cat. no.
C011-2-1; Nanjing Jiancheng Bioengineering Institute) according to
the manufacturer's protocols. The random urine of the mice was
collected and urine protein was determined using a Bradford Protein
Assay Kit (Nanjing KeyGen Biotech Co., Ltd.). Urinary albumin was
determined using an Albumin Assay kit (cat. no. E038-1-1; Nanjing
Jiancheng Bioengineering Institute).
Histopathological analysis of kidney
samples
The mice were anesthetized with sodium pentobarbital
(200 mg/kg i.p.) before being sacrificed. The kidneys of the mice
were collected and fixed in 4% paraformaldehyde at room temperature
for 24 h. Subsequently, the kidneys were embedded in paraffin, cut
into 3-µm-thick sections, dehydrated in an ascending ethanol
gradient and xylene and stained with hematoxylin and eosin
(H&E, at room temperature for 5 min) according to standard
procedures.
For periodic acid-Schiff (PAS) staining, the PAS
staining kit was used according to manufacturer's protocol (cat.
no. D004-1-2; Nanjing Jiancheng Bioengineering Institute). The
sections were first dewaxed with xylene and rehydrated in a
descending ethanol gradient. The sections were then oxidized with
10 g/l period ate for 15 min at 20-25˚C. Next, the sections were
stained with Schiff's reagent for 45 min at 20-25˚C. The 20 g/l
methyl green was used for counterstaining for 15 min at 20-25˚C.
The histopathological analysis of the kidney samples was performed
by two pathologists in a blinded manner with an optical light
microscope (10 fields for each sample, magnification, x20, Olympus
BH-2; Olympus Corporation).
RNA extraction and reverse
transcription-quantitative PCR of IL-12
Total RNA was extracted from peripheral blood
mononuclear cells and kidney tissues of mice using an RNA isolation
kit (cat. no. 9108Q; Takara Bio, Inc.). The peripheral blood
mononuclear cells were isolated from mice by Ficoll-Paque density
gradient centrifugation (cat. no. LTS1092, Tianjin Haoyang
Biological Manufacture, Co., Ltd.) before the samples were
centrifuged at 400 x g for 20 min in 25˚C. The kidney homogenate
was grounded using glass homogenizer. Complementary DNA was
synthesized using PrimeScript™ RTMaster Mix kit (cat. no. RR036Q;
Takara Bio, Inc.) at 37˚C for 15 min and 85˚C for 5 sec. The IL-12
mRNA was detected using TB Green Premix Ex Taq (Tli RNase H Plus)
kit (Takara Bio, Inc.) in an Applied Biosystems 7500 PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The RT-qPCR
cycling program was set at one cycle of pre-denaturation at 95˚C
for 5 min, followed by 40 cycles at 95˚C for 10 sec and 60˚C for 1
min. The primers were synthesized by Takara Bio, Inc. The relative
expression of IL-12 was determined and normalized to the expression
of housekeeping gene GAPDH and calculated with the
2-∆∆Cq assay (20). The primers of mouse IL-12 were as
follows Forward, 5'-ACCCTGACCATCACTGTCAA-3' and reverse,
5'-GTGGAGCAGCAGATGTGAGT-3' and GAPDH forward,
5'-ACAACTTTGGCATTGTGGAA-3' and reverse,
5'-GATGCAGGGATGATGTTCTG-3'.
Statistical analysis
Data are presented as the mean ± SEM. For the
comparison of means between two groups, two-tailed unpaired t-tests
were performed. Categorical variables are presented as frequencies
and percentages. Fisher's exact test was used to compare
categorical variables, such as the sex distribution. Correlations
between values were analyzed using Spearman's test. All statistical
calculations were performed using GraphPad Prism software (version
7.0; GraphPad Software, Inc.). P<0.05 was considered to indicate
a statistically significant difference.
Results
Characteristics of patients with
SLE
A total of 30 patients diagnosed with SLE were
recruited in the present study. Table
I summarizes the sex, age, SLE disease history and clinical
symptoms of the patients. The mean age was 29.1±7.2 years (range,
21-57 years). The median SLE disease duration since diagnosis was
37 months (range, 1-234 months). The SLEDAI score was 7 (range,
4-23). Among the patients, 76.5% were anti-dsDNA-positive and 86.4%
were anti-antinuclear antibody-positive (Table I). All patients with SLE received
standard therapy, including steroids and immunosuppressive after
presentation in the hospital.
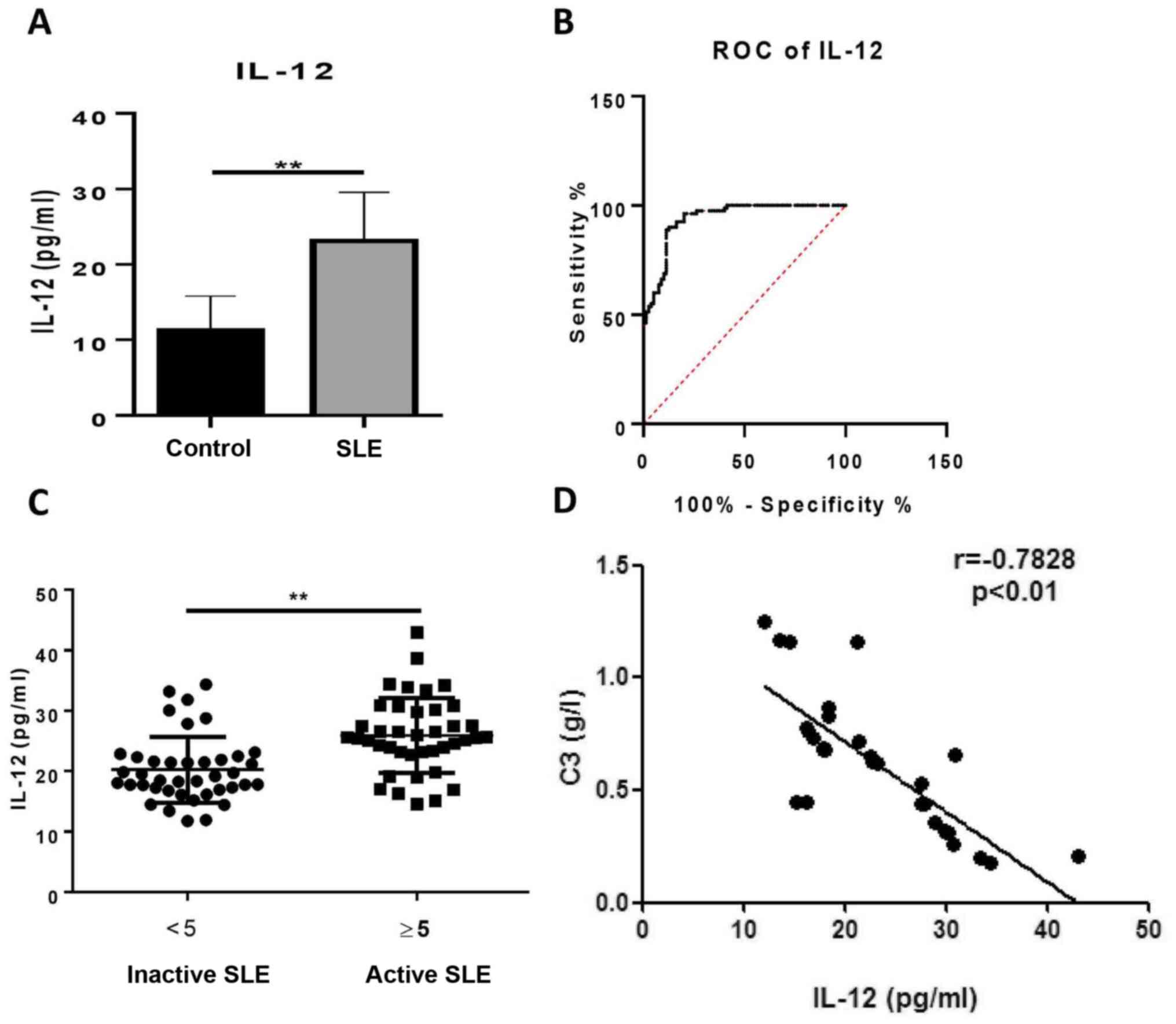
Serum levels of IL-12 are elevated in
patients with SLE
To determine whether IL-12 was involved in the
pathogenesis of SLE, the IL-12 levels in serum from patients with
SLE and healthy controls were measured. The patients with SLE had
significantly increased serum levels of IL-12 compared with healthy
controls (23.09±6.47 vs. 11.26±4.58 pg/ml; Fig. 1A). By constructing an empirical
receiver operating characteristic (ROC) curve, the optimal cut-off
value of 22.41 pg/ml was selected according to the Youden index,
which gave a sensitivity and specificity of 51.25 and 98.75 %,
respectively (Fig. 1B). The area
under the ROC curve was 0.9392. To determine the clinical
significance of IL-12, patients with SLE were divided into active
and inactive groups according to their SLEDAI score. The
correlation between serum IL-12 and SLEDAI was assessed, however no
significant correlation was identified (data not shown). It was
established that the levels of IL-12 in patients with active SLE
(25.93±6.21 pg/ml) were higher than those in patients with inactive
SLE (20.25±5.45 pg/ml; Fig. 1C). As
hypocomplementemia is one of the immunological abnormalities in
patients with SLE (3), the
associations among IL-12, C3 and C4 were evaluated. The results
revealed that serum levels of IL-12 were negatively associated with
C3 in patients with SLE (Fig. 1D),
whilst serum levels of IL-12 exhibited no significant association
with C4 (data not shown). The relationship between IL-12 and titers
of anti-dsDNA antibody was also analyzed. However, no significant
correlation was found (data not shown). These findings indicated
that serum levels of IL-12 may reflect the severity of symptoms in
patients with SLE.
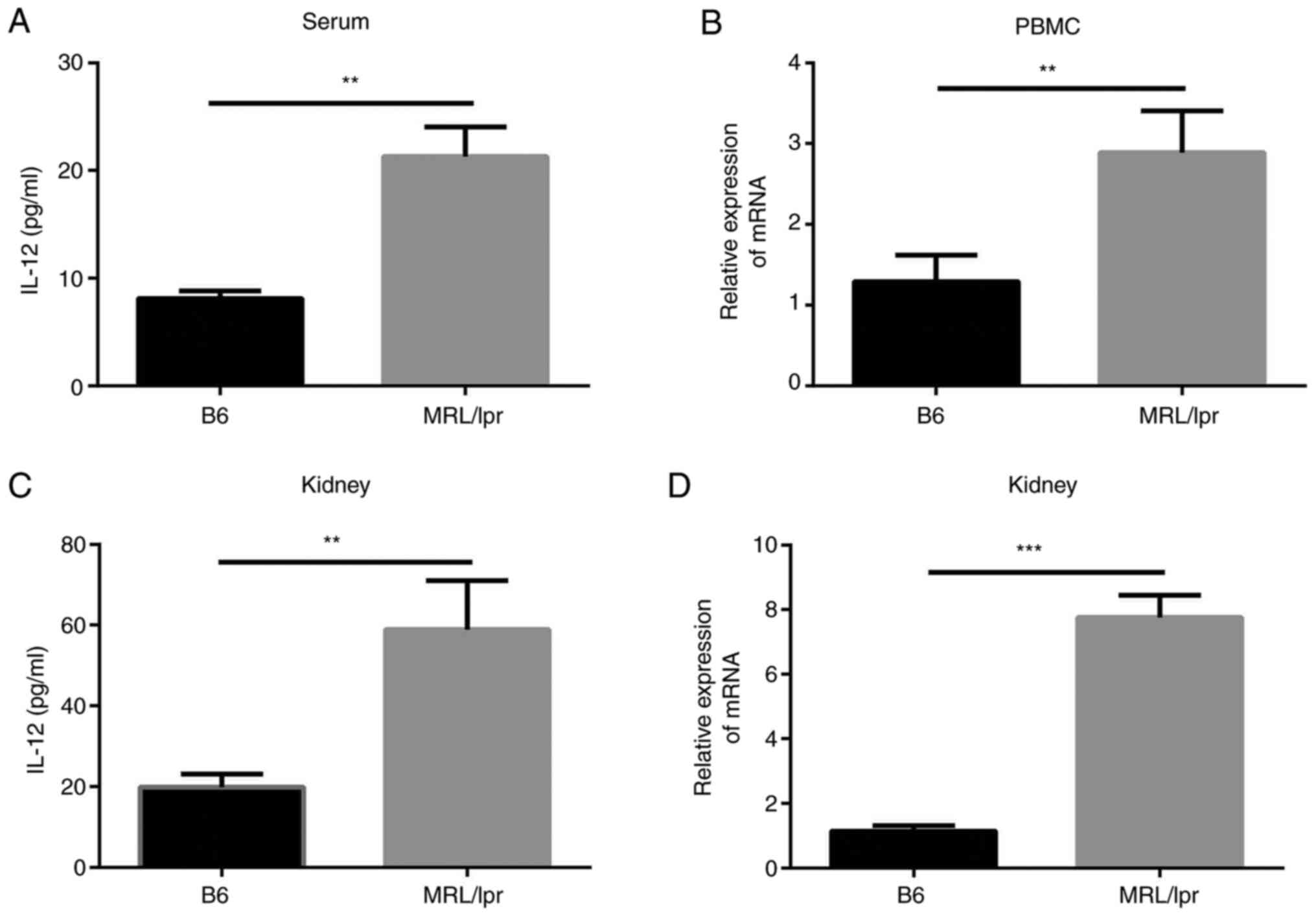
IL-12 levels are increased in a mouse
model of lupus
The levels of IL-12 in the lupus-model
MRL/MpJ-Faslpr mice were examined and compared with
those in control mice. It was identified that the serum levels of
IL-12 were markedly increased in the MRL/MpJ-Faslpr mice
(21.30±2.73 pg/ml) compared with control C57/BL6 mice (8.11±0.72
pg/ml; Fig. 2A). IL-12 mRNA
expression in the peripheral blood mononuclear cells of
MRL/MpJ-Faslpr mice was also increased compared with
that in control mice (Fig. 2B).
Subsequently, the mRNA and protein expression levels of IL-12 in
the kidneys of mice were examined. The results revealed that IL-12
levels in renal homogenate from MRL/MpJ-Faslpr mice
(58.94±6.99 pg/ml) were increased compared with those in control
mice (19.82±1.90 pg/ml; Fig. 2C).
Additionally, IL-12 mRNA expression in kidneys tissue from
MRL/MpJ-Faslpr mice was increased (Fig. 2D). These findings suggested that
IL-12 levels were increased in lupus-model
MRL/MpJ-Faslpr mice in comparison with controls.
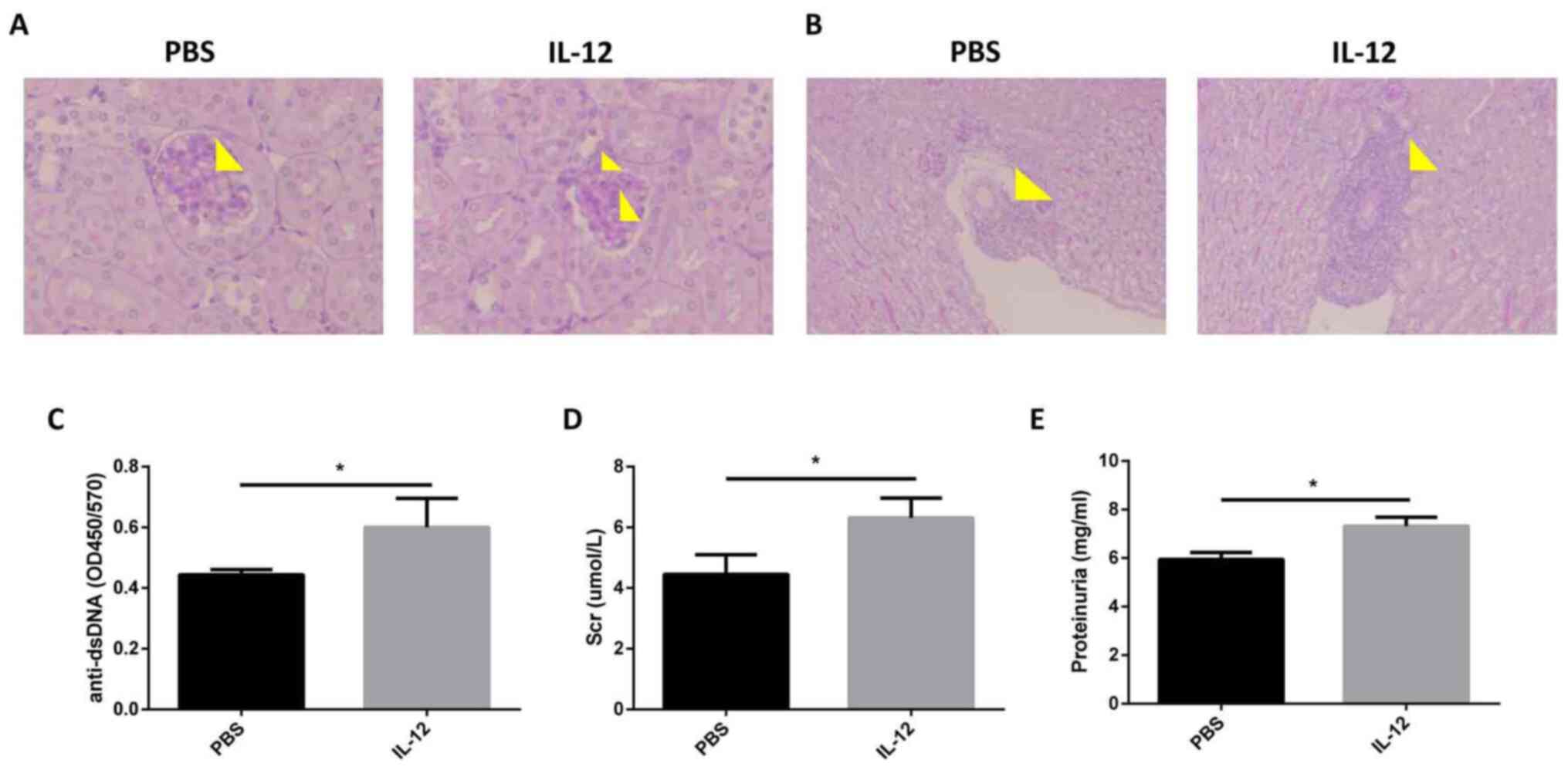
Exogenous IL-12 aggravates LN
MRL/MpJ-Faslpr mice (age, 16 weeks) were
injected with recombinant mouse IL-12. It was revealed that the
pathology of the kidney deteriorated following this injection, as
indicated by increased mesangial cell proliferation and mesangial
matrix deposition in glomeruli (Fig.
3A), and interstitial cellular infiltration (Fig. 3B). Levels of the anti-dsDNA
antibody, one of the hallmarks of SLE (2), were demonstrated to be increased
following exogenous IL-12 treatment in comparison with PBS
treatment (Fig. 3C). Compared with
control treated mice, the MRL/MpJ-Faslpr mice treated
with recombinant murine IL-12 exhibited higher levels of serum
creatinine (4.45±0.37 vs. 6.31±0.38 µmol/l) and proteinuria
(5.95±0.16 vs. 7.32±0.21 mg/ml; Fig.
3D and E). These results
highlighted that exogenous IL-12 treatment exacerbated the SLE-like
symptoms in MRL/MpJ-Faslpr mice.
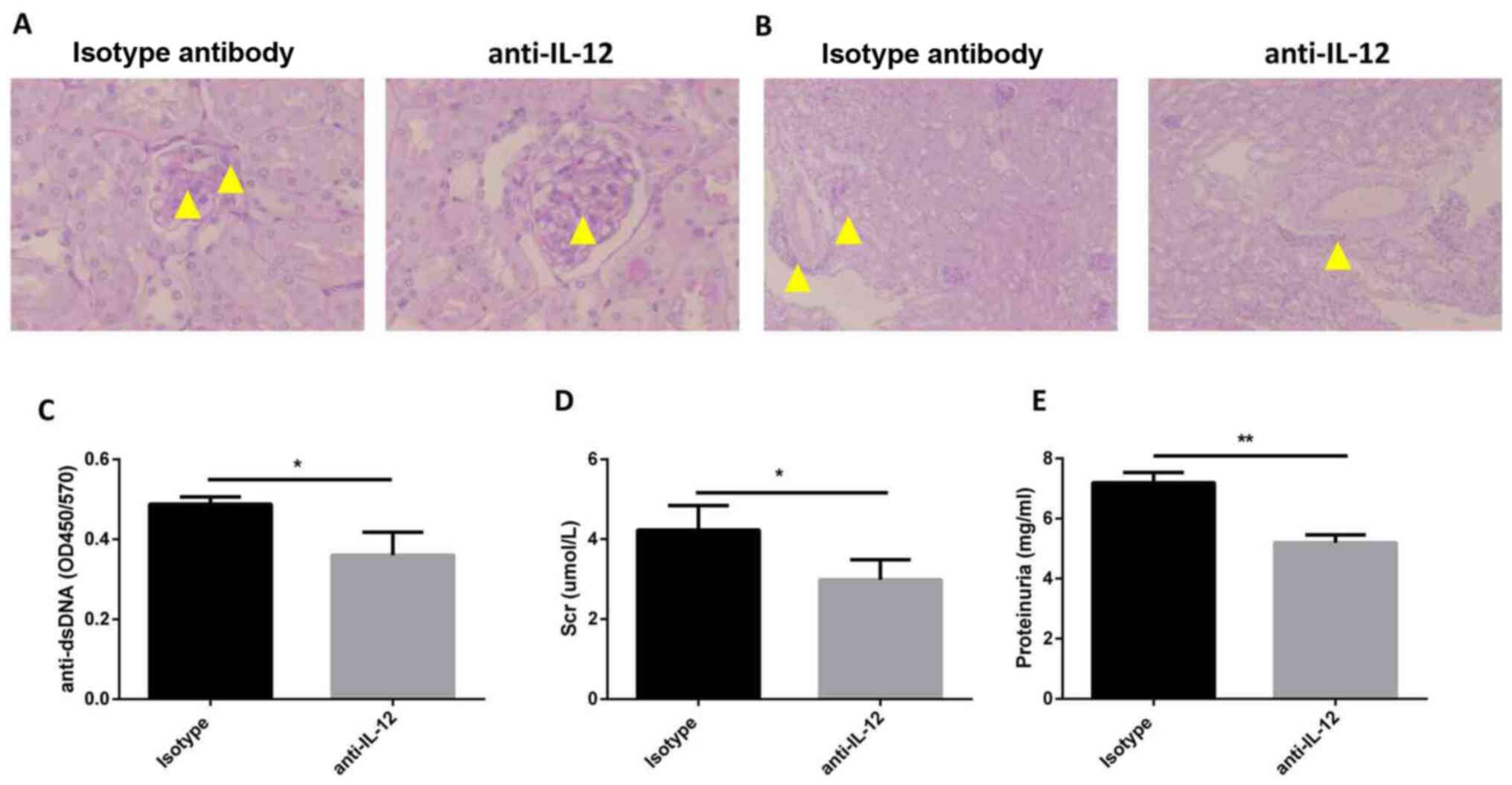
Anti-IL-12 antibody treatment improves
LN
To verify the effects of IL-12 on the pathogenesis
of SLE, MRL/MpJ-Faslpr mice were treated with an
anti-IL-12 antibody. The histological results demonstrated that
anti-IL-12 treatment alleviated the kidney injury, such as
mesangial cell proliferation and mesangial matrix deposition in
glomeruli (Fig. 4A), and
interstitial cellular infiltration (Fig. 4B). Additionally, the serum levels of
anti-dsDNA antibody were identified to be decreased in the
anti-IL-12 treatment group in comparison with the mice treated with
an isotype control (Fig. 4C).
Compared with that in mice in the isotype control group, the
concentration of serum creatinine was decreased in mice in the
anti-IL-12 treatment group (4.23±0.35 vs. 2.99±0.27 µmol/l;
Fig. 4D). In addition, the levels
of proteinuria were reduced in mice in the anti-IL-12 treatment
group in comparison with the isotype control (7.20±0.20 vs.
5.19±0.15 mg/ml; Fig. 4E). These
findings indicated that anti-IL-12 treatment ameliorated LN-like
symptoms in MRL/MpJ-Faslpr mice.
Discussion
The precise role of IL-12 in the pathogenesis of LN
is not fully understood (8,10). The present study revealed that serum
levels of IL-12 were increased in patients with lupus and in
lupus-model mice in comparison with controls. IL-12 reflected the
disease severity of patients with SLE using the SLEDAI score. The
present results suggested that IL-12 may serve an important role in
SLE. Several lines of experimental evidence supported this
conclusion. First, exogenous IL-12 treatment resulted in increased
SLE-like symptoms in MRL/MpJ-Faslpr mice in comparison
with controls. Second, blocking of IL-12 with a monoclonal
anti-IL-12 antibody improved SLE symptoms in
MRL/MpJ-Faslpr mice in comparison with controls.
SLE is a rheumatic disease characterized by loss of
self-tolerance, leading to the development of autoantibodies
against self-antigens. The autoantibodies and self-antigens form an
immune complex and induce organ damage (1,3).
Previous studies have demonstrated that a variety of cytokines are
involved in SLE (2,21). IL-12 is a pro-inflammatory cytokine,
largely produced by antigen-presenting cells (22). The pro-inflammatory capacity of
IL-12 is ascribed to promotion of Th1 cell differentiation.
Furthermore, IL-12 has been linked to innate immunity, as well as
the development of adaptive immunity characterized by the induction
of interferon-γ production (9).
IL-12 has been extensively studied in several animal models of
autoimmune diseases and cancer. Trembleau et al (23) demonstrated that IL-12 accelerates
the onset of autoimmune insulitis and diabetes. Studies have also
demonstrated that exogenous administration of IL-12 causes severe
manifestations of encephalomyelitis symptoms, while treatment with
an anti-IL-12 antibody could ameliorate experimental autoimmune
encephalomyelitis (24,25). Previous studies have revealed that
IL-12-deficient mice are protected from collagen-induced arthritis.
The exogenous administration of IL-12 aggravates the disease, while
inhibition of IL-12 by an anti-IL-12 antibody results in the
reduced severity of collagen-induced arthritis (26,27).
However, the roles and mechanisms of IL-12 in SLE remain
incompletely understood. Studies have demonstrated that IL-12
expression is elevated in the sera of patients with SLE
commensurate with disease activity (11,28).
The present results are consistent with these findings of higher
IL-12 levels in patients with SLE. However, a non-significant
correlation existed between serum IL-12 levels and the SLEDAI.
Patients were divided into two groups according to SLEDAI scores
(<5, inactive patients; ≥5, active patients). The IL-12 levels
in patients with inactive SLE were lower than those in patients
with active SLE, which suggested that IL-12 reflected the disease
severity of patients with SLE. Furthermore, the present study
revealed that the levels of IL-12 were negatively associated with
C3 in serum, which was consistent with the findings of Lauwerys
et al (29). However, Huang
et al (15) reported that
IL-12 specific subunit p35 mRNA expression in untreated and treated
patients with SLE was lower than that in healthy controls and
hypothesized that deficiency of IL-12 may contribute to the
pathogenesis of SLE. Although the reasons for these different
observations remain unknown, this may be because the mRNA
expression was assessed in their study, while protein expression
levels of IL-12 were determined in the present study.
The present study revealed that exogenous IL-12
treatment resulted in increased SLE-like symptoms in
MRL/MpJ-Faslpr mice in comparison with control
treatment. In line with the present findings, Segal et al
(30) found that administration of
IL-12 to aging mice reversed the Th1/T helper 2 cytokine profile
and, therefore, rendered them vulnerable to the induction of
experimental SLE. However, injection of cDNA encoding IL-12 has
been demonstrated to provoke limited amelioration in the
pathogenesis of SLE model mice (31). The differences between the study by
Neumann et al (31) and the
present study may be ascribed to anti-IL-12 activity induced by the
cDNA injection procedure, as the authors described.
In the present study, blocking of IL-12 with a
monoclonal anti-IL-12 antibody improved SLE-like symptoms in
MRL/MpJ-Faslpr mice. A previous study demonstrated that
MRL/MpJ-Faslpr mice with a genetic deficiency in IL-12
have reduced kidney pathology, diminished lymphadenopathy and
prolonged survival (32).
Therefore, these findings indicated that eliminating IL-12 may
reduce systemic pathology in lupus-model MRL/MpJ-Faslpr
mice. Increasing knowledge regarding IL-12 in SLE has led to the
development of a novel therapeutic strategy targeting IL-12. Phase
III clinical trials have demonstrated the efficacy of ustekinumab
(a fully human IgG1κ mAb directed against the p40 subunit of IL-12
and IL-23) in treating moderate to severe plaque psoriasis
(28). In a phase II randomized
controlled trial in patients with active SLE, ustekinumab was
superior to the placebo in terms of SLE Responder Index-4 response
after 24 weeks (25). However,
phase III clinical trials on the efficacy and safety of ustekinumab
in patients with SLE are required.
In summary, the present study demonstrated that an
increase in IL-12 reflected the disease severity in patients with
SLE and mouse models. Exogenous administration of IL-12 aggravated
manifestations of LN and treatment with neutralizing anti-IL-12
antibody ameliorated LN in lupus-model MRL/MpJ-Faslpr
mice in comparison with controls. The present data suggested that
blocking IL-12 may be a therapeutic strategy to halt the
progression of LN. However, there are certain unresolved questions
concerning the mechanisms of IL-12 in LN that need to be
addressed.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Taizhou Research and
Development Program (grant no. TS201809).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL and XS participated in study design, data
collection, data analysis, data interpretation and drafting the
paper. SW, XY and BL participated in patient recruitment, animal
experiments and data collection. XZ supervised the whole research,
designed the study, interpreted the data and wrote the paper. LL
and XZ assessed the authenticity of all the raw data to ensure its
legitimacy. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by Ethics Committee of
Taizhou Hospital Affiliated to Nanjing University of Chinese
Medicine (Taizhou, China). Written informed consents were obtained
from all individuals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anders HJ, Saxena R, Zhao MH, Parodis I,
Salmon JE and Mohan C: Lupus nephritis. Nat Rev Dis Primers.
6(7)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stokes MB and D'Agati VD: Classification
of lupus nephritis: Time for a change? Adv Chronic Kidney Dis.
26:323–329. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Davidson A, Aranow C and Mackay M: Lupus
nephritis: Challenges and progress. Curr Opin Rheumatol.
31:682–688. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Song K, Liu L, Zhang X and Chen X: An
update on genetic susceptibility in lupus nephritis. Clin Immunol.
210(108272)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yang X, Yao G, Chen W, Tang X, Feng X and
Sun L: Exacerbation of lupus nephritis by high sodium chloride
related to activation of SGK1 pathway. Int Immunopharmacol.
29:568–573. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Frangou E, Georgakis S and Bertsias G:
Update on the cellular and molecular aspects of lupus nephritis.
Clin Immunol. 216(108445)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tang Y, Zhang W, Zhu M, Zheng L, Xie L,
Yao Z, Zhang H, Cao D and Lu B: Lupus nephritis pathology
prediction with clinical indices. Sci Rep. 8(10231)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Trinchieri G: Interleukin-12 and the
regulation of innate resistance and adaptive immunity. Nat Rev
Immunol. 3:133–146. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Kang BY, Kim E and Kim TS: Regulatory
mechanisms and their therapeutic implications of interleukin-12
production in immune cells. Cell Signal. 17:665–673.
2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ma CS, Suryani S, Avery DT, Chan A, Nanan
R, Santner-Nanan B, Deenick EK and Tangye SG: Early commitment of
naïve human CD4(+) T cells to the T follicular helper (T(FH)) cell
lineage is induced by IL-12. Immunol Cell Biol. 87:590–600.
2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Teng MWL, Bowman EP, McElwee JJ, Smyth MJ,
Casanova JL, Cooper AM and Cua DJ: IL-12 and IL-23 cytokines: From
discovery to targeted therapies for immune-mediated inflammatory
diseases. Nat Med. 21:719–729. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Sandborn WJ, Gasink C, Gao LL, Blank MA,
Johanns J, Guzzo C, Sands BE, Hanauer SB, Targan S, Rutgeerts P, et
al: CERTIFI Study Group: Ustekinumab induction and maintenance
therapy in refractory Crohn's disease. N Engl J Med. 367:1519–1528.
2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vom Berg J, Prokop S, Miller KR, Obst J,
Kälin RE, Lopategui-Cabezas I, Wegner A, Mair F, Schipke CG, Peters
O, et al: Inhibition of IL-12/IL-23 signaling reduces Alzheimer's
disease-like pathology and cognitive decline. Nat Med.
18:1812–1819. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Ueno H: The IL-12-STAT4 axis in the
pathogenesis of human systemic lupus erythematosus. Eur J Immunol.
50:10–16. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Huang X, Hua J, Shen N and Chen S:
Dysregulated expression of interleukin-23 and interleukin-12
subunits in systemic lupus erythematosus patients. Mod Rheumatol.
17:220–223. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hochberg MC: Updating the American College
of Rheumatology revised criteria for the classification of systemic
lupus erythematosus. Arthritis Rheum. 40:1725–1726. 1997.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Weening JJ, D'Agati VD, Schwartz MM,
Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T,
Ferrario F, et al: International Society of Nephrology Working
Group on the Classification of Lupus Nephritis; Renal Pathology
Society Working Group on the Classification of Lupus Nephritis: The
classification of glomerulonephritis in systemic lupus
erythematosus revisited. Kidney Int. 65:521–530. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li W, Titov AA and Morel L: An update on
lupus animal models. Curr Opin Rheumatol. 29:434–441.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yao G, Qi J, Zhang Z, Huang S, Geng L, Li
W, Chen W, Tang X, Wang S and Sun L: Endothelial cell injury is
involved in atherosclerosis and lupus symptoms in
gld.apoE-/- mice. Int J Rheum Dis. 22:488–496.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-∆ ∆ C(T)) Method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Larosa M, Zen M, Gatto M, Jesus D, Zanatta
E, Iaccarino L, Inês L and Doria A: IL-12 and IL-23/Th17 axis in
systemic lupus erythematosus. Exp Biol Med (Maywood). 244:42–51.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zundler S and Neurath MF: Interleukin-12:
Functional activities and implications for disease. Cytokine Growth
Factor Rev. 26:559–568. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Trembleau S, Penna G, Gregori S,
Giarratana N and Adorini L: IL-12 administration accelerates
autoimmune diabetes in both wild-type and IFN-gamma-deficient
nonobese diabetic mice, revealing pathogenic and protective effects
of IL-12-induced IFN-gamma. J Immunol. 170:5491–5501.
2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Smith T, Hewson AK, Kingsley CI, Leonard
JP and Cuzner ML: Interleukin-12 induces relapse in experimental
allergic encephalomyelitis in the Lewis rat. Am J Pathol.
150:1909–1917. 1997.PubMed/NCBI
|
|
25
|
Constantinescu CS, Wysocka M, Hilliard B,
Ventura ES, Lavi E, Trinchieri G and Rostami A: Antibodies against
IL-12 prevent superantigen-induced and spontaneous relapses of
experimental autoimmune encephalomyelitis. J Immunol.
161:5097–5104. 1998.PubMed/NCBI
|
|
26
|
McIntyre KW, Shuster DJ, Gillooly KM,
Warrier RR, Connaughton SE, Hall LB, Arp LH, Gately MK and Magram
J: Reduced incidence and severity of collagen-induced arthritis in
interleukin-12-deficient mice. Eur J Immunol. 26:2933–2938.
1996.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Malfait AM, Butler DM, Presky DH, Maini
RN, Brennan FM and Feldmann M: Blockade of IL-12 during the
induction of collagen-induced arthritis (CIA) markedly attenuates
the severity of the arthritis. Clin Exp Immunol. 111:377–383.
1998.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Talaat RM, Mohamed SF, Bassyouni IH and
Raouf AA: Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus
erythematosus (SLE) patients: Correlation with disease activity.
Cytokine. 72:146–153. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lauwerys BR, Van Snick J and Houssiau FA:
Serum IL-12 in systemic lupus erythematosus: Absence of p70
heterodimers but presence of p40 monomers correlating with disease
activity. Lupus. 11:384–387. 2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Segal R, Dayan M, Zinger H, Habut B,
Shearer GM and Mozes E: The effect of IL-12 on clinical and
laboratory aspects of experimental SLE in young and aging mice. Exp
Gerontol. 38:661–668. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Neumann D, Tschernig T, Popa D, Schmiedl
A, Pérez de Lema G, Resch K and Martin MU: Injection of IL-12- and
IL-18-encoding plasmids ameliorates the autoimmune pathology of
MRL/Mp-Tnfrsf6lpr mice: Synergistic effect on autoimmune symptoms.
Int Immunol. 18:1779–1787. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kikawada E, Lenda DM and Kelley VR: IL-12
deficiency in MRL-Fas(lpr) mice delays nephritis and intrarenal
IFN-γ expression, and diminishes systemic pathology. J Immunol.
170:3915–3925. 2003.PubMed/NCBI View Article : Google Scholar
|