Introduction
Diabetes mellitus (DM) is a multi-systemic metabolic
disease that is primarily characterized by hyperglycemia (1). Constant hyperglycemia can result in a
series of chronic complications (2), with the gastrointestinal tract being
one of the target organs (2-4).
As one of the most common complications of DM, diabetic enteropathy
has attracted increasing attention; 10-20% of patients experience
gastrointestinal symptoms that primarily manifest as
gastrointestinal dysfunction, such as abdominal distension,
intractable diarrhea and malabsorption, which has a negative impact
on health-related quality of life (5). However, to date, little is known of
the effects of high glucose conditions on intestinal epithelial
cells.
In 1986, Mooradian et al (6) first reported that patients with DM
experienced increased intestinal permeability. Subsequently, other
studies have revealed that intestinal permeability is increased in
patients at different stages of DM, although it is the most obvious
in the early stages of disease (7).
Furthermore, Neu et al (8)
discovered morphological and structural changes to intestinal cells
in DM. Over the past decade, studies on the diabetic intestinal
epithelium have largely focused on damage to the gut tissue from
advanced glycation end-products, impaired myenteric nerve plexus
function due to autonomic neuropathy and fibrosis of the intestinal
muscular layers (9). However, the
pathophysiological mechanisms of action underlying diabetic
intestinal epithelium alterations remain to be fully
elucidated.
Baynes et al have demonstrated that constant high
blood glucose can directly or indirectly trigger
apoptosis-associated pathways through oxidative stress or the
induction of inflammation (10).
Reactive oxygen species (ROS) are generated by several enzymes,
such as NADPH oxidase (NOX), xanthine oxidase, endothelial nitric
oxide synthase and enzymes of the mitochondrial electron transport
system (11). NOX is reportedly the
primary source of cellular ROS, including in epithelial cells
(12). Emerging evidence has
indicated that ROS generation can be blocked by NOX inhibitors,
which has been proposed as a promising antioxidant therapy against
DM (13,14). Therefore in the present study, the
NOX4 inhibitor, GKT137831 was used to inhibit NOX (15). The Janus kinase (JAK)/STAT3 pathway
is an important intracellular signaling pathway, which plays a
prominent role in inflammation and cell survival (16). This pathway can be activated by
various different cytokines, such as TNF-α, IL-1, IL-6 and growth
factors, which are involved in cell proliferation, apoptosis and
migration (17). However, whether
constant hyperglycemia induces intestinal epithelial cell apoptosis
via the NOX4/ROS/JAK/STAT3 signaling pathway remains unclear.
It has been demonstrated that acute glucose
fluctuation plays an important role in the occurrence and
development of DM-associated complications (18). Whether in patients with DM or
healthy subjects, acute blood glucose fluctuations may exert
deleterious effects on vascular endothelial cells (19). The aim of the present study was to
elucidate whether unstable hyperglycemia or glucose fluctuations
promote cell apoptosis. Rat small intestinal epithelial cells
(IEC-6) were used to establish an in vitro diabetic model
and cellular alterations (and their underlying molecular mechanisms
of action) were investigated under high glucose conditions.
Materials and methods
Cell lines, cell culture and
treatment
IEC-6 cells were purchased from the American Type
Culture Collection (cat. no. CRL-1592) and cultured in DMEM
supplemented with 10% fetal bovine serum (HyClone; Cytiva), 100
U/ml penicillin (Sigma-Aldrich; Merck KGaA) and 100 mg/ml
streptomycin (Sigma-Aldrich; Merck KGaA). The cells were maintained
in a humidified incubator at 37˚C in an atmosphere containing 5%
CO2, and the culture medium was replaced every 2
days.
Cells were assigned to five groups and then exposed
to various concentrations of glucose as follows: i) Normal group
(NG), 5 mmol; constant high glucose (CHG) group, 25 mmol; ii)
intermittent high glucose (IHG) group, alternating between 5.0 and
25.0 mmol/l every 8 h; iii) IHG + GKT137831 group, pretreated with
100 nmol/l GKT137831; and finally, iv) the osmotic control group,
which was treated with 25 mmol/l mannose.
Western blotting
Western blotting was conducted according to
previously described methods (20).
IEC-6 cells were lysed in RIPA buffer (Beyotime Institute of
Biotechnology) containing protease inhibitors (Sigma-Aldrich; Merck
KGaA), phosphatase inhibitors (Sigma-Aldrich; Merck KGaA) and PMSF
(Beyotime Institute of Biotechnology). Total protein was extracted
and quantified using a bicinchoninic acid kit (Beyotime Institute
of Biotechnology). Lysed protein (20 µg) was separated by 10%
SDS-PAGE and then transferred to PVDF membranes (Stratagene;
Agilent Technologies, Inc.). The membranes containing proteins were
blocked for 1.5 h in 5% BSA at room temperature. Subsequently, the
membranes were incubated with primary antibodies against NOX4 (cat.
no. ab109225; Abcam), Bcl-2 (cat. no. D17C4; Cell Signaling
Technology, Inc.), Bax (cat. no. 2772; Cell Signaling Technology,
Inc.), cleaved caspase-3 (cat. no. 9664; Cell Signaling Technology,
Inc.), phosphorylated (p-)JAK (cat. no. 66245; Cell Signaling
Technology, Inc.), p-STAT3 (cat. no. 9145; Cell Signaling
Technology, Inc.), JAK (cat. no. 3344; Cell Signaling Technology,
Inc.), STAT3 (cat. no. 9139; Cell Signaling Technology, Inc.) and
GAPDH (cat. no. A19056; ABclonal, Biotech Co., Ltd.) overnight at
4˚C. The aforementioned antibodies were diluted at ratio of
1:1,000. Membranes were then incubated with HRP-conjugated
goat-anti-rabbit IgG (1:10,000; cat. no. AS014; ABclonal, Biotech
Co., Ltd.) and HRP-conjugated goat-anti-mouse IgG secondary
antibodies (1:10,000; cat. no. AS003; ABclonal, Biotech Co., Ltd.)
for 2 h at room temperature. Finally, Protein bands were visualized
by enhanced chemiluminescence detection (Amersham; Cytiva) and
quantified by Image J software (version 1.46, National Institutes
of Health). Target protein expression is presented relative to that
of GAPDH.
Immunocytofluorescence assay
After fixing with 4% formaldehyde (Sigma-Aldrich;
Merck KGaA) at 4˚C for 30 min, the cells were permeabilized with
0.1% Triton X-100 in PBS and then blocked with 10% normal goat
serum (Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature. The cells were subsequently incubated with primary
antibodies against NOX4 (1:100; cat. no. PA5-72816; Thermo Fisher
Scientific, Inc.) at 4˚C overnight, followed by FITC-conjugated
anti-rabbit IgG secondary antibody (1:100) for 1 h at room
temperature (cat. no. A30008; Invitrogen; Thermo Fisher Scientific,
Inc.). Fluorescence was assessed by fluorescence microscopy
(magnification, x400; Leica Microsystems GmbH). Quantification of
fluorescence intensity or positive cell/nuclear ratios was
performed using ImageJ version 1.47 (National Institutes of
Health). The results are presented as the mean ± standard error of
the mean (SEM) from ≥3 independent experiments.
ELISA
The samples were centrifuged at 1,500 x g for 15 min
at 4˚C to obtain the cell supernatant. The expression levels of
TNF-α (cat. no. ER006-96), IL-1 (cat. no. ER008-96) and IL-6 (cat.
no. ER003-96) in the cell supernatant were determined using ELISA
kits (Shanghai ExCell Biology, Inc.) according to the
manufacturers' protocols. The cell supernatant was evaluated using
a multiclan spectrum spectrophotometer (Thermo Fisher Scientific,
Inc.).
Detection of intracellular ROS
generation
Following appropriate group treatments, the cells
were harvested and incubated with the fluorescent probe,
2',7'-dichlorodihydrofluorescein diacetate (0.1%), at 37˚C for 30
min. After resuspension, the fluorescence intensity was analyzed
using a microplate reader (Thermo Fisher Scientific, Inc.).
Detection of malondialdehyde (MDA)
levels
The generation of MDA in the cells was assessed
using an MDA ELISA assay kit (cat. no. S0131S; Beyotime Institute
of Biotechnology). The cells were homogenized with PBS and the MDA
detection working solution was mixed with the cell homogenate.
Subsequently, the mixed solution was heated at 100˚C for 15 min.
The absorbance was then measured at 532 nm using a microplate
reader (Thermo Fisher Scientific, Inc.).
Apoptosis analysis
The apoptotic rates of small intestinal epithelial
cells were assessed by flow cytometry, following a series of
previously described procedures (21). The cells were harvested and stained
with Annexin V-fluorescein isothiocyanate (FITC) and propidium
iodide using the Annexin VFITC Apoptosis Detection kit I (BD
Biosciences), and were then incubated in the dark for 15 min at
room temperature. The cells were subsequently analyzed within 1 h
using BD FACSVerse™ flow cytometer (BD Biosciences) and the FlowJo™
VX10 software (FlowJo LLC) was used to analyze the data.
Statistical analysis
All data were analyzed by one-way analysis of
variance followed by Tukey's post hoc test, which was conducted
using GraphPad 6.0 (GraphPad Software, Inc.) Data are presented as
the means ± SEM and P<0.05 was considered to indicate a
statistically significant difference. All experiments were repeated
at least three times.
Results
Comparison of NOX4 expression between
the various treatment groups
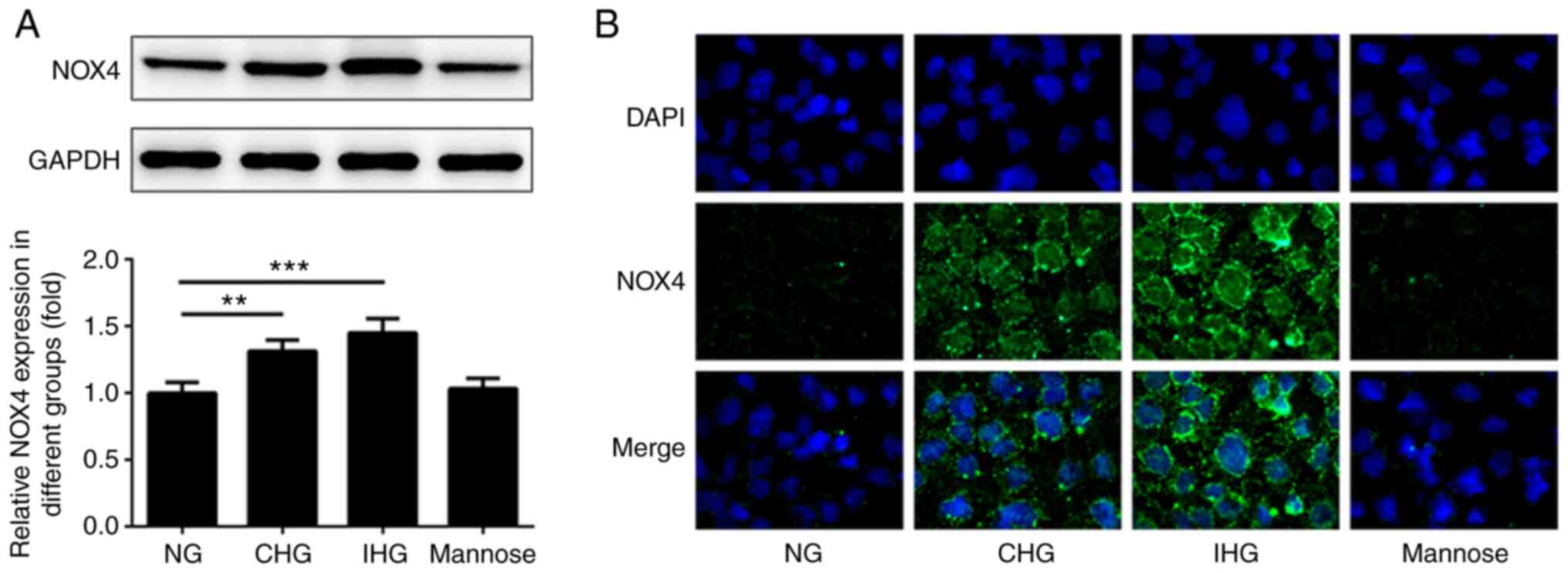
Changes in NOX4 expression in intestinal epithelial
cells were detected under fluctuating glucose concentrations using
western blotting and immunocytofluorescence staining. As indicated
in Fig. 1A-B, the expression levels
of NOX4 in both the CHG and IHG groups were significantly increased
compared with that in the NG group, and to a greater extent in the
IHG group than in the CHG group. Moreover, there were no
significant differences in NOX4 expression levels between the
mannose group and the NG group at 24 h post-treatment. In addition,
the fluorescence intensity (green stain) of NOX4 in the CHG and IHG
groups was increased compared with the NG group. NOX4 expression in
the IHC group was also higher than the CHG group. However, there
was no significant difference between the mannose group and the NG
group.
Comparison of inflammatory cytokine
expression levels between groups
Compared with the NG group, the levels of
inflammatory cytokines (TNF-α, IL-1 and IL-6) in the CHG and IHG
groups were markedly increased, and the levels in the CHG group
where lower than those in the IHG group. Notably, the levels of
TNF-α, IL-1 and IL-6 in the IHG group were decreased following
pretreatment with GKT137831, but were still higher than those in
the NG group. In addition, there was no significant difference
between the mannose group and the NG group (Fig. 2A-C).
Expression of oxidative
stress-associated biomarkers
Oxidative stress is reported to play an important
role in intestinal epithelial cell injury under high glucose
conditions. Compared with the NG group, the levels of oxidative
stress biomarkers [ROS and MDA] were increased in the CHG and IHG
groups, with the highest levels found in the IHG group (Fig. 3A and B). Notably, the levels of ROS and MDA
activity in the IHG + GKT137831 group were lower than those in the
IHG group, but higher than those in the NG group. There was no
significant difference between the mannose group and the NG group.
These results suggested that blood glucose fluctuation exacerbates
oxidative stress in intestinal epithelial cells.
Blood glucose fluctuation induces
apoptosis in intestinal epithelial cells
Flow cytometry was used to detect intestinal
epithelial cell apoptosis following blood glucose fluctuation. As
shown in Fig. 4A, apoptosis in the
IHG and CHG groups was significantly higher than that in the NG
group and was at its highest level in the IHG group; there was no
significant difference between the mannose control and NG groups.
In addition, compared with the IHG group, the level of apoptosis in
the IHG + GKT137831 group was significantly decreased, but remained
higher than that in the NG group. These results indicated that
blood glucose fluctuation aggravates intestinal epithelial cell
apoptosis.
Effects of blood glucose fluctuation
on the expression of apoptosis-associated proteins
Compared with the NG group, the expression levels of
Bax and cleaved caspase 3/caspase 3 was significantly increased in
the CHG and IHG groups, while the expression levels of Bcl-2 was
decreased (Fig. 4B). Furthermore,
Bax and cleaved caspase-3 expression levels were higher in the IHG
group than in the CHG group. In addition, compared with the IHG
group, the levels of Bax and cleaved caspase-3 were significantly
decreased in the IHG + GKT137831 group, but higher than those in
the NG group, and the IHG + GKT137831 group exhibited significantly
increased Bcl-2 expression compared with the IHG group. There were
no significant differences in the levels of Bax, cleaved caspase-3
and Bcl-2 between the mannose group and the NG group.
Role of the JAK/STAT3 pathway in
inflammation and cell survival
To determine the effects of glucose fluctuation on
the JAK/STAT3 pathway in intestinal epithelial cells, p-JAK and
p-STAT3 levels were detected in IEC-6 cells exposed to fluctuating
glucose concentrations. As shown in Fig. 5, the ratios of p-JAK/JAK and
p-STAT3/STAT3 in the CHG and IHG groups were significantly higher
than those in the NG group, though the increase was more pronounced
in the IHG group. Moreover, compared with the IHG group, the ratios
of p-JAK/JAK and p-STAT3/STAT3 in the IHG + GKT137831 group were
significantly decreased, although they remained higher
significantly than those in the NG group. There were no significant
differences in the levels of p-JAK and p-STAT3 between the mannose
group and the NG group. These results indicated that glucose
fluctuation accelerates intestinal epithelial cell apoptosis and
that this is associated with increased phosphorylation of JAK and
STAT3.
Discussion
To the best of our knowledge, the present study is
the first to investigate the changes in intestinal epithelial cells
under high glucose conditions, as well as the associated underlying
pathophysiological mechanisms. When investigating the influence of
acute glucose fluctuations on cells, a high glucose concentration
is consider to be 25 mmol (22,23).
At this concentration, acute glucose fluctuations can induce
inflammation and apoptosis (22,23).
As such, the present study used this concentration for all
experiments. The following results were noted: i) CHG increased the
expression of NOX4, ROS, apoptosis-associated proteins and
inflammatory factors in intestinal epithelial cells, as well as,
ultimately, the number of apoptotic cells, which was exacerbated by
acute glucose fluctuation; ii) a persistent high glucose
concentration upregulated the phosphorylation levels of JAK and
STAT3, which was further increased by acute glucose fluctuation, In
parallel with the points outlined in i); and iii) the levels of
NOX4, ROS, apoptosis-associated proteins, inflammatory factors,
p-JAK and p-STAT3 were markedly downregulated following NOX
inhibition.
Furthermore, the number of apoptotic intestinal
epithelial cells was higher in the CHG group compared with those in
the NG group, suggesting that apoptosis was induced by CHG.
Therefore, it was hypothesized that the hyperglycemia-induced
apoptosis of intestinal epithelial cells may account for the
occurrence and development of diabetic enteropathy, which is
supported by the results of previous studies. In 2003, Quagliaro
et al (24) reported that
high glucose triggered apoptosis in human umbilical vein
endothelial cells. Such effects have also been observed in vascular
endothelial cells, mesangial cells, myocardial cells and pancreatic
islets (21,24,25).
However, the results of previous studies investigating the changes
in intestinal epithelial cells under constant hyperglycemia have
been inconsistent. Other studies have indicated that the
proliferation of various cell types, such as myocardial, mammary
and placental cells, are enhanced under hyperglycemic and
hyperinsulinemic conditions (26-28).
An animal study also revealed that the intestinal epithelium of
rats with hyperglycemia displayed longer villi, increased wet
weight, increased cellular proliferation and hypertrophy within the
crypts (29). In addition, patients
with DM reportedly are at an increased risk of developing
colorectal cancer (30) and
diabetic rats are more susceptible to colorectal tumorigenesis
(31). At present, further
investigation is required to fully elucidate the association
between intestinal epithelial cell injury and CHG.
Hyperglycemia is common in patients with DM, which
can result in oxidative stress and may be responsible for the
emergence and development of complications (32). Increasing clinical evidence has
demonstrated that DM-related complications are more pronounced in
association with peak or post-meal, rather than average blood
glucose levels (25). Furthermore,
in vivo studies have shown that apoptosis, oxidative stress
and pro-inflammatory cytokine release are enhanced by acute blood
glucose fluctuation in human umbilical vein endothelial cells,
vascular endothelial cells, mesangial cells, myocardial cells and
pancreatic islets (33-35),
which has been further confirmed by a number of in vitro
studies (36,37).
The correlation between blood glucose fluctuations
and diabetes complications has received extensive attention in
recent years (38). Compared to
persistent hyperglycemia, fluctuant hyperglycemia has a greater
potential to increase microvascular lesions and the risk of
cardiovascular death (18,39), but the specific mechanism of action
remains unclear. One possible explanation is that oxidative stress
may play an important role in cell damage caused by blood glucose
fluctuations. Studies have shown that the levels of oxidative
stress have no significant correlation with fasting blood glucose,
but have a significant correlation with expression of markers of
acute glucose fluctuation (18).
This suggested that oxidative stress increases more as a result of
larger glucose fluctuations (18).
Additionally, as a result of continuous hyperglycemia, certain
compensation or feedback reactions are induced in cells to
compensate for the constant stimulation (24). However, in the intermittent
hyperglycemic state, it is speculated that such an adaptative
reaction is reduced or does not function properly (24). However, further researches are
required to confirm this speculation.
Oxidative stress promotes redox imbalance and a
marked increase in the generation of ROS and inflammatory
cytokines, which ultimately results in enhanced apoptosis (40). Studies have demonstrated that
oxidative stress can cause inflammation, but no inflammation occurs
during cell apoptosis (41,42). Inflammation will further aggravate
oxidative stress and IL-6 can play an important role through the
JAK/STAT3 pathway (43). In the
present study, oxidative stress was caused by glucose fluctuation.
Therefore, it is believed that the release of inflammatory factors
is partly caused by this glucose fluctuation. Studies have also
shown that NOX4 may lead to necroptosis and thus release
inflammatory factors (44). Whether
acute glucose fluctuations lead to necroptosis requires further
verification in intestinal epithelial cells.
The NOX family proteins are multicomponent enzymes
that are the key source of ROS generation in various cell types, as
well as in rodent diabetic models (45). The NOX family is composed of seven
members, including NOX1 (mainly in colon tissues), NOX2 (primarily
expressed in phagocytes), NOX3 (predominantly in the inner ear),
NOX4, NOX5 (mainly expressed in lymphoid tissues), Duxo1 and Duxo2
(both mainly in the thyroid and bronchus) (46). Of note, NOX4 is also expressed in
intestinal tissue (47,48). NOX4 has also been shown to play a
potentially important role in diabetes and its complications
(49). In addition, the activation
of NOX can also cause inflammation and apoptosis (45). Therefore, the present study examined
the role of NOX4 in inflammation and apoptosis induced by acute
glucose fluctuation in intestinal epithelial cells.
The results of the present study confirmed that the
expression of NOX4, ROS, apoptosis-associated proteins and
inflammatory factors (as well as the number of apoptotic cells) was
raised in the IHG group compared with that in the CHG group,
indicating that acute glucose fluctuation exacerbates intestine
epithelial cell apoptosis. Since the signal transduction cascades
involved in DM are associated with the activation of various
molecules (such as transcription factors, cytokines, hormones and
protein kinases), the present study aimed to investigate the
underlying molecular mechanisms of action involved in the effects
of acute blood fluctuation. The results revealed that high glucose
upregulated the levels of NOX4, ROS, p-JAK and p-STAT3, which was
further exacerbated by acute glucose fluctuation. This effect was
suppressed by a NOX inhibitor, suggesting that acute high glucose
enhances intestinal epithelial cell apoptosis by activating the
NOX4/ROS/JAK/STAT3 signaling pathway. These results were consistent
with those of previous reports (50,51).
The JAK/STAT3 signaling pathway is also reportedly implicated in
the pathophysiology behind DM (52). Studies have demonstrated that, in
diabetic rodents, the apoptosis and abnormal proliferation of
vascular endothelial cells, mesangial cells, myocardial cells and
sensory neurons was promoted by JAK/STAT3 pathway activation,
leading to organ injury and deterioration (52-55).
This organ damage was suppressed and/or ameliorated by
STAT3-knockdown or JAK inhibition (56,57).
However, several studies have also shown that activation of the
JAK/STAT3 pathway protects against myocardial ischemia-reperfusion
injury, myocardial cell apoptosis and inflammation (58,59).
Differences between various animal models and the course of the
disease may explain the effective differences resulting from
JAK/STAT3 pathway activation.
In conclusion, the results of the present study
suggested that CHG triggers intestinal epithelial cell apoptosis
through the NOX4/ROS/JAK/STAT3 signaling pathway, which is
subsequently enhanced by acute glucose fluctuation. The current
study has demonstrated that acute glucose fluctuation inflicts
greater damage to intestinal epithelial cells, further emphasizing
the importance of glucose control.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by Postgraduate Research and
Practice Innovation Program of Jiangsu Province (grant no. KYCX20
1480).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZS and BC designed the study. BC and YJ performed
the literature search and selection. BC, YJ and DL performed the
experiment and analyzed the data. BC and YJ drafted the manuscript.
All authors revised and approved the final manuscript. ZS and BC
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wilson PWF, D'Agostino RB, Helen P, Lisa S
and Meigs JB: Metabolic syndrome as a precursor of cardiovascular
disease and type 2 diabetes mellitus. Circulation. 112:3066–3072.
2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Brownlee M: Biochemistry and molecular
cell biology of diabetic complications. Nature. 414:813–820.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Papatheodorou K, Banach M, Bekiari E,
Rizzo M and Edmonds M: Complications of diabetes 2017. J Diabetes
Res. 2018(3086167)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Snelson M, de Pasquale C, Ekinci EI and
Coughlan MT: Gut microbiome, prebiotics, intestinal permeability
and diabetes complications. Best Pract Res Clin Endocrinol Metab,
2021 (Ahead of print).
|
|
5
|
Talley NJ, Young L, Bytzer P, Hammer J,
Leemon M, Jones M and Horowitz M: Impact of chronic
gastrointestinal symptoms in diabetes mellitus on health-related
quality of life. Am J Gastroenterol. 96:71–76. 2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mooradian AD, Morley JE, Levine AS, Prigge
WF and Gebhard RL: Abnormal intestinal permeability to sugars in
diabetes mellitus. Diabetologia. 29:221–224. 1986.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Meddings JB, Jarand J, Urbanski SJ, Hardin
J and Gall DG: Increased gastrointestinal permeability is an early
lesion in the spontaneously diabetic BB rat. Am J Physiol.
276:951–957. 1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Neu J, Reverte CM, Mackey AD, Liboni K,
Tuhacek-Tenace LM, Hatch M, Li N, Caicedo RA, Schatz DA and
Atkinson M: Changes in intestinal morphology and permeability in
the biobreeding rat before the onset of type 1 diabetes. J Pediatr
Gastroenterol Nutr. 40:589–595. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chocarro-Calvo A, García-Martínez JM,
Ardila-González S, De la Vieja A and García-Jiménez C:
Glucose-induced β-catenin acetylation enhances Wnt signaling in
cancer. Mol Cell. 49:474–486. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Baynes JW and Thorpe SR: Role of oxidative
stress in diabetic complications: A new perspective on an old
paradigm. Diabetes. 43:1–9. 1999.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Babior BM: NADPH oxidase. Curr Opin
Immunol. 16:42–47. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ago T, Kuroda J, Kamouchi M, Sadoshima J
and Kitazono T: Pathophysiological roles of NADPH oxidase/nox
family proteins in the vascular system. Review and perspective.
Circ J. 75:1791–1800. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Asaba K, Tojo A, Onozato ML, Goto A, Quinn
MT, Fujita T and Wilcox CS: Effects of NADPH oxidase inhibitor in
diabetic nephropathy. Kidney Int. 67:1890–1898. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Roe ND, Thomas DP and Ren J: Inhibition of
NADPH oxidase alleviates experimental diabetes-induced myocardial
contractile dysfunction. Diabetes Obes Metab. 13:465–473.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Teixeira G, Szyndralewiez C, Molango S,
Carnesecchi S, Heitz F, Wiesel P and Wood JM: Therapeutic potential
of NADPH oxidase 1/4 inhibitors. Br J Pharmacol. 174:1667–1669.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang JW, Pan YB, Cao YQ, Wang C, Jiang WD,
Zhai WF and Lu JG: Loganin alleviates LPS-activated intestinal
epithelial inflammation by regulating TLR4/NF-κB and JAK/STAT3
signaling pathways. Kaohsiung J Med Sci. 36:257–264.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hirano T, Ishihara KM and Hibi M: Roles of
STAT3 in mediating the cell growth, differentiation and survival
signals relayed through the IL-6 family of cytokine receptors.
Oncogene. 19:2548–2556. 2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Monnier L, Mas E, Ginet C, Michel F,
Villon L, Cristol JP and Colette C: Activation of oxidative stress
by acute glucose fluctuations compared with sustained chronic
hyperglycemia in patients with type 2 diabetes. JAMA.
295:1681–1687. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ceriello A, Esposit K, Piconi L, Ihnat MA,
Thorpe JE, Testa R, Boemi M and Giugliano D: Oscillating glucose is
more deleterious to endothelial function and oxidative stress than
mean glucose in normal and type 2 diabetic patients. Diabetes.
57:1349–1354. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lee MK, Kim IH, Choi YH and Nam TJ: A
peptide from Porphyra yezoensisstimulates the proliferation of
IEC-6 cells by activating the insulin-like growth factor I receptor
signaling pathway. Int J Mol Med. 35:533–538. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shen JT, Li YS, Xia ZQ, Wen SH, Yao X,
Yang WJ, Li C and Liu KX: Remifentanil preconditioning protects the
small intestine against ischemia/reperfusion injury via intestinal
δ- and µ-opioid receptors. Surgery. 159:548–559. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ying C, Wang S, Lu Y, Chen L, Mao Y, Ling
H, Cheng X and Zhou X: Glucose fluctuation increased mesangial cell
apoptosis related to AKT signal pathway. Arch Med Sci. 15:730–737.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hsieh CF, Liu CK, Lee CT, Yu LE and Wang
JY: Acute glucose fluctuation impacts microglial activity, leading
to inflammatory activation or self-degradation. Sci Rep.
9(840)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Quagliaro L, Piconi L, Assaloni R,
Martinelli L, Motz E and Ceriello A: Intermittent high glucose
enhances apoptosis related to oxidative stress in human umbilical
vein endothelial cells: The Role of Protein Kinase C and
NAD(P)H-Oxidase Activation. Diabetes. 52:2795–2804. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cho JH, Chang SA, Kwon HS, Choi YH, Ko SH,
Moon SD, Yoo SJ, Song KH, Son HS, Kim HS, et al: Long-term effect
of the Internet-based glucose monitoring system on HbA1c reduction
and glucose stability: A 30-month follow-up study for diabetes
management with a ubiquitous medical care system. Diabetes Care.
29:2625–2631. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu Q, Huang QX, Lou FC, Zhang L and Hou
WK, Yu S, Xu H, Wang Q, Zhang Y and Hou WK: Effects of glucose and
insulin on the H9c2 (2-1) cell proliferation may be mediated
through regulating glucose transporter 4 expression. Chin Med J
(Engl). 126:4037–4042. 2013.PubMed/NCBI
|
|
27
|
Lopez R, Arumugam A, Joseph R, Monga K,
Boopalan T, Agullo P, Gutierrez C, Nandy S, Subramani R, de la Rosa
JM and Lakshmanaswamy R: Hyperglycemia enhances the proliferation
of non-tumorigenic and malignant Mammary epithelial cells through
increased leptin/IGF1R signaling and activation of AKT/mTOR. PLoS
One. 8(e79708)2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ozmen A, Unek G, Kipmen-Korgun D and
Korgun ET: The PI3K/Akt and MAPK-ERK1/2 pathways are altered in STZ
induced diabetic rat placentas. Histol Histopathol. 29:743–756.
2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Adachi T, Mori C, Sakurai K, Shihara N,
Tsuda K and Yasuda K: Morphological changes and increased sucrase
and isomaltase activity in small intestines of insulin-deficient
and type 2 diabetic rats. Endocr J. 50:271–279. 2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rasool S, Kadla SA, Rasool V and Ganai BA:
A comparative overview of general risk factors associated with the
incidence of colorectal cancer. Tumor Biology. 34:2469–2476.
2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hata K, Kubota M, Shimizu M, Moriwaki H,
Kuno T, Tanaka T, Hara A and Hirose Y: Monosodium glutamate-induced
diabetic mice are susceptible to azoxymethane-induced colon
tumorigenesis. Carcinogenesis. 33:702–707. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
King GL and Loeken MR:
Hyperglycemia-induced oxidative stress in diabetic complications.
Histochem Cell Biol. 122:333–338. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wu N, Shen H, Liu H, Wang Y, Bai Y and Han
P: Acute blood glucose fluctuation enhances rat aorta endothelial
cell apoptosis, oxidative stress and pro-inflammatory cytokine
expression in vivo. Cardiovasc Diabetol. 15(109)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sharma A, Tate M, Mathew G, Vince JE,
Ritchie RH and Haan JB: Oxidative stress and NLRP3-inflammasome
activity as significant drivers of diabetic cardiovascular
complications: Therapeutic implications. Front Physiol.
9(114)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lal MA, Brismar H, Eklöf AC and Aperia A:
Role of oxidative stress in advanced glycation end product-induced
mesangial cell activation. Kidney Int. 61:2006–2014.
2002.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang W, Zhao S, Li Y, Peng G and Han P:
Acute blood glucose fluctuation induces myocardial apoptosis
through oxidative stress and nuclear factor-ĸB activation.
Cardiology. 124:11–17. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Xue B, Wang L, Zhang Z, Wang R, Xia XX,
Han PP, Cao LJ, Liu YH and Sun LQ: Puerarin may protect against
Schwann cell damage induced by glucose fluctuation. J Nat Med.
71:472–481. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xia J, Hu S, Xu J, Hao H, Yin C and Xu D:
The correlation between glucose fluctuation from self-monitored
blood glucose and the major adverse cardiac events in diabetic
patients with acute coronary syndrome during a 6-month follow-up by
WeChat application. Clin Chem Lab Med. 56:2119–2124.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Glucose tolerance and mortality.
Comparison of WHO and American Diabetes Association diagnostic
criteria. The DECODE study group. European Diabetes Epidemiology
Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic
criteria in Europe. Lancet. 354:617–621. 1999.PubMed/NCBI
|
|
40
|
Curtin JF, Donovan M and Cotter TG:
Regulation and measurement of oxidative stress in apoptosis. J
Immunol Methods. 265:49–72. 2002.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Nikoletopoulou V, Markaki M, Palikaras K
and Tavernarakis N: Crosstalk between apoptosis, necrosis and
autophagy. Biochim Biophys Acta. 1833:3448–3459. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hussain T, Tan B, Yin Y, Blachier F,
Tossou MC and Rahu N: Oxidative stress and inflammation: What
Polyphenols Can Do for Us? Oxid Med Cell Longev.
2016(7432797)2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shen Y, Zhang Q, Huang Z, Zhu J, Qiu J, Ma
W, Yang X, Ding F and Sun H: Isoquercitrin delays denervated soleus
muscle atrophy by inhibiting oxidative stress and inflammation.
Front Physiol. 11(988)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Meng XM, Ren GL, Gao L, Yang Q, Li HD, Wu
WF, Huang C, Zhang L, Lv XW and Li J: NADPH oxidase 4 promotes
cisplatin-induced acute kidney injury via ROS-mediated programmed
cell death and inflammation. Lab Invest. 98:63–78. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sedeek M, Montezano AC, Hebert RL, Gray
SP, Di Marco E, Jha JC, Cooper ME, Jandeleit-Dahm K, Schiffrin EL,
Wilkinson-Berka JL and Touyz RM: Oxidative stress, Nox isoforms and
complications of diabetes-potential targets for novel therapies. J
Cardiovasc Transl Res. 5:509–518. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sedeek M, Nasrallah R, Touyz RM and Hebert
RL: NADPH oxidases, reactive oxygen species, and the kidney: Friend
and foe. J Am Soc Nephrol. 24:1512–1518. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chu FF, Esworthy RS, Shen B, Gao Q and
Doroshow JH: Dexamethasone and Tofacitinib suppress NADPH oxidase
expression and alleviate very-early-onset ileocolitis in mice
deficient in GSH peroxidase 1 and 2. Life Sci.
239(116884)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lindquist RL, Bayat-Sarmadi J, Leben R,
Niesner R and Hauser AE: NAD(P)H oxidase activity in the small
intestine is predominantly found in enterocytes, not professional
phagocytes. Int J Mol Sci. 19(1365)2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yan J, Wang C, Jin Y, Meng Q, Liu Q, Liu
Z, Liu K and Sun H: Catalpol ameliorates hepatic insulin resistance
in type 2 diabetes through acting on AMPK/NOX4/PI3K/AKT pathway.
Pharmacol Res. 130:466–480. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sun L, Li W, Li W, Xiong L, Li G and Ma R:
Astragaloside IV prevents damage to human mesangial cells through
the inhibition of the NADPH oxidase/ROS/Akt/NF-κB pathway under
high glucose conditions. Int J Mol Med. 34:167–176. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Chen F, Qian LH, Deng B, Liu ZM, Zhao Y
and Le YY: Resveratrol protects vascular endothelial cells from
high glucose-induced apoptosis through inhibition of NADPH oxidase
activation-driven oxidative stress. CNS Neurosci Ther. 19:675–681.
2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chowdhury SR, Saleh A, Akude E, Smith DR,
Morrow D, Tessler L, Calcutt NA and Fernyhough P: Ciliary
neurotrophic factor reverses aberrant mitochondrial bioenergetics
through the JAK/STAT pathway in cultured sensory neurons derived
from streptozotocin-induced diabetic rodents. Cell Mol Neurobiol.
34:643–649. 2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Li Q, Lin Y, Wang S, Zhang L and Guo L:
GLP-1 inhibits high-glucose-induced oxidative injury of vascular
endothelial cells. Sci Rep. 7(8008)2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Liu M, Yan L, Liang B, Li Z, Jiang Z, Chu
C and Yang J: Hydrogen sulfide attenuates myocardial fibrosis in
diabetic rats through the JAK/STAT signaling pathway. Int J Mol
Med. 41:1867–1876. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Marrero MB, Banes-Berceli AK, Stern DM and
Eaton DC: Role of the JAK/STAT signaling pathway in diabetic
nephropathy. Am J Physiol Renal Physiol. 290:F762–F768.
2006.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wang X, Shaw S, Amiri F, Eaton DC and
Marrero MB: Inhibition of the Jak/STAT signaling pathway prevents
the high glucose-induced increase in tgf-beta and fibronectin
synthesis in mesangial cells. Diabetes. 51:3505–3509.
2002.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Yoshikawa H, Matsubara K, Qian GS, Jackson
P, Groopman JD, Manning JE, Harris CC and Herman JG: SOCS-1, a
negative regulator of the JAK/STAT pathway, is silenced by
methylation in human hepatocellular carcinoma and shows
growth-suppression activity. Nat Genet. 28:29–35. 2001.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Das A, Salloum FN, Durrant D, Ockaili R
and Kukreja RC: Rapamycin protects against myocardial
ischemia-reperfusion injury through JAK2-STAT3 signaling pathway. J
Mol Cell Cardiol. 53:858–869. 2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Sun X, Chen RC, Yang ZH, Sun GB, Wang M,
Ma XJ, Yang LJ and Sun XB: Taxifolin prevents diabetic
cardiomyopathy in vivo and in vitro by inhibition of oxidative
stress and cell apoptosis. Food Chem Toxicol. 63:221–232.
2014.PubMed/NCBI View Article : Google Scholar
|