Introduction
Systemic vasculitis (SV) is an autoimmune disease
involving various types of vessels and its primary characteristics
are vascular wall injury and necrosis as a result of inflammation
(1). However, the pathogenesis
behind SV is not fully understood. Although SV has received
increasing attention, as SV can occur in patients of all ages, the
clinical manifestations are complex and diverse, and there is a
lack of specific markers (2).
Therefore, diagnosing and estimating the disease activity of SV is
difficult. If left untreated, SV progresses to permanent organ
damage, resulting in poor health, poor quality of life, premature
death and other manifestations, which ultimately result in a heavy
socio-economic burden (3).
Therefore, identifying novel markers for estimating the disease
activity in patients with SV is important (4,5).
Red blood cell distribution width (RDW), routinely
reported as a parameter of the standard automated complete blood
count, is a reflection of the variability in the erythrocyte size
in the circulation (6). In the past
few decades, RDW has been widely used in clinical practice as a
tool for analyzing and identifying types of anemia (7). In recent years, the value of RDW has
attracted increasing attention and its application scope has become
increasingly extensive (8). RDW is
considered as an inflammatory related index and research has
revealed that it has a potential for predicting the overall
mortality in a variety of human inflammatory diseases (9,10). It
has also been indicated that increased RDW is associated with
autoimmune diseases, such as rheumatoid arthritis and systemic
lupus erythematosus (10-13).
However, the role of RDW in assessing the disease activity,
especially in polyarteritis nodosa (PAN), is not completely
understood. Therefore, the present study aimed to investigate
whether RDW was increased in patients with SV and could serve as a
reliable marker to evaluate disease activity.
Patients and methods
Study subjects
A total of 287 patients with SV who received
diagnosis and treatment at the People's Hospital of Xinjiang Uygur
Autonomous Region (Urumqi, China) between January 2010 and December
2016 were included as the disease group (Female, n=147, Male,
n=140; Age, 49.02±16.52 years). Patients were diagnosed with SV
according to the 2012 revised International Chapel Hill Consensus
Conference classification criteria or the 1990 American College of
Rheumatology (14-18).
Inclusion criteria for the classification of Churg-Strauss syndrome
(CSS) (14): i) Asthma (history of
wheezing or diffuse high-pitched rales on expiration); ii)
Eosinophilia (Eosinophils >10% on white blood cell differential
count); iii) mononeuropathy or polyneuropathy [development of
mononeuropathy, multiple mononeuropathies or polyneuropathy (i.e.
glove/stocking distribution) attributable to a systemic
vasculitis]; iv) non-fixed pulmonary infiltrates [migratory or
transitory pulmonary non-fixed infiltrates on radiographs (not
pulmonary infiltrates, including fixed infiltrates, attributable to
systemic vasculitis)]; v) paranasal sinus abnormality (history of
acute or chronic paranasal sinus pain or tenderness or radiographic
opacification of the paranasal sinuses); and vi) extravascular
eosinophils (biopsy, including artery, arteriole or venule, showing
accumulations of eosinophils in extravascular areas). For purposes
of classification, a patient should have CSS ≥ four of these six
criteria. Criteria for the classification of Wegener's
granulomatosis (WG) (15): i) Nasal
or oral inflammation (development of painful or painless oral
ulcers, purulent or bloody nasal discharge); ii) abnormal chest
radiograph (chest radiograph showing the presence of nodules, fixed
infiltrates or cavities); iii) urinary sediment [Microhematuria
(>5 red blood cells per high power field) or red cell casts in
urine sediment]; iv) granulomatous inflammation on biopsy
[histological changes showing granulomatous inflammation within the
wall of an artery or in the perivascular or extravascular area
(artery or arteriole)]; For purposes of classification, a patient
should have WG ≥ two of these four criteria are present. There is
no uniform standard for the diagnosis of microscopic polyangiitis
(MPA), which must be distinguished from PAN and WG before
diagnosis. The following conditions contribute to the diagnosis of
MPA (16): i) Middle-aged and
elderly (≥45 years), but more commonly seen in men; ii) renal
involvement (proteinuria, hematuria or/and acute renal
insufficiency); iii) pulmonary involvement (cough, hemoptysis,
dyspnea, pulmonary inflammation and pulmonary renal syndrome); iv)
with gastrointestinal (including nausea, vomiting, gastrointestinal
bleeding, ischemic abdominal pain, ulcer, intestinal perforation,
vascular infarction, intestinal obstruction, melena, hematochezia
and peritonitis), heart (including chest pain, heart murmur,
palpitation, valve disease, angina pectoris, myocardial infarction,
congestive heart failure, hypertension, pericarditis, pericardial
effusion and cardiomyopathy), eyes (including exophthalmos, eye
pain, optic nerve and eye muscle damage, blurred vision, vision
loss, conjunctivitis, corneal ulcer, episcleritis, iritis, retinal
vasculitis, visual impairment, ischemic retinopathy or hypertensive
retinopathy), ears (including conductive deafness and sensorineural
deafness), joints (including joint pain, myalgia, muscle pain,
arthritis, intermittent movement disorder of upper and lower limbs)
and other organs involved in the performance of the whole body; v)
ANCA (indirect fluorescence immunoassay was used for detection)
positive; and vi) renal and lung biopsy is helpful in diagnosis for
MPA. Criteria for the classification of PAN (17): i) Weight loss ≥4 kg (loss of ≥4 kg
body weight since illness began, not due to dieting or other
factors); ii) Livedo reticularis (mottled reticular pattern over
the skin of portions of the extremities or torso); iii) testicular
pain or tenderness (pain or tenderness of the testicles not due to
infection, trauma or other causes); iv) myalgias, weakness or leg
tenderness [diffuse myalgias (excluding shoulder and hip girdle) or
weakness of muscles or tenderness of leg muscles]; v)
mononeuropathy or polyneuropathy (development of mononeuropathy,
multiple mononeuropathies or polyneuropathy); vi) diastolic blood
pressure (BP) >90 mm Hg (development of hypertension with the
diastolic BP >90 mm Hg); vii) Elevated blood urea nitrogen (BUN)
creatinine (elevation of BUN >40 mg/dl or creatinine >1.5
mg/dl not due to dehydration or obstruction); viii) hepatitis B
viral infection (Presence of hepatitis B surface antigen or
antibody in serum); ix) arteriographic abnormality (arteriogram
showing aneurysms or occlusions of the visceral arteries, not due
to arteriosclerosis, fibromuscular dysplasia, or other
noninflammatory causes); and x) biopsy of the small or medium-sized
artery containing PAN (histological changes showing the presence of
granulocytes or mononuclear granulocytes). For classification
purposes, a patient should have PAN ≥ three of these 10 criteria.
Criteria for the classification of Takayasu arteritis (TA)
(18): i) Age at disease onset ≤40
years (development of symptoms or findings related to TA at age ≤40
years); ii) claudication of extremities (development and worsening
of fatigue and discomfort in muscles of ≥ one extremity whilst in
use, especially the upper extremities); iii) Decreased brachial
artery pulse (decreased pulsation of one or both of the brachial
arteries); iv) BP difference >10 mm Hg (Difference of >10 mm
Hg in systolic blood pressure between arms); v) Bruit over
subclavian arteries or aorta (bruit audible on auscultation over
one or both of the subclavian arteries or abdominal aorta); vi)
arteriogram abnormality (arteriographic narrowing or occlusion of
the entire aorta, its primary branches or large arteries in the
proximal upper or lower extremities, not due to arteriosclerosis,
fibromuscular dysplasia, or similar causes; changes usually focal
or segmental). For purposes of classification, a patient would be
diagnosed with Takayasu arteritis if ≥ three of these six criteria
are present. Exclusion criterion: Patients with secondary
vasculitis, systemic lupus erythematosus, rheumatoid arthritis,
malignancy, infection or with any other coexisting renal disease,
including anti-glomerular basement membrane nephritis, IgA
nephropathy, diabetic nephropathy or lupus nephritis.
A further 64 age- and sex-matched healthy controls
(HC) were included as the control group (female, n=34, male, n=30;
age, 48.13±11.03 years). All participants signed informed consent
and the study protocol was approved by the Ethics Committee of
Xinjiang People's Hospital (Urumqi, China).
Data collection and measurements
All clinical data were obtained from the medical
records of patients with SV during hospitalization. The following
laboratory parameters were assessed: Hematological parameters,
high-sensitivity C-reactive protein (Hs-CRP), serum creatinine
(Scr), erythrocyte sedimentation rate (ESR). RDW, white blood cell
(WBC), red blood cell (RBC), hemoglobin (HB) and platelet counts
were measured by electrical impedance testing method, products of
Sysmex diagnostic and using a Sysmex XN 9000 Hematology analyzer
(Sysmex GmbH). ESR was measured by using a Model Minitor-100 (light
emitting diodes and photocells were used to detect the change of
light transmittance at the interface between red blood cells and
plasma, where the ESR value was obtained). Hs-CRP was performed
using a turbid metric method and diagnostic products of Olympus
diagnostic, using an Olympus AU-2700 Chemistry Analyzer (Olympus
Corporation). Scr was measured by enzyme colorimetry method,
products of Olympus diagnostic and using an Olympus AU-2700
Chemistry Analyzer (Olympus Corporation).
Definitions of disease activity
Disease activity was measured using the third
version of the Birmingham Vasculitis Activity Score (BVAS)
(19). Active disease was defined
as a BVAS ≥1, whereas inactive disease was defined as BVAS=0.
Definitions of kidney injury
The presence of increased Scr, proteinuria and/or
hematuria was taken to indicate kidney injury. Increased Scr was
defined as Scr >84 µmol/l or >104 µmol/l for female and male
patients, respectively. Proteinuria was defined as >1+ in the
routine urine collection or in a 24-h urine collection, containing
≥150 mg protein. Hematuria was defined as ≥ five red blood cells
per high-power field (light microscopy; magnification, x400;
Olympus Corporation) in urine sediment (20,21).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 20.0; IBM Corp). Continuous variables are
presented as the mean ± SD or median (interquartile range).
Categorical variables are presented as the number (%). Comparisons
among groups were analyzed using an independent sample Student's
t-test or one-way ANOVA. Multiple comparison tests were performed
using the Student-Newman-Keuls test. The Kruskal-Wallis test was
used to test data that was not normally distributed. Multiple
comparison tests were performed using the Dunn's post hoc test.
Categorical variables were evaluated using the χ2 test.
Binary logistic regression was used to identify independent factors
of disease activity in patients with SV. Correlation between
numerical data was calculated using Spearman's or Pearson's
correlation coefficient. A receiver-operating characteristic (ROC)
curve was used to determine a cut-off value with the highest
sensibility and specificity for estimating the disease activity in
patients with SV. In addition, to further improve sensitivity or
specificity, multiple biomarkers were used for combined diagnosis,
and binary logistic regression analysis and ROC curves were
established (22). P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of patients with SV
and HC
A total of 287 patients with SV and 64 HCs were
recruited. Among the patients with SV, 170 were diagnosed as
anti-neutrophil cytoplasmic antibody associated vasculitis (AAV),
73 were diagnosed with PAN and 44 were diagnosed with TA) Among
them, 193 patients had active SV and 94 had inactive SV. Moreover,
149 patients displayed kidney injury and 138 patients did not
display kidney injury. Demographic and clinical characteristics of
patients and HCs are presented in Tables I and II. There were no significant differences
in the age and sex distribution of patients with SV and HCs (all
P>0.05).
 | Table IDemographic and clinical
characteristics in systemic vasculitis patients and healthy
controls. |
Table I
Demographic and clinical
characteristics in systemic vasculitis patients and healthy
controls.
| Parameters | SV, n=287 | HC, n=64 | P-valuec | AAV (n=170) | PAN (n=73) | TA (n=44) | P-valued |
|---|
| Age, years | 49.02±16.52 | 48.13±11.03 | 0.597 |
56.29±15.52a |
41.36±10.89a,b | 34.00±11.85 | <0.001 |
| Female, n (%) | 147 (51.2) | 34 (53.1) | 0.783 | 82
(48.2)a | 34
(46.6)a | 31 (70.5) | 0.021 |
| WBC,
x109/l | 8.36±3.21 | 6.25±1.43 | <0.001 | 8.90±3.48 |
7.31±2.21b | 7.95±3.04 | 0.001 |
| RBC,
x109/l | 4.21±0.88 | 4.78±0.50 | <0.001 |
3.87±0.93a |
4.73±0.53b | 4.67±0.65 | <0.001 |
| HB, g/l | 122.13±27.88 | 144.73±12.45 | <0.001 |
113.40±28.67a |
138.31±18.41b | 132.79±14.56 | <0.001 |
| PLT,
x109/l | 238.23±89.56 | 251.33±66.80 | 0.189 | 231.29±91.02 | 237.10±69.54 | 262.45±105.88 | 0.154 |
| RDW, % | 14.13±1.73 | 12.67±0.66 | <0.001 |
14.50±1.82a |
13.52±1.57b | 13.87±1.26 | <0.001 |
| ESR, mm/h | 31.52±25.49 | 11.13±8.11 | <0.001 |
39.27±27.79a |
18.60±11.89b | 25.63±23.55 | <0.001 |
| Hs-CRP, mg/l | 23.76±46.10 | 1.82±2.61 | <0.001 |
36.41±56.22a |
4.31±5.91b | 8.13±14.18 | <0.001 |
| Scr, mg/dl | 204.28±240.26 | 63.50±17.27 | <0.001 |
276.96±287.90a |
100.55±43.87b | 91.62±41.90 | <0.001 |
| BVAS | 9.54±6.68 | - | - |
10.82±6.88a |
8.11±5.67b | 7.00±6.34 | <0.001 |
 | Table IIDemographic and clinical
characteristics of patients with active stage, inactive stage and
healthy controls. |
Table II
Demographic and clinical
characteristics of patients with active stage, inactive stage and
healthy controls.
| Parameters | Active, n=193 | Inactive, n=94 | HC, n=64 |
P-valuec |
|---|
| Age, years | 51.91±16.31 |
43.15±15.57a,b | 48.13±11.03 | <0.001 |
| Female, n (%) | 92 (47.7) | 55 (58.5) | 34 (53.1) | 0.217 |
| WBC,
x109/l |
8.79±3.32b |
7.45±2.76a,b |
6.25±1.43a | <0.001 |
| RBC,
x109/l |
4.06±0.96b |
4.50±0.61a,b |
4.78±0.50a | <0.001 |
| HB, g/l |
117.01±29.56b |
132.93±20.17a,b |
144.73±12.45a | <0.001 |
| PLT,
x109/l | 235.44±93.82 | 244.03±80.18 | 251.33±66.80 | 0.402 |
| RDW, % |
14.45±1.82b |
13.48±1.30a,b |
12.67±0.66a | <0.001 |
| ESR, mm/h |
36.53±26.69b |
20.83±18.78a,b |
11.13±8.11a | <0.001 |
| Hs-CRP, mg/l |
28.85±50.59b |
13.52±33.35a,b |
1.82±2.61a | <0.001 |
| Scr, mg/dl |
265.21±271.04b |
76.39±31.12a |
63.50±17.27a | <0.001 |
RDW in patients with SV and HC
RDW in the SV group was significantly increased
compared with matched HCs (14.13±1.73 vs. 12.67±0.66; P<0.05;
Fig. 1A). RDW was higher in the
active stage group compared with the inactive stage group
(14.45±1.82 vs. 13.48±1.30; P<0.05; Fig. 1B). In addition, the RDW was
significantly different between patients with kidney injury and
patients without kidney injury (14.35±1.84 vs. 13.89±1.56;
P<0.05; Fig. 1C). In the
subgroup analysis, the RDW of each subgroup, including TA, PAN and
AAV, was higher compared with the HC group (AAV vs. HC, 14.50±1.82
vs. 12.67±0.66, P<0.05; PAN vs. HC, 13.52±1.57 vs. 12.67±0.66,
P<0.05; TA vs. HC, 13.87±1.26 vs. 12.67±0.66, P<0.05).
Moreover, RDW was increased in patients with AAV compared with
patients with PAN or TA (AAV vs. PAN, 14.50±1.82 vs. 13.52±1.57,
P<0.05; AAV vs. TA, 14.50±1.82 vs. 13.87±1.26, P<0.05;
Fig. 1D). RDW was not significantly
different between the patients with PAN and patients with TA
(13.52±1.57 vs. 13.87±1.26; P>0.05; Fig. 1D).
Correlation between RDW and other
parameters in patients with SV
The relationship between RDW and laboratory
parameters in patients with SV was assessed. The results indicated
that there was positive correlation between RDW and BVAS (r=0.291;
P<0.001), Hs-CRP (r=0.372; P<0.001), ESR (r=0.212; P=0.001),
Scr (r=0.215; P<0.001) and WBC (r=0.213; P<0.001) (Fig. 2A-E). By contrast, the opposite
result was obtained between RDW and HB (r=-0.365; P<0.001;
Fig. 2F). Multivariate logistic
regression analysis suggested that RDW [odds ratio (OR) =1.500; 95%
confidence interval (CI)=1.101-2.042; P=0.010] and Scr [OR=1.024;
95% CI=1.013-1.045; P<0.001] were independently associated with
patients with active SV (Table
III).
 | Figure 2Correlations of RDW with BVAS, Hs-CRP,
ESR, Scr, WBC and HB. The correlations between RDW and (A) BVAS,
(B) Hs-CRP, (C) ESR, (D) Scr, (E) WBC and (F) HB were analyzed in
patients with systemic vasculitis. BVAS, Birmingham Vasculitis
Activity Score; ESR, erythrocyte sedimentation rate; HB,
hemoglobin; Hs-CRP, high-sensitivity C-reactive protein; RDW, red
blood cell distribution width; Scr, serum creatinine; WBC, white
blood cell. |
 | Table IIIMultivariate logistic regression
analysis of patients with active stage versus inactive stage. |
Table III
Multivariate logistic regression
analysis of patients with active stage versus inactive stage.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variable | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Age | 1.035 | 1.018-1.052 | <0.001 | 0.984 | 0.957-1.012 | 0.261 |
| Female | 0.639 | 0.388-1.052 | 0.078 | 0.599 | 0.255-1.406 | 0.239 |
| WBC | 1.167 | 1.062-1.283 | 0.001 | 1.136 | 0.959-1.346 | 0.140 |
| HB | 0.977 | 0.966-0.987 | <0.001 | 1.009 | 0.986-1.033 | 0.435 |
| PLT | 0.999 | 0.996-1.002 | <0.001 | 0.998 | 0.993-1.004 | 0.536 |
| RDW | 1.533 | 1.260-1.865 | <0.001 | 1.500 | 1.101-2.042 | 0.010 |
| ESR | 1.031 | 1.017-1.046 | <0.001 | 1.018 | 0.991-1.045 | 0.190 |
| Hs-CRP | 1.010 | 1.002-1.018 | 0.018 | 0.994 | 0.979-1.009 | 0.413 |
| Scr | 1.022 | 1.014-1.031 | <0.001 | 1.024 | 1.013-1.045 | <0.001 |
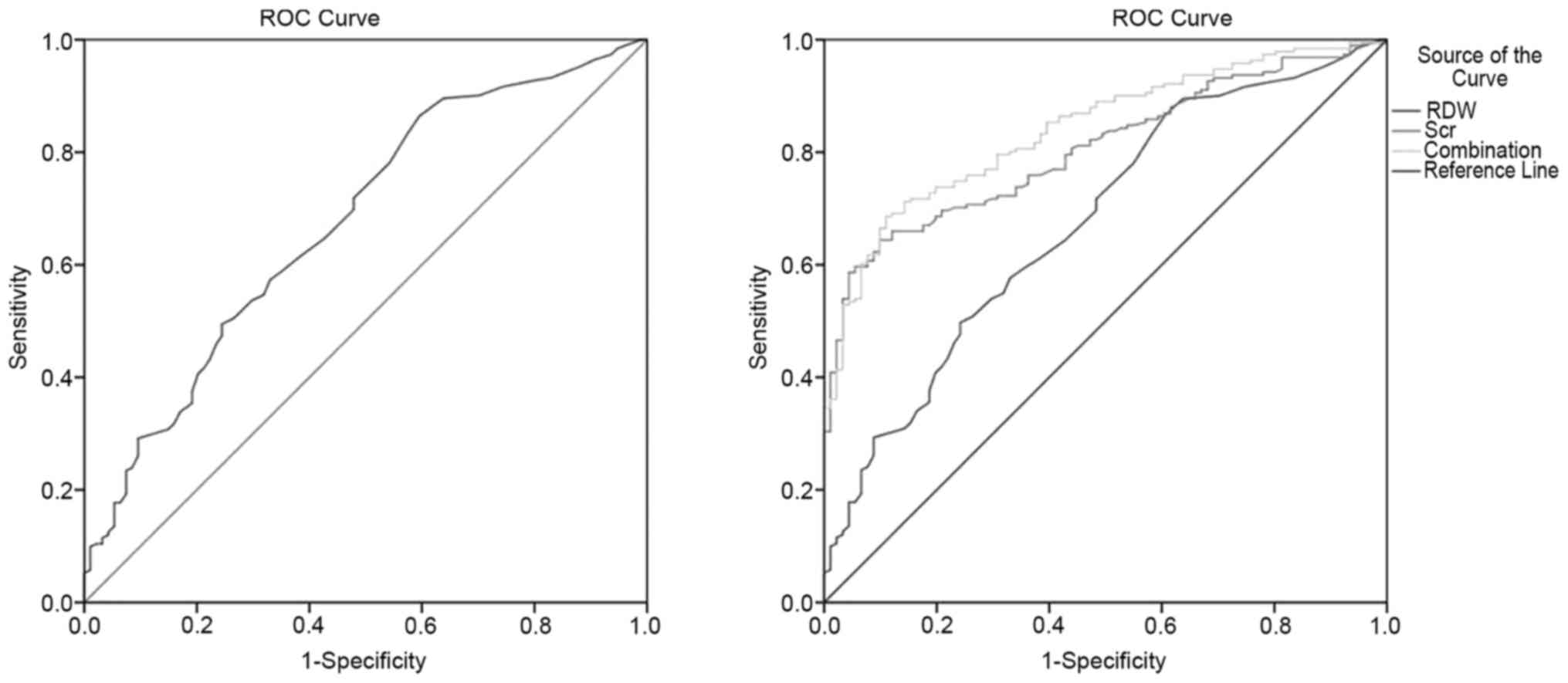
ROC curve analysis to identify optimal
cutoff values of RDW
When assessing the presence of disease activity with
RDW in patients with SV, a cut-off value of 13.65, with 57.3%
sensitivity and 67.0% specificity was observed according to ROC
curve analysis [area under the curve (AUC)=0.68; 95% CI=0.61-0.74;
Fig. 3A]. Furthermore, binary
logistic regression and ROC curves assessed the combined diagnostic
efficiency of multiple parameters. The combination of RDW and Scr,
after adjusting by the regression coefficient of the binary
logistic regression, had 68.6% sensitivity and 88.9% specificity
for diagnosing patients with active SV (AUC=0.84; 95% CI=0.80-0.89;
Fig. 3B).
Discussion
The extent of disease activity or relapse serves an
important role in the early individualized therapy and prognostic
assessment of SV (23). However,
the assessment of disease activity and relapses in patients with SV
is difficult (24). Although
angiography and biopsies are used to assess disease activity and
relapses in patients with SV, both strategies display imperfect
sensitivity due to sampling error and often contain inconclusive
findings (25). Furthermore, the
strategies are expensive, invasive, clinically risky and not easily
tolerated by all patients (26).
Therefore, improved tests and useful markers are required to assess
disease activity and relapses in patients with SV. RDW is an
indicator of inflammation that has feasibility as a prognostic
parameter in various diseases (7).
In the clinic, RDW is easily obtained as part of a routine blood
examination and provides valuable information in a number of
diseases without adding additional financial burden on the patients
(8). Recent studies have discovered
a relationship between subsets of SV and RDW (27,28);
therefore, the present study investigated whether RDW could serve a
role as a marker for estimating the disease activity of SV.
The results of the present study suggested that RDW
was significantly elevated in patients with active SV compared with
patients with inactive SV or HCs. The correlation analysis
indicated that there was positive correlation between RDW and BVAS,
Hs-CRP, ESR and WBC. As the P-value was low and the correlation
coefficient was <0.4, it is unlikely that there was a strong
correlation. A potential explanation for this is that the present
study was retrospective; therefore, further longitudinal studies
are required to verify the results of the present study. In
addition, RDW was found to be an independent factor for patients
with active SV, which was indicated by multivariate logistic
regression analysis. The results suggested that RDW could be a
reflection of disease activity in patients with SV. As
aforementioned, there was a connection between RDW and SV subsets.
RDW has been reported to be a potential marker to evaluate disease
activity in other forms of vasculitis, including Behçet disease, TA
and granulomatosis with polyangiitis (29-31),
which was consistent with the results of the present study. In
addition, RDW in patients with inactive SV was raised compared with
the HCs. The results indicated a potential persistent state of
hidden inflammation or tissue/vascular wall injury, but further
investigation is required to verify this finding.
Several studies have assessed renal function and
extent of renal damage with RDW in various diseases (32-34).
The present study investigated whether RDW was related to kidney
injury in SV. The results indicated that RDW was elevated in
patients with SV with kidney injury compared with patients with SV
without kidney injury. The correlation analysis suggested that
there was a positive correlation between RDW and Scr, as well as a
negative correlation between RDW and HB. Zhu et al (27) indicated that RDW was increased in
patients with TA with anemia compared with patients with TA without
anemia and control subjects, which was consistent with the present
study. However, Kim et al (30) indicated that RDW was not
significantly associated with HB, which may be related to the sex
and age variations between the enrolled cohorts.
Subgroup analysis of the type of SV was also
performed in the present study. The results indicated that RDW was
significantly higher in patients with AAV, PAN and TA compared with
HCs, which suggested that RDW may participate in the pathogenesis
behind SV subsets. However, the exact role of RDW requires further
investigation. Additionally, RDW was higher in patients with AAV
compared with patients with PAN and TA. A potential explanation may
be due to the relatively higher disease activity of patients with
AAV. To further assess the disease activity in patients with SV,
ROC analysis was conducted. The results suggested that the optimal
cut-off value of RDW was 13.65 with 57.3% sensitivity and 67.0%
specificity. To improve the accuracy and efficiency of diagnosis,
identification of patients with active SV is important. Using RDW
and Scr as combinatorial markers to construct ROC curves using
binary logistic regression indicated that RDW combined with Scr had
an advantage compared with RDW alone, with 68.6% sensitivity and
88.9% specificity. Therefore, the combination achieved higher
values for assessing patients with active SV.
At present, the exact pathogenesis behind the
relationship between RDW and SV is not completely understood. A
previous study indicated that RDW levels might be affected by
inflammatory cytokines, such as IL-1, IL-6 and TNF-α (12). Inflammatory cytokines may affect the
function of bone marrow, inhibit the maturation of erythrocytes and
increase the RDW (35). Therefore,
higher RDW levels may reflect the underlying inflammatory state
caused by chronic inflammation, thereby transforming the
intracellular homeostasis of red blood cells and impairing the
maturation of red blood cells (36). Collectively, the aforementioned
findings suggested that cytokines and RDW serve an important role
in the pathogenesis of SV.
The results of the present study suggested that RDW
may serve as a suitable biomarker for assessing the disease
activity of patients with SV and its subgroups. However, the
present study had a number of limitations. Firstly, due to the
limitations of cross-sectional research, the present study was
unable to derive the causal relationship between RDW and SV.
Therefore, prospective studies are required to investigate whether
RDW predicts the prognosis of SV. Secondly, the present study
included patients with anemia, but serum iron, vitamin B12 and
folic acid levels were not recorded. Therefore, some of the
patients may have had anemia due to vitamin B12 and mineral
deficiencies, thus affecting RDW levels. Finally, the relationship
between RDW and other sensitive inflammatory markers, such as
TNF-α, IL-1 and IL-6, was not evaluated.
In conclusion, the results of the present study
suggested that RDW might serve as a potential marker for disease
activity and kidney injury in patients with SV. Moreover, the
combination of RDW and Scr may have a higher value when assessing
the risk of disease activity in patients with SV. However, further
studies are required to investigate the exact role of RDW in the
pathogenesis of SV.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the National Natural
Science Foundation of Xinjiang (grant no. 2018D01C117).
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
JH and BZ analyzed the data and wrote the
manuscript. XC, ShanL and ShasL performed the data collection from
medical records and participated in the data analysis. QZ, XC and
TW participated in the study design and revised the manuscript. XC,
TW, XA and AA performed the data collection from medical records.
XC and TW revised the manuscript critically for important
intellectual content. QZ and TW confirm the authenticity of all the
raw data. NL conceived and helped design the study, revised the
manuscript and approved the final version of the manuscript. JH and
BZ contributed equally to this study, and should be regarded as
co-first authors. All authors reviewed and approved the final
manuscript.
Ethics approval and consent to
participate
All participants have signed an informed consent
form the study protocol was approved by the ethics committee of
Xinjiang People's Hospital (Urumqi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Elefante E, Bond M, Monti S, Lepri G,
Cavallaro E, Felicetti M, Calabresi E, Posarelli C, Talarico R,
Quartuccio L and Baldini C: One year in review 2018: Systemic
vasculitis. Clin Exp Rheumatol. 36 (Suppl 111):S12–S32.
2018.PubMed/NCBI
|
|
2
|
Perez-Alamino R and Maldonado-Ficco H: New
insights on biomarkers in systemic vasculitis. Curr Rheumatol Rep.
17(12)2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Trieste L, Palla I, Baldini C, Talarico R,
D'Angiolella L, Mosca M and Turchetti G: Systemic vasculitis: How
little we know about their societal and economic burden. Clin Exp
Rheumatol. 30 (Suppl 73):S154–S156. 2012.PubMed/NCBI
|
|
4
|
Benarous L, Terrier B, Laborde-Casterot H,
Bérezné A, Dunogué B, Cohen P, Puéchal X, Mouthon L, Bensefa-Colas
L and Guillevin L: French Vasculitis Study Group (FVSG).
Employment, work disability and quality of life in patients with
ANCA-associated vasculitides. The EXPOVAS study. Clin Exp
Rheumatol. 35 (Suppl 103):S40–S46. 2017.PubMed/NCBI
|
|
5
|
Barra LJ, Bateman EA, Rohekar S, Pagnoux C
and Moradizadeh M: Assessment of work limitations and disability in
systemic vasculitis. Clin Exp Rheumatol. 34 (Suppl 97):S111–S114.
2016.PubMed/NCBI
|
|
6
|
Yousefi B, Sanaie S, Ghamari AA,
Soleimanpour H, Karimian A and Mahmoodpoor A: Red cell distribution
width as a novel prognostic marker in multiple clinical studies.
Indian J Crit Care Med. 24:49–54. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Salvagno GL, Sanchis-Gomar F, Picanza A
and Lippi G: Red blood cell distribution width: A simple parameter
with multiple clinical applications. Crit Rev Clin Lab Sci.
52:86–105. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lippi G, Mattiuzzi C and Cervellin G:
Learning more and spending less with neglected laboratory
parameters: The paradigmatic case of red blood cell distribution
width. Acta Biomed. 87:323–328. 2016.PubMed/NCBI
|
|
9
|
Hu Z, Sun Y, Wang Q, Han Z, Huang Y, Liu
X, Ding C, Hu C, Qin Q and Deng A: Red blood cell distribution
width is a potential prognostic index for liver disease. Clin Chem
Lab Med. 51:1403–1408. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Patel KV, Ferrucci L, Ershler WB, Longo DL
and Guralnik JM: Red blood cell distribution width and the risk of
death in middle-aged and older adults. Arch Intern Med.
169:515–523. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hu ZD, Chen Y, Zhang L, Sun Y, Huang YL,
Wang QQ, Xu YL, Chen SX, Qin Q and Deng AM: Red blood cell
distribution width is a potential index to assess the disease
activity of systemic lupus erythematosus. Clin Chim Acta.
425:202–205. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
He Y, Liu C, Zeng Z, Ye W, Lin J and Ou Q:
Red blood cell distribution width: A potential laboratory parameter
for monitoring inflammation in rheumatoid arthritis. Clin
Rheumatol. 37:161–167. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu H, Fu S, Wang W, Zhang Q, Hu J, Gao L,
Zhu W and Gong F: Predictive value of red blood cell distribution
width for coronary artery lesions in patients with Kawasaki
disease. Cardiol Young. 26:1151–1157. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Masi AT, Hunder GG, Lie JT, Michel BA,
Bloch DA, Arend WP, Calabrese LH, Edworthy SM, Fauci AS, Leavitt
RY, et al: The American college of rheumatology 1990 criteria for
the classification of Churg-Strauss syndrome (allergic
granulomatosis and angiitis). Arthritis Rheum. 33:1094–1100.
1990.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Leavitt RY, Fauci AS, Bloch DA, Michel BA,
Hunder GG, Arend WP, Calabrese LH, Fries JF, Lie JT, Lightfoot RW
Jr, et al: The American college of rheumatology 1990 criteria for
the classification of Wegener's granulomatosis. Arthritis Rheum.
33:1101–1107. 1990.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jennette JC, Falk RJ, Bacon PA, Basu N,
Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen
EC, et al: 2012 Revised international chapel hill consensus
conference nomenclature of vasculitides. Arthritis Rheum. 65:1–11.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lightfoot RW Jr, Michel BA, Bloch DA,
Hunder GG, Zvaifler NJ, McShane DJ, Arend WP, Calabrese LH, Leavitt
RY, Lie JT, et al: The American college of rheumatology 1990
criteria for the classification of polyarteritis nodosa. Arthritis
Rheum. 33:1088–1093. 1990.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Arend WP, Michel BA, Bloch DA, Hunder GG,
Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY, Lie JT, Lightfoot
RW Jr, et al: The American college of rheumatology 1990 criteria
for the classification of Takayasu arteritis. Arthritis Rheum.
33:1129–1134. 1990.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mukhtyar C, Lee R, Brown D, Carruthers D,
Dasgupta B, Dubey S, Flossmann O, Hall C, Hollywood J, Jayne D, et
al: Modification and validation of the Birmingham vasculitis
activity score (version 3). Ann Rheum Dis. 68:1827–1832.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li N, Zhu B, Zhu Q, Heizati M, Wu T, Wang
G, Yao X, Luo Q and Liu S and Liu S: Serum lysosomal-associated
membrane protein-2 levels are increased in small and medium-vessel
vasculitis, especially in polyarteritis nodosa. Clin Exp Rheumatol.
37 (Suppl 117):S79–S85. 2019.PubMed/NCBI
|
|
21
|
Liu S, Li N, Zhu Q, Zhu B, Wu T, Wang G,
Liu S and Luo Q: Increased serum MCP-1 levels in systemic
vasculitis patients with renal involvement. J Interferon Cytokine
Res. 38:406–412. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhu B, Li N, Zhu Q, Wu T, Heizati M, Wang
G, Yao X, Luo Q, Liu S, Liu S and Hong J: Association of serum high
mobility group box 1 levels with disease activity and renal
involvement in patients with systemic vasculitis. Medicine
(Baltimore). 98(e14493)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Eleftheriou D and Brogan PA: Therapeutic
advances in the treatment of vasculitis. Pediatr Rheumatol Online
J. 14(26)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Katsuyama T, Sada KE and Makino H: Current
concept and epidemiology of systemic vasculitides. Allergol Int.
63:505–513. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hatemi G, Esatoglu SN and Yazici Y:
Biomarkers in vasculitis. Curr Opin Rheumatol. 30:30–35.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Langford CA: Vasculitis. J Allergy Clin
Immunol. 125 (Suppl 2):S216–S225. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhu X, Zhang M, Lan F, Wei H, He Q, Li S
and Qin X: The relationship between red cell distribution width and
the risk of Henoch-Schönlein purpura nephritis. Br J Biomed Sci.
75:30–35. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kim DS, Shin D, Kim TG, Kim SH, Kim DY,
Kim SM and Lee MG: Red blood cell distribution width as a useful
indicator to predict systemic vasculitis in patients with cutaneous
vasculitis. Rheumatol Int. 35:719–725. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu Q, Dang AM, Chen BW, Lv NQ, Wang X and
Zheng DY: The association of red blood cell distribution width with
anemia and inflammation in patients with Takayasu arteritis. Clin
Chim Acta. 438:205–209. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kim HJ, Yoo J, Jung SM, Song JJ, Park YB
and Lee SW: Red blood cell distribution width can predict
vasculitis activity and poor prognosis in granulomatosis with
polyangiitis. Yonsei Med J. 59:294–302. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Aksoy ŞN, Savaş E, Sucu M, Kisacik B, Kul
S and Zengin O: Association between red blood cell distribution
width and disease activity in patients with Behçet's disease. J Int
Med Res. 43:765–773. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li ZZ, Chen L, Yuan H, Zhou T and Kuang
ZM: Relationship between red blood cell distribution width and
early-stage renal function damage in patients with essential
hypertension. J Hypertens. 32:2450–2455. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang M, Zhang Y, Li C and He L:
Association between red blood cell distribution and renal function
in patients with untreated type 2 diabetes mellitus. Ren Fail.
37:659–663. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ujszaszi A, Molnar MZ, Czira ME, Novak M
and Mucsi I: Renal function is independently associated with red
cell distribution width in kidney transplant recipients: A
potential new auxiliary parameter for the clinical evaluation of
patients with chronic kidney disease. Br J Haematol. 161:715–725.
2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang W, Liu J, Yang YH, Zhai ZG, Wang C
and Wang J: Red cell distribution width is increased in chronic
thromboembolic pulmonary hypertension. Clin Respir J. 10:54–60.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Afsar B, Saglam M, Yuceturk C and Agca E:
The relationship between red cell distribution width with
erythropoietin resistance in iron replete hemodialysis patients.
Eur J Intern Med. 24:e25–e29. 2013.PubMed/NCBI View Article : Google Scholar
|