Introduction
Stroke is an acute cerebral blood circulation
disorder, which is the second deadliest and the first most
disabling disease worldwide (1).
Ischemic stroke accounts for ~85% of cases of stroke, and the main
causes of ischemic stroke include middle cerebral artery thrombosis
and acute obstruction caused by thrombosis from other sources
(2). Diabetes is a group of
metabolic diseases characterized by hyperglycemia (3). Sustained hyperglycemia and long-term
metabolic disorders can lead to systemic organ and tissue damage,
dysfunction and failure (4).
Diabetes is also a high-risk factor for ischemic stroke (5). The probability of ischemic stroke in
individuals with diabetes is three times higher than that in
non-diabetic individuals (6).
Furthermore, patients with diabetic stroke have a faster course of
disease, higher mortality and worse prognosis than those with other
stroke mechanistic subtypes (7).
Although there have been numerous studies on different classes of
neuroprotectants, including excitatory amino acids, antioxidants,
and anti-inflammatory and lipid regulation agents, no drug has been
able to exhibit a clear therapeutic effect in clinical practice
(8-10).
At present, stem cell transplantation is a novel therapeutic
strategy for ischemic stroke (11,12).
However, research on this therapy for ischemic stroke with
diabetes-associated diseases is lacking.
Endothelial progenitor cells (EPCs), as the
progenitor cells of endothelial cells, serve essential roles in the
repair of endothelial injury or dysfunction and the formation of
new blood vessels (13). Generally,
EPCs are found in the stem cells of bone marrow tissues, and in
small amounts in the peripheral blood of healthy organisms
(14). When peripheral blood
vessels are damaged, EPCs in bone marrow quickly mobilize into the
blood circulation under the influence of chemokines and home to the
endothelium to assist in the repair of damage due to ischemia,
hypoxia or injury (15). EPCs also
have the potential to proliferate, and can be directionally
differentiated into mature vascular endothelial cells (VECs), or
induced to secrete vasogenic growth factors to activate peripheral
mature endothelial cells and accelerate the repair of damaged VECs
(16,17). EPC transplantation has been
demonstrated to be effective in promoting angiogenesis following
ischemic stroke in animal models, contributing to the formation of
an enriched tubular environment conducive to neurogenesis, thus
accelerating the recovery of nerve function (18-20).
However, the potential role and mechanism of EPCs in type 2
diabetes with ischemic stroke have not been fully elucidated.
Matrix metalloproteinases (MMPs) are a family of
Zn2+-dependent proteases secreted by connective tissues
that can degrade components of the extracellular matrix (ECM)
(21). Studies have identified that
MMPs participate in a variety of biological processes, including
the inflammatory response, tumor metastasis and cell migration
(22,23). In addition, MMPs and their
inhibitors have been demonstrated to be associated with the
progression of ischemic stroke and atherosclerosis (24-26).
It has been reported that after ischemic stroke, MMPs can disrupt
the blood-brain barrier and affect a series of inflammatory
cascades (27). Therefore, the
targeting of MMPs is considered a potential approach for the
treatment of ischemic stroke, and MMP inhibitors may serve as a
safe and effective therapy for ischemic stroke.
In the present study, the effects of a combination
of EPCs and an MMP inhibitor, BT-94, in diabetic ischemic stroke
were explored through a series of in vitro and in
vivo experiments.
Materials and methods
Animals
A total of 32 male C57/BL6 mice weighing 25-30 g
were purchased from the Model Animal Research Center of Nanjing
University at 6 weeks of age. All mice received free access to food
and water for a week at room temperature with a 12-h light/dark
cycle and relative humidity of 40-70% to adapt to the new
laboratory environment. For DM mice model, mice fed by high-fat
diet (60% standard diet, 20% lard, 10% yolk powder and 10%
saccharose) for 8 weeks and subsequently injected intraperitoneally
with 30 mg/kg STZ. Meanwhile, the other mice sequentially fed with
a standard diet for collection EPCs. All experiments using animals
were approved by the Institutional Animal Ethics Committee of
Guizhou Medical University and conducted according to Animal Care
Guidelines for the Care and Use of Animals from Guizhou Medical
University.
Isolation and culture of EPCs
Cell culture dishes were coated with fibronectin
(Sigma-Aldrich; Merck KGaA) and incubated at 37˚C for 1 h. Mice
were anesthetized using 2% pentobarbital sodium (45 mg/kg,
intraperitoneal injection; cat. no. 1063180500; Merck KGaA). After
sacrificing the mice by cervical dislocation, the limbs were
removed and the muscles shaved off to reveal the bones. The bone
marrow cavity was exposed and cells were collected from the cavity
and rinsed with PBS. The cell suspension was added as an upper
layer to 5 ml lymphocyte separation medium (Sigma-Aldrich; Merck
KGaA), and density gradient centrifugation (550 x g, 20 min, 37˚C)
was performed until the marrow cavity fluid was divided into four
layers. The fog-like white layer, which was the mononuclear cell
layer, was gently sucked out using a straw, and the cells were
cultured using endothelial growth medium™-2 (cat. no. CC-3162;
Lonza Group, Ltd.) containing 5% fetal bovine serum (FBS; cat. no.
10099-141; Gibco; Thermo Fisher Scientific, Inc.) at 37˚C with 5%
CO2. The EPCs were cultured for 7 days for use in the
subsequent experiments.
Detection of Dil-labeled acetylated
low-density lipoprotein (Dil-ac-LDL) and FITC-lectin-Ulex europaeus
agglutin (UEA)-1
After washing the EPCs with PBS three times on day
10 of incubation, the EPCs were cultured in M199 medium (Gibco;
Thermo Fisher Scientific, inc.) containing 12 µg/ml Dil-ac-LDL
(cat. no. BT-902; Biomedical Technologies; Alfa Aesar) at 37˚C in
an incubator with 5% CO2 for 4 h. After washing, the
EPCs were fixed with 2% paraformaldehyde for 20 min at 4˚C and 10
µg/ml FITC-lectin-UEA-1 (cat. no. L9006; Sigma-Aldrich; Merck KGaA)
was added and incubated at 37˚C for 4 h. After sealing with neutral
resin, the labeled cells were observed and images captured using a
laser confocal microscope (Olympus Corporation).
Immunofluorescence (IF) assay
EPCs were inoculated in 24-well plates and incubated
for 3 days, after which they were washed with PBS to remove any
detached cells. Then, adherent cells were fixed with 10% formalin
for 15 min at room temperature to ensure that the EPCs were
completely adherent to the well. The EPCs were blocked using 5%
bovine serum albumin (BSA; Sigma-Aldrich; Merck KGaA) for 1 h at
room temperature and incubated with the following antibodies from
Abcam at 4˚C overnight: Anti-CD34 (1:10,000; rabbit; cat. no.
ab81289; Abcam), anti-CD133 (1:2,000; rabbit; cat. no. ab222782;
Abcam), anti-VEGFR2 (1:1,000; rabbit; cat. no. ab134191; Abcam) and
anti-von Willebrand factor (vWF; 1:1,000; rabbit; cat. no.
ab154193; Abcam). The EPCs were then processed with Alexa
Fluor® 488 antibodies (1:100; cat. no. sc-516248; Santa
Cruz Biotechnology, Inc.) for 1 h at room temperature. After
nuclear staining with DAPI (1:500; cat. no. D9564; Sigma-Aldrich;
Merck KGaA) at room temperature for 5 min, a fluorescence
microscope was used for visualization.
Construction of an oxygen-glucose
deprivation/reoxygenation (OGD/R) cell model
HT22 mouse hippocampal cells (cat. no. SCC129; Merck
KGaA) were grown in high-glucose DMEM (Gibco; Thermo Fisher
Scientific, Inc.) with 10% FBS, 2 mM glutamine, 100 U/ml penicillin
and 100 µg/ml streptomycin. The OGD/R model was constructed
following the protocol described in previous studies (28-31).
The HT22 cells were incubated in glucose-free DMEM (Gibco; Thermo
Fisher Scientific, Inc.) under hypoxic conditions (1%
O2, 94% N2, 5% CO2) at 37˚C for 2
h, and then cultured in normal DMEM under normal oxygen conditions
(95% air, 5% CO2) for 24 h. HT22 cells cultured in
normal oxygen conditions were used as a control.
Establishment of the middle cerebral
artery occlusion (MCAO) model
Based on previous research (32), the aforementioned DM mice were
anesthetized by the intraperitoneal injection of 2% pentobarbital
sodium (45 mg/kg). The common carotid artery, internal carotid
artery and external carotid artery were separated, and blood flow
to the internal and common carotid arteries was briefly blocked.
This was achieved by inserting a heated nylon thread into the
bifurcation of the common carotid artery and into the internal
carotid artery. The heated nylon wire was quickly advanced to the
bifurcation of the internal carotid artery and common carotid
artery by ~10 mm and knotted. The body temperature of the mice was
maintained at 37˚C during the procedure, and the thread plug was
removed after 60 min.
Experimental groups
After the DM-MCAO model was established for 24 h,
mice were randomly divided into four groups as follows: DM-MCAO
model group (n=8); DM-MCAO + EPCs (n=8), in which 1x106
EPCs were administered to MCAO model mice through the right
internal carotid artery; DM-MCAO + BB-94 (cat. no. S7155; Selleck
Chemicals) (n=8), in which BB-94 (50 mg/kg) was administered
intraperitoneally to MCAO model mice once a day for 7 days; and
DM-MCAO + EPCs + BB-94 (n=8).
Modified neurological severity score
(mNSS)
With reference to a previous study (33), scoring was performed using an
18-point mNSS system to evaluate the neurological function of the
mice, where 0 is a normal score, and 18 is the maximal deficit
score. The mNSS assessment includes four neurological tests,
namely, a fine motor function measurement scale, sensory ability
test, beam balance test, and absence of reflection and abnormal
motion. Data were analyzed using a Kruskal-Wallis test followed by
Steel-Dwass tests to compare between multiple groups.
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) assay
The OGD/R model cells were collected and adjusted to
a concentration of 1x105 cells/ml. Then, 100 µl
cells/well were inoculated into 96-well plates. Different
concentrations of BB-94 (0, 5, 10, 20, 30 and 40 mM; 20 µl) were
added to each well. In another experiment, OGD/R model cells were
treated with 1,000 EPCs or/and BB-94 (5 mM). For each group, 6
duplicate wells were set. The cells were cultured for 48 h at 37˚C.
The supernatant was then discarded and 20 µl MTT (10 mg/ml) was
added to each well. After 4 h at 37˚C, 150 µl dimethyl sulfoxide
was added and the plate was oscillated for 10 min. The absorbance
value at 490 nm was measured using a microplate reader.
Flow cytometric analysis
OGD/R cells were collected and incubated with
various concentrations of BB-94 (0, 5, 10, 20, 30 and 40 mM) for 48
h. The apoptosis rate in each group was then assessed using Annexin
V/FITC double staining with a FACSCalibur Flow Cytometry System
(each, BD Biosciences) according to the manufacturer's
instructions. The results were analyzed using FlowJo v.8.0 software
(Tree Star, Inc.).
Enzyme-linked immunosorbent assays
(ELISAs)
For assessment of the in vitro experiment,
the culture supernatants of OGD/R model cells treated with EPCs
or/and BB-94 (5 mM) were collected for analysis; for the in
vivo experiment, serum was collected from the MCAO model mice
treated with EPCs or/and BB-94 (50 mg/kg). The aforementioned
samples were prepared for ELISA according to the instructions of
the ELISA kits. Superoxide dismutase (SOD), reactive oxygen species
(ROS), malondialdehyde (MDA) and D-lactate dehydrogenase (D-LDH)
were quantified using a mouse SOD ELISA kit (cat. no. E-EL-M2398),
ROS Assay kit (cat. no. E-BC-K138), MDA ELISA kit (cat. no.
E-EL-0060c) and mouse D-LDH ELISA kit (cat. no E-EL-M0419c), all
from Elabscience.
Collection of brain tissue
samples
Mice in each group were anesthetized with an
intraperitoneal injection of 2% pentobarbital sodium (45 mg/kg) and
then decapitated. The brain tissues were removed and immediately
used for later experiments.
Reverse transcription-quantitative PCR
(RT-qPCR) assay
The obtained brain tissues (20 mg) were added to 1
ml TRIzol® reagent (cat. no. 15596026; Thermo Fisher
Scientific, Inc.) and the tissues were cut up and ground. Total
mRNAs were isolated from the tissues according to the
manufacturer's protocol. Total RNA was isolated from the OGD/R
model cells also using TRIzol® (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
The purity and content of total RNA were determined by UV
spectrophotometry. A Reverse Transcription kit (Takara Bio, Inc.)
was used to synthetize cDNA from the RNA according to the
manufacturer's protocol. The following temperature protocol was
used: 37˚C for 15 min (reverse transcription reaction) and 85˚C for
5 sec (reverse transcriptase inactivation reaction). The levels of
MMP-2, MMP-8, MMP-9 and tissue inhibitor of metalloproteinases-1
(TIMP-1) were examined using SYBR-Green PCR Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions for PCR was as follows: 95˚C for 3 min, followed by 40
cycles of denaturation at 95˚C for 10 sec, followed by annealing
and extension at 58˚C for 30 sec. The relative expression level was
calculated using the 2-ΔΔCq method (34). The primers used are displayed in
Table I. GAPDH was used as the
reference gene.
 | Table ISequences of primers used in
quantitative PCR. |
Table I
Sequences of primers used in
quantitative PCR.
| Gene | Sequence
(5'-3') |
|---|
| GAPDH | Forward:
TGTTCGTCATGGGTGTGAAC |
| GAPDH | Reverse:
ATGGCATGGACTGTGGTCAT |
| MMP-2 | Forward:
CCCTCCCCTGATGCTGATACT |
| MMP-2 | Reverse:
GTCACTGTCCGCCAAATAAACC |
| MMP-8 | Forward:
CTGTTGAAGGCCTAGAGCTGCTG CTCC |
| MMP-8 | Reverse:
GATCTTCTCTTCAAACTCT ACCC |
| MMP-9 | Forward:
CCCTGGAGACCTGAGAACCA |
| MMP-9 | Reverse:
AACCATAGCGGTACAGGTATTCCT |
| TIMP-1 | Forward:
CTGGCATCCTCTTGTTGCTATC |
| TIMP-1 | Reverse:
AACGCTGGTATAAGGTGGTCTC |
Western blot assay
Ground brain tissues (20 mg) were added to RIPA
lysis buffer (cat. no. P0013B; Beyotime Institute of Biotechnology)
containing protease inhibitor, and total proteins were extracted by
centrifugation at 14,000 x g for 10 min at 4˚C. The OGD/R cells
(1x106/well) were washed once with ice cold PBS and
lysed with RIPA lysis buffer on ice for 30 min, after which the
total protein was also extracted using RIPA lysis buffer (Beyotime
Institute of Biotechnology). An Ultra-Bradford Protein Assay kit
(Sangon Biotech Co., Ltd.) was used to determine the concentration
of proteins. The proteins (30 µg/lane) were subjected to 10%
SDS-PAGE, and then transferred onto a PVDF membrane (Roche
Diagnostics). After blocking the PVDF membrane with 5% BSA for 1 h,
the membrane was incubated with primary antibodies overnight at
4˚C. The secondary antibody HRP-labeled goat anti-rabbit IgG
(dilution 1:5,000; cat. no. ab6721; Abcam) was then applied to
react at room temperature for 1 h. After visualization of the bands
using an ECL reagent (cat. no. sc-2048; Santa Cruz Biotechnology,
Inc.), the results were recorded using a gel imaging system (Kodak
film developer; FUJIFILM Wako Pure Chemical Corporation) and
analyzed using ImageJ software (version 7.0; National Institutes of
Health). The primary antibodies targeted MMP-2 (1:1,000; cat. no.
ab97779), MMP-9 (1:1,000; cat. no. ab38898), MMP-8 (1:500; cat. no.
ab53017), TIMP-1 (1:1,000; cat. no. ab211926) and GAPDH (1:2,000;
cat. no. ab9482), and were all acquired from Abcam.
H&E staining
After fixing the brain tissues with 4%
paraformaldehyde at 4˚C overnight, the tissues were dehydrated with
gradient ethanol, made transparent with xylene (cat. no. 534056;
Sigma-Aldrich; Merck KGaA) and embedded with paraffin. The tissues
were then sliced into 3-µm slices. After dewaxing, the slices were
processed with xylene and gradient ethanol. The sections were
stained with hematoxylin (cat. no. HHS32; Sigma-Aldrich; Merck
KGaA) for 10 min at room temperature and then treated with 1%
hydrochloric acid ethanol. After washing, the slices were stained
with 0.5% eosin (cat. no. 6766007; Thermo Fisher Scientific, Inc.)
for 5 min at room temperature, dehydrated with 70, 85, 95 and 100%
ethanol, and made transparent with xylene twice. After treatment
with neutral gum, the stained sections were observed under a light
microscope.
TUNEL staining
After brain tissues in each group were fixed
following the above methods, the sections were dewaxed and tested
for apoptosis using a TUNEL assay. The specific procedures for
TUNEL staining were performed according to the instructions
provided with the TUNEL kit (cat. no. MK1020; Wuhan Boster
Biological Technology, Ltd.). TUNEL-positive cells were those with
brown granules in the nucleus.
Statistical analysis
All data from the current study are presented as the
mean ± standard deviation based on three repetitions. All results
were calculated using GraphPad Prism Software (version Prism 7;
GraphPad Software, Inc.). Differences were analyzed by one-way
ANOVA followed by Tukey's post-hoc test. For mNSS analysis, data
were analyzed using the Kruskal-Wallis test and Steel-Dwass tests
were performed to compare multiple groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Culture and identification of
EPCs
EPCs were isolated from mouse bone marrow via
density gradient centrifugation. After 1 and 7 days of incubation,
the morphology of the EPCs was observed using a microscope.
Following 1 day of culture, the EPCs were round, small and
suspended in the medium; however, after 7 days of culture, the
number of EPCs was markedly increased, and the cells exhibited
fusiform or polygonal morphology, and were adherent to the well
(Fig. 1A). The Dil-ac-LDL and
FITC-lectin-UEA-1 fluorescent staining results revealed that the
cytoplasm of EPCs took up Dil-ac-LDL (red) and the membranes of
EPCs bound with FITC-Lectin-UEA-1 (green), suggesting that EPCs
were differentiating (Fig. 1B and
C). Furthermore, the results of IF
staining assays demonstrated the presence of surface markers for
EPCs, namely CD34, CD133, VEGFR2 and vWF (Fig. 1D-G). These results indicate that
EPCs were successfully isolated from mouse bone marrow.
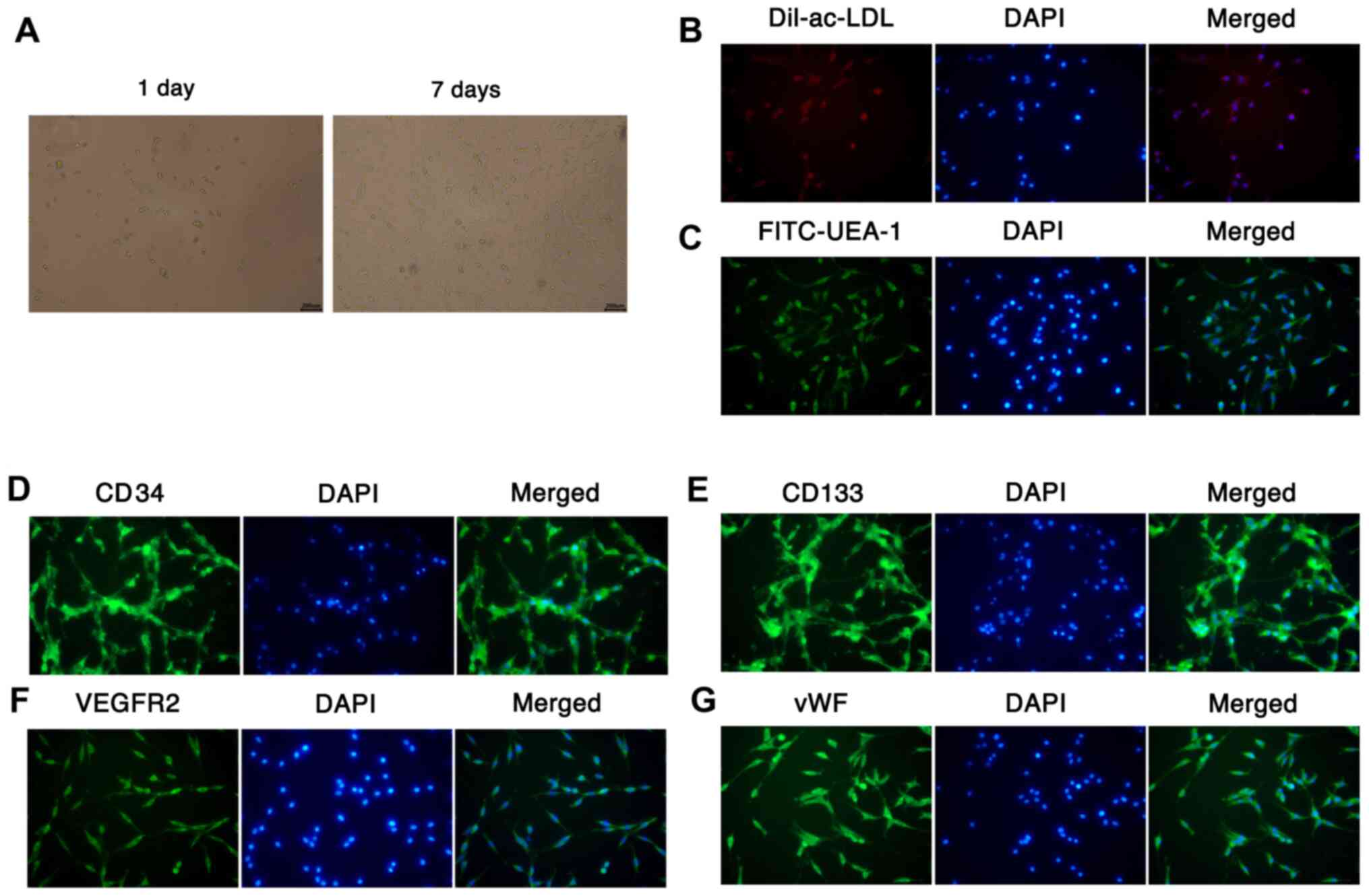 | Figure 1Culture and identification of EPCs.
(A) EPCs were isolated from mouse bone marrow by density gradient
centrifugation, and the morphology of the cultured EPCs was
observed under a microscope after 1 and 7 days (magnification,
x200). Fluorescent staining was conducted to identify the abilities
of EPCs to (B) take up Dil-ac-LDL and (C) bind UEA-l
(magnification, x200; scale bar, 200 µm). The expression of (D)
CD34 (E), CD133, (F) VEGFR2 and (G) vWF was examined using
immunofluorescence assays with DAPI nuclear staining
(magnification, x200). EPCs, endothelial progenitor cells;
Dil-ac-LDL, Dil-labeled acetylated low-density lipoprotein; UEA-1,
Ulex europaeus agglutin-1; vWF, von Willebrand factor. |
MMP inhibitor BB-94 accelerates
proliferation and prevents apoptosis in OGD/R model cells in a
dose-dependent manner
The possible effects of the MMP inhibitor BB-94 in
stroke were next investigated. Firstly, an OGD/R cell model was
established, and the OGD/R cells were stimulated with 0, 5, 10, 20,
30 and 40 mM BB-94 for 48 h. As shown in Fig. 2A, BB-94 significantly increased the
proliferation of OGD/R cells, with 20 mM BB-94 exhibiting the
strongest effect. The proliferation of the OGD/R cells began to
decrease when the BB-94 concentration reached 30 or 40 mM. which
suggests that high doses of BB-94 might have a certain toxic effect
on the proliferating activity of OGD/R cells (P<0.01).
Additionally, flow cytometric analysis revealed that cell apoptosis
was significantly attenuated in BB-94-treated OGD/R cells compared
with untreated OGD/R cells (P<0.05; Fig. 2B and C). To further analyze the possible effect
of BB-94 on MMPs in OGD/R model cells, the OGD/R model cells were
treated with 20 mM BB-94 and analyzed using RT-qPCR. The results
demonstrated that the expression levels of MMP-2, MMP-9 and MMP-8
were significantly reduced, and the expression of TIMP-1 was
significantly increased in BB-94-treated OGD/R cells compared with
untreated OGD/R cells (P<0.05; Fig.
2D). Similarly, the western blotting results also showed that
BB-94 significantly downregulated MMP-2, MMP-9 and MMP-8 and
upregulated TIMP-1 expression in OGD/R cells (P<0.01; Fig. 2E). These results certified that
BB-94 exhibited protective effects in OGD/R model cells.
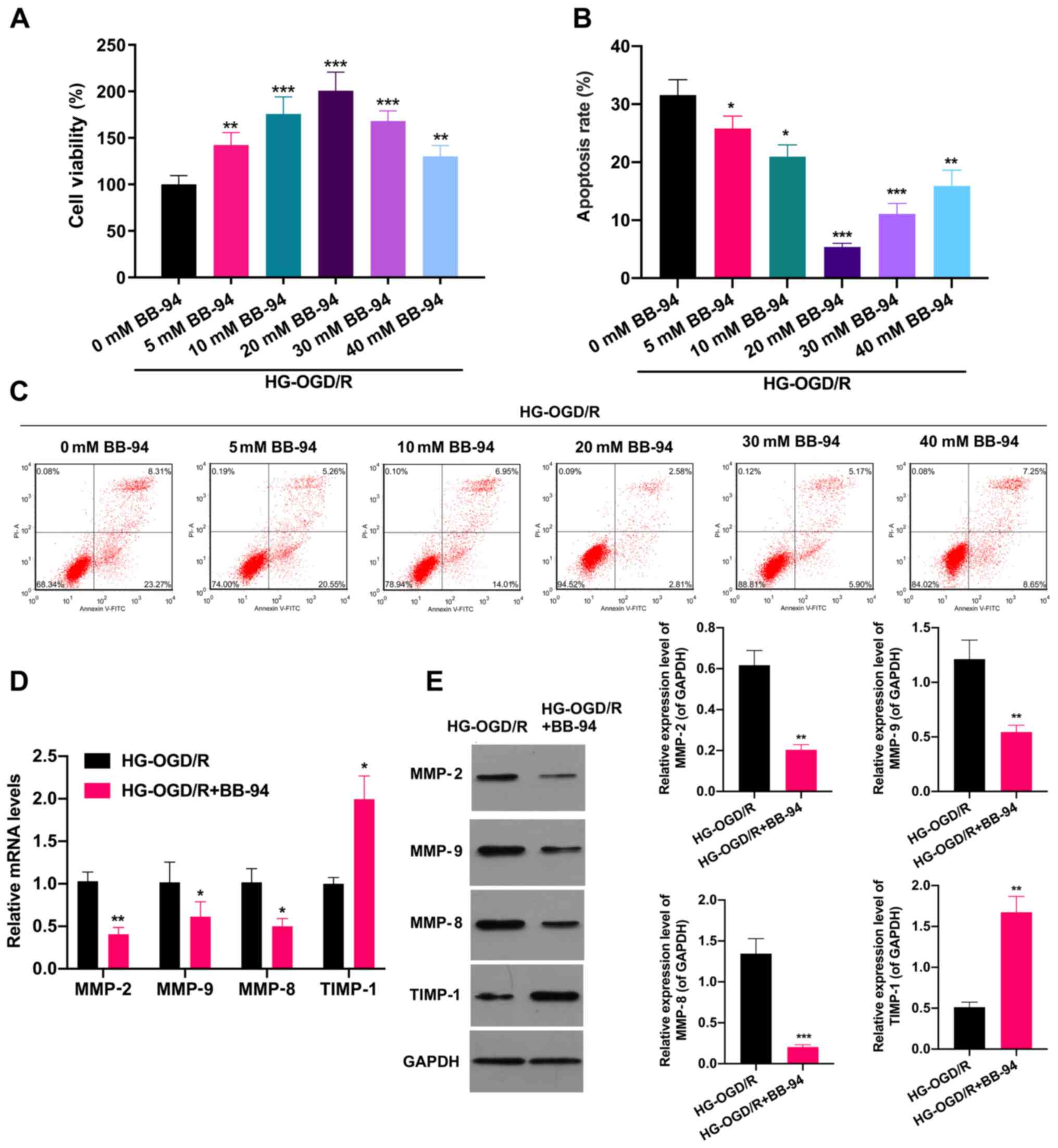 | Figure 2MMP inhibitor BB-94 accelerated the
proliferation of OGD/R model cells and prevented their apoptosis.
An OGD/R cell model was established, and the cells were stimulated
with 0, 5, 10, 20, 30 or 40 mM BB-94 for 48 h. (A) Cell
proliferation was evaluated using an MTT assay. (B and C) Flow
cytometry was applied to monitor the effect of BB-94 on the
apoptosis of the OGD/R cells. (B) The apoptosis rate was calculated
and (C) representative flow cytometry plots are shown.
*P<0.05, **P<0.01,
***P<0.001 vs. 0 mM BB-94, (D) OGD/R model cells were
treated with 20 mM BB-94, and reverse transcription-quantitative
PCR analysis was utilized to examine the levels of MMP-2, MMP-9,
MMP-8 and TIMP-1. (E) Western blotting analysis was also performed
to evaluate MMP-2, MMP-9, MMP-8 and TIMP-1 expression in the 20 mM
BB-94-treated OGD/R cells. *P<0.05,
**P<0.01, ***P<0.001 vs. HG-OGD/HR.
MMP, matrix metalloproteinase; HG-, high glucose; OGD/R,
oxygen-glucose deprivation/reoxygenation; TIMP-1, tissue inhibitor
of metalloproteinases 1. |
Combined application of EPCs and BB-94
induces proliferation and alleviates oxidative damage in OGD/R
model cells
The impacts of EPCs and BB-94 on the proliferation
and oxidative damage of OGD/R model cells were subsequently
investigated. MTT analysis demonstrated that EPCs or BB-94 each
significantly facilitated the proliferation of OGD/R model cells
(P<0.05), and that EPCs used in combination with BB-94 promoted
the proliferation of OGD/R model cells more strongly than each
treatment alone (P<0.001; Fig.
3A). As shown in Fig. 3B, ELISA
results revealed that SOD levels were markedly reduced and ROS, MDA
and LDH levels were notably elevated in the EPCs or BB-94 groups
compared with the control OGD/R group (P<0.001); furthermore,
the combination of EPCs and BB-94 induced a further reduction of
SOD levels and significant increases of ROS, MDA and LDH levels
(P<0.01). The impacts of EPCs alone and in combination with
BB-94 on MMPs were also studied; EPCs and/or BB-94 were applied to
the OGD/R model cells. The levels of MMP-2, MMP-9, MMP-8 and TIMP-1
were determined through RT-qPCR and western blotting. The results
showed that MMP-2, MMP-9 MMP-8 expression levels were significantly
reduced and TIMP-1 expression was significantly raised in the EPCs
or BB-94 groups compared with the control OGD/R group. In addition,
compared with the individual treatment groups, the combination of
EPCs and BB-94 further downregulated MMP-2, MMP-9 and MMP-8, and
upregulated TIMP-1 expression in the OGD/R model cells (P<0.05;
Fig. 3C and D). These results reveal that the
combination of BB-94 and EPCs had a significant protective effect
on the OGD/R model cells.
 | Figure 3Combined application of EPCs and
BB-94 induces proliferation and alleviates oxidative damage in
OGD/R model cells. (A) OGD/R model cells were treated with EPCs
or/and BB-94 (5 mM) and an MTT assay was used to evaluate the
proliferation of the cells. (B) The levels of SOD, ROS, MDA and LDH
were assessed using ELISAs. (C) The mRNA expression levels of
MMP-2, MMP-9, MMP-8 and TIMP-1 were confirmed by reverse
transcription-quantitative PCR in OGD/R cells treated with EPCs
and/or 5 mM BB-94. (D) MMP-2, MMP-9, MMP-8 and TIMP-1 protein
levels were certified through western blotting analysis in OGD/R
cells following treatment with EPCs and/or 5 mM BB-94.
*P<0.05, **P<0.01,
***P<0.001 vs. the OGD/R group;
#P<0.05, ##P<0.01,
###P<0.001 vs. the OGD/R + EPCs group;
&P<0.05, &&P<0.01,
&&&P<0.001 vs. the OGD/R + BB-94 group.
EPCs, endothelial progenitor cells; HG-, high glucose; OGD/R,
oxygen-glucose deprivation/reoxygenation; SOD, superoxide
dismutase; ROS, reactive oxygen species; MDA, malondialdehyde; LDH,
lactate dehydrogenase; MMP, matrix metalloproteinase; TIMP-1,
tissue inhibitor of metalloproteinases 1. |
EPCs alone and combined with BB-94
prominently attenuate MCAO-induced cerebral I/R injury
On the basis of the roles of EPCs and BB-94 in OGD/R
model cells, the effects of EPCs and BB-94 on MCAO-induced cerebral
I/R injury were further investigated. MCAO model mice were
successfully established and the mice were treated with EPCs and/or
BB-94. The neurological deficit scores were significantly reduced
in the MCAO + EPCs or MCAO + BB-94 groups compared with the control
MCAO group, and the combination of EPCs and BB-94 further lowered
the neurological deficit scores in the MCAO mice compared with
those of mice treated with EPCs or BB-94 alone (P<0.05; Fig. 4A). Additionally, H&E staining
revealed that the nerve cells in the brain tissues of MCAO model
mice were scattered, and the cells appeared to be loose with
evident edema. Treatment with EPCs alone or combined with BB-94
markedly attenuated this abnormal change in the morphological
structure of the brain tissues, and this attenuation was most
evident in the MCAO rats treated with a combination of EPCs and
BB-94 (Fig. 4B). Moreover, through
TUNEL analysis, treatment with EPCs or BB-94 alone was found to
observably reduce the number of TUNEL-positive cells, and the
combined treatment with EPCs and BB-94 further lowered the number
of TUNEL-positive cells in the MCAO model mice compared with either
EPCs or BB-94 alone (Fig. 4C).
These results indicate that EPCs alone and in combination with
BB-94 had a marked protective role against cerebral I/R injury in
MCAO mice.
EPCs and BB-94 notably alleviate
oxidative damage and downregulate MMPs in MCAO model mice
Whether EPCs and BB-94 have an effect on oxidative
damage and the expression levels of MMPs/TIMP-1 in MCAO model mice
were further determined. The ELISA results indicated that either
EPCs or BB-94 alone significantly reduced the SOD concentration and
elevated the ROS, MDA and LDH levels in the brain tissues of the
MCAO model mice, and the combined treatment with EPCs and BB-94
further enhanced the effects of EPCs or BB-94 on SOD, ROS, MDA and
LDH levels in the mice (P<0.001; Fig. 5A). In addition, RT-qPCR results
showed that either EPCs or BB-94 alone markedly downregulated
MMP-2, MMP-9 and MMP-8 and upregulated TIMP-1 expression, and the
changes in the expression of these genes in the brain tissues of
the MCAO model mice were further enhanced when EPCs and BB-94 were
used in combination (P<0.05; Fig.
5B). In addition, the western blot results exhibited the same
trends as the RT-qPCR results (P<0.05; Fig. 5C). These results indicate that EPCs
alone and combined with BB-94 significantly inhibited oxidative
damage and the expression of MMPs in MCAO model mice.
 | Figure 5EPCs and BB-94 notably alleviate
oxidative damage and downregulate MMPs in MCAO model mice. (A)
ELISAs were used to determine the changes of SOD, ROS, MDA and LDH
levels in MCAO model mice treated with EPCs and/or BB-94. After
treating the mice with EPCs and/or BB-94, MMP-2, MMP-9, MMP-8 and
TIMP-1 expression levels were confirmed in the brains of the mice
using (B) reverse transcription-quantitative PCR and (C) western
blotting. GAPDH served as an internal control.
*P<0.05, **P<0.01,
***P<0.001 vs. the MCAO group; #P<0.05,
##P<0.01, ###P<0.001 vs. the MCAO +
EPCs group; &P<0.05,
&&P<0.01,
&&&P<0.001 vs. the MCAO + BB-94 group.
EPCs, endothelial progenitor cells; MMP, matrix metalloproteinase;
DM-, diabetes mellitus; MCAO, middle cerebral artery occlusion;
SOD, superoxide dismutase; ROS, reactive oxygen species; MDA,
malondialdehyde; LDH, lactate dehydrogenase; TIMP-1, tissue
inhibitor of metalloproteinases 1. |
Discussion
Diabetes is an independent risk factors for stroke,
and the mortality rate from cerebrovascular complications in
patient with diabetes is 2-4-fold higher than that in non-diabetic
individuals (35). The onset time
of stroke in patients with type 2 diabetes is 10 years earlier than
that in non-diabetic individuals (36). The main pathological changes
observed in patients with diabetes and ischemic stroke include
macrovascular and microvascular diseases (37). The pathogenesis of diabetic stroke
mainly includes fat and lipoprotein metabolic disorders, insulin
resistance, hypertension, endothelium-dependent vasomotor
dysfunction, microvascular lesions and genetic changes (38,39).
Studies have demonstrated that EPCs have significant effects on
vascular repair (17,40). In diabetic retinopathy, EPCs have
been shown to contribute to the remodeling of blood vessels by
participating in the formation of vascular structures, thereby
increasing the normal functioning of vascular systems (41,42).
However, diabetes can reduce the number and function of EPCs
(43). Traditionally, EPCs are
isolated from the bone marrow and are verified as EPCs by testing
for the presence of specific surface markers (44,45).
CD133 and CD34 are known as markers for hematopoietic stem cells
and they are gradually lost during the differentiation and
maturation of EPCs (46). VEGFR2 is
also a specific marker for endothelial cells. Therefore,
CD34+CD113+VEGFR2+ expressing
cells are generally identified as EPCs (47). Moreover, endothelial cells produce
vWF in the cytoplasm and specifically take up Dil-ac-LDL and UEA-1,
which can also serve as specific markers for endothelial
identification (48,49). In the present study, EPCs were
extracted from the bone marrow cavities of mice, and the successful
extraction of EPCs was demonstrated through measurements of
Dil-ac-LDL and UEA-1 uptake, and CD34, CD133, VEGFR2 and vWF
expression. In addition, an OGD/R cell model and MCAO mouse model
were successfully established.
MMPs are a family of metal-dependent proteolytic
enzymes with similar structures and common biochemical properties
(50). The ECM is the main
component of the vascular wall (51). Research has shown that MMPs are able
to degrade all ECM components, with the exception of
polysaccharides such as collagen and elastin, and are key enzymes
in the extracellular degradation of ECM (52). MMPs and TIMPs together constitute a
vital system for regulating the dynamic balance of the ECM
(53). In this system, MMP-9 is a
widely studied and active MMP, and TIMP-1 is a specific inhibitor
of MMP-9(54). MMP-2 is a key
enzyme involved in substrate degradation, and can specifically
degrade the main components of the basal membrane of the arterial
walls (55,56). Diabetes mellitus (DM) is a chronic
disease comprising lifestyle-associated insulin resistance and/or
abnormal insulin secretion. Previous studies have shown that an
increase in insulin-secreting pancreatic islet β-cells is a common
feature of DM progression (57,58).
Recent studies have shown that increased MMPs contribute to the
progression of DM by inhibiting islet β-cell apoptosis through an
integrin-mediated Akt/BAD pathway (59,60).
Other studies have revealed that the high expression of MMP-9 is
associated with poor prognosis and recovery for ischemic stroke
(61,62); MMP-8 has a close association with
ischemic stroke (26); and MMP-2
polymorphism is associated with the occurrence risk of stroke
(63). However, the impacts of MMPs
on the progression of diabetic ischemic stroke have not been fully
elucidated.
BB-94 is a synthetic MMP inhibitor (64). It has a competitive inhibitory
effect on MMPs due the similarity of its chemical structure with
that of MMP enzyme restriction sites (65). Previous studies have reported the
ability of BB-94 to inhibit multiple disease processes (66), including pancreatitis (67), abdominal aortic aneurysm (64), glioblastoma (68) and breast cancer (69). It has also been shown that BB-94
does not directly affect the viability of cancer cells; instead, it
reduces the release of collagenase from these cells (70). Furthermore, acute and long-term
toxicological experiments indicate that BB-94 has no toxic effects
on animals (71). Despite BB-94
having shown strong promising preclinical data, it failed its phase
I trial due to unforeseen side effects (72). This was likely due to the poor
solubility of BB-94, which resulted in local toxicity associated
with a high dose of the drug administered intraperitoneally
(71). Consistent with this, the
results of the present study revealed that the proliferation of
OGD/R cells was inhibited when the concentration of BB-94 increased
to ≥30 mM, which indicates that high doses of BB-94 may have a
toxic effect on cells. Moreover, the present study showed that
BB-94 significantly induced the proliferation of OGD/R model cells,
and also inhibited their apoptosis. It also revealed the ability of
BB-94 to markedly downregulate MMP-2, MMP-9 and MMP-8, and
upregulate TIMP-1 expression in OGD/R model cells.
The present study demonstrated that the combined
application of EPCs and BB-94 prominently accelerated the
proliferation of OGD/R model cells and alleviated oxidative damage
in these cells. It also certified that EPCs and BB-94 significantly
alleviated cerebral I/R injury in MCAO model mice, and markedly
reduced oxidative damage and the expression of certain MMPs in
these mice. These results suggest that EPCs can change the dynamic
balance of the ECM, and indicate that the combined application of
EPCs and BB-94 significantly ameliorated the brain damage induced
by diabetic ischemic stroke.
In summary, the present study successfully isolated
and identified EPCs from mice, and established an OGD/R cell model
and MCAO mice model. Using these models, the study demonstrated
that EPCs alone or combined with BB-94 have protective effects
against ischemic stroke that are associated with the reduction of
MMP expression.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the National Natural
Science Foundation of China (grant. no. 81860321). The funding body
played a role in the design of the study and editing the
manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DZ, ZH and TH designed and performed the
experiments; DZ, ZH, XZ and YZ performed the literature research,
research design and manuscript editing. TH performed manuscript
editing. DZ and ZH confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This experiments involving animal were approved by
the Institutional Animal Ethics Committee of Guizhou Medical
University and performed according to Animal Care Guidelines for
the Care and Use of Animals from Guizhou Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guzik A and Bushnell C: Stroke
epidemiology and risk factor management. Continuum (Minneap Minn).
23:15–39. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Randolph SA: Ischemic stroke. Workplace
Health Saf. 64(444)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sreedharan R and Abdelmalak B: Diabetes
mellitus: Preoperative concerns and evaluation. Anesthesiol Clin.
36:581–597. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Echouffo-Tcheugui JB and Garg R:
Management of hyperglycemia and diabetes in the emergency
department. Curr Diab Rep. 17(56)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hill MD: Stroke and diabetes mellitus.
Handb Clin Neurol. 126:167–174. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shindo A and Tomimoto H: Diabetes and
ischemic stroke. Brain Nerve. 66:107–119. 2014.PubMed/NCBI(In Japanese).
|
|
7
|
Chen R, Ovbiagele B and Feng W: Diabetes
and stroke: Epidemiology, pathophysiology, pharmaceuticals and
outcomes. Am J Med Sci. 351:380–386. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Campos AC, Fogaca MV, Sonego AB and
Guimaraes FS: Cannabidiol, neuroprotection and neuropsychiatric
disorders. Pharmacol Res. 112:119–127. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lavaur J, Le Nogue D, Lemaire M, Pype J,
Farjot G, Hirsch EC and Michel PP: The noble gas xenon provides
protection and trophic stimulation to midbrain dopamine neurons. J
Neurochem. 142:14–28. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lavaur J, Lemaire M, Pype J, Nogue DL,
Hirsch EC and Michel PP: Xenon-Mediated neuroprotection in response
to sustained, low-level excitotoxic stress. Cell Death Discov.
2(16018)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bernstock JD, Peruzzotti-Jametti L, Ye D,
Gessler FA, Maric D, Vicario N, Lee YJ, Pluchino S and Hallenbeck
JM: Neural stem cell transplantation in ischemic stroke: A role for
preconditioning and cellular engineering. J Cereb Blood Flow Metab.
37:2314–2319. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Boncoraglio GB, Ranieri M, Bersano A,
Parati EA and Del Giovane C: Stem cell transplantation for ischemic
stroke. Cochrane Database Syst Rev. 5(CD007231)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chong MS, Ng WK and Chan JK: Concise
review: Endothelial progenitor cells in regenerative medicine:
Applications and challenges. Stem Cells Transl Med. 5:530–538.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang JX, Pan YY, Wang XX, Qiu YG and Mao
W: Endothelial progenitor cells in age-related vascular remodeling.
Cell Transplant. 27:786–795. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Emontzpohl C, Simons D, Kraemer S,
Goetzenich A, Marx G, Bernhagen J and Stoppe C: Isolation of
endothelial progenitor cells from healthy volunteers and their
migratory potential influenced by serum samples after cardiac
surgery. J Vis Exp. 14(55192)2017.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Guerra G, Perrotta F and Testa G:
Circulating endothelial progenitor cells biology and regenerative
medicine in pulmonary vascular diseases. Curr Pharm Biotechnol.
19:700–707. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rana D, Kumar A and Sharma S: Endothelial
progenitor cells as molecular targets in vascular senescence and
repair. Curr Stem Cell Res Ther. 13:438–446. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Esquiva G, Grayston A and Rosell A:
Revascularization and endothelial progenitor cells in stroke. Am J
Physiol Cell Physiol. 315:C664–C674. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li Y, Chang S, Li W, Tang G, Ma Y, Liu Y,
Yuan F, Zhang Z, Yang GY and Wang Y: cxcl12-Engineered endothelial
progenitor cells enhance neurogenesis and angiogenesis after
ischemic brain injury in mice. Stem Cell Res Ther.
9(139)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ma F, Morancho A, Montaner J and Rosell A:
Endothelial progenitor cells and revascularization following
stroke. Brain Res. 1623:150–159. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jablonska-Trypuc A, Matejczyk M and
Rosochacki S: Matrix metalloproteinases (MMPs), the main
extracellular matrix (ECM) enzymes in collagen degradation, as a
target for anticancer drugs. J Enzyme Inhib Med Chem. 31:177–183.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Omran OM and Thabet M: Gelatinases a and B
expression in human colorectal cancer in upper egypt: A
clinicopathological study. Ultrastruct Pathol. 36:108–116.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cui N, Hu M and Khalil RA: Biochemical and
biological attributes of matrix metalloproteinases. Prog Mol Biol
Transl Sci. 147:1–73. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Back M, Ketelhuth DF and Agewall S: Matrix
metalloproteinases in atherothrombosis. Prog Cardiovasc Dis.
52:410–428. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lin HF, His E, Huang LC, Liao YC, Juo SH
and Lin RT: Methylation in the matrix metalloproteinase-2 gene is
associated with cerebral ischemic stroke. J Investig Med.
65:794–799. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Palm F, Pussinen PJ, Safer A,
Tervahartiala T, Sorsa T, Urbanek C, Becher H and Grau AJ: Serum
matrix metalloproteinase-8, tissue inhibitor of metalloproteinase
and myeloperoxidase in ischemic stroke. Atherosclerosis. 271:9–14.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chaturvedi M and Kaczmarek L: Mmp-9
inhibition: A therapeutic strategy in ischemic stroke. Mol
Neurobiol. 49:563–573. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tasca CI, Dal-Cim T and Cimarosti H: In
vitro oxygen-glucose deprivation to study ischemic cell death.
Methods Mol Biol. 125:197–210. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liang X, Liu X, Lu F, Zhang Y, Jiang X and
Ferriero DM: HIF1α signaling in the endogenous protective responses
after neonatal brain hypoxia-ischemia. Dev Neurosci. 40:617–626.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Novak AE, Jones SM and Elliott JP:
Induction of the HIF pathway: Differential regulation by chemical
hypoxia and oxygen glucose deprivation. bioR. xiv(525006)2019.
|
|
31
|
Zhang Y, Liu D, Hu H, Zhang P, Xie R and
Cui W: HIF-1α/BNIP3 signaling pathway-induced-autophagy plays
protective role during myocardial ischemia-reperfusion injury.
Biomed Pharmacother. 120(109464)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu C, Kong X, Wu X, Wang X, Guan H, Wang
H, Wang L, Jin X and Yuan H: Alleviation of A disintegrin and
metalloprotease 10 (ADAM10) on thromboangiitis obliterans involves
the HMGB1/RAGE/ NF-kappaB pathway. Biochem Biophys Res Commun.
505:282–289. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M
and Chopp M: Therapeutic benefit of intravenous administration of
bone marrow stromal cells after cerebral ischemia in rats. Stroke.
32:1005–1011. 2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lee YK and Lee JA: Role of the mammalian
ATG8/LC3 family in autophagy: Differential and compensatory roles
in the spatiotemporal regulation of autophagy. BMB Rep. 49:424–430.
2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hardigan T, Ward R and Ergul A:
Cerebrovascular complications of diabetes: Focus on cognitive
dysfunction. Clin Sci (Lond). 130:1807–1822. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Khan S, Shafique L and Miah M: Risk
factors and patterns of stroke among diabetic and non-diabetic
patients. Imperial Journal of Interdisciplinary Research 3,
2017.
|
|
37
|
Mohammedi K, Woodward M, Hirakawa Y,
Zoungas S, Williams B, Lisheng L, Rodgers A, Mancia G, Neal B,
Harrap S, et al: Microvascular and macrovascular disease and risk
for major peripheral arterial disease in patients with type 2
diabetes. Diabetes Care. 39:1796–1803. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Alloubani A, Saleh A and Abdelhafiz I:
Hypertension and diabetes mellitus as a predictive risk factors for
stroke. Diabetes Metab Syndr. 12:577–584. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Boehme AK, Esenwa C and Elkind MS: Stroke
risk factors, genetics, and prevention. Circ Res. 120:472–495.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Rodriguez-Carrio J, Lopez P and Suarez A:
Endothelial progenitor cells as mediators of the crosstalk between
vascular repair and immunity: Lessons from systemic autoimmune
diseases. Curr Med Chem. 25:4478–4496. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lois N, McCarter RV, O'Neill C, Medina RJ
and Stitt AW: Endothelial progenitor cells in diabetic retinopathy.
Front Endocrinol (Lausanne). 5(44)2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Shao Y, Li X, Wood JW and Ma JX:
Mitochondrial dysfunctions, endothelial progenitor cells and
diabetic retinopathy. J Diabetes Complications. 32:966–973.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wils J, Favre J and Bellien J: Modulating
putative endothelial progenitor cells for the treatment of
endothelial dysfunction and cardiovascular complications in
diabetes. Pharmacol Ther. 170:98–115. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Schmeisser A, Garlichs CD, Zhang H, Eskafi
S, Graffy C, Ludwig J, Strasser RH and Daniel WG: Monocytes
coexpress endothelial and macrophagocytic lineage markers and form
cord-like structures in matrigel under angiogenic conditions.
Cardiovasc Res. 49:671–680. 2001.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Peichev M, Naiyer AJ, Pereira D, Zhu Z,
Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA and Rafii
S: Expression of VEGFR-2 and AC133 by circulating human CD34(+)
cells identifies a population of functional endothelial precursors.
Blood. 95:952–958. 2000.PubMed/NCBI
|
|
46
|
Takahashi T, Kalka C, Masuda H, Chen D,
Silver M, Kearney M, Magner M, Isner JM and Asahara T: Ischemia-
and cytokine-induced mobilization of bone marrow-derived
endothelial progenitor cells for neovascularization. Nat Med.
5:434–438. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
47
|
He S, Pant D, Schiffmacher A, Bischoff S,
Melican D, Gavin W and Keefer C: Developmental expression of
pluripotency determining factors in caprine embryos: Novel pattern
of NANOG protein localization in the nucleolus. Mol Reprod Dev.
73:1512–1522. 2006.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wagner DD, Olmsted JB and Marder VJ:
Immunolocalization of von Willebrand protein in Weibel-Palade
bodies of human endothelial cells. J Cell Biol. 95:355–360.
1982.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Heeschen C, Aicher A, Lehmann R,
Fichtlscherer S, Vasa M, Urbich C, Mildner-Rihm C, Martin H, Zeiher
AM and Dimmeler S: Erythropoietin is a potent physiologic stimulus
for endothelial progenitor cell mobilization. Blood. 102:1340–1346.
2003.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Nagase H, Visse R and Murphy G: Structure
and function of matrix metalloproteinases and TIMPs. Cardiovasc
Res. 69:562–573. 2006.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Theocharis AD, Skandalis SS, Gialeli C and
Karamanos NK: Extracellular matrix structure. Adv Drug Deliv Rev.
97:4–27. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Bonnans C, Chou J and Werb Z: Remodelling
the extracellular matrix in development and disease. Nat Rev Mol
Cell Biol. 15:786–801. 2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Paiva KBS and Granjeiro JM: Matrix
metalloproteinases in bone resorption, remodeling, and repair. Prog
Mol Biol Transl Sci. 148:203–303. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wei H, Wang S, Zhen L, Yang Q, Wu Z, Lei
X, Lv J, Xiong L and Xue R: Resveratrol attenuates the blood-brain
barrier dysfunction by regulation of the MMP-9/TIMP-1 balance after
cerebral ischemia reperfusion in rats. J Mol Neurosci. 55:872–879.
2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Toth M, Sohail A and Fridman R: Assessment
of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol
Biol. 878:121–135. 2012.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Dong H, Diao H, Zhao Y, Xu H, Pei S, Gao
J, Wang J, Hussain T, Zhao D, Zhou X and Lin D: Overexpression of
matrix metalloproteinase-9 in breast cancer cell lines remarkably
increases the cell malignancy largely via activation of
transforming growth factor beta/SMAD signalling. Cell Prolif.
52(e12633)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Hongwei Y, Ruiping C, Yingyan F, Guanjun
Z, Jie H, Xingyu L, Jie T, Zhenghong L, Qin G, Junfeng H and Heng
Z: Effect of Irbesartan on AGEs-RAGE and MMPs systems in rat type 2
diabetes myocardial-fibrosis model. Exp Biol Med (Maywood).
244:612–620. 2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Song W and Ergul A: Type-2
diabetes-induced changes in vascular extracellular matrix gene
expression: Relation to vessel size. Cardiovasc Diabetol.
5(3)2006.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Nishihama K, Yasuma T, Yano Y,
D'Alessandro-Gabazza CN, Toda M, Hinneh JA, Tonto PB, Takeshita A,
Totoki T, Mifuji-Moroka R, et al: Anti-Apoptotic activity of human
matrix metalloproteinase-2 attenuates diabetes mellitus.
Metabolism. 82:88–99. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
de de Morais EF, Dantas AN, Pinheiro JC,
Leite RB, Barboza CA, de Vasconcelos Gurgel BC and de Almeida
Freitas R: Matrix metalloproteinase-8 analysis in patients with
periodontal disease with prediabetes or type 2 diabetes mellitus: A
systematic review. Arch Oral Biol. 87:43–51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Zhong C, Yang J, Xu T, Xu T, Peng Y, Wang
A, Wang J, Peng H, Li Q, Ju Z, et al: Serum matrix
metalloproteinase-9 levels and prognosis of acute ischemic stroke.
Neurology. 89:805–812. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Abdelnaseer MM, Elfauomy NM, Esmail EH,
Kamal MM and Elsawy EH: Matrix metalloproteinase-9 and recovery of
acute ischemic stroke. J Stroke Cerebrovasc Dis. 26:733–740.
2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Fatar M, Stroick M, Steffens M, Senn E,
Reuter B, Bukow S, Griebe M, Alonso A, Lichtner P, Bugert P, et al:
Single-Nucleotide polymorphisms of MMP-2 gene in stroke subtypes.
Cerebrovasc Dis. 26:113–119. 2008.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Nosoudi N, Nahar-Gohad P, Sinha A,
Chowdhury A, Gerard P, Carsten CG, Gray BH and Vyavahare NR:
Prevention of abdominal aortic aneurysm progression by targeted
inhibition of matrix metalloproteinase activity with
batimastat-loaded nanoparticles. Circ Res. 117:e80–e89.
2015.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Walz W and Cayabyab FS: Neutrophil
infiltration and matrix metalloproteinase-9 in lacunar infarction.
Neurochem Res. 42:2560–2565. 2017.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Singh T, Jayaram B and Adekoya OA:
Computational approaches to matrix metalloprotease drug design.
Methods Mol Biol. 1579:273–285. 2017.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Wu Z, Mulatibieke T, Niu M, Li B, Dai J,
Ye X, He Y, Chen C, Wen L and Hu G: Inhibition of matrix
metalloproteinase with BB-94 protects against caerulein-induced
pancreatitis via modulating neutrophil and macrophage activation.
Gastroenterol Res Pract. 28(8903610)2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Dong F, Eibach M, Bartsch JW, Dolga AM,
Schlomann U, Conrad C, Schieber S, Schilling O, Biniossek ML,
Culmsee C, et al: The metalloprotease-disintegrin ADAM8 contributes
to temozolomide chemoresistance and enhanced invasiveness of human
glioblastoma cells. Neuro Oncol. 17:1474–1485. 2015.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Gautam J, Banskota S, Lee H, Lee YJ, Jeon
YH, Kim JA and Jeong BS: Down-Regulation of cathepsin S and matrix
metalloproteinase-9 via src, a non-receptor tyrosine kinase,
suppresses triple-negative breast cancer growth and metastasis. Exp
Mol Med. 50:1–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Sledge GW Jr, Qulali M, Goulet R, Bone EA
and Fife R: Effect of matrix metalloproteinase inhibitor batimastat
on breast cancer regrowth and metastasis in athymic mice. J Natl
Cancer Inst. 87:1546–1551. 1995.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Wojtowicz-Praga S, Low J, Marshall J, Ness
E, Dickson R, Barter J, Sale M, McCann P, Moore J, Cole A and
Hawkins MJ: Phase I trial of a novel matrix metalloproteinase
inhibitor batimastat (BB-94) in patients with advanced cancer.
Invest New Drugs. 14:193–202. 1996.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Winer A, Adams S and Mignatti P: Matrix
metalloproteinase inhibitors in cancer therapy: Turning past
failures into future successes. Mol Cancer Ther. 17:1147–1155.
2018.PubMed/NCBI View Article : Google Scholar
|



















