Introduction
Rectal carcinoma (RC) is a common intestinal
malignant tumor belonging to the colorectal cancer class. RC is
ranked third and fourth worldwide in terms of global tumor
morbidity and mortality, respectively, with an increasing overall
prevalence and a trend towards an earlier onset of disease
(1,2). With the improvement of living
conditions, constant changes in diet structure and lifestyle, and
the accelerated pace of work, there has been an increased intake of
high-fat, high-protein and low-fiber foods, resulting in dietary
disorders (3). RC has become the
second most prevalent malignant tumor of the digestive tract behind
by gastric cancer (4,5). Diagnosis of RC is limited by the
unclear early symptoms; therefore, diagnosis and treatment of RC
are usually delayed, and most patients have already reached
advanced stages at the time of diagnosis (6). The 5-year survival rate of RC is
relatively low (7). The
pathogenesis of RC has not been fully elucidated as it involves
multiple factors, processes and genes (8,9).
Understanding the pathogenesis behind RC is essential to develop
early diagnosis procedures and to improve the prognosis, thus a
greater understanding of RC pathogenesis is key to improving the
survival rate and quality of life of patients.
Aminoacylase-1 (ACY-1) is a zinc-binding enzyme that
hydrolyzes N-acetyl amino acids into free amino acids and acetic
acid, and is expressed at abnormally high levels in various
malignant tumor tissues. It has been shown that ACY-1 is
overexpressed in liver cancer and that ACY-1 expression is
associated with tumor proliferation, invasion and metastasis
(10,11). Human epidermal growth factor
receptor-2 (HER2) is a proto-oncogene which is expressed in healthy
adults, but its expression increases in more than one-third of
tumors (12). HER2 can promote cell
proliferation by inhibiting apoptosis, enhancing tumor cell
invasiveness, promoting tumor angiogenesis and lymphangiogenesis
and promoting tumor occurrence and development (13). In particular, HER2 gene
overexpression is closely associated with breast cancer and can be
used for clinical monitoring and indicating prognosis (14). Tumor necrosis factor (TNF)-related
apoptosis-inducing ligand (TRAIL) is a newly discovered member of
the TNF family (15) and can
rapidly promote large-scale apoptosis in transformed cells,
virus-infected cells and tumor cells (16,17).
Therefore, the aim of the present study was to investigate the
effect of the ACY-1 gene on HER2 and TRAIL expression in RC.
Materials and methods
Research objective
A total of 48 RC patients, who had received surgery
in the First People's Hospital Xianyang City between May and
December 2017, were enrolled for the present study. This included
26 males and 22 females with an average age of 55.8±7.9 (range,
41-75) years. There were 18 cases of mucinous adenocarcinoma, 20
cases of papillary adenocarcinoma, and 10 cases of signet ring cell
carcinoma. The inclusion criteria were as follows (6): First time treatment with no adjuvant
therapy such as chemotherapy, radiotherapy or biological therapy
prior to the surgery. The exclusion criteria were as follows
(6): Previous severe heart, lung,
liver or kidney failure; cerebrovascular disease; other malignant
tumor types; autoimmune diseases; infectious diseases; unable to
cooperate with the study; and incomplete clinical data. The study
was approved by the Medical Ethics Committee of The First People's
Hospital Xianyang City, and the patients or their families had
signed informed consent forms. Specimens were collected of
intraoperative rectal cancer and adjacent tissue (at least 3 cm
from tumor tissue), and stored in liquid nitrogen. Some samples
were prepared with 4% neutral formalin solution at 4˚C for 15 min
for immunohistochemistry.
Main reagents and instruments
The rectal cancer cell lines HT29 and SW620 were
purchased from the American Type Culture Collection and the
authenticity was verified by Suzhou Genetic Testing Biotechnology
Co., Ltd. These cell lines, as well as the normal colorectal
mucosal epithelial fetal human cell line (American Type Culture
Collection) were cryopreserved by our laboratory. DMEM, EDTA, FBS,
penicillin and streptomycin were purchased from HyClone; GE
Healthcare Life Sciences. DMSO and MTT powder were purchased from
Gibco; Thermo Fisher Scientific, Inc. Trypsin was purchased from
Sigma-Aldrich; Merck KGaA. The PVDF membrane was purchased from
Pall Life Sciences. All western blotting-related chemical reagents
were purchased from Beyotime Institute of Biotechnology. ECL
reagents were purchased from GE Healthcare. Rabbit anti-human HER2
monoclonal antibody, rabbit anti-human TRAIL monoclonal antibody,
rabbit anti-human ACY-1 monoclonal antibody, and mouse anti-rabbit
horseradish peroxidase (HRP)-conjugated IgG secondary antibody were
purchased from Cell Signaling Technology, Inc. The
immunohistochemical streptavidin-perosidase kit (cat. no. SA1098)
was purchased from Boster Biological Technology. The RNA extraction
kit and the reverse transcription kit were purchased from Axygen;
Corning Inc. The caspase-3 activity assay kit (cat. no. 556485) was
purchased from BD Biosciences. The Labsystem Version 1.3.1
microplate reader was purchased from Bio-Rad Laboratories, Inc. The
ABI 7900 HT Real-time PCR machine was purchased from Applied
Biosystems; Thermo Fisher Scientific, Inc. Other commonly used
reagents were purchased from Sangon Biotech Co., Ltd.
Immunohistochemistry
Tissue samples were prepared and sectioned to a
thickness of 5 µm. Paraffin sections were immunohistochemically
stained using the SP kit following the manufacturer's instructions.
After blocking with 10% normal FBS at room temperature for 30 min
to block endogenous peroxidase, the sections were incubated with
ACY-1 monoclonal antibodies (1:1,000) at 37˚C for 1 h. Washing was
performed with PBS solution three times, and the sections were
incubated with biotinylated goat anti-mouse IgG secondary
antibodies (cat. no. ab6788; Abcam; 11,000) at room temperature for
20 min. Next, the sections were incubated with streptavidin-biotin
complex to amplify the signal at room temperature for 20 min. After
3,3'-diaminobenzidine staining, the sections were counterstained
with hematoxylin for 1 min at room temperature, dehydrated with
serial gradients of ethanol, and sealed. The sections were then
visualized using a light microscope (magnification, x400). Positive
expression was determined by the number of cells that contained
brown/yellow granules. Samples that contained no brown/yellow
stained cells were defined as negative, samples with <50% of
cells stained brown/yellow in five random field were defined as
positive (+) and when ≥50% of cells were stained brown-yellow,
samples were defined as strong positive (++).
Cell culture and grouping
Cells were resuspended in 1 ml fresh DMEM and topped
up with 4 ml of fresh DMEM at 37˚C and 5% CO2 prior to
seeding. Cells were seeded in 6-well plates at 1x105
cells/cm2 in high-glucose DMEM (containing 10% FBS, 100
U/ml penicillin and 100 µg/ml streptomycin) at 37˚C with 5%
CO2. The cells in the 3rd-8th generation and logarithmic
growth phase were used for experiments. HT29 cells were randomly
divided into three group: A control group; a small interfering RNA
(siRNA) (Sigma-Aldrich; Merck KGaA) negative control group
(scramble group); and an ACY-1 siRNA (Sigma-Aldrich; Merck KGaA)
group.
Liposomal transfection
ACY-1 siRNA was transfected into HT29 or SW620
cells. The ACY-1 siRNA sequence was as follows: Forward,
5'-GATAAATGGACTTGGAGAACAG-3' and reverse,
5'-TAGACATGGAGACTTGACAGACT-3'. The siRNA negative control primer
sequence was: Forward, 5'-ATTCACCTGCCATGTAT-3' and reverse,
5'-GAACACTAATGTTGACAG-3'. Cells were grown to 70-80% confluence.
The ACY-1 siRNA or negative control siRNA was added to 200 µl of
serum-free DMEM medium and mixed at room temperature for 15 min.
The Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)
was mixed with 20 nM ACY-1 siRNA or negative control siRNA and
incubated for 30 min at room temperature. The mixture together with
1.6 ml serum-free DMEM, was incubated with the cells at 37˚C with
5% CO2 for 6 h. The cell culture medium was replaced
with DMEM containing serum and cultured for 48 h before
experimental research.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from tissues or cells using
TRIzol® reagent and reverse-transcribed to cDNA using
High Capacity cDNA Reverse Transcription Kit (Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions
(25˚C for 10 min, 37˚C 120 for min and at 85˚C for 5 min). The
primers were designed by Primer Premier 6.0 software (Premier
Biosoft) and synthesized by Invitrogen; Thermo Fisher Scientific,
Inc. (Table I). The qPCR reaction
was performed using Fast SYBR Green Master Mix (Thermo Fisher
Scientific, Inc.) with 35 cycles of 92˚C for 30 sec, 58˚C for 45
sec and 72˚C for 35 sec. GAPDH was used as a reference gene. The
relative expression was calculated using the 2ΔΔCq
method (18).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Forward (5'-3') | Reverse (5'-3') |
|---|
| GAPDH | ACCAGGTATCTTGG
TTG | TAACCATGTCAGC
GTGGT |
| ACY-1 | AGTCGACCACTCC
ACAGT | GATTCTGTGGTCA
CTATATT |
MTT assay
HT29 and SW620 cells in the logarithmic growth phase
were trypsinized and seeded onto a 96-well plate at 3,000
cells/well. The cells were randomly divided into the control group,
scramble group and ACY-1 siRNA group, with three replicates per
group. After treatment, the cells were given 20 µl of 5 g/l MTT
solution for 4 h in the incubator. Next, 150 µl DMSO/well was
applied and the solution was shaken for 10 min. After the purple
crystals were fully dissolved, the absorbance (A) was measured at
570 nm to calculate cell viability rate. Cell viability rate was
calculated with the following formula: Cell viability rate=sample A
value/control A value x100%. The experiment was repeated three
times (n=3).
Caspase-3 activity detection
The cells were trypsinized and centrifuged at 4˚C at
600 x g for 5 min. Next, the cells were lysed with cell lysis
buffer [10 mM Tris-HCl; 10 mM
NaH2PO4/NaHPO4 (pH 7.5); 130 mM
NaCl; 1% Triton X-100; 10 mM sodium pyrophosphate] on ice for 15
min and centrifuged at 4˚C and 20,000 x g for 5 min. Subsequently,
2 mM Ac-DEVD-AMC (included in the caspase-3 activity kit) was
added, the optical density (OD) was measured at 450 nm to calculate
the caspase-3 activity.
Western blot analysis
Cells were treated with RIPA buffer (150 mM NaCl, 1%
NP-40, 0.1% SDS, 2 µg/ml Aprotinin, 2 µg/ml Leupeptin, 1 mM PMSF,
1.5 mM EDTA, and 1 mM sodium vanadate) and quantified by BCA assay.
In total, 40 µg of protein was electrophoresed using 10% SDS-PAGE.
The gel was transferred to a PVDF membrane by semi-dry transfer at
150 mA for 1.5 h. After blocking with 5% skim milk at room
temperature for 1 h, the membrane was incubated with ACY-1, HER2,
TRAIL and β-actin primary antibodies (1:1,000, 1:2,000; 1:1,500 and
1:1,000, respectively) at 4˚C overnight. The membranes were then
incubated with goat anti-rabbit secondary antibodies (1:2,000) in
the dark for 30 min at room temperature. The labelled membrane was
imaged using chemiluminescence reagent for 1 min and analyzed by
image processing system software and Quantity one software (version
4.52; Bio-Rad Laboratories, Inc.). β-actin was used as a loading
control. The experiment was repeated four times (n=4).
Statistical analysis
All data analyses were performed using SPSS 19.0
software (IBM Corp.). The measurement data are presented as the
mean ± SD and compared by one-way ANOVAs with post hoc LSD tests.
The enumeration data are depicted as percentages and were compared
by χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
IHC analysis of ACY-1 expression in RC
tissue and adjuvant tissue
Immunohistochemistry was used to detect ACY-1
expression in RC and adjacent tissue. ACY-1 expression was
significantly increased in rectal cancer tumor tissue, with
positive staining in the cell membrane and cytoplasm. However, the
adjacent tissue presented negative for ACY-1 expression (-)
(P<0.05) (Fig. 1; Table II).
 | Table IIACY-1 expression in RC and adjuvant
tissue. |
Table II
ACY-1 expression in RC and adjuvant
tissue.
| | ACY-1 |
|---|
| Tissue type | (-) | (+ or ++) | Positive rate
(%) |
|---|
| Adjacent tissue | 42 | 4 | 8.33 |
| RC | 2 | 46 | 95.83a |
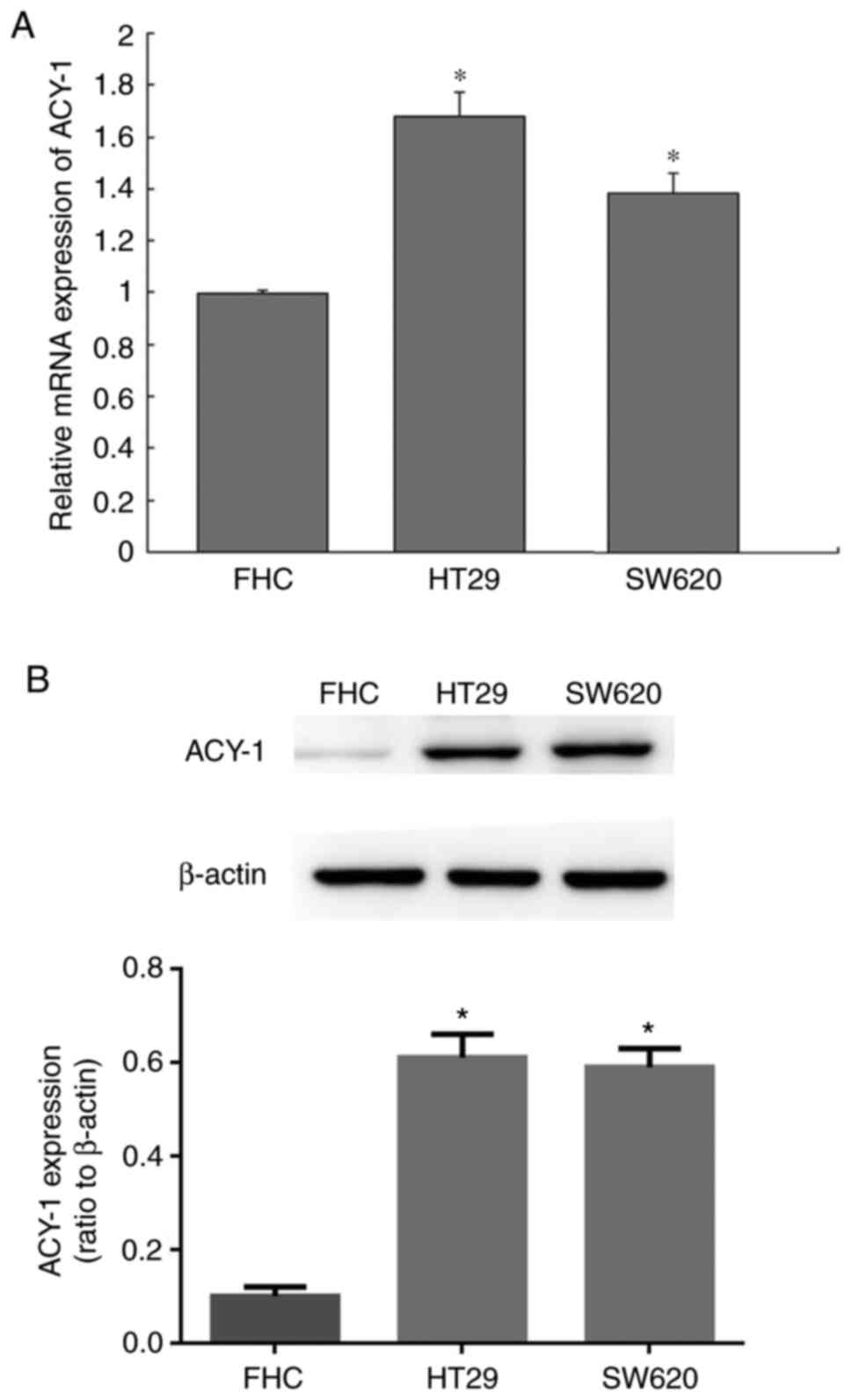
ACY-1 expression in RC cell lines
ACY-1 gene and protein expression levels were
measured between rectal cancer HT29 or SW620 cells and normal
colorectal mucosal epithelial cells. It was found that ACY-1 gene
and protein expression levels were upregulated in HT29 and SW620
cells compared with control cells (P<0.05; Fig. 2).
Impact of ACY-1 siRNA on ACY-1 mRNA
and protein expression in HT29 cells
The effect of siRNA on ACY-1 mRNA and protein
expression levels was detected by RT-qPCR. The gene and protein
expression levels of ACY-1 were significantly reduced after siRNA
treatment (P<0.05; Fig. 3).
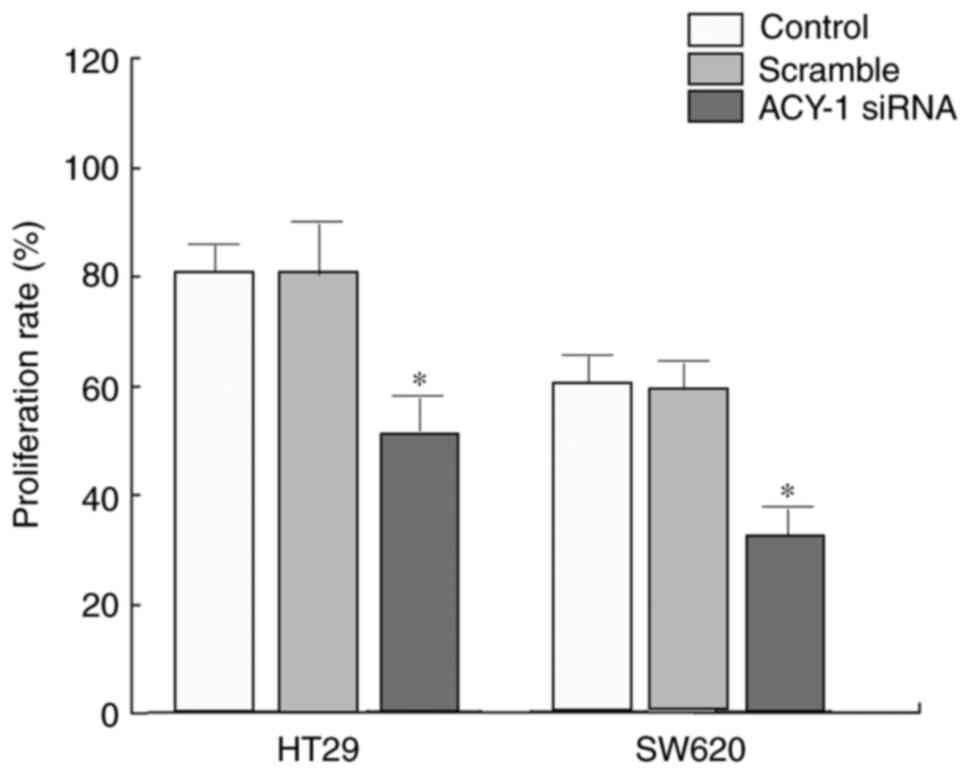
Influence of ACY-1 siRNA on HT29 cell
proliferation
The effect of ACY-1 siRNA on the proliferation of
HT29 or SW620 cells was detected using MTT assays. siRNA
transfection significantly inhibited HT29 or SW620 cell
proliferation compared with control transfection (P<0.05;
Fig. 4).
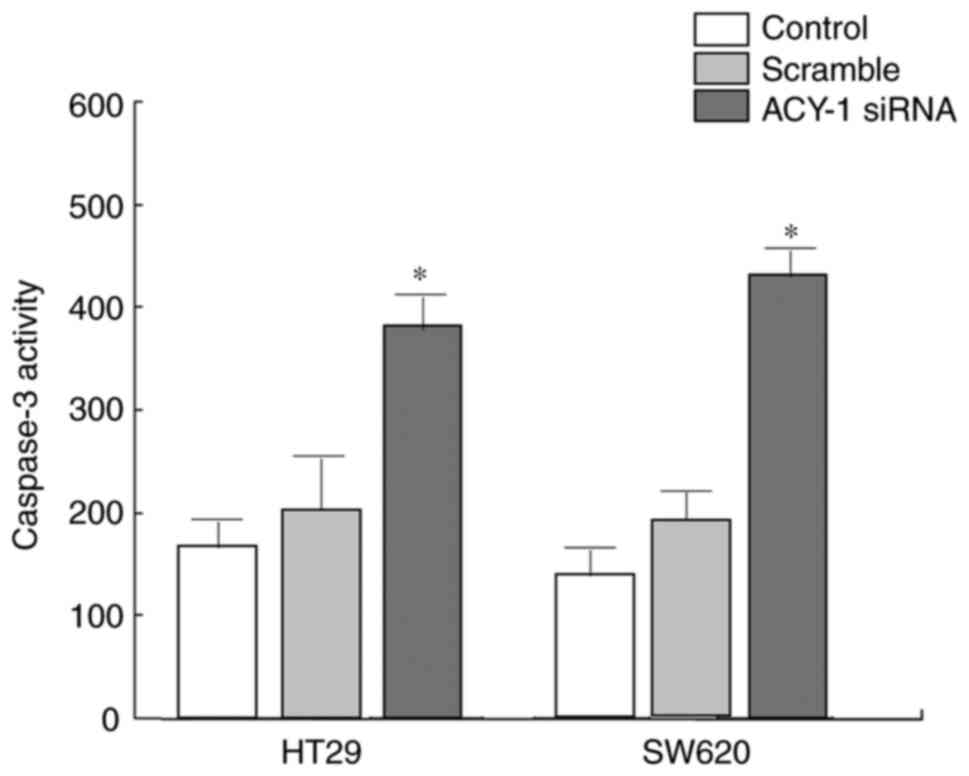
Effect of ACY-1 siRNA on caspase-3
activity in HT29 cells
The effect of ACY-1 siRNA on the activity of
caspase-3 in HT29 or SW620 cells was detected using the caspase-3
activity kit. It was revealed that siRNA significantly enhanced
caspase-3 activity in HT29 or SW620 cells compared with control
treatment (P<0.05; Fig. 5).
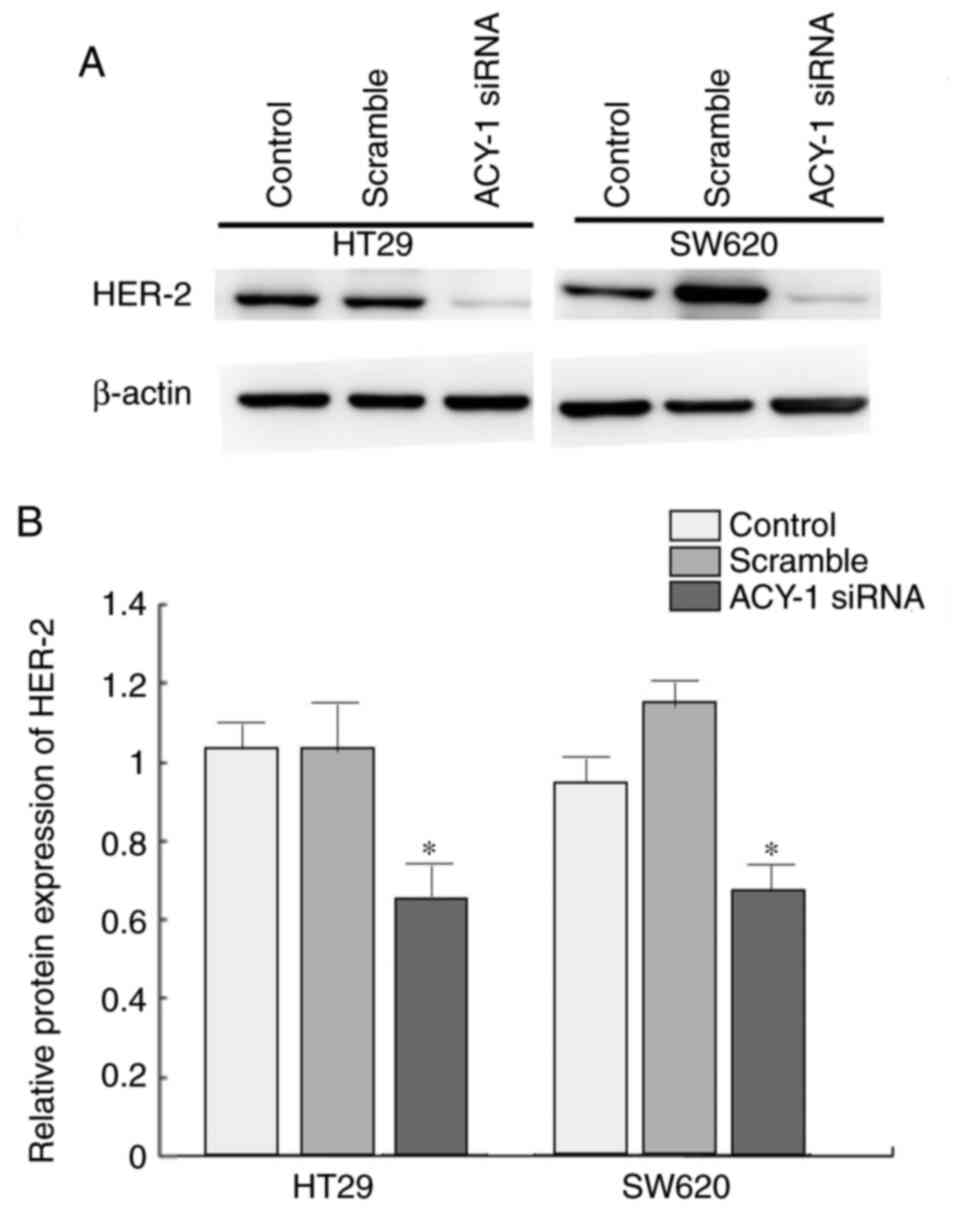
Impact of ACY-1 siRNA on HER2
expression in HT29 cells
Western blotting was performed to analyze the effect
of ACY-1 siRNA on HER2 expression in HT29 or SW620 cells. It was
demonstrated that siRNA against ACY-1 significantly suppressed HER2
expression compared with the control group (P<0.05; Fig. 6).
Influence of ACY-1 siRNA on TRAIL
expression in HT29 cells
Western blotting was performed to analyze the effect
of ACY-1 siRNA on TRAIL expression in HT29 or SW620 cells. ACY-1
siRNA significantly increased TRAIL expression compared with the
control group (P<0.05; Fig.
7).
Discussion
A previous study has investigated the expression
profile of ACY-1 in renal transplantation and as an indicator of
prognosis (19). As one of the
acylated amino acid active enzymes, ACY-1 is mainly expressed in
the cell membrane and cytoplasm. It can acetylate α-acylated amino
acids and enhance protein stability in cells (20). There are relatively few studies on
the role of ACY-1 in tumors. It has been previously found that
ACY-1 expression is decreased in liver cancer and renal cell
carcinoma (21-23);
however, the expression of ACY-1 in rectal cancer has not been
clarified. In the present study, ACY-1 expression was shown to be
significantly increased in rectal cancer tissues and cells,
suggesting that it may be associated with the occurrence and
development of rectal cancer.
The proto-oncogene HER2, also known as the neu gene
or the c-erbB-2 gene, is involved in several physiological
processes, such as cell proliferation and differentiation (24). However, under the influence of
external factors, the HER2 gene is abnormally expressed, which may
lead to the abnormal activation of its related protein product
P185, inducing tumorigenesis and metastasis (25). TRAIL is an apoptosis-inducing
ligand; the binding of TRAIL to its receptor promotes the caspase-3
protease cascade and induces apoptosis (26). The present study demonstrated that
ACY-1 siRNA decreased the expression of ACY-1 at the gene and
protein level, which significantly downregulated the expression
levels of HER2, elevated TRAIL expression, suppressed cell
proliferation and upregulated caspase-3 activity in HT29 and SW620
cells. This indicates that ACY-1 siRNA in rectal cancer may enhance
TRAIL expression by inhibiting HER2 expression, thereby inhibiting
tumor cell proliferation and promoting tumor cell apoptosis. In
further research, it is necessary to explore the specific target
and related mechanisms of ACY-1, and provide relevant evidence for
its use in the clinical diagnosis and treatment of RC.
In conclusion ACY-1 expression is increased in
rectal cancer tissue. Targeting the ACY-1 gene can regulate the
expression of both HER2 and TRAIL which may inhibit the occurrence
and development of rectal cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZX and YH performed the experiments and analyzed the
data. ZY designed the study and wrote the manuscript.
Ethics approval and consent to
participate
The current study was approved by The First People’s
Hospital Xianyang City and consent was obtained for
participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pratap Singh A, Kumar A, Dhar A, Agarwal S
and Bhimaniya S: Advanced colorectal carcinoma with testicular
metastasis in an adolescent: A case report. J Med Case Rep.
12(304)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hu YH, Wei JW, Chang H, Xiao WW, Lin JZ,
Cai MY, Cai PQ, Kong LH, Chen G, Pan ZZ, et al: The high pCR rate
of sandwich neoadjuvant treatment in locally advanced rectal cancer
may translate into a better long-term survival benefit: 5-year
outcome of a Phase II clinical trial. Cancer Manag Res.
10:4363–4369. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hu FB: Globalization of diabetes: The role
of diet, lifestyle, and genes. Diabetes Care. 34:1249–1257.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Guedj N, Maggiori L, Pote N, Norkowski E,
Cros J, Bedossa P and Panis Y: Distal intramural and tumor spread
in the mesorectum after neoadjuvant radiochemotherapy in rectal
cancer: About 124 consecutive patients. Hum Pathol. 52:164–172.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pedziwiatr M, Pisarska M, Kisielewski M,
Major P, Mydlowska A, Rubinkiewicz M, Winiarski M and Budzynski A:
ERAS protocol in laparoscopic surgery for colonic versus rectal
carcinoma: Are there differences in short-term outcomes? Med Oncol.
33(56)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kong I, Vorunganti S, Patel M, Farrell T,
Timotin E, Quinlan-Davidson S, Pond G, Sur R and Hunter R:
Prospective comparison of rectal dose reduction during
intracavitary brachytherapy for cervical cancer using three rectal
retraction techniques. Brachytherapy. 15:450–455. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yu E, DiPetrillo TA, Ramey S and Leonard
KL: Comparison of endorectal ultrasound versus pelvic magnetic
resonance imaging for radiation treatment planning in locally
advanced rectal cancer. Pract Radiat Oncol. 5:e451–e455.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hernández J, Molins L, Fibla JJ, Heras F,
Embún R and Rivas JJ: Grupo Español de Metástasis Pulmonares de
Carcinoma Colo-Rectal (GECMP-CCR) de la Sociedad Española de
Neumología y Cirugía Torácica (SEPAR). Role of major resection in
pulmonary metastasectomy for colorectal cancer in the Spanish
prospective multicenter study (GECMP-CCR). Ann Oncol. 27:850–855.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Simkens GA, van Oudheusden TR, Braam HJ,
Wiezer MJ, Nienhuijs SW, Rutten HJ, van Ramshorst B and de Hingh
IH: Cytoreductive surgery and HIPEC offers similar outcomes in
patients with rectal peritoneal metastases compared to colon cancer
patients: A matched case control study. J Surg Oncol. 113:548–553.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Balamurugan TS, Huang CH, Chang PC and
Huang ST: Electrochemical molecular switch for the selective
profiling of cysteine in live cells and whole blood and for the
quantification of aminoacylase-1. Anal Chem. 90:12631–12638.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Caira S, Iannelli A, Sciarrillo R,
Picariello G, Renzone G, Scaloni A and Addeo P: Differential
representation of liver proteins in obese human subjects suggests
novel biomarkers and promising targets for drug development in
obesity. J Enzyme Inhib Med Chem. 32:672–682. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Meyer HJ, Gundermann P, Hohn AK, Hamerla G
and Surov A: Associations between whole tumor histogram analysis
parameters derived from ADC maps and expression of EGFR, VEGF, Hif
1-alpha, Her-2 and Histone 3 in uterine cervical cancer. Magn Reson
Imaging. 57:68–74. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schizas D: Should addition of five years
of ovarian suppression to tamoxifen be ‘must’ for hormone receptor
positive and HER-2 positive breast cancer under the age of 35? J
BUON. 23(1201)2018.PubMed/NCBI
|
|
14
|
Tabak SA, Khalifa SE and Fathy Y: HER-2
immunohistochemical expression in bone sarcomas: A new hope for
osteosarcoma patients. Open Access Maced J Med Sci. 6:1555–1560.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Radke DI, Ling Q, Hasler R, Alp G,
Ungefroren H and Trauzold A: Downregulation of TRAIL-receptor 1
increases TGFβ type II receptor expression and TGFβ signalling via
microRNA-370-3p in pancreatic cancer cells. Cancers (Basel).
10(E399)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang Z, Zhang M, Lv X, Fan J, Zhang J, Sun
J and Shen Y: GroEL/ES mediated the in vivo recovery of TRAIL
inclusion bodies in Escherichia coli. Sci Rep.
8(15766)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Byun HS, Zhou W, Park I, Kang K, Lee SR,
Piao X, Park JB, Kwon TK, Na M and Hur GM: C-27-carboxylated
oleanane triterpenoids up-regulate TRAIL DISC assembly via p38 MAPK
and CHOP-mediated DR5 expression in human glioblastoma cells.
Biochem Pharmacol. 158:243–260. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
He X, Hong Y, Wang X, Zhang X, Long J, Li
H, Zhang B, Chen S, Liu Q, Li H, et al: Identification and clinical
significance of an elevated level of serum aminoacylase-1
autoantibody in patients with hepatitis B virus-related liver
cirrhosis. Mol Med Rep. 14:4255–4262. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ali S and Sheerin NS: Biomarkers of acute
injury: Predicting the long-term outcome after transplantation.
Kidney Int. 84:1072–1074. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wei X, Li J, Xie H, Ling Q, Wang J, Lu D,
Zhou L, Xu X and Zheng S: Proteomics-based identification of the
tumor suppressor role of aminoacylase 1 in hepatocellular
carcinoma. Cancer Lett. 351:117–125. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Welberry Smith MP, Zougman A, Cairns DA,
Wilson M, Wind T, Wood SL, Thompson D, Messenger MP, Mooney A,
Selby PJ, et al: Serum aminoacylase-1 is a novel biomarker with
potential prognostic utility for long-term outcome in patients with
delayed graft function following renal transplantation. Kidney Int.
84:1214–1225. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhong Y, Onuki J, Yamasaki T, Ogawa O,
Akatsuka S and Toyokuni S: Genome-wide analysis identifies a tumor
suppressor role for aminoacylase 1 in iron-induced rat renal cell
carcinoma. Carcinogenesis. 30:158–164. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Iqbal N and Iqbal N: Human epidermal
growth factor receptor 2 (HER2) in cancers: Overexpression and
therapeutic implications. Mol Biol Int. 2014(852748)2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yang M, Fang X, Li J, Xu D, Xiao Q, Yu S,
Hu H, Weng S, Ding K and Yuan Y: Afatinib treatment for her-2
amplified metastatic colorectal cancer based on patient-derived
xenograft models and next generation sequencing. Cancer Biol Ther.
20:391–396. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Choi SA, Lee C, Kwak PA, Park CK, Wang KC,
Phi JH, Lee JY, Chong S and Kim SK: Histone deacetylase inhibitor
panobinostat potentiates the anti-cancer effects of mesenchymal
stem cell-based sTRAIL gene therapy against malignant glioma.
Cancer Lett. 442:161–169. 2019.PubMed/NCBI View Article : Google Scholar
|