Introduction
Non-alcoholic fatty liver disease (NAFLD) is the
most prevalent liver disorder in advanced countries, and primarily
involves the dysregulation of liver metabolism and inflammation.
The term NAFLD covers a broad spectrum of potentially serious
conditions, including hepatic steatosis and non-alcoholic
steatohepatitis (NASH), and may result in hepatic cirrhosis and
hepatocellular carcinoma (HCC) (1,2). As
the progressive form of NAFLD, 15-25% of cases of NASH progress to
cirrhosis within 10-15 years. NASH-associated cirrhosis carries a
high risk for HCC, and it has been reported that 30-50% of HCC
cases occur in patients with non-cirrhotic NASH (3). In 2011, NASH was predicted to become
the predominant indication for liver transplantation within the
next decade (4). Furthermore, other
NASH-associated complications include hypertension, diabetes
mellitus and cardiovascular disease (CVD) that also have associated
increased risks of morbidity and mortality (3,5).
Therefore, pharmaceutical experts are eager to develop novel
therapeutic approaches for NASH. However, even though more than 35
drugs have been investigated in preclinical and clinical studies,
there are as yet no approved therapies for NASH (6). An understanding of the pathogenesis of
NAFLD is fundamental to the development of new therapeutic
approaches, as it facilitates the identification of potential
therapeutic targets.
NASH is reported to be associated with inappropriate
functioning of the farnesoid X nuclear receptor (FXR), which is
activated by bile acid (BA) in direct proportion to its
lipophilicity, and serves a critical role in BA, lipid and
carbohydrate metabolism (7). When
lipophilic BAs bind to and activate the FXR, insulin sensitivity is
increased and hepatic gluconeogenesis and circulating triglycerides
are decreased. The FXR is principally expressed in the liver,
intestine and kidney (7).
Obeticholic acid (OCA) is a potent, selective FXR agonist, which is
a promising new drug for NASH, based on preclinical studies of
acute hepatic inflammation and fibrosis (8,9) as
well as clinical studies (10,11).
OCA has shown the ability to ameliorate the histological features
of NASH in the Farnesoid X nuclear ligand OCA for non-cirrhotic,
non-alcoholic steatohepatitis (FLINT) study (11) and to mitigate hepatic fibrosis in
the REGENERATE study (12).
However, as noted in the FLINT study (11) and a number of studies using animals
(13,14), OCA can adversely affect plasma
lipoprotein profiles via the elevation of total cholesterol (TC)
and low-density lipoprotein cholesterol (LDL-C) levels and
reduction of high-density lipoprotein cholesterol (HDL-C) levels.
This significant drawback has impeded the introduction of OCA into
clinical practice. To address and potentially overcome this
obstacle, we hypothesized that the use of statins as lipid-lowering
agents would prevent the changes in cholesterol levels associated
with OCA.
Statins are effective cholesterol-lowering drugs
widely used in the treatment and prevention of CVD. They can reduce
LDL-C (‘bad’ cholesterol) by 20-60%, lower triglyceride (TG) levels
and slightly increase HDL (‘good’ cholesterol) (15,16).
In addition, guidelines published in 2018(3) recommended the use of statins in the
management of dyslipidemia, a common comorbidity of NASH.
Nevertheless, the use of statins in certain types of liver disease
remains controversial, because statins can induce liver injury,
characterized by elevated alanine aminotransferase (ALT) and
aspartate aminotransferase (AST) levels in some patients.
Therefore, statins should not be administered to patients with
liver failure or liver decompensation. However, liver toxicity
should be of little concern in patients with non-cirrhotic NASH, in
whom statins are expected to be efficacious in treating
hyperlipemia. Therefore, the present study examined a therapeutic
strategy using a combination of OCA and simvastatin (SIM) for
combating high-fat diet (HFD)-induced NASH in mice. The aim of the
study was to explore the mechanisms and effects of a combination of
OCA and SIM in the HFD-induced NASH model.
Materials and methods
Experimental animals and reagents
A total of 30 male C57BL/6J mice (age, 8 weeks;
weight, 23.68±1.16 g; purchased from Beijing HFK Bioscience Co.
Ltd.) were bred and housed as previously described (17). NASH was induced by a HFD comprising
0.2% cholesterol and 42% calories from fat (TD.88137 adjusted
calories diet; Envigo Tekland). OCA (cat. no. OCA-161101) was a
gift from North China Pharmaceutical Group Corp. SIM tablets (20
mg/tablet; national medicine permission number, H20083840) were
purchased from Shandong Lukang Pharmaceutical Group Saite Co.,
Ltd.
Experimental design
The C57BL/6J mice were randomly divided into five
groups (n=6/group): i) control group, mice fed normal chow diet;
ii) HFD group, mice fed the HFD; iii) HFD + OCA group, mice fed the
HFD with OCA (10 mg/kg/day) provided as a dietary admixture; iv)
HFD + SIM group, mice fed the HFD diet supplemented with SIM (20
mg/kg/day); v) HFD + OCA + SIM group, mice fed the HFD supplemented
with OCA (10 mg/kg/day) and SIM (20 mg/kg/day). The mice were
provided with ad libitum access to water and food. At the end of
the 16-week experiment, the mice were sacrificed by cervical
dislocation performed under diethyl ether anesthesia by a trained
individual. The successful induction of anesthesia by diethyl ether
prior to cervical dislocation was confirmed by observation of the
following parameters: Respiration decreased in frequency and
increased in depth, eyelid and cornea reflexes disappeared, muscle
tension and the reflex response reduced and no response to pain or
other stimulation was exhibited. Death was confirmed by checking
for a lack of heartbeat, pupillary response to light and
respiration. Following sacrifice, blood samples and liver tissues
were collected for analysis. The livers and abdominal adipose
tissue were isolated and weighed in order to calculate the ratio of
liver weight to body weight (Lw/Bw) and the ratio of abdominal
adipose tissue weight to body weight (Aw/Bw). Livers were fixed in
10% formalin at room temperature for 48 h for histological analysis
or snap-frozen in liquid nitrogen followed by storage at -80˚C in a
freezer until required. The blood samples were centrifuged at 1,000
x g for 10 min at 4˚C. The serum supernatants were collected,
divided into aliquots and stored at -80˚C for subsequent
biochemical analyses on the serum biochemical indices. All animal
care and experimental protocols were in accordance with the Animal
Management Rules of the Ministry of Health of the People's Republic
of China. The animal protocol was approved by the Ethics Committee
of the Third Hospital of Hebei Medical University.
Measurement of serum biochemical
indices
Serum ALT, AST, TC, TG and LDL levels were
quantitated by an enzymatic kinetic method using an automatic
biochemical analyzer (Olympus AU2700; Olympus Corporation)
according to the manufacturer's instructions.
Histological examination
The paraffin-embedded liver sections (5 µm thick)
were treated with hematoxylin solution for 5 mins and eosin
solution for 1 min for Hematoxylin and eosin (H&E) staining at
room temperature. The liver sections were scored for hepatic
steatosis and inflammation as described previously using Brunt's
criteria and the histological scoring system for NAFLD issued by
the Pathology Committee of the Nonalcoholic Steatohepatitis
Clinical Research Network (18).
Briefly, steatosis was expressed as the percentage of the total
liver section affected by microvesicular or macrovesicular
steatosis. Hepatic inflammation was analyzed by counting the number
of inflammatory foci per field at x200 magnification in five
overlapping fields per specimen using a light microscope. Liver
samples were scored independently for NASH severity by two
pathologists who were blinded to the source of the samples.
Western blot analysis
Total proteins were extracted from liver tissue
using radio-immunoprecipitation buffer (Wanleibio Co., Ltd.) and
the protein concentration was evaluated using a bicinchoninic acid
kit (Wanleibio Co., Ltd.). Then, ~40 µg sample proteins/lane were
separated using 10 or 12% SDS-PAGE gel and transferred onto
polyvinylidene difluoride membranes (EMD Millipore) by
electroblotting. The membranes were blocked for 60 min at room
temperature in a buffer containing 0.1% Tween-20 and 5% milk, and
then incubated overnight at 4˚C with primary antibodies against FXR
(ab235094; Abcam), small heterodimeric partner (SHP; ab186874;
Abcam), cytochrome P450 family 7 subfamily A member 1 (CYP7A1;
ab65596; Abcam) and β-actin (WL01845; Wanleibio Co., Ltd.). After
washing, the membranes were incubated with HRP-conjugated secondary
antibodies (WLA023; Wanleibio Co., Ltd.) for 1 h at room
temperature, and then the protein bands were visualized using the
enhanced chemiluminescence reagent (Wanleibio Co., Ltd.). β-actin
served as a loading control. The intensity of each protein band was
quantified using ImageJ 1.46r software (National Institutes of
Health).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated and extracted from frozen
liver tissues using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). cDNA was synthesized from the RNA using a
PrimeScript™ RT reagent kit (RR037A; Takara Bio, Inc.) according to
the instructions suggested by the manufacturer. qPCR was performed
on an ABI 7500 Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) using TB Green™ Premix Ex Taq™ II (RR820A;
Takara Bio, Inc.). The thermocycling conditions used were as
follows: Initial denaturation at 95˚C for 30 sec, followed by 40
cycles of denaturation at 95˚C for 5 sec, and finally, annealing
and extension at 60˚C for 34 sec. The expression levels of the
target mRNAs were normalized against the endogenous reference gene
glyceraldehyde-phosphate dehydrogenase. The relative amount of each
gene was measured using the 2-ΔΔCq method (19). All RT-qPCR reactions were conducted
in triplicate. The primers used for RT-qPCR are shown in Table I.
 | Table IPrimers for quantitative polymerase
chain reaction. |
Table I
Primers for quantitative polymerase
chain reaction.
| Genes | Forward primer
(5'-3') | Reverse primer
(5'-3') |
|---|
| FXR (NR1H4) |
GCTAATGAGGACGACAGCGAAGG |
GTCTGTTGGTCTGCCGTGAGTTC |
| SHP (NR0B2) |
GTCCGACTATTCTGTATGCACT |
CTACTGTCTTGGCTAGGACATC |
| CYP7A1 |
GTGATGTTTGAAGCCGGATATC |
TTTATGTGCGGTCTTGAACAAG |
| BSEP (ABCB11) |
ATGAAGCCATTGCCGACCAGATG |
GACTGACAGCGAGAATCACCAAGG |
| IL-6 |
ACTTCCATCCAGTTGCCTTCTTGG |
TTAAGCCTCCGACTTGTGAAGTGG |
| TNF-α |
GCGACGTGGAACTGGCAGAAG |
GCCACAAGCAGGAATGAGAAGAGG |
| SREBP1 |
TAGAGCATATCCCCCAGGTG |
GGTACGGGCCACAAGAAGTA |
| FASN |
AAGGACCTGTCTAGGTTTGATGC |
TGGCTTCATAGGTGACTTCCA |
| GAPDH |
GGCATGGACTGTGGTCATGAG |
TGCACCACCAACTGCTTAGC |
Statistical analysis
Each experiment was repeated 3 times. All data are
presented as the mean ± standard deviation. Statistical analysis
was carried out by one-way analysis of variance followed by Tukey's
post hoc tests for evaluating differences between groups using IBM
SPSS Statistics 26 (IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
OCA combined with SIM ameliorates NASH
histological features in a HFD-induced mouse model
C57BL/6J mice were fed a HFD for 16 weeks to
establish a NASH mouse model. The characteristic histological
features of NASH were confirmed by H&E staining, which included
micro- and macrovesicular steatosis, ballooning degeneration and
the massive infiltration of cells (Fig.
1A).
The HFD group exhibited a significantly increased
inflammation score compared with the control group (P<0.05;
Fig. 1B). All three intervention
groups exhibited significantly lower liver inflammation scores
compared with the HFD group (P<0.05). Moreover, in the HFD + OCA
and HFD + OCA + SIM groups, the liver inflammation scores were
markedly reduced compared with that of the HFD + SIM group
(P<0.05), and no significant difference was observed between the
HFD + OCA and HFD + OCA + SIM groups. The steatosis scores mirrored
the inflammation scores. Notably, the combined administration of
OCA and SIM to the HFD-fed mice significantly alleviated the
steatosis score compared with those of the two monotherapy groups
(P<0.05), whereas no statistically significant difference in
steatosis score was observed between the HFD + SIM and HFD
groups.
Co-administration of OCA and SIM
prevents body weight gain in HFD-fed mice
As weight loss is a factor that can improve the
clinical and metabolic features of NASH (20), the changes in the body weights of
the mice were carefully recorded. The body weight increase was
significantly higher in the HFD-fed mice compared with the control
group (P<0.05; Table II and
Fig. 2A). The mice in the HFD + OCA
group gained a similar amount of body weight to those in the HFD
group, whereas SIM alone significantly reduced body weight gain
compared with that of the HFD group (P<0.05). Furthermore, the
co-administration of OCA and SIM significantly prevented body
weight gain compared with that of the HFD and HFD + OCA groups
(P<0.05).
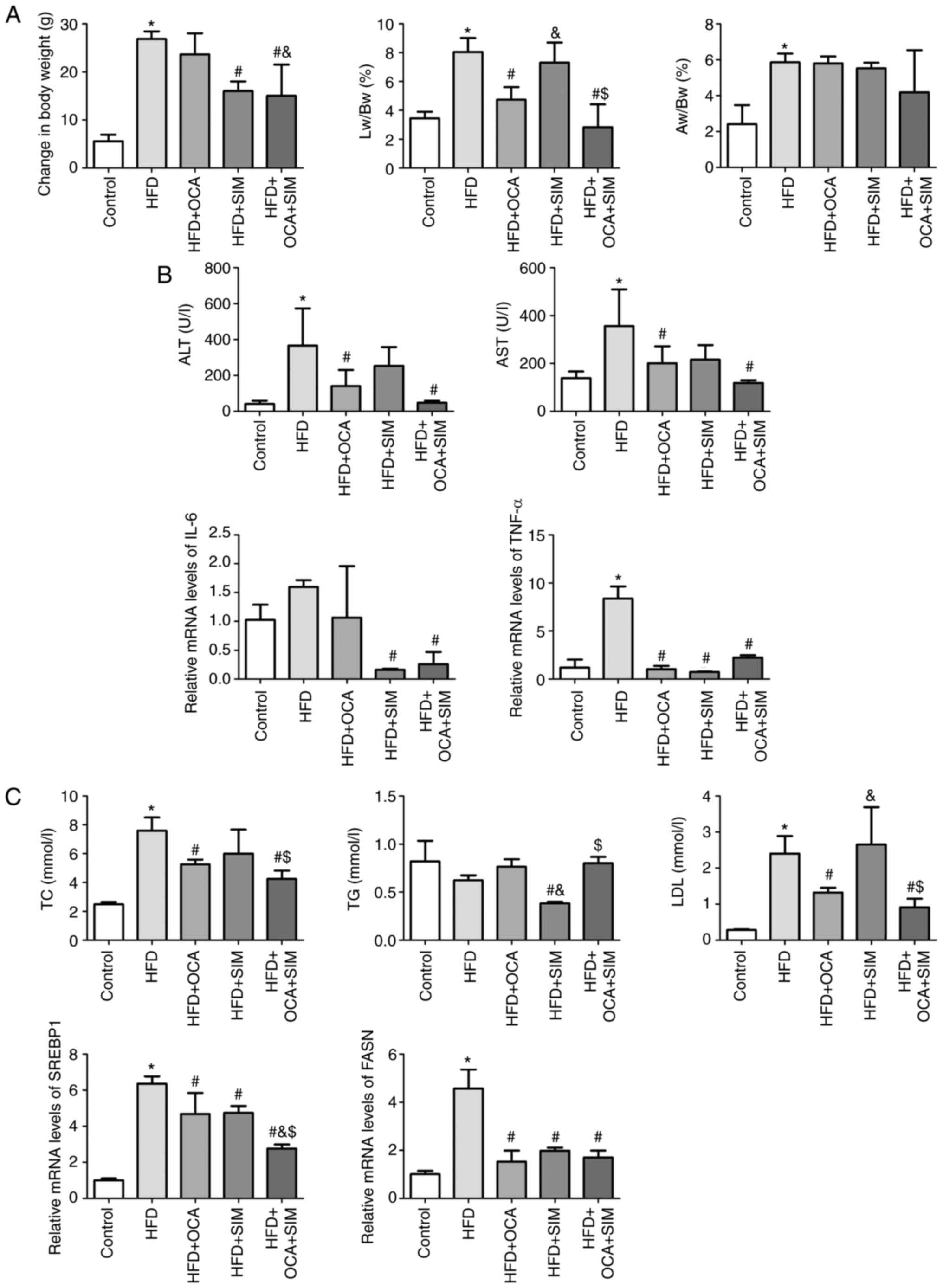 | Figure 2Co-administration of OCA and SIM
prevents body weight gain, alleviates the pro-inflammatory response
and liver damage and downregulates lipid metabolism in HFD-fed
mice. (A) Changes in weights, Lw/Bw ratios and Aw/Bw ratios in the
five groups. (B) Markers of liver damage, the inflammatory response
and (C) lipid metabolism were evaluated. Data are presented as the
mean ± SD (n=6/group). HFD, high-fat diet; OCA, obeticholic acid;
SIM, simvastatin; Lw/Bw, ratio of liver weight to body weight;
Aw/Bw, ratio of abdominal adipose tissue weight to body weight;
ALT, alanine transaminase; AST, aspartate aminotransferase; IL-6,
interleukin-6; TNF-α, tumor necrosis factor-α; TC, total
cholesterol; TG, triglyceride; LDL, low-density lipoprotein;
SREBP1, sterol regulatory element binding protein-1; FASN, fatty
acid synthase. *P<0.05 vs. control group;
#P<0.05 vs. HFD group; &P<0.05 vs.
HFD + OCA group; $P<0.05 vs. HFD + SIM group. |
 | Table IIEffect of HFD and experimental
treatments on body weight and the Lw/Bw and Aw/Bw ratios of
C57BL/6J mice. |
Table II
Effect of HFD and experimental
treatments on body weight and the Lw/Bw and Aw/Bw ratios of
C57BL/6J mice.
| Variable | Control | HFD | HFD + OCA | HFD + SIM | HFD + OCA +
SIM | F-value | P-value |
|---|
| Body weight gain
(g) | 5.60±1.34 |
26.83±1.60a | 23.67±4.37 |
16.00±2.00b |
15.00±6.52b,c | 25.327 | <0.001 |
| Lw/Bw (%) | 3.46±0.44 |
8.05±0.96a |
4.74±0.87b |
7.31±1.39c |
2.83±1.59b,d | 23.055 | <0.001 |
| Aw/Bw (%) | 2.41±1.07 |
5.86±0.49a | 5.79±0.39 | 5.53±0.31 | 4.18±2.35 | 7.725 | 0.001 |
The Lw/Bw and Aw/Bw ratios were calculated in order
to determine the weight changes of the liver and abdominal adipose
tissue. The Lw/Bw and Aw/Bw ratios in the HFD group were
significantly increased compared with those in the control group
(P<0.05), indicating that the weight of the liver and the
abdominal adipose tissue increased in NASH. When compared with the
HFD group, the HFD + OCA group exhibited a decreased Lw/Bw ratio
(P<0.05) but no difference in the Aw/Bw ratio, while the HFD +
SIM group exhibited no significant difference in Lw/Bw or Aw/Bw
ratios. Notably, the HFD + OCA + SIM group presented a prominent
and significant reduction in Lw/Bw ratio compared with the HFD and
HFD + SIM groups (P<0.05), but no significant difference
compared with the HFD + OCA group.
Co-administration of OCA and SIM
alleviates the pro-inflammatory response and liver damage in
NASH
The inflammatory response of the liver was assessed
via the measurement of serum ALT and AST levels. The HFD group
showed significantly increased levels of ALT and AST compared with
the control group (P<0.05; Table
III and Fig. 2B). In the HFD +
OCA and HFD + OCA + SIM groups, the increases in ALT and AST
induced by the HFD were significantly reversed. Furthermore, the
combination of OCA and SIM appeared to exhibit a stronger effect
than OCA alone, but the difference between the HFD + OCA and HFD +
OCA + SIM groups did not reach statistical significance. Although
the HFD + SIM group exhibited a slight but non-significant
reduction in ALT and AST levels compared with the HFD group, the
results suggest that SIM is safe to administer to subjects with
NASH in this model.
 | Table IIIEffect of HFD and experimental
treatments on serum transaminases and circulating lipids. |
Table III
Effect of HFD and experimental
treatments on serum transaminases and circulating lipids.
| Variable | Control | HFD | HFD + OCA | HFD + SIM | HFD + OCA +
SIM | F-value | P-value |
|---|
| ALT (U/l) | 40.00±18.68 |
365.83±206.62a |
139.67±90.28b | 253.33±104.04 |
47.80±11.21b | 7.481 | 0.001 |
| AST (U/l) | 138.80±27.67 |
355.67±153.72a |
201.17±70.80b | 216.00±60.92 |
118.60±11.24b | 6.322 | 0.002 |
| TC (mmol/l) | 2.49±0.16 |
7.60±0.91a |
5.26±0.32b | 6.00±1.67 |
4.25±0.57b,c | 33.172 | <0.001 |
| TG (mmol/l) | 0.82±0.21 | 0.62±0.05 | 0.76±0.08 |
0.38±0.02b,d |
0.80±0.07c | 9.794 | <0.001 |
| LDL (mmol/l) | 0.29±0.02 |
2.40±0.49a |
1.32±0.13b |
2.65±1.04d |
0.91±0.24b,c | 15.610 | <0.001 |
To further investigate the anti-inflammatory
mechanism of OCA and/or SIM therapies, the expression levels of the
pro-inflammatory cytokines interleukin-6 (IL-6) and tumor necrosis
factor-α (TNF-α) were assessed by RT-qPCR. Compared with the
control group, the HFD-fed mice exhibited increased mRNA expression
of IL-6, which was attenuated somewhat by the administration of
OCA; however, neither of these changes were statistically
significant. The HFD + SIM and HFD + OCA + SIM groups presented
significant reductions in the mRNA expression levels of IL-6 when
compared with the HFD group (P<0.05). With regard to TNF-α, the
mRNA expression level in the HFD group was increased compared with
that in the control group (P<0.05). By sharp contrast, the three
interventions significantly reduced the mRNA expression levels of
TNF-α relative to those in the HFD group (P<0.05).
Effect of OCA and/or SIM on lipid
metabolism
Lipid metabolism is a key determinant of NASH, and
OCA has been documented to have an adverse effect on plasma
lipoprotein profiles in patients, as characterized by elevated TC
and LDL-C levels and reduced HDL-C levels. Therefore, serum
lipoproteins level were examined and the mRNA expression levels of
sterol regulatory element binding protein-1 (SREBP1) and fatty acid
synthase (FASN) were measured by RT-qPCR. The HFD-fed mice were
found to have significantly elevated levels of TC and LDL
(P<0.05), and a non-significant reduction in the levels of TGs
compared with those in the control group (Table III and Fig. 2C). These changes in serum
lipoproteins were attenuated by the administration of OCA and/or
SIM. The levels of TGs in the HFD + SIM group were significantly
decreased compared with those in the HFD, HFD + OCA and HFD + OCA +
SIM groups (P<0.05). However, a modest increase in TC and LDL
levels was observed in the HFD + SIM group compared with the HFD +
OCA and HFD + OCA + SIM groups.
The mice in the HFD group presented a significant
increase in the mRNA expression levels of SREBP1 and FASN compared
with those in the control group, and these increases were
attenuated by the administration of OCA or SIM alone or in
combination (P<0.05). Notably, no difference was observed in the
FASN mRNA expression levels of the three intervention groups, but
the HFD + OCA + SIM group exhibited a significant reduction in the
mRNA expression level of SREBP1 compared with the HFD + OCA and HFD
+ SIM groups (P<0.05).
Effect of OCA and/or SIM on the FXR
signaling pathway
OCA is a potent, selective FXR agonist. To determine
whether SIM enhances the effect of OCA on the FXR signaling
pathway, the hepatic expression levels of FXR and its downstream
genes SHP, CYP7A1 and bile salt export pump (BSEP) were
assessed.
No significant difference in the protein expression
levels of FXR was observed between the HFD and control groups
(Fig. 3A), while the protein
expression levels of FXR in the HFD + OCA and HFD + SIM groups were
significantly increased compared with those in the HFD group
(P<0.05). Notably, the FXR protein level in the HFD + SIM group
was significantly higher than that in the HFD + OCA group
(P<0.05), while it was markedly and significantly reduced in the
HFD + OCA + SIM group compared with the HFD, HFD + OCA and HFD +
SIM groups (P<0.05). The mRNA expression level of FXR was
detected by RT-qPCR. The HFD group presented a significant decrease
in FXR mRNA expression (P<0.05), and the FXR mRNA levels in the
HFD + OCA and HFD + OCA + SIM groups were significantly higher than
those in the HFD and HFD + SIM groups (P<0.05).
SHP is a downstream target gene of FXR (21). The SHP protein expression level of
the HFD-fed mice was slightly lower than that of the control group,
but the difference was not significant. The protein expression
levels of SHP in the three intervention groups were significantly
increased compared with those in the HFD group (P<0.05).
Importantly, the HFD + SIM group exhibited a higher protein level
of SHP compared with the HFD + OCA and HFD + OCA + SIM groups
(P<0.05). The trends in SHP mRNA expression levels were similar
to those of SHP protein, with the exception that the SHP mRNA level
in the HFD + SIM group appeared to be lower than those of the HFD +
OCA and HFD + OCA + SIM groups (Fig.
3B).
CYP7A1 is the rate-limiting enzyme in cholesterol
synthesis, which can be regulated by FXR and its target gene SHP in
the liver (22). In the present
study, the protein expression level of CYP7A1 in the HFD group was
increased compared with that in the control group (P<0.05); the
mRNA expression level of CYP7A1 was also increased, albeit not
significantly. In comparison with the HFD group, the HFD + OCA
group showed a significant reduction in the expression of CYP7A1 at
the protein level (P<0.05), but no difference at the mRNA level.
The HFD + SIM and HFD + OCA + SIM groups were found to have
significantly increased protein expression levels of CYP7A1
compared with the HFD and HFD + OCA groups (P<0.05), but no
significant difference in the mRNA expression levels of CYP7A1 was
observed (Fig. 3C).
The mRNA expression levels of BSEP were detected by
RT-qPCR. As shown in Fig. 3D, the
mRNA expression level of BSEP was slightly reduced in the HFD-fed
mice. However, the mice in the HFD + OCA and HFD + OCA + SIM groups
had significantly increased BSEP mRNA expression levels compared
with those in the HFD and HFD + SIM groups (P<0.05). Also, the
BSEP level in the HFD + OCA + SIM group was higher than that in the
HFD + OCA group (P<0.05).
Discussion
OCA is an FXR agonist that has been approved by the
US Food and Drug Administration for use in patients with primary
biliary cirrhosis, either in combination with ursodeoxycholic acid
(UDCA) for patients with an inadequate response to UDCA alone, or
as a monotherapy in patients who are intolerant to UDCA. The FXR
regulates the synthesis of BA by cholesterol catabolism. Although
OCA can stimulate FXR expression, increase the hepatic expression
of SHP and consequently suppress the expression of CYP7A1 to reduce
the rate of BA synthesis in the liver, it has yet to be approved
for the treatment of NASH. Although numerous clinical studies,
including randomized clinical trials, have confirmed the
effectiveness of OCA in ameliorating NASH (10,11),
its side effect of elevating cholesterol levels has prevented its
progression to the clinic. Statins are competitive inhibitors of
the enzyme 3-hydroxy-3-methylglutaryl coenzyme A
(HMG-CoA)-reductase. Via this mechanism, SIM directly inhibits the
formation of mevalonate from HMG-CoA and thus also inhibits the
biosynthesis of cholesterol (23).
SIM is mainly used in the clinical treatment of hyperlipidemia and
effectively prevents the occurrence of cardiovascular and
cerebrovascular adverse events such as coronary heart disease. We
hypothesized that SIM may be able to counteract the side effects of
OCA and perhaps even promote the effectiveness of OCA in the
treatment of NASH.
Our previous study (24) demonstrated that a HFD induces NASH
in mice. Continuous feeding with a HFD triggers severe endoplasmic
reticulum stress in the liver, and hepatocytes fail to adapt to
this stress; they are unable to maintain normal homeostatic levels
of unfolded or misfolded proteins, leading to hepatocyte apoptosis
and inflammation. In the present study, treatment with OCA and SIM
was demonstrated to prevention HFD-induced liver injury in the
mouse model of NASH. The results support the combination of OCA and
SIM as a novel therapy for NASH, which is in line with findings of
the randomized Combination of OCA and statins for monitoring of
lipids (CONTROL) trial (25). In
the CONTROL trial, a phase II placebo-controlled patient study, the
OCA-induced increases in LDL in patients with NASH were mitigated
with atorvastatin, and the combination of OCA and atorvastatin was
generally safe and well-tolerated. In the present study, the
co-administration of OCA and SIM resulted in lower steatosis scores
than were obtained using OCA or SIM monotherapies, while OCA with
or without SIM markedly reduced the hepatic inflammation score
compared with those of the HFD and HFD + SIM groups. Serum ALT and
AST levels were examined as they are indicators of the inflammatory
response associated with liver damage. The use of OCA with or
without SIM significantly prevented the HFD-induced increase of ALT
and AST levels, and the co-administration of OCA and SIM exhibited
the strongest effect. Although the HFD + SIM group exhibited
minimal reductions in ALT and AST levels compared with the HFD
group, the results indicate that the administration of SIM in
subjects with NASH is safe in this model. IL-6 and TNF-α were
examined as representative inflammatory factors. Hu et al
(26) investigated INT-767, a
recently identified dual FXR/TGR5 agonist, in a rat model of NASH
and demonstrated that treatment with INT-767 significantly
alleviated HFD-induced NASH and attenuated the pro-inflammatory
response via the suppression of TNF-α and the NF-κB signaling
pathway. The results of the present study were consistent with
this, and showed that OCA alone or in combination with SIM was able
to attenuate liver inflammation in a mice NASH model and
downregulate the expression levels of IL-6 and TNF-α.
The optimization of metabolic risk factors and
participation in diet management and exercise are key to the
management of NASH, and weight loss alone can improve the histology
of NASH. Based on the FXR ligand OA in NASH treatment (FLINT)
trial, a post hoc subgroup analysis was performed by Hameed et
al (27), which showed that OCA
and weight loss had additive beneficial effects on the liver
enzymes ALT and AST, and on histological features of disease
activity in NASH. Based on these findings from the FLINT trial, the
body weights of the mice were carefully documented in the present
study, and it was observed that OCA administration exhibited no
significant effect on body weight gain. This is in concordance with
a previous study on NASH models using male wild-type C57BL/6J mice
and Lep ob/ob (ob/ob-NASH) mice (28). By contrast, the administration of
SIM alone and the co-administration of OCA and SIM significantly
prevented body weight gain. The Lw/Bw and Aw/Bw ratios in the HFD
group were significantly higher than those in the control group,
indicating that liver and abdominal adipose tissue weights
increased in NASH. Interestingly, the Lw/Bw ratio was only
prominently reduced in the HFD + OCA + SIM group compared with the
HFD and HFD + SIM groups. Relative to the HFD group, the HFD + OCA
group exhibited a lower Lw/Bw ratio, but no difference in the Aw/Bw
ratio, and the HFD + SIM group exhibited only slightly decreased
Lw/Bw and Aw/Bw ratios compared with those of the HFD group. A
suggested interpretation of these data is that OCA principally
reduces liver weight, whereas SIM has the ability to reduce liver
and abdominal adipose tissue weight as well as overall body weight.
Therefore, SIM may enhance the efficacy of OCA in the treatment of
NASH by reducing overall body, liver and abdominal adipose tissue
weights.
The original intention of the present study was to
investigate whether SIM is able to prevent or reverse the adverse
effect of OCA on plasma lipoproteins. In the C57BL/6J mice fed with
a HFD for 16 weeks, significantly elevated levels of TC and LDL
were observed, but the levels of TG were reduced, which is not
consistent with lipid metabolism in humans. These changes in plasma
lipoproteins were inhibited in mice whose HFD was supplemented with
OCA alone or in combination with SIM. Although serum lipid
metabolism in mice is not completely consistent with clinical
studies, the examination of liver histology in the present study
demonstrated that OCA significantly reduced the steatosis score in
the mouse model of NASH. Moreover, the mRNA expression levels of
SREBP1 and FASN were detected by RT-qPCR, and the results showed
that treatment with OCA alone or with SIM reduced the mRNA
expression levels of SREBP1 and FASN, which are the essential gene
transcription factors in lipogenesis.
It has been demonstrated that BAs are endogenous
ligands for FXR, and by activating FXR, they induce SHP, which in
turn suppresses CYP7A1 gene expression and thereby reduces the rate
of BA synthesis in the liver (29).
OCA is able to stimulate the expression of FXR, induce SHP and
suppress the expression of CYP7A1. In the present study, HFD-fed
mice showed no significant difference in the protein expression
levels of FXR and SHP, but a marked increase in CYP7A1 protein
expression compared with the control group. Regarding mRNA
expression, the HFD reduced the levels of FXR and SHP, and induced
CYP7A1 levels, which is entirely consistent with previously
published studies (26,30). FXR is a member of the nuclear
receptor superfamily, and is a ligand-activated transcriptional
regulator, harboring DNA- and ligand-binding domains (7). Therefore, it seems possible that FXR
may exert an effect at the transcriptional level.
The administration of OCA significantly elevated the
expression of FXR and SHP at the mRNA and protein levels and
reduced the CYP7A1 protein level. These results are consistent with
a mechanism involving the FXR signaling pathway. The administration
of SIM did not increase the FXR mRNA level compared with that in
the HFD group, but elevated the SHP mRNA level and reduced the
CYP7A1 mRNA level. The HFD + SIM group showed significantly
elevated protein levels of FXR, SHP and CYP7A1, but decreased
expression of the corresponding mRNAs compared with those in the
HFD + OCA group. There has been considerable research into statins
and the FXR pathway. For example, in a study using cultured Hep3B
cells, pravastatin enhanced FXR and CYP7A1 mRNA expression, and
prevented cholesterol gallstone formation in human hepatocytes by
increasing FXR, LXRα and CYP7A1(31). In addition, in another study
atorvastatin was shown to induce CYP7A1 in mice by suppressing FXR
signaling in the liver and intestine (32). The mechanisms underlying the effect
of statins on FXR and CYP7A1 remain to be clarified, but are
outside the scope of the present study. Furthermore, it must be
noted that the present study is limited by the lack of control
groups comprising OCA and/or SIM-treated mice fed a normal
diet.
In conclusion, the combination of OCA and SIM
ameliorated the histological features of NASH and prevented body
weight gain in an HFD-induced mouse model. The co-administration of
OCA and SIM suppressed the levels of IL-6, TNF-α, SREBP1 and FASN,
and alleviated the pro-inflammatory response and liver steatosis in
NASH. Although the exact mechanisms of the combined therapy require
further verification, the results of the study suggest that the
combination of OCA and SIM may be an effective pharmacotherapy for
NASH.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Key Science and
Technology Research Project of Health and Family Planning
Commission of Hebei Province (grant no. 20190639) and the Key
Research and Development Program of Hebei Province (grant no.
19277779D). The funders had no role in the study design, data
collection and analysis, decision to publish or preparation of the
manuscript.
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
YMN designed the research, WCL, YGZ, LBK and QSZ
performed the experiments, SXZ, RQW and WGR analyzed the data, and
WCL and SXZ wrote the manuscript. WCL and YMN confirmed the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All animal care and experimental protocols were in
accordance with the Animal Management Rules of the Ministry of
Health of the People's Republic of China. The animal protocol was
approved by the Ethics Committee of the Third Hospital of Hebei
Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rinella ME: Nonalcoholic fatty liver
disease: A systematic review. JAMA. 313:2263–2273. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Diehl AM and Day C: Cause, pathogenesis,
and treatment of nonalcoholic steatohepatitis. N Engl J Med.
377:2063–2072. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
National Workshop on Fatty Liver and
Alcoholic Liver Disease, Chinese Society of Hepatology, Chinese
Medical Association; Fatty Liver Expert Committee, Chinese Medical
Doctor Association. Guidelines of prevention and treatment for
nonalcoholic fatty liver disease: A 2018 update. Zhonghua Gan Zang
Bing Za Zhi. 26:195–203. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
4
|
Charlton MR, Burns JM, Pedersen RA, Watt
KD, Heimbach JK and Dierkhising RA: Frequency and outcomes of liver
transplantation for nonalcoholic steatohepatitis in the United
States. Gastroenterology. 141:1249–1253. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lonardo A, Nascimbeni F, Mantovani A and
Targher G: Hypertension, diabetes, atherosclerosis and NASH: Cause
or consequence? J Hepatol. 68:335–352. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ratziu V, Goodman Z and Sanyal A: Current
efforts and trends in the treatment of NASH. J Hepatol. 62 (Suppl
1):S65–S75. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lefebvre P, Cariou B, Lien F, Kuipers F
and Staels B: Role of bile acids and bile acid receptors in
metabolic regulation. Physiol Rev. 89:147–191. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Verbeke L, Mannaerts I, Schierwagen R,
Govaere O, Klein S, Vander Elst I, Windmolders P, Farre R, Wenes M,
Mazzone M, et al: FXR agonist obeticholic acid reduces hepatic
inflammation and fibrosis in a rat model of toxic cirrhosis. Sci
Rep. 6(33453)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang DG, Zhang C, Wang JX, Wang BW, Wang
H, Zhang ZH, Chen YH, Lu Y, Tao L, Wang JQ, et al: Obeticholic acid
protects against carbon tetrachloride-induced acute liver injury
and inflammation. Toxicol Appl Pharmacol. 314:39–47.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mudaliar S, Henry RR, Sanyal AJ, Morrow L,
Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe
E, et al: Efficacy and safety of the farnesoid X receptor agonist
obeticholic acid in patients with type 2 diabetes and nonalcoholic
fatty liver disease. Gastroenterology. 145:574–582.e1.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Neuschwander-Tetri BA, Loomba R, Sanyal
AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy
S, Diehl AM, Hameed B, et al: Farnesoid X nuclear receptor ligand
obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis
(FLINT): A multicentre, randomised, placebo-controlled trial.
Lancet. 385:956–965. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ratziu V, Sanyal AJ, Loomba R, Rinella M,
Harrison S, Anstee QM, Goodman Z, Bedossa P, MacConell L,
Shringarpure R, et al: REGENERATE: Design of a pivotal, randomised,
phase 3 study evaluating the safety and efficacy of obeticholic
acid in patients with fibrosis due to nonalcoholic steatohepatitis.
Contemp Clin Trials. 84(105803)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Briand F, Brousseau E, Quinsat M, Burcelin
R and Sulpice T: Obeticholic acid raises LDL-cholesterol and
reduces HDL-cholesterol in the Diet-Induced NASH (DIN) hamster
model. Eur J Pharmacol. 818:449–456. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Papazyan R, Liu X, Liu J, Dong B, Plummer
EM, Lewis RD II, Roth JD and Young MA: FXR activation by
obeticholic acid or nonsteroidal agonists induces a human-like
lipoprotein cholesterol change in mice with humanized chimeric
liver. J Lipid Res. 59:982–993. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
McTaggart F and Jones P: Effects of
statins on high-density lipoproteins: A potential contribution to
cardiovascular benefit. Cardiovasc Drugs Ther. 22:321–338.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Maron DJ, Fazio S and Linton MF: Current
perspectives on statins. Circulation. 101:207–213. 2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nan YM, Fu N, Wu WJ, Liang BL, Wang RQ,
Zhao SX, Zhao JM and Yu J: Rosiglitazone prevents nutritional
fibrosis and steatohepatitis in mice. Scand J Gastroenterol.
44:358–365. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kleiner DE, Brunt EM, Van Natta M, Behling
C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS,
Unalp-Arida A, et al: Design and validation of a histological
scoring system for nonalcoholic fatty liver disease. Hepatology.
41:1313–1321. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang HJ, Pan LL, Ma ZM, Chen Z, Huang ZF,
Sun Q, Lu Y, Han CK, Lin MZ, Li XJ, et al: Long-term effect of
exercise on improving fatty liver and cardiovascular risk factors
in obese adults: A 1-year follow-up study. Diabetes Obes Metab.
19:284–289. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hoeke MO, Heegsma J, Hoekstra M, Moshage H
and Faber KN: Human FXR regulates SHP expression through direct
binding to an LRH-1 binding site, independent of an IR-1 and LRH-1.
PLoS One. 9(e88011)2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chambers KF, Day PE, Aboufarrag HT and
Kroon PA: Polyphenol effects on cholesterol metabolism via bile
acid biosynthesis, CYP7A1: A review. Nutrients.
11(2588)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Goldstein JL and Brown MS: Regulation of
the mevalonate pathway. Nature. 343:425–430. 1990.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Li D, Zhao D, Du J, Dong S, Aldhamin Z,
Yuan X, Li W, Du H, Zhao W, Cui L, et al: Heme oxygenase-1
alleviated non-alcoholic fatty liver disease via suppressing
ROS-dependent endoplasmic reticulum stress. Life Sci.
253(117678)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pockros PJ, Fuchs M, Freilich B, Schiff E,
Kohli A, Lawitz EJ, Hellstern PA, Owens-Grillo J, Van Biene C,
Shringarpure R, et al: CONTROL: A randomized phase 2 study of
obeticholic acid and atorvastatin on lipoproteins in nonalcoholic
steatohepatitis patients. Liver Int. 39:2082–2093. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hu YB, Liu XY and Zhan W: Farnesoid X
receptor agonist INT-767 attenuates liver steatosis and
inflammation in rat model of nonalcoholic steatohepatitis. Drug Des
Devel Ther. 12:2213–2221. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hameed B, Terrault NA, Gill RM, Loomba R,
Chalasani N, Hoofnagle JH and Van Natta ML: NASH CRN. Clinical and
metabolic effects associated with weight changes and obeticholic
acid in non-alcoholic steatohepatitis. Aliment Pharmacol Ther.
47:645–656. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tolbol KS, Kristiansen MN, Hansen HH,
Veidal SS, Rigbolt KT, Gillum MP, Jelsing J, Vrang N and Feigh M:
Metabolic and hepatic effects of liraglutide, obeticholic acid and
elafibranor in diet-induced obese mouse models of biopsy-confirmed
nonalcoholic steatohepatitis. World J Gastroenterol. 24:179–194.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kim SG, Kim BK, Kim K and Fang S: Bile
acid nuclear receptor Farnesoid X receptor: Therapeutic target for
nonalcoholic fatty liver disease. Endocrinol Metab (Seoul).
31:500–504. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Carino A, Biagioli M, Marchianò S,
Fiorucci C, Zampella A, Monti MC, Scarpelli P, Ricci P, Distrutti E
and Fiorucci S: Ursodeoxycholic acid is a GPBAR1 agonist and resets
liver/intestinal FXR signaling in a model of diet-induced dysbiosis
and NASH. Biochim Biophys Acta Mol Cell Biol Lipids.
1864:1422–1437. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Byun HW, Hong EM, Park SH, Koh DH, Choi
MH, Jang HJ, Kae SH and Lee J: Pravastatin activates the expression
of farnesoid X receptor and liver X receptor alpha in Hep3B cells.
Hepatobiliary Pancreat Dis Int. 13:65–73. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Fu ZD, Cui JY and Klaassen CD:
Atorvastatin induces bile acid-synthetic enzyme Cyp7a1 by
suppressing FXR signaling in both liver and intestine in mice. J
Lipid Res. 55:2576–2586. 2014.PubMed/NCBI View Article : Google Scholar
|

















