Introduction
Artificial hip arthroplasty is used extensively in
replacement of damaged hips. To affix the artificial hip to the
native bone, both cement and non-cement methods are used. Bone
cement fixation is particularly suitable for aged patients with
osteoporosis, as the early stability is generally good (1). With the development of new bone cement
technology, the use of bone cement in artificial hip replacements
is now more commonly accepted in clinics (2-4).
However, bone cement in the proximal femoral medullary cavity
(PFMC) can cause potential complications, such as high intraosseous
pressure in the proximal femur and systemic bone cement
implantation syndrome, including hypotension, arrhythmia, severe
hypoxemia, myocardial infarction and increased pulmonary artery
pressure (5-7).
Previous studies have shown that a cement-fixed artificial joint
prosthesis can lead to long-term occlusion of the medullary cavity
and the destruction of intramedullary blood vessels (8). In addition, Yoon et al
(9) have confirmed that the use of
bone cement in total hip arthroplasty increases the risk of deep
infection.
There are negative effects of bone cement on tissue
around the cement at both the proximal and the distal end of the
femur (10). In previous clinical
observations, it was noted that some patients had pain and
discomfort of the knee joint after artificial bone cement hip
replacement (11), though the
specific mechanisms underlying this were unclear. Our previous
study found that blocking of PFMC with bone cement was associated
with a significant decrease in blood circulation and bone metabolic
rate of the distal femur (10).
However, to the best of our knowledge, the effects of bone cement
on bone mineral density (BMD), intraosseous pressure (IOP),
articular cartilage and subchondral bone of the distal femur have
not been explicitly reported. Therefore, in the present study, the
effects of bone cement in the PFMC on the distal femur in rabbit
models were assessed by measuring the BMD and IOP of the distal
femur and observing changes in articular cartilage and subchondral
bone structure using light microscopy (LM) and transmission
electron microscopy (TEM).
Materials and methods
Ethical approval
The Institutional Animal Care and Use Committee
approved the use of rabbits in this study, and the study protocol
was approved by The Ethics Review Board of Guangxi Medical
University (Nanning, China). The study complied with the National
Institutes of Health Guide for Care and Use of Laboratory Animals
(Publication No. 85-23) (12).
Animals and surgery
A total of 32 New Zealand white rabbits (weight,
2.6-3.5 kg; age, ~6 months; 16 males and 16 females) were purchased
from the Animal Center of Guangxi Medical University (Nanning,
China). Rabbits were housed in individual cages at constant
temperature (18-22˚C) and humidity (40-60%) on a 12-h light-dark
cycle with free access to food and water and were monitored every
24 h.
The experimental rabbits were randomly numbered 1-32
and the left hind limb of the odd-numbered rabbits and the right
hind limb of the even numbered rabbits was selected as the
experimental side, whereas the other hind limb of the rabbit was
labeled as the control side by the principal investigator; the
surgeons and the independent researchers were both blinded to the
identity of the rabbit.
Surgery was performed as described in a previous
report (13): First, rabbits were
anesthetized with an intramuscular injection of ketamine (50 mg/kg)
and xylazine (10 mg/kg). The fur at the bilateral surgical site of
the hind legs was then shaved after 9% sodium sulfide treatment,
the operation field was further disinfected using iodophor and then
covered with a surgical drape. The rabbits were placed on the
surgical table on prone position. Using the third trochanter of the
bilateral femur of the experimental rabbit as the central mark, a
4-cm long arc incision was made. The lateral part of the trochanter
was excised by a bone saw, and the entrance of the medullary cavity
was established with a probe. The PFMC was created by repeatedly
reaming with a medullary cavity file until the cavity reached about
3/5 of the length of the femur. Polymethyl methacrylate (PMMA)
solution was prepared by mixing two parts PMMA powder and one part
PMMA liquid according to manufacturer's instructions (weight ratio,
2:1; DePuy International Ltd.). PMMA solution (2 ml) was injected
into the medullary cavity of the right femur, which was then filled
with the dough-like bone cement. The left femur was used as the
control (without any bone cement). The wounds were washed with
sterile saline, and the tissues were sutured to close the incision.
After surgery, each rabbit received one injection of antibiotics,
including 1 ml gentamicin and 400,000 units of penicillin sodium.
At the 4th or 16th week after operation, rabbits (n=16 rabbits per
time point) were anesthetized with an intramuscular injection of
ketamine (50 mg/kg) and xylazine (10 mg/kg) and euthanized by air
embolization by intravenously injecting 40-50 ml of air into the
auricle vein. Death was confirmed by respiratory and cardiac
arrest, pupil diffusion and disappearance of the nerve reflex.
IOP measurement
At the 4th or 16th week after operation, the rabbits
were fixed on a custom-made operating table, and the skin and
muscles were cut to expose the femoral medial condyle. At 1 cm
above the femoral medial condyle, a bone puncture needle was
inserted into the PFMC, and the needle was immediately connected to
the pressure-measuring device tightly. Once the connection was
established, the recording system was turned on and the pressure
data recording was started (14).
After the pressure signal was converted into an electrical signal,
the pressure signal was input into a specially designed computer
system to amplify and process, and the pressure data and curves
could then be displayed (15). The
IOP was recorded by a Physiological Pressure Transducer (SP844;
MEMSCAP SA).
H&E staining and LM
examination
The articular cartilage of the bilateral distal
femur was harvested (including the subchondral bone). A 1 cm long
cancellous bone mass of femoral condyle was selected near the
articular surface. After rinsing the specimens with normal saline,
the specimens were immediately fixed in 10% formaldehyde for 24-48
h at room temperature, followed by EDTA decalcification, washing
with water and dehydration using a gradient of ethanol solutions
(from 70-100%). Following treatment with xylene I and xylene II for
30 min at room temperature the transparent tissues were paraffin
embedded and sliced into 4-µm thick sections using EM UC7
ultramicrotome (Leica Microsystems GmbH). H&E staining was
carried out according to standard procedures (16). Briefly, slides were immersed in
filtered Harris hematoxylin, rinsed with water, immersed in 0.3%
ammonium hydroxide and rinsed with water. Subsequently, the
sections were stained with eosin staining solution for 2-3 min,
followed by 70 and 90% alcohol dehydration for 10 min. Dehydrated
slides were immersed in xylene and mounted using
Permount™ mounting medium (Thermo Fisher Scientific,
Inc.) and cover slips. Slides were observed under a light
microscope (model, BX53; Olympus Corporation) after sealing.
TEM
The cancellous bone mass of the femoral condyle
(including the articular cartilage and facial segment) near the
articular surface was fixed for 2 h at room temperature with 2%
glutaraldehyde (Panreac Química SLU) and 2.5% paraformaldehyde at
pH 7.4 with 0.1 M sodium cacodylate, rinsed with phosphate buffer
and fixed in 1% osmium-acid solution. After rinsing, the bone mass
was dehydrated using an ascending acetone series (50, 70, 90 and
100%), soaked in a mixture of acetone/encapsulated solution,
embedded in Epons12 embedding agent, and then sliced into 50-nm
thick sections using an EM UC7 ultramicrotome (Leica Microsystems
GmbH). The ultrastructure of cartilage and subchondral bone was
observed using a JEM-1230 transmission electron microscope (JEOL
Ltd.) according to standard procedures (17).
Bone mineral density (BMD)
measurement
The cancellous bone mass of femoral condyle
(including articular cartilage and facial subdivision) near the
articular surface was selected for BMD measurement. Specifically, a
0.5 cm length piece of intact cancellous bone of femoral condyle, 1
cm above the femoral condyle and close to the proximal articular
surface, was used for BMD examination. The specimen was scanned
using a high-resolution mode on a Hologic QDR 4500 fan-beam bone
densitometer (DXA, Hologic, Inc.). The method was adapted from
previous reports (18,19).
Statistical analysis
All numerical data are presented as the mean ±
standard deviation. All statistical analyses were performed with
SPSS18.0 statistical software (SPSS Inc.). Comparisons of BMD and
IOP were analyzed by two-way ANOVA with Dunnett's post hoc test.
P<0.05 was considered to represent a statistically significant
difference.
Results
Surgery
All animals tolerated the surgical procedure well.
At 5 days post-operation, 2 rabbits had a minor infection at the
incision site, but this healed well following treatment with
antibiotics following debridement.
Gross anatomical morphology
observation and X-ray of femurs in rabbits post-surgery
From the gross anatomical analysis of the femoral
specimens, it was observed that the bone cement-filled bone marrow
cavity was very tight without obvious gaps (Fig. 1). The presence of a bone cement
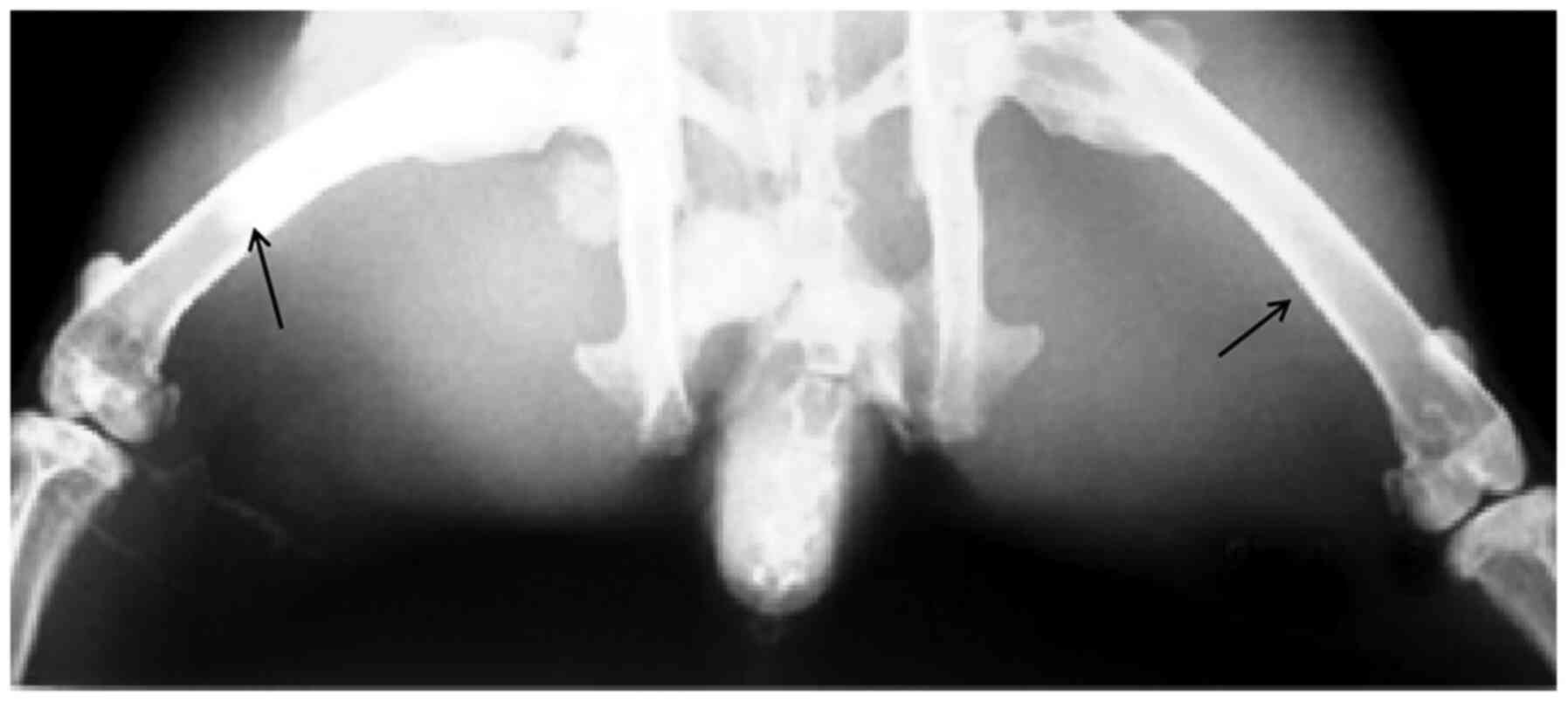
shadow could be seen in X-ray images (Fig. 2); the upper middle femur of the
experimental side of the rabbit was filled with cement, whereas the
upper middle femur of the control side was not filled with cement,
which demonstrated that the bone cement was filled to the middle
segment of femur.
Evaluation of cartilage and bone
tissue structural features by LM
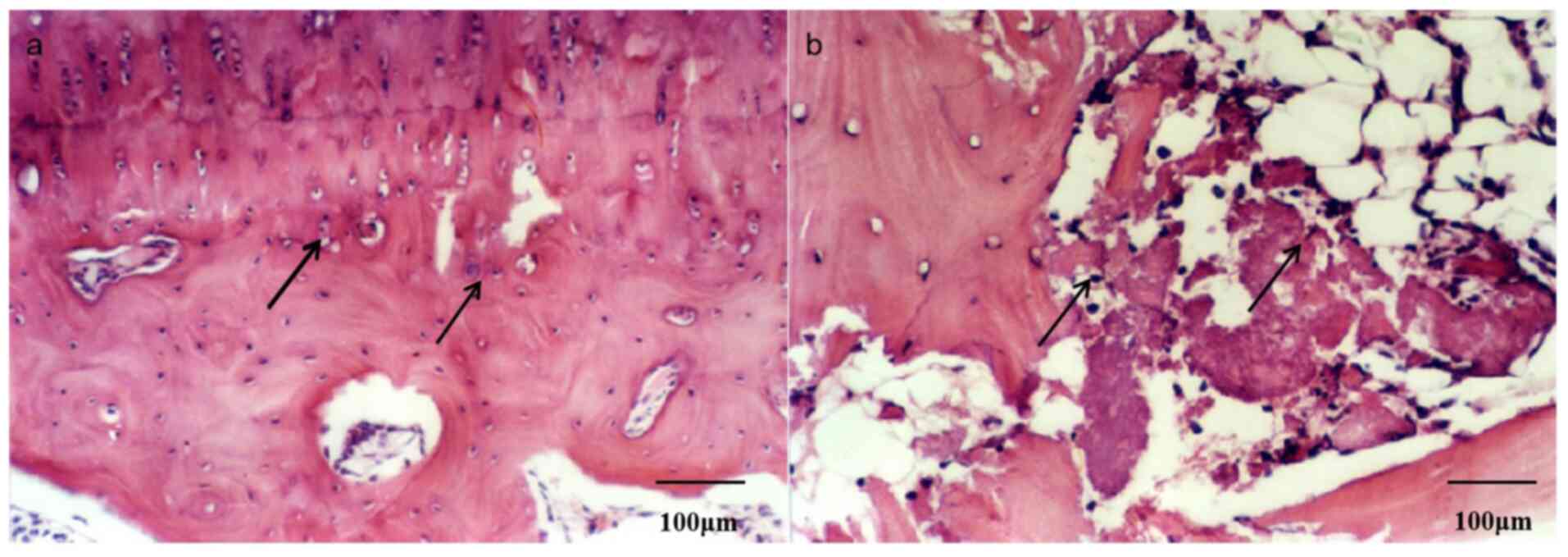
At the 16th week post-surgery, it was found that the
cartilage surface was smooth, chondrocytes were arranged in
columnar shape and that the distribution of chondrocytes and the
size of the nucleus was uniform on the control side (Fig. 4a). In control animals, the
trabecular structure of the subchondral bone was normal, the bone
cells were regularly arranged, the structure of bone was largely
intact, and the lamellar line is clear (Fig. 3a). By contrast, chondrocytes in the
experimental group were thinner and the surface layer was almost
absent with a typical irregular moth-eaten shape (Fig. 4b). The arrangement of chondrocytes
was disordered, the quantity was reduced, and a large number of
chondrocytes were necrotic in the experimental group (Fig. 4b). A large number of empty bone
lacunae in the subchondral trabeculae, a decrease in the number of
bone cells, a disappearance of lamellar lines and broken trabecular
structures were observed (Fig.
3b).
Evaluation of cartilage and bone
tissue ultrastructural features by TEM
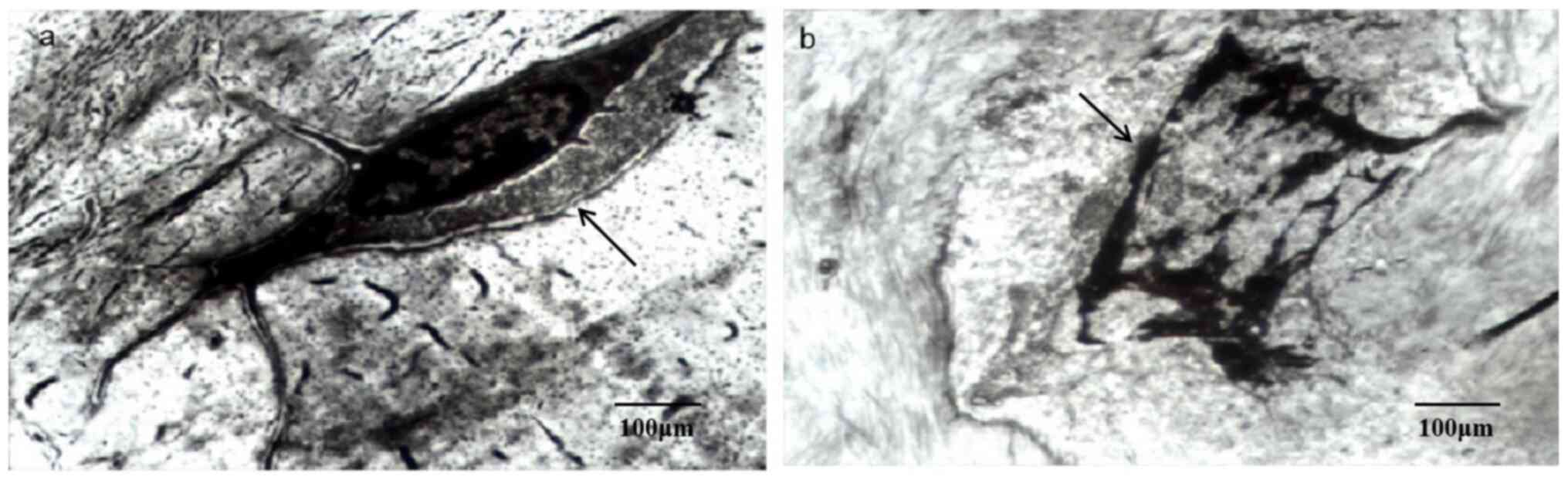
At the 16th week after intervention, the nuclei of
some chondrocytes in the experimental group showed typical features
of pyknosis and some cell and nuclear membranes could not be
distinguished (Fig. 5b); overall,
the general structure of the chondrocytes was disorganized, with
necrotic and disintegrated cytoplasm (Fig. 5b). Similarly, the size of bone cells
in the experimental group was reduced and the cell edges were
unclear (Fig. 6b); the continuity
of cell membrane and nuclear membranes was interrupted, with a
disappearance of cytoplasmic components and the high-density
substances in cells was increased (Fig.
6b). By contrast, the chondrocytes of the control group were
more round-shaped, with an abundant rough endoplasmic reticulum and
developed Golgi complex in the cytoplasm (Fig. 5a); the nucleus was large and
complete and chromatin was abundant and evenly distributed
throughout (Fig. 5a). Bone cells in
the control group were located in the lacuna of bone with a flat
oval shape, rich cytoplasm and endoplasmic reticulum, scattered
free ribosomes, moderate mitochondria and well-developed Golgi
complex (Fig. 6a).
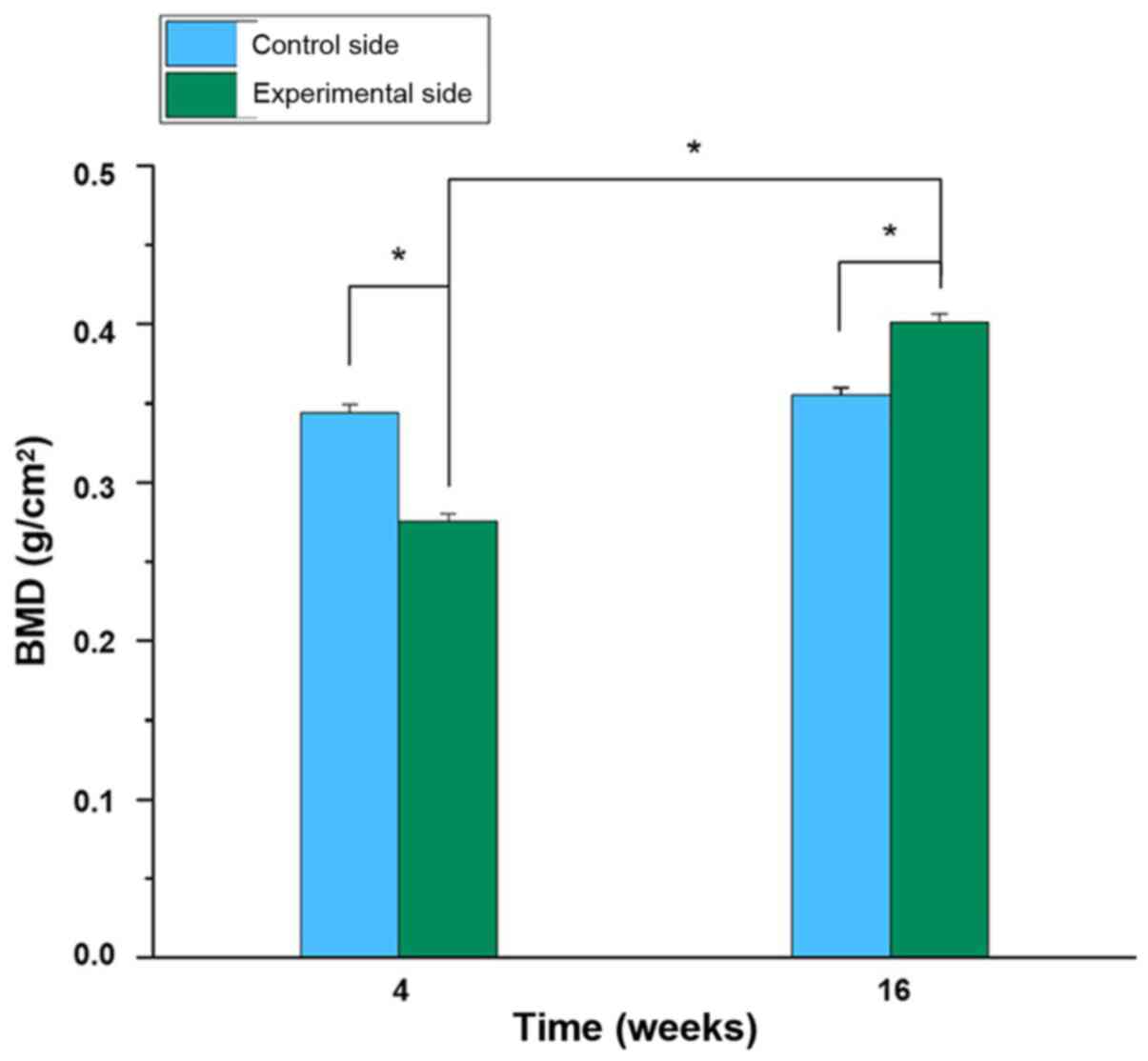
Comparison of BMD of femurs between
the two groups post-surgery
At the 4th week following operation, the BMD of the
distal femur in the experimental group (0.275±0.005) was
significantly lower compared with that in the control group
(0.344±0.06) (P<0.05; Fig. 7).
However, at the 16th week post-operation, the BMD of the distal
femur of the experimental group (0.401±0.006) was significantly
higher compared with the BMD at the 4th week (P<0.05), and also
higher than the control group at the 16th week (0.355±0.005)
(P<0.05). By contrast, there was no significant difference in
BMD between the 4th and 16th week after operation in the control
group (P>0.05; Fig. 7).
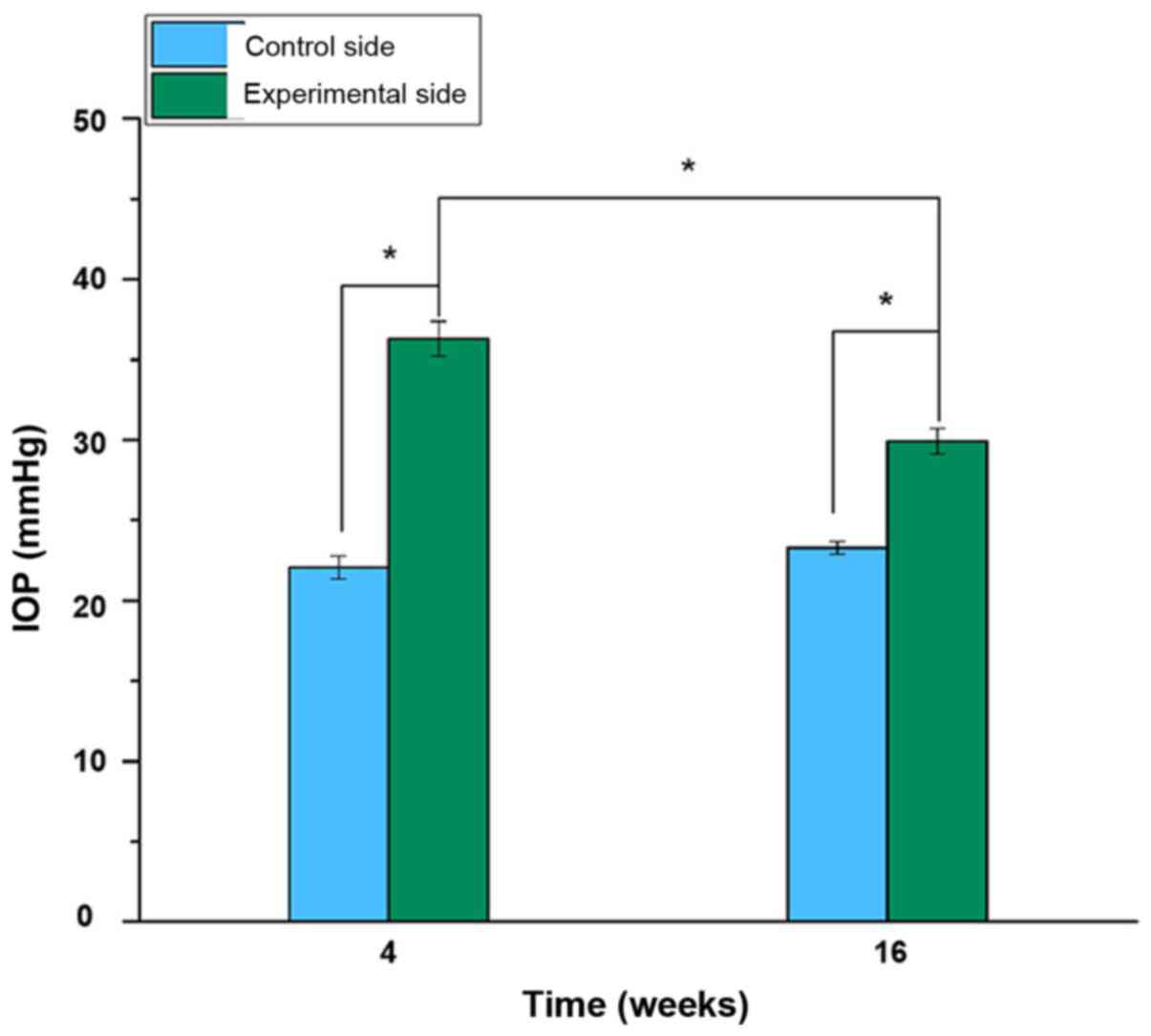
Comparison of IOP between the two
groups post-surgery
At the 4th week post-operation, the IOP of the
distal femur in the experimental group (36.483±1.005) was
significantly higher compared with that in the control group
(21.972±0.640) (P<0.05; Fig. 8).
However, at the 16th week following surgery, the IOP of the distal
femur on the experimental side (29.858±0.733) was lower compared
with that at the 4th week after the operation (P<0.05), but
still higher compared with the control group at the 16th week
(23.204±0.359) (P<0.05). No significant difference in IOP was
identified between the 4th and 16th week after operation in the
control group (P>0.05; Fig.
8).
Discussion
Bone cement is widely used in hip replacements, but
the potential clinical complications of its use have been largely
unrecognized or ignored (4,5). In the present study, a rabbit model of
the PFMC was successfully established, the cavity blocked with bone
cement and the BMD and IOP of the distal femur measured; structural
changes in the articular cartilage and subchondral bone of the
distal femur were also observed. The BMD of the distal femur
decreased significantly at 4 weeks following bone cement treatment
but was increased at week 16 following surgery, and this was
significantly higher compared with the control group BMD. The IOP
of the distal femur increased significantly after intervention and
continued to be significantly higher compared with the control
group. LM and TEM analyses showed that distal femur articular
cartilage and subchondral bone were severely affected by the
specific intervention, which suggested that the effect of bone
cement on BMD, IOP, articular cartilage and subchondral bone of the
distal femur should be considered when considering a hip
replacement with bone cement fixation.
IOP is the pressure produced by the blood flow of
the phalanx in the intramedullary cavity or the interosseous space
and is the most reliable index to gauge intraosseous hemodynamics
and the state of intraosseous circulation (20). Porsch et al (21) used modern bone cement implantation
technology to set up pressure measuring devices in different
positions of femur. They observed that bone cement and prosthesis
implantation could lead to a significant increase in IOP (21). Welch et al (22) and Otto and Matis (23) also found that intramedullary filling
with cement could lead to long-term high pressure in bone in animal
experiments. These studies confirmed that the intramedullary
pressure of the distal femur increased significantly after bone
cement implantation, and it continued to be significantly higher
than that of the control group. This may be due to the long-term
occlusion of the medullary cavity caused by the implantation of
bone cement, which not only reduces the volume of the medullary
cavity, but also destroys blood circulation in the bone and the
pulp during the filling process of bone cement. This could further
cause a series of hemodynamic changes resulting in increased IOP.
The damage caused by bone cement implantation is a complicated one,
and it likely takes a long time to repair. Therefore,
intramedullary high pressure will exist for a long time after bone
cement implantation (22,23). In turn, this increase in IOP could
reduce blood flow in the bone marrow, which could lead to further
dysfunction of the intraosseous vein reflux and a compression of
the surrounding tissue, and this congestion may further reduce
arterial blood flow, eventually causing irreversible subchondral
ischemic necrosis (24).
The results of the present study indicated that bone
cells in the subchondral bone of the proximal femur were affected
by cement filling, including necrotic chondrocytes and disrupted
bone microstructures. At the 16th week of bone cement occlusion,
the cartilage layer of the distal femoral articular surface was
thinner, the arrangement of chondrocytes was disordered and the
number of cells significantly lowered. Although the underlying
mechanism of this pathological change is still unknown, it can be
hypothesized that tissue metabolic disorders owing to disrupted
blood circulation might be the major cause. In addition, a number
of other factors, such as necrosis of subchondral bone and
accumulation of metabolites, could have aggravated the cartilage
damage and resulted in degeneration of the distal joint (25,26). A
series of changes caused by toxicity, fever and osteolysis of bone
cement may also accelerate the degeneration of the distal femoral
articular surface. Further studies are needed to determine the
precise contributions of these pathological processes to changes in
IOP, BMD and bone and cartilage histology.
BMD refers to bone mineral content per unit bone
tissue, which is a standard parameter to quantify bone quality
(27). It can be used as a
parameter to gauge the influence of cemented artificial joint
implantation on the bone quality (28). For example, when van Loon et
al (29) used dual energy X-ray
absorptiometry to measure bone density in patients with a loosening
prosthetic following artificial joint replacement, it was found
that the loss of bone mass stimulated by bone cement was the main
cause of the artificial joint loosening. Similarly, Kramhoft et
al (30) also found that bone
cement implantation reduced BMD and inhibited bone reconstruction
and mineralization in dogs. The present results indicated that the
BMD of the distal femur decreased significantly after bone cement
blocked the PFMC, which may be due to the dual effects of high IOP
and the actions of bone cement itself. It is well known that the
implantation of bone cement into the medullary cavity leads to high
IOP, which increases hypoxia at the distal end of the femur
(31). This, in turn, could
seriously affect bone and cartilage metabolism in the distal femur
and result in a large loss of local bone mass (10).
In addition, particles from bone cement implantation
can cause histochemical reactions, stimulating macrophages and
giant cells to secrete a variety of factors related to bone
resorption; therefore, bone cement might promote bone resorption in
different ways (32). However, the
results of the present study suggested that over time, BMD of the
distal femur gradually increased and eventually exceeded normal BMD
levels. This, at first glance, might be treated as good news, but
further analysis found that this might not be a desirable outcome.
In fact, this phenomenon may be due to abnormal bone repair and
reconstruction, which can result in bone deposition and bone
sclerosis (33). One previous study
also reported an increase in BMD under pathological conditions
(34).
The limitations of the present study include: i)
Observation was for only 16 weeks, which may not be long enough to
model artificial joint surgery; therefore, future study of the
long-term effects of bone cement on the distal femur are warranted;
ii) only a limited number of parameters were assessed in this
study; therefore, more clinically relevant parameters are warranted
for future studies; and iii) species-specific and model-specific
features might argue against the clinical relevance of the present
model to clinical hip replacements in humans. Future studies will
test this hypothesis and other clinically associated or relevant
models are warranted. Overall, the present data support the idea
that cement treatment could potentially increase IOP, disrupt the
BMD and perturb the local articular cartilage and subchondral bone
structure.
In summary, blockage of the PFMC with bone cement
results in an increase in the IOP of the distal femur, changes in
BMD and damage to the subchondral bone and articular cartilage.
Further studies are warranted to further confirm this finding in
the clinic and to investigate the mechanisms underlying how bone
cement occlusion in the PFMC exactly affects the distal femur.
Acknowledgements
Not applicable.
Funding
Funding: This project was supported by The Self-financing
Research Project of Guangxi Zhuang Autonomous Region Health
Commission (grant no. Z20190244) and The National Natural Science
Foundation of China (grant no. 81860274).
Availability of data and materials
All data generated and/or analyzed during the
present study are included in this published article.
Authors' contributions
GPL and LCX contributed to the study design,
manuscript preparation and data collection. GPL and LCX performed
all the experiments. MZ performed the data analysis. JMZ
contributed to the conception and design of the study. GPL and LCX
confirmed the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The Institutional Animal Care and Use Committee
approved the use of rabbits in this study, and the study protocol
was approved by The Ethics Review Board of Guangxi Medical
University (Nanning, China). The study complied with the National
Institutes of Health Guide for Care and Use of Laboratory Animals
(Publication No. 85-23).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Blankstein M, Lentine B and Nelms NJ: The
use of cement in hip arthroplasty: A contemporary perspective. J Am
Acad Orthop Surg. 28:e586–e594. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Beckmann NA, Bitsch RG, Gondan M,
Schonhoff M and Jaeger S: Comparison of the stability of three
fixation techniques between porous metal acetabular components and
augments. Bone Joint Res. 7:282–288. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Amirouche F, Solitro G, Broviak S,
Goldstein W, Gonzalez M and Barmada R: Primary cup stability in THA
with augmentation of acetabular defect. A comparison of healthy and
osteoporotic bone. Orthop Traumatol Surg Res. 101:667–673.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kim SC, Ohashi H and Oonishi H and Oonishi
H: Histologic findings at 14 and 18 years after cemented total hip
arthroplasty with interface bioactive bone cement technique. J
Arthroplasty. 22:1067–1069. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Singh V, Bhakta P, Zietak E and Hussain A:
Bone cement implantation syndrome: A delayed postoperative
presentation. J Clin Anesth. 31:274–277. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vanderstappen J, Simon JP and Bellemans J:
Radiographic analysis of a bone plug in 275 primary cemented total
hip arthroplasties. Acta Orthop Belg. 78:350–356. 2012.PubMed/NCBI
|
|
7
|
Motobe T, Hashiguchi T, Uchimura T,
Yamakuchi M, Taniguchi N, Komiya S and Maruyama I: Endogenous
cannabinoids are candidates for lipid mediators of bone cement
implantation syndrome. Shock. 21:8–12. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Morda M, Pini S, Celli F, Casella F,
Parchi P, Piolanti N, Marchetti S and Scaglione M: Bone cement
implantation syndrome: A thromboelastographic study of the effect
of bone cement on coagulation. J Biol Regul Homeost Agents.
31:121–127. 2017.PubMed/NCBI
|
|
9
|
Yoon BH, Ha YC, Lee YK and Koo KH:
Postoperative deep infection after cemented versus cementless total
hip arthroplasty: A meta-analysis. J Arthroplasty. 30:1823–1827.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xi LC, Li HY, Zhang M and Huang SC:
Effects of bone cement filling in rabbit proximal femoral medullary
cavity on distal femoral blood flow and metabolism. J Int Med Res.
46:5237–5244. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Robertson C, Coutts F and Bell J:
Investigation of anterior knee pain after total hip replacement: A
pilot study. Physiother Res Int. 12:25–28. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Bartlett DH and Silk SB: Office of
laboratory animal welfare comments. Zebrafish. 13:563–564.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ni GX, Lu WW, Chiu KY, Li ZY, Fong DY and
Luk KD: Strontium-containing hydroxyapatite (Sr-HA) bioactive
cement for primary hip replacement: An in vivo study. J Biomed
Mater Res B Appl Biomater. 77:409–415. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Miyanishi K, Yamamoto T, Irisa T,
Yamashita A, Jingushi S, Noguchi Y and Iwamoto Y: Bone marrow fat
cell enlargement and a rise in intraosseous pressure in
steroid-treated rabbits with osteonecrosis. Bone. 30:185–190.
2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Beverly M and Murray D: Factors affecting
intraosseous pressure measurement. J Orthop Surg Res.
13(187)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pan S, Deng X, Sun S, Lai X, Sun L, Li Q,
Xiang L, Zhang L and Huang Y: Black tea affects obesity by reducing
nutrient intake and activating AMP-activated protein kinase in
mice. Mol Biol Rep. 45:689–697. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Karawya S, Said DG, Salaheldin MM and Zaky
I: Impact of intravitreal injection of bevacizumab (Avastin) on
rabbit's choroid and retina. Middle East Afr J Ophthalmol.
15:67–72. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Khoo BC, Brown K, Cann C, Zhu K, Henzell
S, Low V, Gustafsson S, Price RI and Prince RL: Comparison of
QCT-derived and DXA-derived areal bone mineral density and T
scores. Osteoporos Int. 20:1539–1545. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pennypacker BL, Oballa RM, Levesque S,
Kimmel DB and Duong LT: Cathepsin K inhibitors increase distal
femoral bone mineral density in rapidly growing rabbits. BMC
Musculoskelet Disord. 14(344)2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Beverly M, Mellon S, Kennedy JA and Murray
DW: Intraosseous pressure during loading and with vascular
occlusion in an animal model. Bone Joint Res. 7:511–516.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Porsch M, Schmidt J, Brimmers P, Menne A
and Merkle W: Intrafemoral pressure measurement in different cement
removal procedures during hip prosthesis replacement
operations-experimental study with cadaver femora. Biomed Tech
(Berl). 43:53–57. 1998.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
22
|
Welch RD, Johnston CE II, Waldron MJ and
Poteet B: Bone changes associated with intraosseous hypertension in
the caprine tibia. J Bone Joint Surg Am. 75:53–60. 1993.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Otto K and Matis U: Changes in
cardiopulmonary variables and platelet count during anesthesia for
total hip replacement in dogs. Vet Surg. 23:266–273.
1994.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Blankstein M, Widmer D, Gotzen M,
Hofmann-Fliri L, Richards RG, Gueorguiev B and Windolf M:
Assessment of intraosseous femoral head pressures during cement
augmentation of the perforated proximal femur nail antirotation
blade. J Orthop Trauma. 28:398–402. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li C, Kotha S and Mason J: Evaluation of
the effects of implant materials and designs on thermal necrosis of
bone in cemented hip arthroplasty. Biomed Mater Eng. 13:419–428.
2003.PubMed/NCBI
|
|
26
|
Radin EL and Rose RM: Role of subchondral
bone in the initiation and progression of cartilage damage. Clin
Orthop Relat Res. 34–40. 1986.PubMed/NCBI
|
|
27
|
Fonseca H, Moreira-Gonçalves D, Coriolano
HJ and Duarte JA: Bone quality: The determinants of bone strength
and fragility. Sports Med. 44:37–53. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Peitgen DS, Innmann MM, Merle C,
Gotterbarm T, Moradi B and Streit MR: Periprosthetic bone mineral
density around uncemented titanium stems in the second and third
decade after total hip arthroplasty: A DXA study after 12, 17 and
21 years. Calcif Tissue Int. 103:372–379. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
van Loon CJ, de Waal Malefijt MC, Buma P,
Verdonschot N and Veth RP: Femoral bone loss in total knee
arthroplasty. A review. Acta Orthop Belg. 65:154–163.
1999.PubMed/NCBI
|
|
30
|
Kramhoft M, Bodtker S, Nimb L and Jensen
JS: Variation of cortical hypertrophy depending on the medullary
filling material. An experimental study of canine tibial diaphysis.
J Arthroplasty. 8:555–560. 1993.PubMed/NCBI
|
|
31
|
Diehl K: Bone stress and reconstruction of
the bone at the alloarthroplasty of the upper end of the femur with
cement bonding (author's transl). Arch Orthop Unfallchir. 83:9–28.
1975.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
32
|
Nakashima Y, Sun DH, Trindade MC, Chun LE,
Song Y, Goodman SB, Schurman DJ, Maloney WJ and Smith RL: Induction
of macrophage C-C chemokine expression by titanium alloy and bone
cement particles. J Bone Joint Surg Br. 81:155–162. 1999.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Karsdal MA, Bay-Jensen AC, Lories RJ,
Abramson S, Spector T, Pastoureau P, Christiansen C, Attur M,
Henriksen K, Goldring SR and Kraus V: The coupling of bone and
cartilage turnover in osteoarthritis: Opportunities for bone
antiresorptives and anabolics as potential treatments? Ann Rheum
Dis. 73:336–348. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lajeunesse D and Reboul P: Subchondral
bone in osteoarthritis: A biologic link with articular cartilage
leading to abnormal remodeling. Curr Opin Rheumatol. 15:628–633.
2003.PubMed/NCBI View Article : Google Scholar
|