1. Introduction
Patients presenting with malignancies have a high
risk of developing acute kidney injury (AKI) secondary to receiving
chemotherapy, exposure to contrast agents used in medical imaging,
radiation therapy, tumor lysis syndrome, hypotension or caused by
the direct effects of the malignancy. AKI is a frequent
complication in cancer patients and is associated with increased
morbidity and mortality. Because a significant percentage of cancer
patients with AKI eventually require renal replacement therapy
(RRT), it is important to know which method of RRT is appropriate
depending on the context (intermittent or continuous hemodialysis
or plasmapheresis), what is the optimal time of initiation and
whether or not it can improve outcomes in terms of recovery of
kidney function and increasing survival. There are few data in the
literature that provide conclusive information regarding the
appropriate time of dialysis initiation and discontinuation and on
the prognosis of these patients, especially for patients with
multiple system organ failure or with uncontrolled cancer. Thus,
the benefit of RRT must be evaluated according to the prognosis of
the patient, by a multidisciplinary team including a nephrologist,
oncologist and intensivist.
AKI in patients with malignancies can be caused by:
i) The direct effects of the malignancy (lymphoma infiltration of
the kidney), ii) Hematopoietic stem cell transplantation, iii)
Chemotherapy-related injury (tumor lysis syndrome), iv)
Drug-associated nephrotoxicity (acute tubular injury), v)
Obstructive nephropathy due to urothelial or retroperitoneal
cancers, vi) Nephrectomy (in the case of kidney cancer) which
increases the risk of renal failure and the need for dialysis
(1-3),
vii) Volume depletion secondary to vomiting as a side effect of
cancer treatment, viii) Paraneoplastic syndromes which can
compromise the renal function: Syndrome of inappropriate secretion
of antidiuretic hormone (SIADH), hypercalcemia (4), and tumor lysis syndrome that may
require dialysis in extreme situations.
Risk factors associated with the development of AKI
in cancer patients include: Female sex, older age, diabetes
mellitus, the presence of underlying chronic kidney disease,
hypotension and inadequate renal perfusion (5).
2. Definition of AKI
In the last few decades, different definitions of
AKI have been applied, the most used being those taking into
consideration the urine output and serum creatinine. The multitude
of definitions has made it difficult to compare data from different
studies regarding AKI. The KDIGO workgroup on AKI standardized
these variations into a single definition and a staging system that
is being used today (6) (Table I).
 | Table IDefinition and staging of AKI
(6). |
Table I
Definition and staging of AKI
(6).
| Stage | Serum
creatinine | Urine output |
|---|
| 1 | >1.5-1.9 times
baseline or >0.3 mg/dl increase | <0.5 ml/kg/h for
6-12 h |
| 2 | ≥2-2.9 times
baseline | <0.5 ml/kg/h for
≥2 h |
| 3 | >3 times
baseline or >4 mg/dl increase or Renal replacement therapy
initiation or In patients <18 years, a decrease in eGFR to
<35 ml/min per 1.73 m2 | <0.3 ml/kg/h for
>24 h or Anuria for >12 h |
3. Epidemiology of AKI in cancer
patients
A Danish population-based cohort study reported the
incidence of AKI in cancer patients followed up for more than a
period of 7 years (7). Of 1.2
million individuals, 44,116 developed a malignancy. The risk of AKI
was 17.5% during the first year after cancer diagnosis and the
overall 5-year risk of AKI was 27.0%. The highest incidence rates
were in patients with cancer localized in the kidney (44%), biliary
tract, liver, pancreas, and in patients with multiple myeloma.
Among patients with renal impairment, 7% had severe AKI and 5%
required RRT.
High AKI incidence rates are also noted in men with
prostate cancer (29%) and urinary bladder cancer (36.3%), but also
in women with ovarian (36.2%) or uterus/cervical cancers
(26.7/28.6%) (7). Considering that
prostate cancer is the second most commonly diagnosed cancer in men
in Europe, accounting for 10-15% of all diagnosed cancers (8-10)
and the most frequent neoplastic pathology in men of age over 60
years (11), and also that uterus
and cervical cancers are a major health issue for European women,
with the biggest morbidity and mortality rates among Romanians
(12), we need to provide access to
optimal treatment and the current health programs must be adapted
for a better prevention of neoplastic disease and its
complications-AKI and the need for renal replacement in these
patients, with the most appropriate method of dialysis and the
optimal duration and dose of therapy.
4. Indications for kidney replacement
therapy in AKI
The standard indications for RRT initiation in the
acute setting are well known: Fluid overload resistant to diuretic
therapy, refractory acid-base and hydroelectrolytic disorders,
uremic pericarditis, pleuritis, encephalopathy or progressive
neuropathy (Table II) (13,14).
It remains uncertain whether earlier initiation of such therapy can
improve outcomes in terms of the recovery of kidney function and
decreasing mortality in cancer patients.
 | Table IIIndications for initiation of RRT
(adapted from ref. 13,14). |
Table II
Indications for initiation of RRT
(adapted from ref. 13,14).
| Indications for RRT
initiation |
|
Anuria
<50 ml/12 h |
|
Hyperkalemia
(K >6.5 mEq/l) |
|
Severe
acidosis (pH <7.2) |
|
Uremia
(>30 mmol/l) |
|
Uremia
complications: Pleuritis, pericarditis, encephalopathy, progressive
neuropathy, bleeding |
|
Severe
hypercalcemia refractory to pharmacologic treatment |
|
Dysnatremia
(Na >155/<120 mmol/l) |
|
Severe tumor
lysis syndrome |
|
Severe
rhabdomyolysis |
|
Overdose of
a dialyzable substance (alcohol, aspirin) |
| RRT, renal
replacement therapy. |
5. Renal replacement therapies
Dialysis principles
There is no ideal method of RRT. The ideal method of
kidney replacement therapy should allow control of the
intravascular and extravascular volume, correction of acid-base
imbalances, correction of uremia, an effective removal of toxins,
while also promoting recovery of renal function, increasing
survival without complications.
Blood purification through the artificial kidney is
governed by physical forces: Diffusion and convection
(ultrafiltration) exerted on the surface of the dialyzer membrane,
a semipermeable membrane through which the patient's blood is
brought into contact with the dialysis fluid. There are two methods
used to correct the acid-base and electrolyte imbalances and remove
toxins and excess fluid: Dialysis (method that uses diffusion) and
ultrafiltration (method that uses hemofiltration) (15).
In the case of continuous or intermittent dialysis,
the mechanism of solute transport is diffusion-the passage of
solvents from one compartment to another through a semipermeable
membrane, according to a concentration gradient and depending on
time, molecular mass of the substances passing through it and
membrane pore size. By diffusion we achieve a very good clearance
for small molecules, below 500 Da: Urea, creatinine, ions. The
transport of solutes between the two compartments is also the
result of the Brownian motion. Thus, larger molecules, such as
mediators of inflammation [interleukin (IL)-6, tumor necrosis
factor (TNF)α] that are found in high amounts in critically ill
septic patients or in patients in shock, will collide with the
semipermeable membrane less often due to their slower movement in
the liquid medium, which will cause a deficient clearance of these
molecules (15).
When using convection, solutes pass from one
compartment to another through the semipermeable membrane according
to a pressure gradient created by a pump. Negative pressure is
being applied in the dialysate compartment and water is being
dragged through the membrane accompanied by solutes. This is the
mechanism used in hemofiltration. The clearance of a molecule is
the product between the ultrafiltration rate and the selectivity
(separation factor); thus, to increase the clearance when the
selectivity is low, one must increase the ultrafiltration rate
(15).
Modalities of RRT in patients with
malignancies and AKI
There are 3 methods of RRT used in patients with
malignancies and acute renal impairment (Table III). These include: i) Continuous
renal replacement therapy (CRRT): Continuous veno-venous
hemofiltration (CVVH), continuous veno-venous hemodialysis (CVVHD),
continuous veno-venous hemodiafiltration (CVVHDF), slow continuous
ultrafiltration (SCUF); ii) Intermittent renal replacement therapy
(IRRT): Intermittent hemodialysis (IHD), intermittent
hemodiafiltration (IHDF), intermittent isolated ultrafiltration
(IUF); and iii) Hybrid therapy: Sustained (slow) low efficiency
daily dialysis (SLEDD/SLEDD-F), prolonged intermittent renal
replacement therapy (PIRRT) (16,17).
 | Table IIIComparison of the different methods
of renal replacement therapy. |
Table III
Comparison of the different methods
of renal replacement therapy.
| | Continuous
therapies | Intermittent
therapies | Hybrid
therapies |
|---|
| Time (h/day) | 24 | 4 | 8-12 |
| Blood flow rate
(ml/min) | 15-300 | 300-400 | 150-300 |
| Dialysate flow rate
(ml/min) | 30-60 | 600-800 | 100 |
| Replacement fluid
flow rate (ml/min) | 30-60 | - | 100 |
| Dialysis | Yes | Yes | Yes |
| Hemofiltration | Yes | No | Yes |
| Efficiency | Low-Moderate | High | Moderate |
| Hemodynamic
stability | High | Low | High |
| Cost | ↑↑↑ | ↑ | ↑↑ |
Hybrid therapies use the standard dialyzer, the
difference being time. The hemodialysis session last for 4 h, while
SLEDD lasts for 12 h. The continuous renal replacement therapies
take place in intensive care units, using special dialysis machines
(16).
Intermittent therapies
IRRT are used in hemodynamically stable patients.
They are performed on regular dialysis machines, are relatively
cheaper and have a series of advantages which include the
possibility of establishing a more flexible schedule of
sessions-which offers advantage over patient transport and
administration of dialyzed drugs, quick correction of acid-base and
electrolyte imbalances and have a lower risk of bleeding (16).
They also have certain disadvantages, the best known
of them being intradialytic hypotension and cerebral edema
(18).
Intradialytic hypotension occurs by intravascular
volume reduction through the ultrafiltration process and can cause
coronary ischemia, intestinal ischemia and increases the renal
injury. Risk factors for intradialytic hypotension include: Left
ventricular hypertrophy with systolic or diastolic dysfunction,
valvulopathies, pericardial involvement, uremic neuropathy, severe
anemia, predialysis systolic blood pressure less than 100 mmHg,
poor nutritional status, high volume ultrafiltration and age over
65(18).
Cerebral edema, causing a range of neurologic
symptoms that form the dialysis disequilibrium syndrome, occurs
particularly when a patient is first started on dialysis. It is
caused by the rapid reduction during hemodialysis sessions in blood
levels of osmotically active substances, which makes the plasma
more hypotonic compared to the brain tissue, favoring the passage
of water from the blood to the brain and the appearance of
symptoms: Nausea, vomiting, headache, and dizziness (18,19).
Another mechanism by which cerebral edema occurs is related to the
decrease in urea transporters and the overexpression of aquaporins
in the brain (19). Risk factors
for cerebral edema in the first dialysis sessions are: BUN >175
mg/dl, rapid decrease in urea level, pre-existing neurological
disorders, hyponatremia and liver diseases (18-20).
Continuous therapies
CRRT is recommended in critically ill patients:
Hemodynamically unstable, septic, shock patients and mechanically
ventilated patients (Table IV)
(21). The sessions take place in
intensive care units, using special dialysis machines. Although the
costs are higher, they offer a series of advantages. The advantages
include: A slower decrease in intravascular volume and slower
solute clearance which improves hemodynamic stability, thus
favoring recovery of renal function, and also ensures a better and
more predictable control of the blood parameters and volume, a more
stable intracranial pressure and finally ensures a better clearance
of cytokines (16,21).
 | Table IVIndications for CRRT and SLEDD. |
Table IV
Indications for CRRT and SLEDD.
| Indications for
CRRT and SLEDD |
|---|
| Shock: |
|
Cardiac SOFA
score >2 |
|
Intra-aortic
balloon pump |
| Extracorporeal
membrane oxygenator (ECMO) |
| Cerebral edema |
| Hepatic
failure |
| Refractory
hypervolemia |
| Rhabdomyolysis |
| Tumor lysis
syndrome |
| Severe
hypercatabolism |
| Hyperammonemia |
| CRRT, continuous
renal replacement therapy; SLEDD, sustained low efficiency daily
dialysis; SOFA, sequential organ failure assessment. |
The disadvantages of continuous therapies include:
Prolonged patient immobilization and transport problems, increased
costs of dialysis fluids and supplies, increased risk of
coagulation of dialysis circuits and the use of high doses of
anticoagulants-the latter increasing the risk of thrombocytopenia
and bleeding, due to prolonged exposure to heparin (16,18,21).
Hybrid therapies
Hybrid therapies (SLEDD, SLEDD-F and PIRRT) combine
the advantages of continuous and intermittent therapies. These
include: Good clearance for small molecules, improved hemodynamic
stability because of slower ultrafiltration, need of lower doses of
anticoagulants, lower costs because the sessions can take place on
standard dialysis machines (22,23).
The major advantage is the flexibility in terms of session duration
and its intensity (the ultrafiltration rate can be high, but it can
also be adjusted according to the patient's needs). A series of
studies that have compared SLEDD to CRRT did not find significant
differences between the two methods regarding the hemodynamic
parameters measured (mean blood pressure, systemic vascular
resistance and LV ejection fraction) (24-26).
In addition, the clearance for urea and creatinine was similar
(16).
Peritoneal dialysis in cancer patients
with AKI
Peritoneal dialysis is being used as RRT in AKI
patients only under very specific conditions. This can be useful in
hemodynamically unstable patients, in those with high risk of
bleeding or with fragility syndrome, but it is less efficient than
blood purification techniques, in regards to solute clearance and
excess fluid removal (16,21,27).
6. Choosing the appropriate therapy
(IRRT/CRRT), optimal time of initiation and dose/prognosis
relationship
Although there are arguments in favor of higher
doses of therapy and better prognosis (28,29),
there is still not enough evidence for the superiority of one
therapy over another (30). There
are large variations in practice and the subject remains open and
intensely debated.
Ronco et al (29) published in 2000 a prospective,
randomized study of 425 patients with AKI who were treated with
CVVH. They were divided into 3 groups according to the
ultrafiltration volumes (ml/bw/h) and followed the survival rate.
The group with the lowest dose of ultrafiltration had the lowest
survival. He concluded that an increase in the rate of
ultrafiltration improved survival significantly and recommend that
ultrafiltration should be prescribed according to patient's
bodyweight (bw).
Currently there are contradictory data regarding the
relationship between the type of chosen therapy (IRRT or CRRT) and
prognosis. Kellum et al (31) published the results of a
meta-analysis of 13 studies comparing the effects of intermittent
vs. continuous therapy. The primary endpoint was in-hospital
mortality. He found no significant differences between the two
methods. There were a few studies that compared groups of equal
severity of illness at baseline (time of enrollment) and adjusting
for study quality and severity of illness, mortality was lower in
patients treated with CRRT (31).
Later, Tonelli et al published a
meta-analysis of 6 trials, which showed no difference between the
two types of therapies (continuous and intermittent) in terms of
mortality (32). Of these, only 4
studies (33-36)
had data on improving renal function and their analysis showed no
significant differences between the two methods.
Another important therapeutical aspect in patients
with AKI is the decision concerning the timing of initiating RRT,
respectively the effects of early dialysis on survival, recovery of
renal function and the number of days spent in intensive care unit.
Early initiation of renal replacement therapy may have some
advantages in achieving more rapidly a state of euvolemia,
electrolyte and acid-base rebalancing and removal of
proinflammatory and other toxins from circulation (21). On the other hand, it also has some
side effects which include catheter-related infections, hypotensive
episodes, and bleeding (21). There
were two major trials published in 2016 that investigated whether
early renal replacement therapy decreases mortality in critically
ill patients with AKI (37). The
Early versus Late Initiation of Renal Replacement Therapy in
Critically Ill Patients with AKI (ELAIN) trial (38) and The Artificial Kidney Initiation
in Kidney Injury (AKIKI) trial (39) are two studies that have brought
contradictory results; the former showed that early initiation of
RRT (within 8 h of diagnosis of KDIGO stage 2) significantly
reduced 90-day mortality compared with delayed initiation of RRT
(within 12 h of stage 3 AKI). AKIKI showed no significant
difference in 60-day mortality between early and delayed RRT.
Patients should be treated individually based on
their characteristics and physician's experience (Table V) (17). Continuous therapy is the best method
in hemodynamically unstable patients on at least two vasopressors
or respiratory support, those with cerebral edema and
craniocerebral trauma and those with severe sepsis (6,16,21,40).
Hybrid therapies are indicated rather as transitional therapies to
intermittent therapies in patients with progressively reduced doses
of vasopressors, when mechanically ventilated patients are
extubated or for critically ill patients, hemodynamically unstable
and at high risk of bleeding (after surgery or anticoagulant
therapy) (6,16,21).
Intermittent therapies in AKI should be reserved for
life-threatening conditions that require rapid correction (eg
severe hyperkalemia) (6,16,21). A
special situation is tumor lysis syndrome and rhabdomyolysis which
can be treated by IRRT/CRRT combinations (21,41).
 | Table VChoice of renal replacement therapy
according to the associated clinical conditions (modified from ref.
17). |
Table V
Choice of renal replacement therapy
according to the associated clinical conditions (modified from ref.
17).
| | Life-threatening
conditions | Hypervolemia | Hemodynamic
instability | Cerebral edema |
|---|
| First option | IRRT |
CRRT/SLEDD/PIRRT |
CRRT/SLEDD/PIRRT | CRRT/PD |
| Second option | PIRRT | IRRT | PD | PIRRT |
| Third option | CRRT | PD | IRRT | IRRT |
| Fourth option | PD | - | - | - |
7. Extracorporeal blood purification
techniques in oncology
Plasmapheresis is an extracorporeal procedure used
in oncology. This is a process in which the liquid part of the
blood, or plasma, is separated from the blood cells. Typically, the
plasma is replaced with another solution such as fresh frozen
plasma or 5% albumin solution. Plasmapheresis can be intermittent
or continuous; there are ‘high-volume’ or ‘ultrahigh-volume’
hemofiltration therapies (42).
Plasmapheresis is used in the oncology field for
paraneoplastic syndromes with neurological manifestations, Eaton
Lambert myasthenic syndrome, paraproteinemias, myelomas, peripheral
neuropathies related to paraproteinemias, cytokine release syndrome
from sepsis (42).
Patients diagnosed with multiple myeloma usually
present to the nephrologist with myelomatous nephropathy, amyloid
infiltration of the kidney, or direct tubular light chain toxicity.
These patients have a much lower survival rate at 1 year compared
to those with normal kidney function. Rapid reduction of light
chains is the most important step in the treatment. Early reduction
in these chains is associated with an increased rate of renal
function recovery (43-45).
In addition to general measures, chemotherapy and
stem cell transplantation, recently there is a special interest in
extracorporeal purification techniques such as dialysis or
plasmapheresis regarding the fact that the renal distress is
directly consistent with the serum and urinary level of these light
chains (44,46).
In 2011 Hutchison et al (44) published a study of 39 patients from
2 large university centers (Birmingham and Rochester) with
histopathological diagnosis of myelomatous nephropathy and AKI, who
received either chemotherapy and extensive hemodialysis with
protein-permeable dialysis (HF-HD) or chemotherapy and
plasmapheresis. The results emphasized that a 60% reduction in
light chains by day 21 of diagnosis was associated with recovery of
renal function in 80% of cases. Thus, there is an increased
interest in the use of extracorporeal therapies for rapidly
decreasing the serum level of light chains. Only one randomized
trial using plasmapheresis for myelomatous nephropathy has been
reported. Clark et al (45)
published a study of 97 patients with multiple myeloma and presumed
myelomatous nephropathy, who were randomly assigned to conventional
therapy plus 5 to 7 plasma exchanges of 50 ml per kg of body weight
of 5% human serum albumin for 10 days or conventional therapy
alone. The investigators found no evidence that the use of
plasmapheresis improved the survival rate and recovery of renal
function. Chemotherapy with melphalan, prednisone and
cyclophosphamide was the standard of care in these patients, but
the use of new nongenotoxic chemotherapy (bortezomib, thalidomide
and lenalidomide) increased interest in extracorporeal treatment of
light chains, especially by high cut-off hemodialysis (HCO-HD),
which uses high cut-off (HCO) membranes that enables the removal of
large molecule, up to 60 kDa. However, these membranes allow the
passage of plasma proteins, such as albumin, an unwanted loss.
These membranes allow for the removal of higher-molecular-weight
molecules, such as mediators of sepsis/inflammation or
rhabdomyolysis or the removal of nephrotoxic light chains of
immunoglobulins, but they have the disadvantage of losing albumin
so they are used only for a limited number of sessions (46).
All this information leads to a complex, combined
treatment of chemotherapy and hemodialysis.
In the largest study of patients with multiple
myeloma and AKI requiring hemodialysis, conducted by Hutchison
et al (47), 67 patients
from several countries were treated with HCO-HD, most of them being
treated also with bortezomib-based chemotherapy or thalidomide. A
total of 63% of patients recovered their renal function. Predictors
associated with renal function recovery were the reduction of light
chains by day 12 and 21 of treatment and the time until the
initiation of hemodialysis. Unfortunately, patients had high
cut-off hemodialysis (HCO-HD) together with chemotherapy, while the
study had no control group for comparison. Even though the results
were promising in terms of reducing light chains, they did not
answer the fundamental question of whether hemodialysis with HCO
membranes has benefits in addition to bortezomib-based
chemotherapy. This requires randomized trials and there are no
prospective randomized controlled group studies published. There
are two major trials, a British trial (EuLITE) and a French trial
(MYRE), whose results are somewhat contradictory. Both studies
enrolled patients with myelomatous nephropathy and AKI and tested
the effect of lowering the serum level of light chains on the
recovery of renal function. Patients received chemotherapy and
conventional hemodialysis or chemotherapy and HCO-HD; at 3 months
of treatment there were no significant differences between the two
groups of patients regarding independence from dialysis (48,49).
However, the MYRE study showed differences at 6 and 12 months
(50) in favor of HCO-HD in
recovering renal function, but the group of patients was not large
enough.
HCO-HD may not yet be recommended as a routine
treatment for these patients considering the lack of large-scale
studies.
We still need to deal with the high mortality rate
and high morbidity among these patient and new therapeutic
solutions are needed despite the progress in the development of
hemodialysis regarding survival and quality of life.
Online hemodiafiltration is increasingly used. This
method uses high-flow membranes and combines diffusion with
convection, with large volumes of ultrafiltration for medium-high
molecular-weight molecules (since their purification is dependent
on a large convection volume). Thus, a study was published in
2011(51) comparing the efficacy of
online hemodiafiltration with high-flow hemodialysis in patients
with multiple myeloma and AKI. The study included 27 patients and
showed a higher extraction capacity by hemodiafiltration vs.
hemodialysis for both K and λ light chains, although the clearance
capacity increased proportionally to the volume of
substitution.
Online hemodiafiltration shows good results, but it
is still suboptimal and unsatisfactory, with high mortality and
cardiovascular morbidity, so that new therapeutic strategies are
needed, one of them being extended hemodialysis. This is a process
by which diffusion and convection are combined inside a special
dialyzer, equipped with a medium cut-off (MCO) membrane. These
recently produced MCO membranes with intermediate porosity (between
HF and HCO) have certain favorable characteristics such as higher
permeability for medium molecules and much lower albumin loss
compared to HCO membranes. MCO membrane filtration resembles quite
well that of the normal kidney (52-54).
Extended hemodialysis (HDx) is the latest
advancement in efficiency and simplification. In HDx the convective
transport required to remove medium to large MW solutes is the
result of a complex mechanism hidden inside the MCO dialyzer
membrane. Manufacturers have reduced the thickness of the
semipermeable membrane and the fiber inner diameter, which improved
the membrane's permeability and efficiency and solute transport
(larger number of fibers per dialyzer making it more compact)
(53-56).
Reducing the fiber inner diameter increases the wall shear rate
with a cleaning effect at the blood membrane interface, which
improves the solute transport (53-56).
The combination of hydraulic permeability and geometric structure
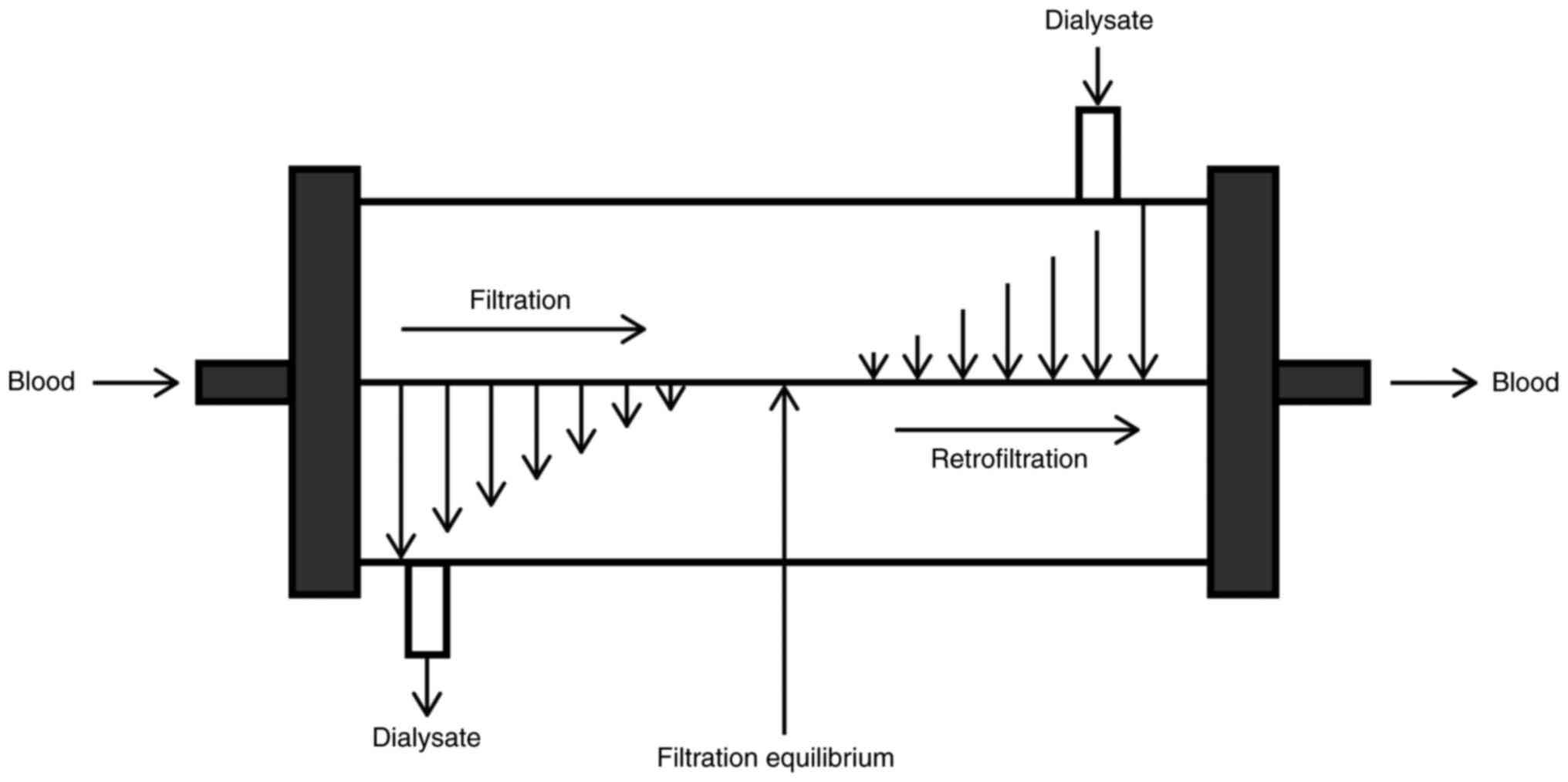
of the fiber increases the process of internal filtration and back
filtration. Thus, this mechanism allows a large volume of
convection inside the dialyzer, where the filtration takes place in
the proximal part and back filtration compensates for the excessive
ultrafiltration rate in the distal part (Fig. 1) (53,55,56).
Randomized trials are still needed for definitive
conclusion. Given the high cost of MCO filters, online
hemodiafiltration is reserved for a limited number of sessions for
certain types of patients, such as those with multiple myeloma.
8. Conclusions
Acute kidney injury is a common complication among
patients with a malignancy of various types, which may require
renal replacement therapy. The adequate management of this special
group of patients requires the establishment of the most
appropriate type of therapy, timing of initiation, the optimal
duration and dose of therapy, because all of these aspects
influence the recovery of renal function, quality of life and
mortality rate of the patients. While waiting for large randomized
trials to be published, we have to focus on personalized therapy
based on clinical and laboratory characteristics, patient's
decision and experience of the nephrologist-oncologist-intensive
care team.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All information is documented by relevant
references.
Authors' contributions
Conceptualization was achieved by GL, ML, IA, GF,
AA, EM, MB, RC and GI. Methodology was the responsibility of ML,
GL, RC and GI. Resources were acquired by AA, EM, GF and MB. Data
curation was carried out by GL, MB, AA and R.C. Writing of the
original draft preparation was done by ML, GL and GI; writing,
review and editing were the responsibility of ML, GL, IA, GF, AA,
MB, EM, RC and GI. Supervision was carried out by GI. All authors
read and agreed to the published version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Finkel K, Perazella MA and Cohen E: Acute
kidney injury-Incidence, Pathogenesis and Outcomes. In:
Onco-Nephrology. 1st edition. Elsevier, Amsterdam, pp270-274,
2019.
|
|
2
|
Bratu OG, Cherciu AI, Bumbu A, Lupu S,
Marcu DR, Ionita-Radu F, Manea M, Furau C, Diaconu CC and Mischianu
DL: Retroperitoneal tumours-treatment and prognosis of tumour
recurrence. Rev Chim. 70:190–194. 2019.
|
|
3
|
Marcu DR, Ionita-Radu F, Iorga LD, Manea
M, Socea B, Scarneciu I, Isvoranu G, Costache R, Diaconu CC and
Bratu OG: Vascular involvement in primary retroperitoneal tumors.
Rev Chim. 70:445–448. 2019.
|
|
4
|
Draghici T, Negreanu L, Bratu OG, Stoian
AP, Socea B, Neagu TP, Stanescu AM, Manuc D and Diaconu CC:
Paraneoplastic syndromes in digestive tumors: A review. Rom
Biotechnol Lett. 24:813–819. 2019.
|
|
5
|
Perazella MA: Renal vulnerability to drug
toxicity. Clin J Am Soc Nephrol. 4:1275–1283. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kidney Disease: Improving Global Outcomes
(KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for
Acute Kidney Injury. Kidney Int Suppl. 1:1–138. 2012.
|
|
7
|
Christiansen CF, Johansen MB, Langeberg
WJ, Fryzek JP and Sørensen HT: Incidence of acute kidney injury in
cancer patients: A Danish population-based cohort study. Eur J
Intern Med. 22:399–406. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
International Agency for Research on
Cancer: Europe-Global cancer Observatory. Globoscan 2018.
https://gco.iarc.fr/today/data/factsheets/populations/908-europe-fact-sheets.pdf.
Accessed October 28, 2020.
|
|
9
|
Bratu OG, Diaconu CC, Mischianu DLD,
Constantin T, Stanescu AM, Bungau SG, Ionita-Radu F and Marcu RD:
Therapeutic options in patients with biochemical recurrence after
radical prostatectomy. Exp Ther Med. 18:5021–5025. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bratu O, Spînu D, Oprea I, Popescu R,
Marcu D, Farcaș C, Dinu M and Mischianu D: Complications of radical
retropubicprostatectomy-our experience. Romanian J Military Med.
118:23–25. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Marcu D, Spinu D, Mischianu D, Socea B,
Oprea I and Bratu OG: Oncological follow-up after radical
prostatectomy. Romanian J Military Med. 120:39–42. 2017.
|
|
12
|
Tataru AL, Furau G, Afilon J, Ionescu C,
Dimitriu M, Bratu OG, Tit DM, Bungau A and Furau C: The situation
of cervical cancers in the context of female genital cancer
clustering and burden of disease in Arad County. Romania J Clin
Med. 8(96)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lameire N, Van Biesen W and Vanholder R:
Acute renal failure. Lancet. 365:417–430. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Finkel KW and Kala J: Renal replacement
therapy in critically Ill cancer patients. In: Oncologic Critical
Care. Nates J and Price K (eds). Springer, Cham, pp1-12, 2019.
|
|
15
|
Daugirdas JT: Physiologic principles and
urea kinetic modeling. In: Handbook of Dialysis. 5th edition.
Daugirdas JT, Blake PG and Ing TS (eds). Wolters Kluwer,
Philadelphia, pp34-65, 2015.
|
|
16
|
Fathima N, Kashif T, Janapala RN, Jayaraj
JS and Qaseem A: Single-best choice between intermittent vs.
continuous renal replacement therapy: A review. Cureus.
11(e5558)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
PIRRT may help you improve resource
utilization, patient care, early patient mobilization programs.
https://www.nxstage.com/hcp/therapies/pirrt/. Accessed
September 14, 2020.
|
|
18
|
Sherman RA, Daugirdas JT and Ing TS:
Complications during hemodialysis. In: Handbook of Dialysis. 5th
edition. Daugirdas JT, Blake PG and Ing TS (eds). Wolters Kluwer,
Philadelphia, pp215-236, 2015.
|
|
19
|
Trinh-Trang-Tan MM, Cartron JP and Bankir
L: Molecular basis for the dialysis disequilibrium syndrome:
Altered aquaporin and urea transporter expression in the brain.
Nephrol Dial Transplant. 20:1984–1988. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mistry K: Dialysis disequilibrium syndrome
prevention and management. Int J Nephrol Renovasc Dis. 12:69–77.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Finkel KW and Waguespack DR: Renal
replacemet therapies. In: Onco-Nephrology. 1st edition. Finkel K,
Perazella MA and Cohen E (eds). Elsevier, Amsterdam, pp290-298,
2019.
|
|
22
|
Fliser T and Kielstein JT: Technology
insight: Treatment of renal failure in the intensive care unit with
extended dialysis. Nat Clin Pract Nephrol. 2:32–39. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Berbece AN and Richardson RMA: Sustained
low-efficiency dialysis in the ICU: Cost, anticoagulation, and
solute removal. Kidney Int. 70:963–968. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Baldwin I, Naka T, Koch B, Fealy N and
Bellomo R: A pilot randomized controlled comparison of continuous
venovenous hemofiltration and extended daily dialysis with
filtration: Effect on small solutes and acid-base balance.
Intensive Care Med. 33:830–835. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kielstein JT, Kretschmer U, Ernst T, Hafer
C, Bahr MJ, Haller H and Fliser D: Efficacy and cardiovascular
tolerability of extended dialysis in critically ill patients: A
randomized controlled study. Am J Kidney Dis. 43:342–349.
2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Schwenger V, Weigand MA, Hoffmann O, Dikow
R, Kihm LP, Seckinger J, Miftari N, Schaier M, Hofer S, Haar C, et
al: Sustained low efficiency dialysis using a single-pass batch
system in acute kidney injury-a randomized interventional trial:
The REnal replacement therapy study in intensive care unit
patiEnts. Crit Care. 16(R140)2012.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Cullis B, Abdelraheem M, Abrahams G, Balbi
A, Cruz DN, Frishberg Y, Koch V, McCulloch M, Numanoglu A, Nourse
P, et al: Peritoneal dialysis for acute kidney injury. Perit Dial
Int. 34:494–517. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Schiffl H, Lang SM and Fischer R: Daily
hemodialysis and the outcome of acute renal failure. N Engl J Med.
346:305–310. 2002.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ronco C, Bellomo R, Homel P, Brendolan A,
Dan M, Piccinni P and La Greca G: Effects of different doses in
continuous veno-venous haemofiltration on outcomes of acute renal
failure: A prospective randomised trial. Lancet. 356:26–30.
2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Uchino S: The epidemiology of acute renal
failure in the world. Curr Opin Crit Care. 12:538–543.
2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kellum JA, Angus DC, Johnson JP, Leblanc
M, Griffin M, Ramakrishnan N and Linde-Zwirble WT: Continuous
versus intermittent renal replacement therapy: A meta-analysis.
Intensive Care Med. 28:29–37. 2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tonelli M, Manns B and Feller-Kopman D:
Acute renal failure in the intensive care unit: A systematic review
of the impact of dialytic modality on mortality and renal recovery.
Am J Kidney Dis. 40:875–885. 2002.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sandy D, Moreno L, Lee J and Paganini EP:
A randomized stratified, dose equivalent comparison of continuous
veno-venous hemodialysis (CVVHD) vs intermittent hemodialysis (IHD)
support in ICU acute renal failure patients (ARF). J Am Soc
Nephrol. 9(225A)1998.
|
|
34
|
John S, Griesbach D, Baumgartel M,
Weihprecht H, Schmieder RE and Geiger H: Effects of continuous
haemofiltration vs intermittent haemodialysis on systemic
haemodynamics and splanchnic regional perfusion in septic shock
patients: A prospective, randomized clinical trial. Nephrol Dial
Transplant. 16:320–327. 2001.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mehta RL, McDonald BR, Gabbai FB, Pahl M,
Pascual MT, Farkas A and Kaplan RM: Collaborative Group for
Treatment of ARF in the ICU. A randomized clinical trial of
continuous vs intermittent dialysis for acute renal failure. Kidney
Int. 60:1154–1163. 2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Uehlinger DE, Jakob SM, Eichelberger M, et
al: A randomized, controlled single center study for the comparison
of continuous renal replacement therapy with intermittent
hemodialysis in critically ill patients with acute renal failure. J
Am Soc Nephrol. 12(278A)2001.
|
|
37
|
Leaf DE and Waika SS: IDEAL-ICU in
context. Clin J Am Soc Nephrol. 14:1264–1267. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zarbock A, Kellum JA, Schmidt C, Van Aken
H, Wempe C, Pavenstädt H, Boanta A, Gerß J and Meersch M: Effect of
early vs delayed initiation of renal replacement therapy on
mortality in critically ill patients with acute kidney injury: The
ELAIN randomized clinical trial. JAMA. 315:2190–2199.
2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gaudry S, Hajage D, Schortgen F,
Martin-Lefevre L, Pons B, Boulet E, Boyer A, Chevrel G, Lerolle N,
Carpentier D, et al: for the AKIKI study group: initiation
strategies for renal-replacement therapy in the intensive care
unit. N Eng J Med. 375:122–133. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Teo BW, Messer JS, Chua HR, How P and
Demirjian S: Continuous Renal Replacement Therapies. In: Handbook
of Dialysis. 5th edition. Daugirdas JT, Blake PG and Ing TS (eds).
Wolters Kluwer, Philadelphia, pp268-304, 2015.
|
|
41
|
Rastegar M, Kitchlu A and Shirali AC:
Tumor lysis syndrome. In: Onco-Nephrology. 1st edition. Daugirdas
JT, Blake PG and Ing TS (eds). Elsevier, Amsterdam, pp275-280,
2019.
|
|
42
|
Kiprov DD, Sanchez A and Pusey C:
Therapeutic apheresis. In: Handbook of Dialysis. 5th edition.
Daugirdas JT, Blake PG and Ing TS (eds). Wolters Kluwer,
Philadelphia, pp333-359, 2015.
|
|
43
|
Favà A, Fulladosa X, Montero N, Draibe J,
Torras J, Gomà M and Cruzado JM: Treatment of multiple myeloma with
renal involvment: the nephrologist's view. CKD. 11:777–785.
2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hutchison CA, Cockwell P, Stringer S,
Bradwell A, Cook M, Gertz MA, Dispenzieri A, Winters JL, Kumar S,
Rajkumar SV, et al: Early reduction of serum-free light chains
associates with renal recovery in myeloma kidney. J Am Soc Nephrol.
22:1129–1136. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Clark WF, Stewart AK, Rock GA, Sternbach
M, Sutton DM, Barrett BJ, Heidenheim AP, Garg AX and Churchill DN:
Canadian Apheresis Group. Plasma exchange when myeloma presents as
acute renal failure: A randomized, controlled trial. Ann Intern
Med. 143:777–784. 2005.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Gondouin B and Hutchison CA: High cut-off
dialysis membranes: Current uses and future potential. Adv Chronic
Kidney Dis. 18:180–187. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hutchison CA, Heyne N, Airia P, Schindler
R, Zickler D, Cook M, Cockwell P and Grima D: Immunoglobulin free
light chain levels and recovery from myeloma kidney on treatment
with chemotherapy and high cut-off haemodialysis. Nephrol Dial
Transplant. 27:3823–3828. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Finkel K and Fabbrini P: Paolo fabbrini.
High cut-off hemodialysis for myeloma cast nephropathy-do we
finally have an answer? J Onco-Nephrol. 1:67–70. 2017.
|
|
49
|
Hutchison CA, Cook M, Heyne N, Weisel K,
Billingham L, Bradwell A and Cockwell P: European trial of free
light chain removal by extended haemodialysis in cast nephropathy
(EuLITE): A randomised control trial. Trials. 9(55)2008.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Bridoux F, Carron PL, Pegourie B,
Alamartine E, Augeul-Meunier K, Karras A, Joly B, Peraldi MN,
Arnulf B, Vigneau C, et al: Effect of high-cutoff hemodialysis vs
conventional hemodialysis on hemodialysis independence among
patients with myeloma cast nephropathy: A randomized clinical
trial. JAMA. 318:2099–2110. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Vallée AG, Chenine L, Leray-Moragues H,
Patrier L, Cognot C, Cartron G, Cristol JP and Canaud B: Online
high-efficiency haemodiafiltration achieves higher serum free light
chain removal than high-flux haemodialysis in multiple myeloma
patients: Preliminary quantitative study. Nephrol Dial Transplant.
26:3627–3633. 2011.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kirsch AH, Lyko R, Nilsson LG, Beck W,
Amdahl M, Lechner P, Schneider A, Wanner C, Rosenkranz AR and
Krieter DH: . Performance of hemodialysis with novel medium cut-off
dialyzers. Nephrol Dial Transplant. 1:165–172. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zweigart C, Boschetti-de-Fierro A, Hulko
M, Nilsson LG, Beck W, Storr M and Krause B: Medium cut-off
membranes-closer to the natural kidney removal function. Int J
Artif Organs. 40:328–334. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Krishnasamy R, Hawley CM, Jardine MJ,
Roberts MA, Cho YJ, Wong MG, Heath A, Nelson CL, Sen A, Mount PF,
et al: Design and methods of the REMOVAL-HD study: A tRial
evaluating Mid cut-Off value membrane clearance of albumin and
light chains in haemoDialysis patients. BMC Nephrol.
19(89)2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Ronco C, Marchionna N, Brendolan A, Neri
M, Lorenzin A and Rueda AJ: Expanded haemodialysis: Expanded
haemodialysis: From operational mechanism to clinical results.
Nephrol Dial Transplant. 33 (Suppl 3):iii41–iii47. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Pérez-García R and Alcázar R: The dialyser
in the year 2017: Much more than a membrane. Nefrologia. 38:4–7.
2018.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|