Introduction
Chronic atrophic gastritis (CAG) is a common
digestive-tract disease characterized by gastric gland atrophy,
decreased number of gastric glands and chronic inflammation
(1). The typical clinical symptoms
of patients with CAG include reduced appetite, nausea and acid
reflux (2). Helicobacter
pylori (H. pylori) infection, smoking, drinking and
immune abnormalities may lead to the development of CAG (3). Furthermore, CAG is a precancerous
state of gastric cancer (GC). Therefore, effective and timely
treatment of CAG may help prevent the occurrence of GC (4,5).
Proton pump inhibitors, antibiotics, folic acid, vitamins and
gastric mucosal protective agents are frequently used as
conventional treatments for patients with CAG (6). However, there is a lack of specific
drugs that may reverse the atrophy of patients with CAG in clinical
practice.
Jianpiyiqi formula, also called Weiwei No. 1, is a
type of Traditional Chinese Medicine (TCM) formula and is used to
treat CAG in clinical settings (7).
Jianpiyiqi formula is not a commercial product and there is
currently a lack of effective commercial formulas for treating CAG
in the clinic. Jianpiyiqi formula is composed of 12 Chinese
medicinal herbal components/ingredients: Dangshen, Baizhu, Fuling,
Gancao, Chenpi, Banxia, Muxiang, Sharen, Ezhu, Baihuasheshecao,
Yunmushi and Yujin (Table I). It is
mainly used for patients with CAG with a weak spleen and stomach,
qi stagnation and blood stasis according to the theory of TCM
(8). However, the pharmacological
mechanisms of action of the Jianpiyiqi formula for improving CAG
remained elusive. In the present study, a CAG rat model induced by
N-methyl-N'-nitro-N-nitrosoguanidine and ranitidine was constructed
to assess the molecular pharmacology of the Jianpiyiqi formula.
 | Table IComponents of Jianpiyiqi formula. |
Table I
Components of Jianpiyiqi formula.
| Chinese name | Latin or English
name | Plant parts | Amount (g) | Proportion w/w
(%) |
|---|
| Dangshen | Codonopsis
pilosula (Franch.) Nannf. | Root | 15 | 10.27 |
| Baizhu | Atractylis
macrocephala (Koidz.) Hand.-Mazz. | Rhizoma | 10 | 6.85 |
| Fuling | Poria cocos
(Schw.) Wolf | Sclerotia | 10 | 6.85 |
| Gancao | Glycyrrhiza
uralensis Fisch | Rhizoma | 3 | 2.05 |
| Chenpi | Citrus
reticulata Blanco | Peel | 6 | 4.11 |
| Banxia | Pinellia
ternata (Thunb.) Makino | Tuber | 6 | 4.11 |
| Muxiang | Aucklandia
Lappa Decne. | Root | 10 | 6.85 |
| Sharen | Amomum
villosum Lour. | Fruit | 6 | 4.11 |
| Ezhu | Curcuma
aeruginosa Roxb. | Rhizome | 10 | 6.85 |
|
Baihuasheshecao | Oldenlandia
diffusa (Willd.) Roxb. | Whole herb | 30 | 20.55 |
| Yujin | Curcuma
wenyujin Y. H. Chen & C. Ling | Root tuber | 10 | 6.85 |
|
Yunmushia | Mica | NA | 30 | 20.55 |
| Total | NA | NA | 146 | 100.00 |
The gastric mucosa of patients with CAG is
continuously damaged by various pathogenic factors and it cannot be
repaired within a sufficient time period (1). It has been reported that the damage
repair and proliferation of gastric epithelial cells are closely
associated with the Wnt/β-catenin signaling pathway (9-12).
H. pylori infection is associated with the expression of
β-catenin in CAG with intestinal metaplasia (13). GC has been associated with increased
expression of the Wnt/β-catenin signaling pathway, which may
promote abnormal proliferation of gastric gland cells and then
induce tumors (14). To date, a
limited number of studies have investigated the association between
CAG and the Wnt/β-catenin signaling pathway (15). Therefore, the aim of the present
study was to clarify the pharmacological mechanisms of action of
Jianpiyiqi formula in the treatment of CAG via the Wnt/β-catenin
signaling pathway.
Materials and methods
Reagents
N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) was
purchased from Tokyo Chemical Industry Co., Ltd. Ranitidine
hydrochloride was purchased from Shanghai Hengshan Pharmaceutical
Co., Ltd. Liquiritin, hesperidin, lobetyolin, curdione, costunolide
and atractylenolide II were purchased from Chengdu Must
Bio-Technology Co., Ltd. The H&E staining kit and prostaglandin
E2 (PGE2) ELISA kit (cat. no. H099-1) were
purchased from Nanjing Jiancheng Bioengineering Institute. Methanol
and acetonitrile were purchased from EMD Millipore. Ethanol, acetic
acid, xylene and formaldehyde were purchased from Sinopharm
Chemical Reagent Co., Ltd. The gastrin (GAS), pepsin (PP), and
somatostatin (SS) ELISA kits were purchased from Elabscience, Inc.
Wnt1 was purchased from Abcam Co., Ltd. The β-catenin, glycogen
synthase kinase-3β (GSK-3β) and cyclin D1 antibodies and the goat
anti-rabbit and anti-mouse IgG secondary antibodies were purchased
from Cell Signaling Technology, Inc. The PCR primers for Wnt1,
Wnt5a, β-catenin, GSK-3β, cyclin D1, MMP7 and GAPDH were produced
by Nanjing Genscript Biological Technology Co., Ltd.
TRIzol® reagent, Maxima First Strand cDNA Synthesis
Reverse transcription PCR kit and Maxima SYBR Green/ROX
quantitative PCR kit were obtained from Thermo Fisher Scientific,
Inc.
Preparation of Jianpiyiqi formula
water extract
All of the medicinal herbs used to prepare
Jianpiyiqi formula were purchased from Jiangsu Province Integrated
Chinese and Western Medicine Hospital (Nanjing, China). All had
their identity confirmed by Professor Shihui Qian (Chinese Medicine
Plant Resources Laboratory, Jiangsu Province Institute of
Traditional Chinese Medicine) and stored in a dry and ventilated
laboratory cabinet. One batch weighed 146 g and consisted of 12
Chinese medicinal herbs/ingredients: Dangshen, Baizhu, Fuling,
Gancao, Chenpi, Banxia, Muxiang, Sharen, Ezhu, Baihuashshecao,
Yunmushi and Yujin (Table I). The
146-g herbal mixture was placed in a round-bottomed flask and
soaked for 0.5 h in 1.46 liter water. The water containing the
herbs was boiled for 0.5 h continuously. The water extract was
naturally cooled to room temperature was then filtered and
collected for the first time. Subsequently, 0.73 liter water was
added to the round-bottomed flask, followed by continuous boiling
for 0.5 h. The water extract was naturally cooled to room
temperature was filtered and collected for a second time. The twice
collected water extracts were concentrated to 48.7 ml (equivalent
to a drug concentration of 3 g/ml) using a vacuum rotary evaporator
and stored at -80˚C in an ultra-low temperature refrigerator.
Animals
A total of 20 male Sprague Dawley rats [body weight,
200±20 g; age, 6-8 weeks; animal quality certificate no. SCXK (Su)
2019-0001] were purchased from the Laboratory Animal Center of
Nantong University. All rats were kept under a 12-h light/dark
cycle and housed at the Laboratory Animal Center of Jiangsu
Province Institute of Traditional Chinese Medicine (Nanjing,
China). The animals had ad libitum access to food and water
in an environment with a temperature of 20˚C and a humidity of 50%.
Animal experimental operations complied with the Guidelines of
Welfare and Ethics of Laboratory Animals (issued by the General
Administration of Quality Supervision, Inspection and Quarantine of
the P.R. China) and the Guide for the Care and Use of Laboratory
Animals (issued by the US National Institutes of Health). The
animal experiment of the present study was approved by the Ethics
Committee of Jiangsu Province Integrated Chinese and Western
Medicine Hospital (Nanjing, China; approval no.
AEWC-20160810-12).
Establishment of CAG rat model
The 20 male Sprague Dawley rats were randomly
divided into a blank group, model group, positive control drug
group (2.7 mg/kg folate and 13.5 mg/kg teprenone) and Jianpiyiqi
formula group (13.2 g/kg crude drug). A total of 5 rats were in
each group. The rats in the CAG model groups were freely provided
with drinking water containing 150 µg/ml MNNG and with food
containing 0.03% ranitidine. The construction of the CAG rat model
lasted 24 weeks. Subsequently, the rats were administered the drugs
via gavage in positive control drug group and Jianpiyiqi formula
group. Rats in the blank group and model group were gavaged with
sterile water. The experiment was terminated after 8 weeks of
treatment. Body weight and daily food intake were recorded. All
rats were anesthetized by intraperitoneal injection of 30 mg/kg
(body weight) sodium pentobarbital. Subsequently, 20 ml blood was
collected from the abdominal aorta of the live anesthetized rats.
After the blood was collected completely, the rats died naturally
due to the exsanguination.
High-performance liquid chromatography
(HPLC) analysis
Liquiritin, hesperidin, lobetyolin, curdione,
costunolide and atractylenolide II were prepared to a solution of 1
mg/ml in methanol. The six compounds were mixed at equal
proportions for HPLC analysis. Jianpiyiqi formula water extraction
solution (3 g/ml crude drug) was diluted to 1 g/ml solution with
methanol for HPLC analysis. All samples were separated using a
C18 chromatographic column (5 µm; 250x4.6 mm) in a
Waters 2695 HPLC system and detected in a Waters 2489 detector
(Waters Corp.). Mobile phrases were water containing 0.1% formic
acid (solvent A) and acetonitrile (solvent B). The mobile phase
elution procedure was as follows: Solvent A was varied from 95 to
85% from 0 to 30 min, solvent A was varied from 85 to 70% from 30
to 45 min, solvent A was varied from 70 to 30% from 45 to 55 min
and solvent A remained at 30% from 55 to 60 min. The column
temperature was 30˚C, the sample loading volume was 25 µl, the flow
rate was 1 ml/min and the detection wavelength was 230 nm.
H&E staining
The gastric tissues of the rats were fixed in 4%
formalin at 25˚C for 24 h and underwent routine ethanol dehydration
and paraffin embedding. The embedded tissues were cut into 5-µm
slices using a Leica Biosystems RM2245 wheel slicer (Leica
Microsystems GmbH). The slices were baked in an oven at 60˚C for 2
h and were then dewaxed and rehydrated. Subsequently, the slices
were stained with hematoxylin (Nanjing Jiancheng Bioengineering
Institute) at 25˚C for 3 min and eosin (Nanjing Jiancheng
Bioengineering Institute) at 25˚C for 30 sec. The stained slices
were dehydrated with ethanol, cleared with xylene, mounted on glass
slides with neutral balsam and covered with coverslips. The rat
gastric tissues were imaged using the Olympus CKX-41 inverted light
microscope (Olympus Corporation) at x100 magnification. The atrophy
and inflammation of gastric glands were scored according to the
visual analogue scale of the new Sydney system (16,17).
The pathology scoring was carried out independently by two
examiners, and the mean value was finally taken.
ELISA
The contents of GAS (cat. no. E-EL-R0472c;
Elabscience, Inc.), PP (cat. no. E-EL-R0719c; Elabscience, Inc.),
SS (cat. no. E-EL-R0914c; Elabscience, Inc.) and PGE2
(cat. no. H099-1; Nanjing Jiancheng Bioengineering Institute) in
rat serum were detected according to the manufacturer's protocols
for the ELISA kits. The rat serum was added to the coated 96-well
microplate. The antibodies and HRP-conjugated streptomycin were
added to the plate. Subsequently, the 96-well microplate was sealed
with parafilm and incubated at 37˚C for 1 h. The solution in the
wells was then completely discarded. The microplate was washed five
times with washing buffer and incubated with diaminobenzidine (DAB)
chromogenic reagent at 37˚C for 10 min, and reaction termination
solution was then immediately added. The optical density (OD) value
of all wells was measured at the wavelength of 450 nm using a Tecan
M200 pro automatic microplate reader (Tecan Group, Ltd.). According
to the standard curve linear regression equation and the OD values
of the samples in each group, the corresponding sample
concentration was calculated.
Immunohistochemistry (IHC)
The paraffin-embedded stomach tissues were cut into
3-µm slices. After the slices were baked, the slides were submersed
in citrate unmasking solution for antigen retrieval and boiled at
100˚C for 5 min. The slices were incubated with 3% hydrogen
peroxide for 10 min and blocked with 0.5 ml blocking solution for 1
h at room temperature. The slides were coated with 0.2 ml Wnt1
(1:300 dilution; cat. no. ab15251; Abcam), β-catenin (1:300
dilution; cat no. 7074; Cell Signaling Technology, Inc.), GSK-3β
(1:300 dilution; cat no. 7074; Cell Signaling Technology, Inc.) and
cyclin D1 (1:300 dilution; cat no. 7074; Cell Signaling Technology,
Inc.) primary antibodies and incubated overnight at 4˚C. The slices
were then incubated with 0.2 ml HRP-conjugated secondary antibody
(1:500 dilution; cat no. 7074; Cell Signaling Technology, Inc.) in
a humidified chamber at 25˚C for 1 h. The slides were incubated
with 0.5 ml DAB solution for 5 min and the sections were
counterstained with hematoxylin at 25˚C for 30 sec. The slides were
mounted with neutral balsam mounting medium and covered using
coverslips. The rat gastric tissues were imaged using the Olympus
CKX-41 inverted light microscope (Olympus Corporation) at x100
magnification. Staining of the IHC sections was determined using
Image-Pro Plus 6.0 software (Media Cybernetics, Inc.).
Reverse transcription-quantitative
(RT-q)PCR
The total RNA was extracted from rat gastric tissues
using TRIzol reagent. The extracted RNA was reverse transcribed
into the complementary DNA using the Maxima First Strand cDNA
Synthesis Reverse transcription PCR kit according to the
manufacturer's protocol. The reaction conditions were 25˚C for 10
min, 50˚C for 15 min and 85˚C for 5 min. The relative mRNA
expression levels of Wnt1, Wnt5a, β-catenin, GSK-3β, cyclin D1 and
MMP7 were detected using Applied Biosystems StepOnePlus
fluorescence quantitative PCR equipment (Applied Biosystems; Thermo
Fisher Scientific, Inc.) with the Maxima SYBR Green/ROX
quantitative PCR kit according to the manufacturer's protocol. The
thermal cycling conditions were: One cycle of 95˚C for 15 min, then
40 cycles of 95˚C for 15 sec, 60˚C for 30 sec and 72˚C for 30 sec.
The relative mRNA expression was analyzed and calculated using the
2-∆∆Cq method (18),
with the housekeeping gene GAPDH used as a control gene. The
sequences of all qPCR primers are listed in Table II.
 | Table IISequences of primers used for
quantitative PCR. |
Table II
Sequences of primers used for
quantitative PCR.
| Gene name | Forward sequence
(5'-3') | Reverse sequence
(5'-3') | Product length
(bp) |
|---|
| Wnt1 |
AACAGTAGTGGCCGATGGTG |
GGGTTCTGTCGGATCAGTCG | 143 |
| Wnt5a |
CCAGTACCAGTTCCGGCATC |
GCCTATTTGCATCACCCTGC | 82 |
| β-catenin |
CAGATCCCATCCACGCAGTT |
TCTGTGACGGTTCAGCCAAG | 70 |
| Glycogen synthase
kinase-3β |
TCCTTATCCCTCCTCACGCT |
GATGCAGAAGCGGCGTTATT | 118 |
| Cyclin D1 |
CAAGTGTGACCCGGACTGC |
GACCAGCTTCTTCCTCCACTT | 137 |
| MMP7 |
GATGGGCCAGGAAACACTCT |
TCAAAGTGAGCATCTCCGCC | 71 |
| GAPDH |
GTGAAGGTCGGTGTGAACGG |
CCACTTTGTCACAAGAGAAGGC | 76 |
Statistics analysis
Values are expressed as the mean ± standard
deviation. Differences between groups were analyzed using one-way
ANOVA followed by Tukey's post hoc test. P<0.05 was considered
to indicate a statistically significant difference. The data were
analyzed using SPSS 22.0 software (IBM Corp.) and graphs were
plotted using GraphPad Prism 5.0 software (GraphPad Software,
Inc.).
Results
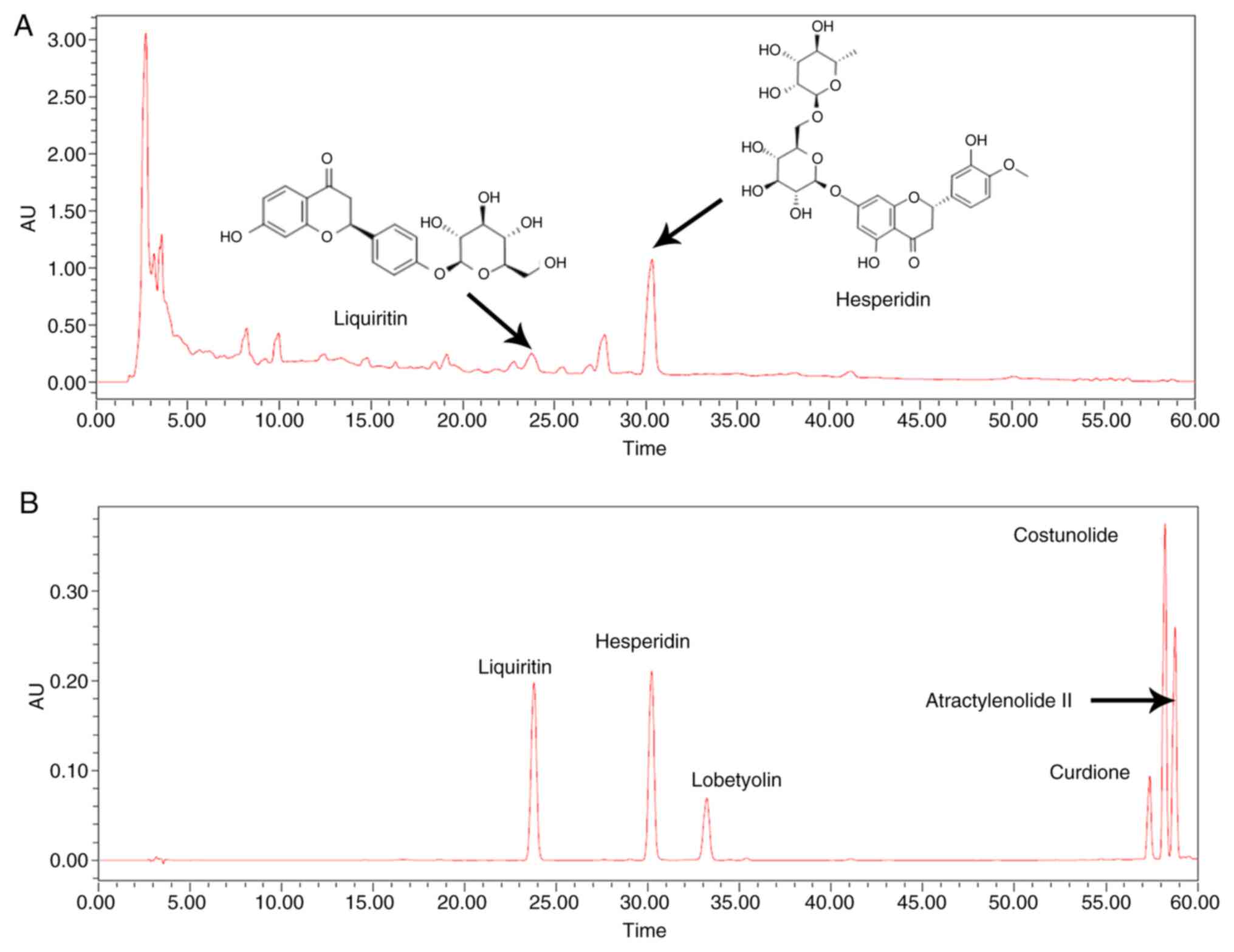
HPLC chromatogram of Jianpiyiqi
formula
The TCM prescription Jianpiyiqi formula is an
extract of 12 Chinese medicinal herbs/ingredients (Table I). The chemical composition of the
formula is complex. In the present study, Jianpiyiqi formula was
prepared and its HPLC chromatogram was examined (Fig. 1A). The HPLC analysis indicated that
relatively high concentrations of liquiritin and hesperidin were
present in the extract (Fig. 1A).
HPLC results showed hesperidin was the major active component of
Jianpiyiqi formula. The six compounds liquiritin, hesperidin,
lobetyolin, curdione, costunolide and atractylenolide II were used
as controls to identify the pharmaceutically relevant components of
the formula (Fig. 1B).
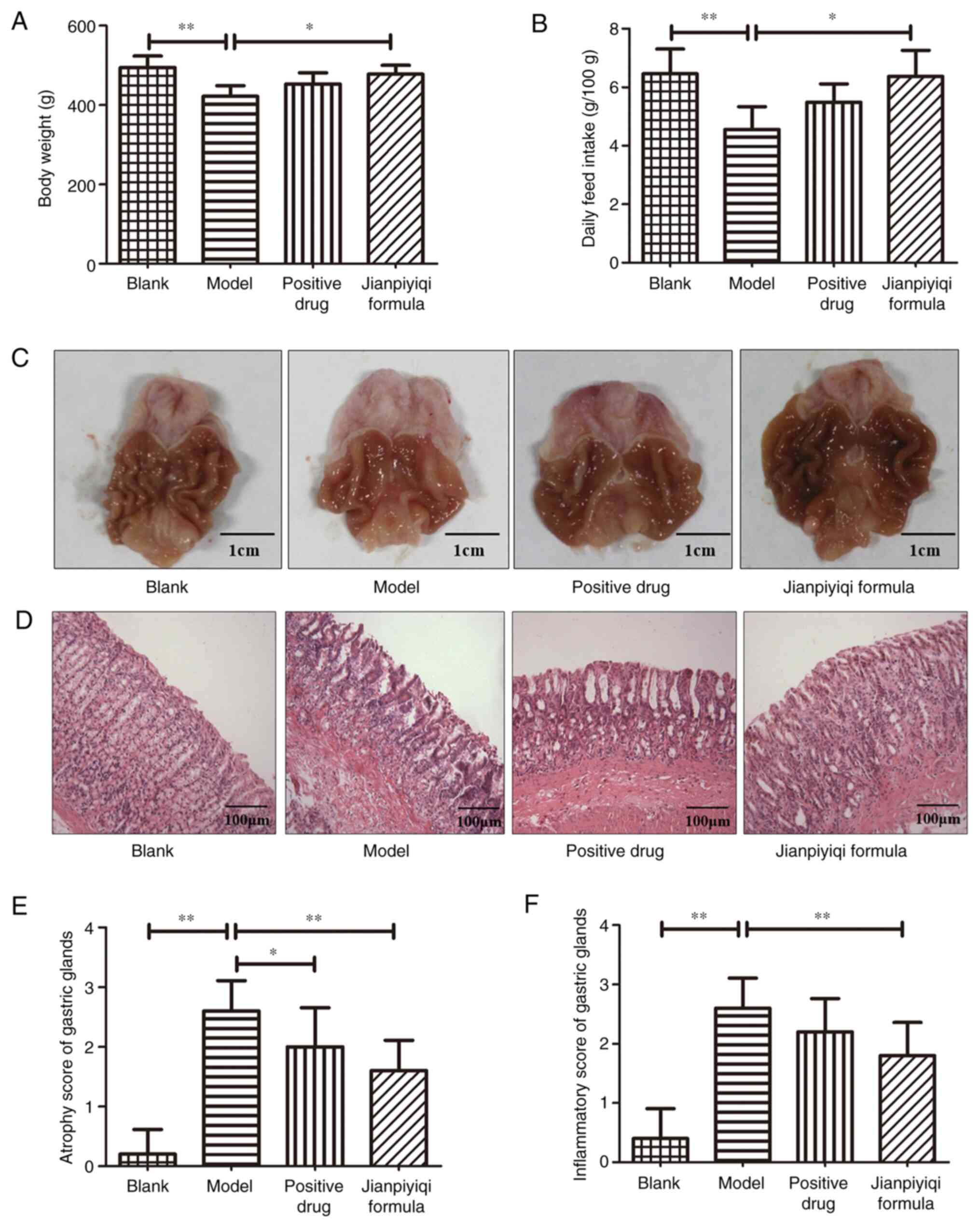
Effect of Jianpiyiqi formula on body
weight, food intake, gastric morphology and pathology of rats with
CAG
After the establishment of the CAG rat model induced
by MNNG and ranitidine, the therapeutic effect of Jianpiyiqi
formula on CAG was evaluated. The experimental results demonstrated
that compared with the blank group, the body weight and feed intake
of the rats were decreased, and gastric mucosal atrophy and
inflammation appeared in the model group. Jianpiyiqi formula
significantly improved the body weight and the food intake of the
rats (Fig. 2A and B). All rat stomachs were cut along the
side of the large curve and gastric morphology was imaged using a
camera. Compared with that in the blank group, the stomach tissue
in the model group appeared slightly paler and had fewer gastric
folds. Furthermore, Jianpiyiqi formula and the positive control
drugs improved gastric morphology (Fig.
2C). Subsequently, the rat gastric tissue was stained with
H&E for pathological observation. Atrophy and inflammation of
gastric tissues were scored according to the new Sydney system
(16). Marked gastric atrophy and
inflammation were observed in the CAG model group. After Jianpiyiqi
formula treatment, the atrophy and inflammation of gastric glands
was improved. Furthermore, the positive control drugs significantly
improved gastric atrophy (Fig.
2D-F).
Effect of Jianpiyiqi formula on GAS,
PP, SS and PGE2 in rats with CAG
Next, the gastric mucosa-related factors were
detected to evaluate the curative effect of Jianpiyiqi formula on
CAG. The contents of GAS, PP, SS and PGE2 in rat serum
were measured via ELISAs. Compared with those in the blank group,
the contents of GAS, PP, SS and PGE2 were decreased in
the CAG rat model group. It was indicated that the positive control
drugs significantly increased the content of SS. In addition,
Jianpiyiqi formula significantly improved the secretion of gastric
mucosa-related factors (GAS, PP, SS and PGE2) in the
serum of CAG rats (Fig. 3).
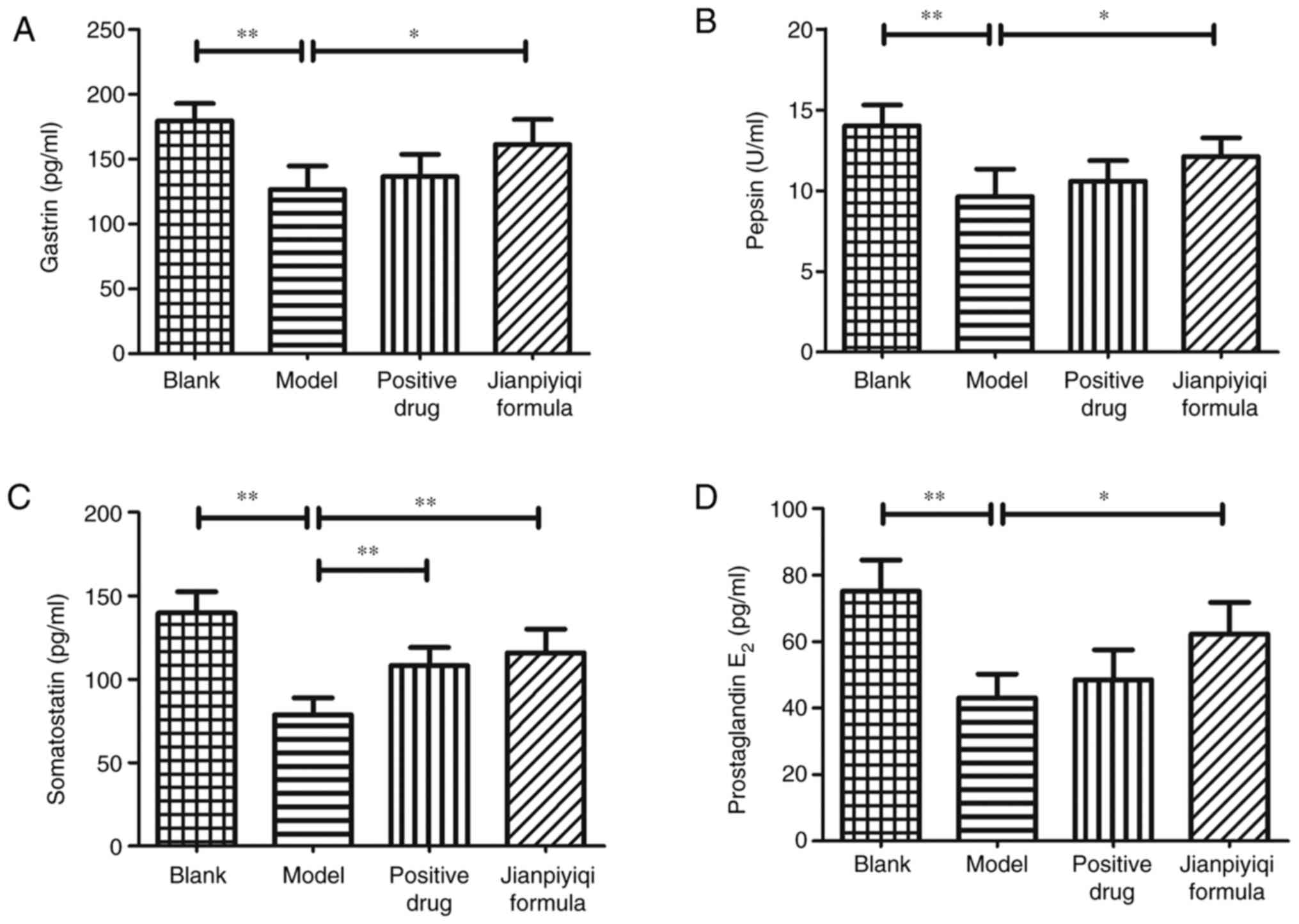 | Figure 3Effect of Jianpiyiqi formula on GAS,
PP, SS and PGE2 in serum of rats with CAG. At the end of
the animal experiment, the rat serum was collected and used to
detect the contents of (A) GAS, (B) PP, (C) SS and (D)
PGE2 according to the ELISA protocol.
*P<0.05 and **P<0.01 vs. model group.
CAG, chronic atrophic gastritis; GAS, gastrin; PP, pepsin; SS,
somatostatin; PGE2, prostaglandin E2. |
Effect of Jianpiyiqi formula on the
protein expression levels of Wnt1, β-catenin, GSK-3β and cyclin D1
in rats with CAG
The Wnt/β-catenin signaling pathway was previously
reported to be closely associated with the efficacy of Jianpiyiqi
formula in rats with CAG (13). The
in situ protein expression levels of Wnt1, β-catenin, GSK-3β
and cyclin D1 in gastric tissues were detected via IHC. The results
indicated that compared with the blank group, the protein
expression levels of Wnt1, β-catenin and cyclin D1 were increased
and the protein expression of GSK-3β was decreased in the model
group. Furthermore, compared with that in the model group, the
protein expression of GSK-3β was significantly increased and the
protein expression levels of Wnt1 and β-catenin were significantly
decreased in the positive control drug group. It was also observed
that Jianpiyiqi formula significantly decreased the protein
expression levels of Wnt1, β-catenin and cyclin D1 and increased
the expression of GSK-3β in rats with CAG (Fig. 4).
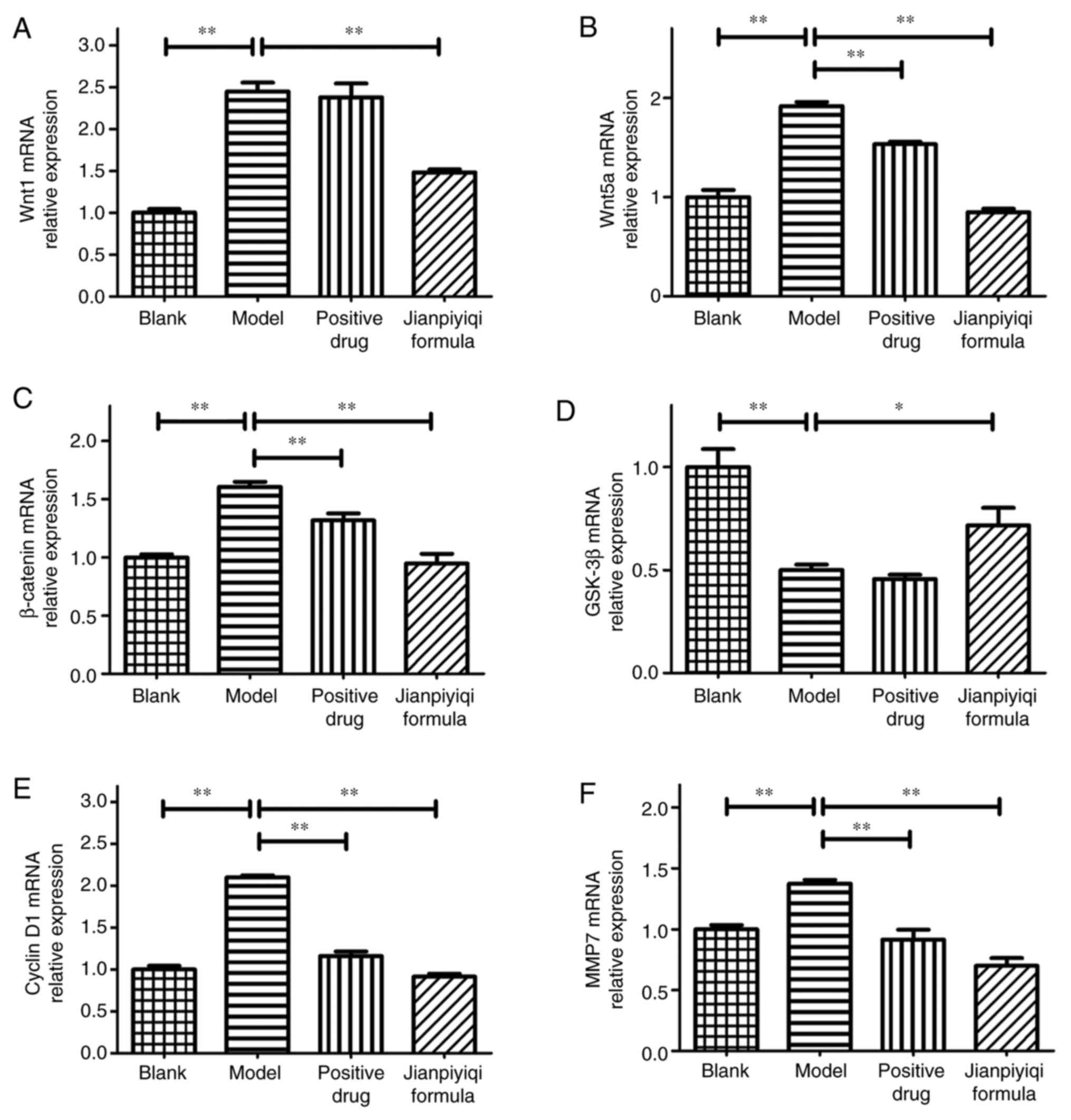
Effect of Jianpiyiqi formula on gene
expression levels of Wnt1, Wnt5a, β-catenin, GSK-3β, cyclin D1 and
MMP7 in rats with CAG
The relative mRNA expression levels of Wnt1, Wnt5a,
β-catenin, GSK-3β, cyclin D1 and MMP7 in rat gastric tissues were
detected via RT-qPCR. The results demonstrated that the gene
expression levels of Wnt1, Wnt5a, β-catenin, cyclin D1 and MMP7
were significantly increased and the gene expression levels of
GSK-3β were significantly decreased in the CAG model group as
compared with those in the blank group. In the positive control
drug group, no evident change in the mRNA expression of GSK-3β was
observed, while the mRNA expression levels of Wnt5a, β-catenin,
MMP7 and cyclin D1 were decreased compared with those in the model
group. Furthermore, Jianpiyiqi formula significantly decreased the
gene expression of Wnt1, Wnt5a, β-catenin, cyclin D1 and MMP7 and
significantly increased the gene expression of GSK-3β compared with
that in the model group (Fig. 5).
Therefore, based on the results of the IHC and RT-qPCR experiments,
it was suggested that Jianpiyiqi formula significantly inhibited
the Wnt/β-catenin signaling pathway in rats with CAG.
Discussion
CAG is frequently accompanied by intestinal
metaplasia and intraepithelial neoplasia, which are considered
precancerous lesions of GC (PLGC) (19). Therefore, it is urgent to identify
novel and effective treatments for CAG. In recent years,
significant progress has been made in the treatment of patients
with CAG using TCM (20,21). Previous in vivo studies have
indicated that TCM formulations are able to improve gastric mucosal
atrophy, including Modified Sijunzi Decoction (22), Weiqi Decoction (23), Banxia Xiexin Decoction (24) and Huangqi Jianzhong Tang (25). Jianpiyiqi formula is a TCM
prescription that may be used to effectively treat CAG in the
clinic (7,8). However, the mechanisms of action of
Jianpiyiqi formula in the treatment of CAG have remained to be
elucidated. The mixture of pharmacologically active components in
the water extract of the formula is complex. To the best of our
knowledge, no previous study reported on the analysis of Jianpiyiqi
formula by HPLC. Thus, the present study first constructed the HPLC
chromatogram of Jianpiyiqi formula for drug quality control and
subsequent investigation of its pharmacological mechanisms.
Liquiritin and hesperidin were identified and major components of
the formula. Although previous studies suggested that liquiritin
and hesperidin have anti-inflammatory and tumor preventive
properties (26,27), the two compounds do not represent
all components of the formula. The effects of liquiritin and
hesperidin on CAG need to be verified by further experiments in the
future.
It has been reported that the Wnt signaling pathway
serves an important role in the proliferation of gastric mucosal
epithelium (12). Furthermore, the
proliferation of gastric mucosal epithelial cells in patients with
CAG is inhibited or delayed (1).
Numerous studies have indicated that the Wnt/β-catenin signaling
pathway is related to GC (11,14).
CAG is a gastric precancerous disease, which is different from GC.
However, limited studies have investigated the relationship between
CAG and the Wnt/β-catenin signaling pathway (13,15).
Therefore, it is worthwhile investigating whether the mechanisms of
action of Jianpiyiqi formula in the treatment of CAG involve the
Wnt/β-catenin signaling pathway.
In the present study, the MNNG-induced CAG rat model
was established (28) and was used
to evaluate the effect of Jianpiyiqi formula on CAG. It was
indicated that the formula significantly improved gastric atrophy
and reduced stomach inflammation. Furthermore, GAS, PP, SS and
PGE2 are closely associated with the development of CAG
(1,4,29-31).
GAS was able to promote the proliferation of gastric mucosal cells
and increase gastric acid and pepsinogen secretion. Furthermore, PP
promoted food digestion, while SS had a strong inhibitory effect on
gastric acid secretion. It has been reported that PGE2
is able to regulate the relaxation and contraction of blood vessels
and exert a protective effect on gastric mucosal cells (29-31).
The present study demonstrated that Jianpiyiqi formula
significantly increased the contents of GAS, PP, SS and
PGE2 in rats with CAG.
The canonical Wnt/β-catenin signaling pathway serves
an important role in regulating gastric cell proliferation
(32,33). Wnt ligands specifically bind to the
Frizzled receptors and then activate the disheveled family
proteins. The activation of disheveled proteins leads to the
inactivation of GSK-3β. As a result of the accumulation and
migration of β-catenin from the cytoplasm to the nucleus, β-catenin
is able to upregulate the expression of related genes (12). Furthermore, Wnt signal transduction
may be closely associated with the pathogenesis of CAG. The present
study reported that the Wnt/β-catenin signaling pathway was
upregulated in the CAG rat model. It was also identified that
Jianpiyiqi formula inhibited the activation of the Wnt/β-catenin
signaling pathway in CAG. Due to the consistency of rat modelling,
the error of rat gene expression in the group was small. There is
currently no gold standard for the treatment of CAG in the clinic.
According to the specific symptoms of patients with CAG,
corresponding treatment drugs are given. Folate, as a vitamin, is
able to improve gastric mucosal damage and teprenone is a gastric
mucosal protective agent (34,35).
In the present study, the positive control drugs selected for the
rat model of CAG were folate and teprenone, as these have a certain
effect to improve gastric atrophy according to previous
experimental results (23,25). Furthermore, the positive control
drugs inhibited the expression levels of Wnt signal-related genes.
Collectively, it was indicated that the therapeutic effect of
Jianpiyiqi formula on CAG was more potent compared with that of the
positive control drugs.
In conclusion, the present study demonstrated that
Jianpiyiqi formula significantly ameliorated gastric gland atrophy
and gastric mucosal inflammation in rats with CAG. Furthermore,
Jianpiyiqi formula was demonstrated to inhibit the Wnt/β-catenin
signaling pathway; the experimental evidence and proposed pathway
are summarized in Fig. 6. According
to the present results, the Wnt signaling pathway may serve an
important role in the transition process from CAG to PLGC. However,
these results should be further confirmed by additional experiments
in the future. Overall, it was suggested that due to its promising
efficacy, Jianpiyiqi formula may be more widely used in the
clinical treatment of CAG.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the National Natural
Science Foundation of China (grant nos. 81804071 and 81573966).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
The animal experiments were performed by ZY and TX.
HPLC analysis was conducted by ZA. Immunohistochemistry and RT-qPCR
were performed by WC and YX. ZY and FZ were the major contributors
to the design of the study and writing of the manuscript. All
authors confirm the authenticity of all the raw data and all
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiment of the present study was
approved by the Ethics Committee of Jiangsu Province Integrated
Chinese and Western Medicine Hospital (Nanjing, China; approval no.
AEWC-20160810-12).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li Y, Xia R, Zhang B and Li C: Chronic
atrophic gastritis: A review. J Environ Pathol Toxicol Oncol.
37:241–259. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rodriguez-Castro KI, Franceschi M, Noto A,
Miraglia C, Nouvenne A, Leandro G, Meschi T, De' Angelis GL and Di
Mario F: Clinical manifestations of chronic atrophic gastritis.
Acta Biomed. 89:88–92. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sung J, Kim N, Lee J, Hwang YJ, Kim HW,
Chung JW, Kim JW and Lee DH: Associations among Gastric Juice pH,
atrophic gastritis, intestinal metaplasia and helicobacter pylori
infection. Gut Liver. 12:158–164. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Lahner E, Zagari RM, Zullo A, Di Sabatino
A, Meggio A, Cesaro P, Lenti MV, Annibale B and Corazza GR: Chronic
atrophic gastritis: Natural history, diagnosis and therapeutic
management. A position paper by the Italian Society of Hospital
Gastroenterologists and Digestive Endoscopists [AIGO], the Italian
Society of Digestive Endoscopy [SIED], the Italian Society of
Gastroenterology [SIGE], and the Italian Society of Internal
Medicine [SIMI]. Dig Liver Dis. 51:1621–1632. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Spence AD, Cardwell CR, McMenamin UC,
Hicks BM, Johnston BT, Murray LJ and Coleman HG: Adenocarcinoma
risk in gastric atrophy and intestinal metaplasia: A systematic
review. BMC Gastroenterol. 17(157)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Annibale B, Esposito G and Lahner E: A
current clinical overview of atrophic gastritis. Expert Rev
Gastroenterol Hepatol. 14:93–102. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Su KL, Zhu FS, Shi T, Xu TT, Zhang X and
Wang C: Clinical observation on treatment of 62 cases of chronic
atrophic gastritis with spleen stomach deficiency syndrome by
Weiwei No.1 granule. Pharmacology and Clinics of Chinese Materia
Medica. 29:154–156. 2013.(In Chinese).
|
|
8
|
Zhu FS: Theoretical study on the treatment
of chronic atrophic gastritis with Jianpi Yiqi Formula. Chinese
Journal of Basic Medicine in Traditional Chinese Medicine.
23:471–472+487. 2017.(In Chinese).
|
|
9
|
McCracken KW, Aihara E, Martin B, Crawford
CM, Broda T, Treguier J, Zhang X, Shannon JM, Montrose MH and Wells
JM: Wnt/beta-catenin promotes gastric fundus specification in mice
and humans. Nature. 541:182–187. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Leushacke M, Tan SH, Wong A, Swathi Y,
Hajamohideen A, Tan LT, Goh J, Wong E, Denil SLIJ, Murakami K and
Barker N: Lgr5-expressing chief cells drive epithelial regeneration
and cancer in the oxyntic stomach. Nat Cell Biol. 19:774–786.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Tan SH and Barker N: Wnt signaling in
adult epithelial stem cells and cancer. Prog Mol Biol Transl Sci.
153:21–79. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Flanagan DJ, Austin CR, Vincan E and
Phesse TJ: Wnt signalling in gastrointestinal epithelial stem
cells. Genes (Basel). 9(178)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yu XW, Xu Q, Xu Y, Gong YH and Yuan Y:
Expression of the E-cadherin/β-catenin/tcf-4 pathway in gastric
diseases with relation to Helicobacter pylori infection: clinical
and pathological implications. Asian Pac J Cancer Prev. 15:215–220.
2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chiurillo MA: Role of the Wnt/β-catenin
pathway in gastric cancer: An in-depth literature review. World J
Exp Med. 5:84–102. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chu A, Yu X, Guo Q, Li Q, Sun M, Yuan Y
and Gong Y: H. pylori slyD, a novel virulence factor, is associated
with Wnt pathway protein expression during gastric disease
progression. Microb Pathog. 148(104428)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dixon MF, Genta RM, Yardley JH and Correa
P: Classification and grading of gastritis. The updated sydney
system. International workshop on the histopathology of Gastritis,
Houston 1994. Am J Surg Pathol. 20:1161–1181. 1996.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Crafa P, Russo M, Miraglia C, Barchi A,
Moccia F, Nouvenne A, Leandro G, Meschi T, De' Angelis GL and Di
Mario F: From Sidney to OLGA: An overview of atrophic gastritis.
Acta Biomed. 89:93–99. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Berger H, Marques MS, Zietlow R, Meyer TF,
Machado JC and Figueiredo C: Gastric cancer pathogenesis.
Helicobacter. 21 (Suppl 1):S34–S38. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fang WJ, Zhang XY, Yang B, Sui SJ, Chen M,
Pan WH, Liao WQ, Zhong M and Wang QC: Chinese herbal decoction as a
complementary therapy for atrophic gastritis: A systematic review
and meta-analysis. Afr J Tradit Complement Altern Med. 14:297–319.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dai YK, Zhang YZ, Li DY, Ye JT, Zeng LF,
Wang Q and Hu L: The efficacy of Jianpi Yiqi therapy for chronic
atrophic gastritis: A systematic review and meta-analysis. PLoS
One. 12(e0181906)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tian G, Wu C, Li J, Liang B, Zhang F, Fan
X, Li Z, Wang Y, Li Z, Liu D, et al: Network pharmacology based
investigation into the effect and mechanism of Modified Sijunzi
Decoction against the subtypes of chronic atrophic gastritis.
Pharmacol Res. 144:158–166. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yin J, Yi J, Yang C, Xu B, Lin J, Hu H, Wu
X, Shi H and Fei X: Weiqi decoction attenuated chronic atrophic
gastritis with precancerous lesion through regulating
microcirculation disturbance and HIF-1α signaling pathway. Evid
Based Complement Alternat Med. 2019(2651037)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ji Q, Yang Y, Song X, Han X and Wang W:
Banxia Xiexin Decoction in the treatment of chronic atrophic
gastritis: A protocol for systematic review and meta-analysis.
Medicine (Baltimore). 99(e22110)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu Y, Jin Z, Qin X and Zheng Q: Urinary
metabolomics research for Huangqi Jianzhong Tang against chronic
atrophic gastritis rats based on 1H NMR and UPLC-Q/TOF
MS. J Pharm Pharmacol. 72:748–760. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wei F, Jiang X, Gao HY and Gao SH:
Liquiritin induces apoptosis and autophagy in cisplatin
(DDP)-resistant gastric cancer cells in vitro and xenograft
nude mice in vivo. Int J Oncol. 51:1383–1394.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ahmadi A, Shadboorestan A, Nabavi SF,
Setzer WN and Nabavi SM: The role of hesperidin in cell signal
transduction pathway for the prevention or treatment of cancer.
Curr Med Chem. 22:3462–3471. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Miwa H, Endo K, Wada R, Hirai S, Hirose M,
Misawa H, Nagahara A, Ohta K, Watanabe S and Sato N: Cellular
proliferation and differentiation in rat atrophic gastric mucosa
induced by N'-methyl-N'-nitro-N-nitrosoguanidine. J Clin
Gastroenterol. 25 (Suppl 1):S116–S121. 1997.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Smith JP, Nadella S and Osborne N: Gastrin
and gastric cancer. Cell Mol Gastroenterol Hepatol. 4:75–83.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Schubert ML: Functional anatomy and
physiology of gastric secretion. Curr Opin Gastroenterol.
31:479–485. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Takeuchi K and Amagase K: Roles of
cyclooxygenase, prostaglandin E2 and EP receptors in mucosal
protection and Ulcer healing in the gastrointestinal tract. Curr
Pharm Des. 24:2002–2011. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Steinhart Z and Angers S: Wnt signaling in
development and tissue homeostasis. Development.
145(dev146589)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhao J, Fan Y, Ye W, Feng W, Hu Y, Cai L
and Lu B: The protective effect of teprenone on aspirin-related
gastric mucosal injuries. Gastroenterol Res Pract.
2019(6532876)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ajeigbe K, Oladejo E, Emikpe B, Asuk A and
Olaleye S: The dual modulatory effect of folic acid supplementation
on indomethacin-induced gastropathy in the rat. Turk J
Gastroenterol. 23:639–645. 2012.PubMed/NCBI View Article : Google Scholar
|