Introduction
Sjogren's syndrome (SS) is a common systemic
autoimmune disease of exocrine glands, particularly the salivary
glands (1). Dry mouth caused by
salivary gland dysfunction is one of the typical features of SS
(2). The pathogenesis and etiology
of SS remain unclear, since complex elements, including genes and
the environment, have been reported to contribute to the
development of this disease (3,4). The
inflammatory process of salivary glands is a common feature in
patients with SS (5), where
inflammation is associated with the persistence of interferon (IFN)
signaling (6).
MicroRNAs (miRNAs/miRs) bind to target transcripts
to suppress translation (7).
Previous studies have demonstrated that miRNAs can regulate several
biological processes, such as the innate immune response (8,9).
miR-155-5p has multiple functions, including the regulation of
tumor development (10), immune
regulation (11) and oxidative
stress (12). Furthermore, due to
its notable regulatory effect on the immune system, miR-155-5p is
closely associated with a variety of immune-related diseases
(13,14), including during rheumatoid arthritis
(15), systemic lupus erythematosus
(16) and SS (2,17,18).
It has been previously reported that miR-155-5p expression is
markedly elevated in the peripheral mononuclear cells of patients
with primary SS (19). However, the
function of miR-155-5p in SS remains unclear, where its potential
effect on salivary glands damaged by SS has not been reported
previously.
Arrestin β2 (ARRB2) is a scaffolding protein of the
arrestin family, which exerts multiple functions, including
promoting angiogenesis, alleviating neuropathic pain and modulating
the sensitivity of cancer cells to chemotherapy drugs (20-22).
Previous studies have demonstrated that ARRB2 also exhibits
anti-inflammatory effects in some inflammatory diseases, such as
colitis and sepsis (23-25).
In addition, ARRB2 has been reported to inhibit NF-κB signaling in
septic and lipopolysaccharide-treated mice (23,26).
Previous studies have demonstrated that NF-κB serve a promoting
role in SS and its complications (27,28).
Therefore, based on these previous findings aforementioned, the
present study hypothesized that miR-155-5p may participate in
SS-induced salivary gland damage by targeting ARRB2.
Materials and methods
Isolation, transfection and treatment
of salivary gland epithelial cells (SGECs)
The present study was approved by the Ethics
Committee of Hongqi Hospital Affiliated to Mudanjiang Medical
University (Mudanjiang, China) and performed in accordance with the
Guidelines for the Care and Use of Laboratory Animals (29).
BALB/c mice, aged 7-8 weeks, weighted 20±2 g (n=30;
15 male and female) were purchased from Beijing Huafukang
Biotechnology Co., Ltd. (http://www.hfkbio.com/). The housing conditions of the
mice were: 12-h light/dark cycle, 25±1˚C, and 45-55% humidity. All
the mice were free access to food and water. Mice were euthanized
by an intraperitoneal injection of sodium pentobarbital (200
mg/kg). In total, five mice were randomly selected from the 30 mice
and the salivary glands were collected from the parotid,
submandibular and sublingual glands. In accordance with previous
research, SGECs were extracted from the salivary glands of five
mice and pooled (30). Briefly, the
salivary glands were washed 2-3 times with PBS. Tissue samples were
minced into fragments (1-2 mm3) and cultured in a petri
dish. Following incubation for 2 h at 37˚C, DMEM/F12 complete
medium (Procell Life Science & Technology Co., Ltd.) was added
to the petri dish and the fragments were further incubated for 72 h
at 37˚C in 5% CO2 and saturated humidity. The culture
medium was replaced every 3 days until the cell density reached
~80%. The isolated SGECs were cultured in DMEM/nutrient mixture
F-12 medium (Procell Life Science & Technology Co., Ltd.)
supplemented with streptomycin (100 µg/ml), epidermal growth factor
(10 ng/ml; Sino Biological), insulin (0.5 mg/ml; Shenyang Bying
Biotechnology Co., Ltd.), hydrocortisone (0.4 mg/ml; Shanghai
Aladdin Biochemical Technology Co., Ltd.) and FBS (3%; Biological
Industries), at 37˚C in 5% CO2. SGECs were identified
via immunocytochemistry staining of cytokeratin (CK) 7, CK8 and
CK19 (31,32).
Transfection
Negative control (NC)/miR-155-5p agomir (25 nmol/l;
Shanghai GenePharma Co., Ltd.) or NC/miR-155-5p antagomir (25
nmol/l; Shanghai GenePharma Co., Ltd.) were transfected into SGECs
for 24 h at 37˚C using Lipofectamine® 3000 reagent (5
µl; Invitrogen; Thermo Fisher Scientific, Inc.). SGECs were treated
with IFN-γ (10 ng/ml, Sino Biological https://www.sinobiological.com/) for 12 h at 37˚C to
induce inflammation as previously described (30). For drug inhibition, SGECs were
transfected with miR-155-5p agomir/agomir NC for 24 h at 37˚C,
followed by treatment with IFN-γ (10 ng/ml) and pyrrolidine
dithiocarbamate (PDTC, 100 uM; Shanghai Aladdin Biochemical
Technology Co., Ltd.) for 12 h. For rescue experiments, ARRB2
overexpression vector (pcDNA3.1; 121.3 ng/µl, Genscript) or
pcDNA3.1 vector (154.6 ng/µl, Genscript) and miR-155-5p agomir were
co-transfected into the SGECs. These concentrations of ARRB2
overexpression vector and its NC were used for co-transfection.
Following transfection for 24 h at 37˚C, the cells were treated
with IFN-γ (10 ng/ml) for 12 h. The sequences of the miR-155-5p
agomir/NC agomir and miR-155-5p antagomir/NC antagomir were:
miR-155-5p agomir sense, 5'-UUAAUGCUAAUUGUGAUAGGGGU-3' and
antisense, 5'-CCCUAUCACAAUUAGCAUUAAUU-3'; NC agomir sense,
5'-UUCUCCGAACGUGUCACGUTT-3' and antisense,
5'-ACGUGACACGUUCGGAGAATT-3'; miR-155-5p antagomir,
5'-ACCCCUAUCACAAUUAGCAUUAA-3' and NC antagomir,
5'-CAGUACUUUUGUGUAGUACAA-3'.
Immunocytochemistry
SGECs (3x104 cells each well) in 12-well
plate were fixed in 4% paraformaldehyde for 15 min at room
temperature and subsequently incubated with 3%
H2O2 for 15 min at room temperature to
inhibit endogenous peroxidase activity. No antigen retrieval was
performed before this. Cells were blocked in 100% normal goat serum
(Beijing Solarbio Science & Technology Co., Ltd.) for 15 min at
room temperature and incubated with primary antibodies against CK7
(1:200, cat. no. A4765; ABclonal Biotech Co., Ltd.), CK8 (1:200,
cat. no. 17514-1-AP; ProteinTech Group, Inc.) and CK19 (1:200, cat.
no. A19040; ABclonal Biotech Co., Ltd.) overnight at 4˚C. Following
the primary antibody incubation, cells were incubated with
horseradish peroxidase (HRP)-conjugated goat anti-rabbit lgG
(1:500, cat. no. 31460; Thermo Fisher Scientific, Inc) for 2 h at
37˚C (Thermo Fisher Scientific, Inc.). DAB and hematoxylin (both
purchased from Beijing Solarbio Science & Technology Co., Ltd.)
were used for color development (5 min at room temperature) and
counterstaining (5 min at room temperature), respectively. The
slides were observed under a light microscope (magnification, x400;
Olympus Corporation).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from IFN-γ-treated SGECs
using TRIPure reagent (cat. no. RP1001; BioTeke Corporation,) and
reverse transcribed into cDNA using M-MLV reverse transcriptase
(cat. no. PR6502; BioTeke Corporation,), dNTPs (Beijing Solarbio
Science & Technology Co., Ltd.), and primers (Genscript, random
hexamers and poly-A were used). The temperature protocol was used
for reverse transcription for miR-155-5p: 37˚C for 30 min, 42˚C for
30 min and 70˚C for 10 min. For interleukin-6 (IL-6), tumor
necrosis factor-α (TNF-α), ARRB2 and β-actin, the temperature
protocol was: 25˚C for 10 min, 42˚C for 50 min and 80˚C for 10 min.
The qPCR was performed using SYBR® Green I nucleic acid
gel stain (cat. no. S9430; Sigma-Aldrich; Merck KGaA) and 2X Power
Taq PCR Master Mix (cat. no. PR1702, BioTeke Corporation). The
temperature protocol was used for qPCR for miR-155-5p was the
following: Initial denaturation at 94˚C for 4 min, followed by 40
cycles of 94˚C for 15 sec, 60˚C for 20 sec and 72˚C for 15 sec. For
IL-6, TNF-α, ARRB2 and β-actin, the thermocycling conditions were
the following: Initial denaturation at 94˚C for 5 min, followed by
40 cycles of 94˚C for 15 sec, 60˚C for 25 sec and 72˚C for 30 sec.
The following primer sequences were used for qPCR: miR-155-5p
forward, 5'-TTAATGCTAATTGTGATAGGGGT-3' and reverse,
5'-GCAGGGTCCGAGGTATTC-3'; 5S rRNA forward,
5'-CTAAAGATTTCCGTGGAGAG-3' and reverse, 5'-TGGTGCAGGGTCCGA
GGTAT-3'; IL-6 forward, 5'-ATGGCAATTCTGATT GTATG-3' and reverse,
5'-GACTCTGGCTTTGTC TTTCT-3'; TNF-α forward, 5'-CAGGCGGTGCCTATG
TCTCA-3' and reverse, 5'-GCTCCTCCACTTGGTGGTTT-3'; ARRB2 forward,
5'-CCATTGTGAAGGAGGGAG-3' and reverse, 5'-GCATTAGGACGAAGGGTAG-3' and
β-actin forward, 5'-CTGTGCCCATCTACGAGGGCTAT-3' and reverse,
5'-TTTGATGTCACGCACGATTTCC-3'. Relative expression levels were
calculated using the 2-ΔΔCq method (33) and normalized to the internal
reference gene β-actin. 5S rRNA served as the internal control for
miRNA expression.
Cell Counting Kit-8 (CCK-8) assay
CCK-8 assay was performed to assess cell viability.
SGECs were seeded into 96-well plates (3x103 cells each
well). Following transfection for 24 h at 37˚C, cells were
incubated with CCK-8 solution for 1 h following the manufacturer's
protocols at 37˚C (10 µl each well; Sigma-Aldrich, Merck KGaA)
before viability was subsequently analyzed at a wavelength of 450
nm, using a microplate reader.
Apoptosis analysis
Early and late apoptotic cells were assessed using
flow cytometry. Cells were seeded into six-well plates at a density
of 1x105 cells/well. Following transfection and
treatment, apoptotic cells were analyzed. Briefly, cells in each
group were collected and resuspended with 195 µl Annexin V-FITC
(Beyotime Institute of Biotechnology). Cells (1x105)
were subsequently treated with 5 µl Annexin V-FITC and 10 µl
propidium iodide (both purchased from Beyotime Institute of
Biotechnology) for 15 min at room temperature. Apoptotic cells were
subsequently detected by flow cytometer (NovoCyte; ACEA Bioscience,
Inc.) and analyzed by NovoExpress (version 1.2.5; ACEA Biosciences,
Inc.).
Immunofluorescence staining
Cell slides were fixed with 4% paraformaldehyde for
15 min at room temperature and then incubated with 0.1% Triton
X-100 for 30 min at room temperature. After blocking with 100%
normal goat serum (Beijing Solarbio Science & Technology Co.,
Ltd.) for 15 min at room temperature, the cell slides were
incubated with the anti-p65 antibody (1:200; total
non-phosphorylated version; cat. no. 10745-1-AP; ProteinTech Group,
Inc.) overnight at 4˚C. After the primary antibody incubation,
cells were treated with Cy3-labeled goat anti-rabbit IgG (1:200,
cat. no. A0516, Beyotime Institute of Biotechnology) for 60 min at
room temperature. After staining the nucleus with DAPI (cat. no.
C1002; Beyotime Institute of Biotechnology) at room temperature,
slides were analyzed with a fluorescence microscope (magnification,
x400; Olympus Corporation).
Western blotting
Total protein was extracted using RIPA lysis buffer
(Beyotime Institute of Biotechnology). Cytoplasmic protein and
nuclear protein were extracted using a nuclear protein extraction
kit (cat. no. P0027; Beyotime Institute of Biotechnology).
Bicinchoninic acid protein assay kit was used to measure the
protein concentration. Equal amounts of protein (20-40 µg) were
separated via SDS-PAGE (10 and 12% gel). The separated proteins
were subsequently transferred onto PVDF membranes (EMD Millipore)
and blocked with 5% non-fat milk for 1 h at room temperature. The
membranes were incubated with primary antibodies against Bcl-2
(1:1,000; cat. no. A19693; ABclonal Biotech Co., Ltd.), Bax
(1:1,000; cat. no. A19684; ABclonal Biotech Co., Ltd), Inhibitor of
NF-κB (I-κB; 1:1,000; cat. no. A11397; ABclonal Biotech Co., Ltd.),
phosphorylated (p-)-I-κB (1:1,000; cat. no. AP0707; ABclonal
Biotech Co., Ltd.), NF-κB p65 (1:1,000; cat. no. AF5006; Affinity
Biosciences), ARRB2 (1:1,000; cat. no. A1171; ABclonal Biotech Co.,
Ltd.), Histone H3 (1:2,000; cat. no. AM8433; Abgent Inc.) and
β-actin (1:1,000; cat. no. sc-47778; Santa Cruz Technology, Inc.)
overnight at 4˚C. Following primary antibody incubation, membranes
were incubated with HRP-conjugated anti-rabbit/mouse lgG (1:5,000;
cat. nos. A0208 and A0216; Beyotime Institute of Biotechnology) at
room temperature for 30 min. Proteins bands were visualized using
an enhanced chemiluminescence reagent solution (Beyotime Institute
of Biotechnology) and analyzed using a Gel-Pro-Analyzer (version
4.0; Beijing Liuyi Biotechnology, Inc.).
Caspase-3 and -9 activities
After the cells were harvested and lysed, caspase-3
detection kit (cat. no. C1116; Beyotime Institute of Biotechnology)
was used to detect caspase-3 activity in the lysates, whilst
caspase-9 activity was measured using a caspase-9 detection kit
(cat. no. BC3890; Beijing Solarbio Science & Technology Co.,
Ltd.). Caspase-3 and -9 activities were subsequently analyzed at a
wavelength of 405 nm detected using a microplate reader (BioTek
Instruments, Inc.).
ELISA
The ELISA kits (Wuhan Boster Biological Technology,
Ltd.), were used to detect the expression levels of IL-6 (cat. no.
EK0411) and TNF-α (cat. no. EK0527) in cell culture supernatant,
according to the manufacturer's protocols. Tetramethylbenzidine
(TMB) substrate solution (Wuhan Boster Biological Technology, Ltd.)
was used to incubate the samples for 20 min at 37˚C in the dark.
TMB stop solution (Wuhan Boster Biological Technology, Ltd.) was
then used to suspend the color reaction. A microplate reader
(BioTek Instruments, Inc.) was used to obtain the optical density
value at a wavelength of 450 nm. The lower detection limit of the
ELISA kits was 15.6 pg/ml.
Dual-luciferase reporter assay
The binding sites between miR-155-5p and ARRB2 were
predicted using TargetScan 7.2 (http://www.targetscan.org/vert_72). Briefly, the
species was ‘Human’, following which the microRNA name ‘miR-155-5p’
was typed in and the ‘submit’ button was clicked. A number of genes
potentially targeted by miR-155-5p can then be obtained. After
searching for ARRB2, the ‘Sites in UTR’ button was clicked to
obtain the targeted binding sequence between miR-155-5p and ARRB2.
The association between miR-155-5p and ARRB2 was detected using
dual-luciferase reporter assay. The mirGLO-ARRB2-3'-UTR-WT and
pmirGLO-ARRB2-3'-UTR-MT plasmids were synthesized by GenScript. The
plasmid (0.5 µg) and miR-155-5p mimic or its NC (25 pmol) were
co-transfected into 293T cells at ~70%. The co-transfection was
mediated by Lipofectamine® 3000 reagent (9 µl;
Invitrogen; Thermo Fisher Scientific, Inc.). Following incubation
for 48 h at 37˚C, luciferase detection kit (cat. no. E1910; Promega
Corporation) was used to detect luciferase activity. The changes in
luciferase activities were measured using a microplate reader
(Tecan Group Ltd.). Firefly luciferase activity was normalized to
that of Renilla luciferase activity.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 7 software (GraphPad Software, Inc.). Data are presented as
the mean ± standard deviation. Student's unpaired t-test was used
to compare differences between two groups, whilst one-way ANOVA
with Tukey's post hoc test was used to compare differences between
multiple groups. P<0.05 was considered to indicate a
statistically significant difference. All experiments were
performed at least in triplicate.
Results
miR-155-5p expression in IFN-γ-treated
SGECs
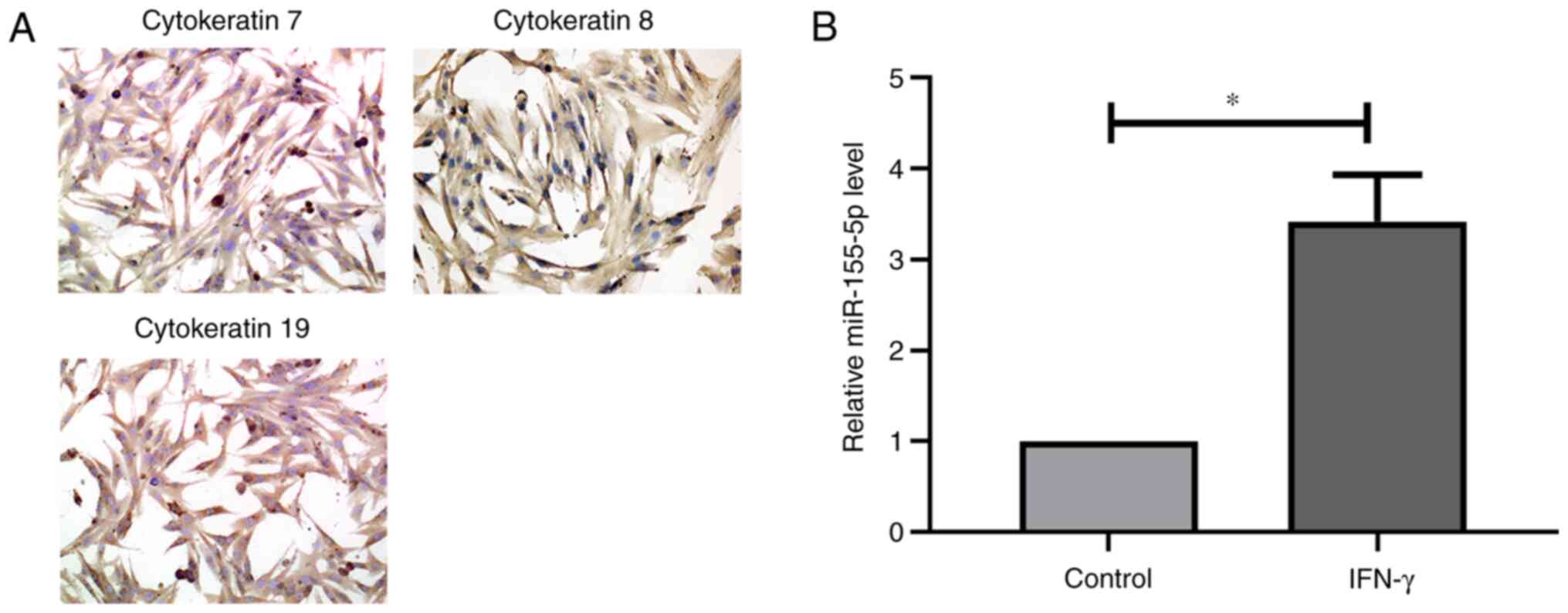
SGECs were phenotyped by immunocytochemistry
staining (Fig. 1A). The results
demonstrated that the isolated cells exhibited strong cytokeratin
expression, including that of epithelial markers CK7, CK8 and CK19,
suggesting that the isolated cells were SGECs. RT-qPCR analysis
demonstrated that miR-155-5p expression was significantly increased
in SGECs following treatment with IFN-γ (Fig. 1B). Taken together, these results
suggest that IFN-γ treatment increases miR-155-5p expression.
miR-155-5p promotes IFN-γ-induced
apoptosis in SGECs
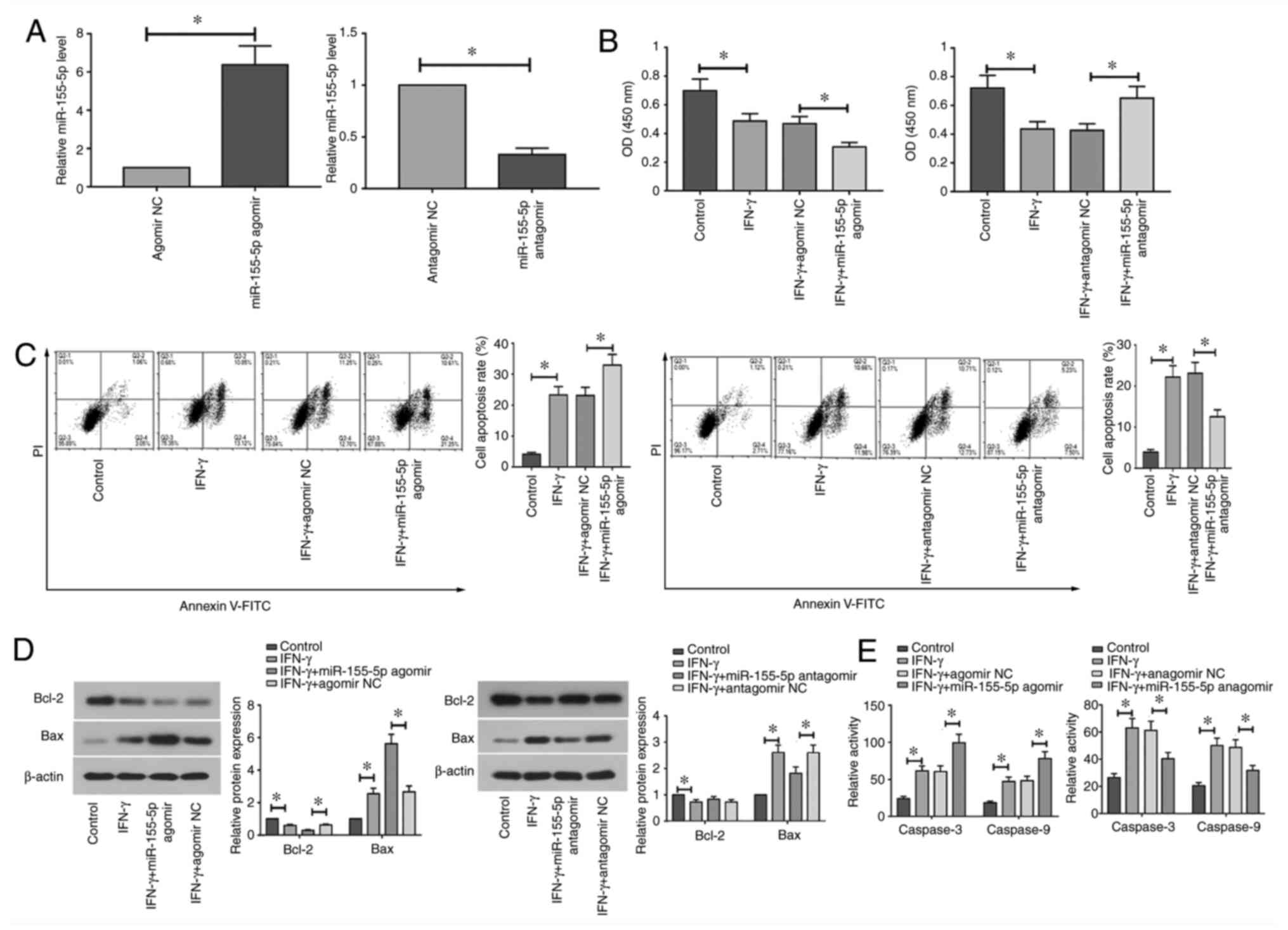
The effects of miR-155-5p knockdown and
overexpression on the apoptosis of IFN-γ-treated SGECs were next
assessed. RT-qPCR analysis demonstrated that miR-155-5p expression
was significantly upregulated following transfection with
miR-155-5p agomir, but was significantly downregulated following
transfection with miR-155-5p antagomir compared with that in their
corresponding NCs (Fig. 2A).
Transfected SGECs were subsequently treated with IFN-γ (10 ng/ml)
for 12 h. Treatment with IFN-γ significantly reduced cell viability
and promoted apoptosis in SGECs (Fig.
2B and C). Overexpression of
miR-155-5p significantly decreased cell viability and induced
apoptosis in IFN-γ-treated SGECs, whereas miR-155-5p knockdown
significantly increased cell viability and inhibited apoptosis
compared with those in their corresponding NCs (Fig. 2B and C). Furthermore, treatment with IFN-γ
significantly decreased Bcl-2 protein expression, but significantly
increased Bax protein expression and the enzyme activity of caspase
3 and 9 in SGECs (Fig. 2D and
E). Compared with those in their
corresponding NCs, overexpression of miR-155-5p significantly
potentiated the effect of IFN-γ on apoptotic protein expression and
caspase 3 and 9 enzyme activity, whilst opposite effects were
observed following the downregulation of miR-155-5p (Fig. 2D and E). Collectively, these results suggest
that the overexpression of miR-155-5p aggravates IFN-γ-induced
apoptosis, whereas miR-155-5p knockdown reversed IFN-γ-induced
apoptosis in SGECs.
miR-155-5p promotes IFN-γ-induced
inflammation in SGECs
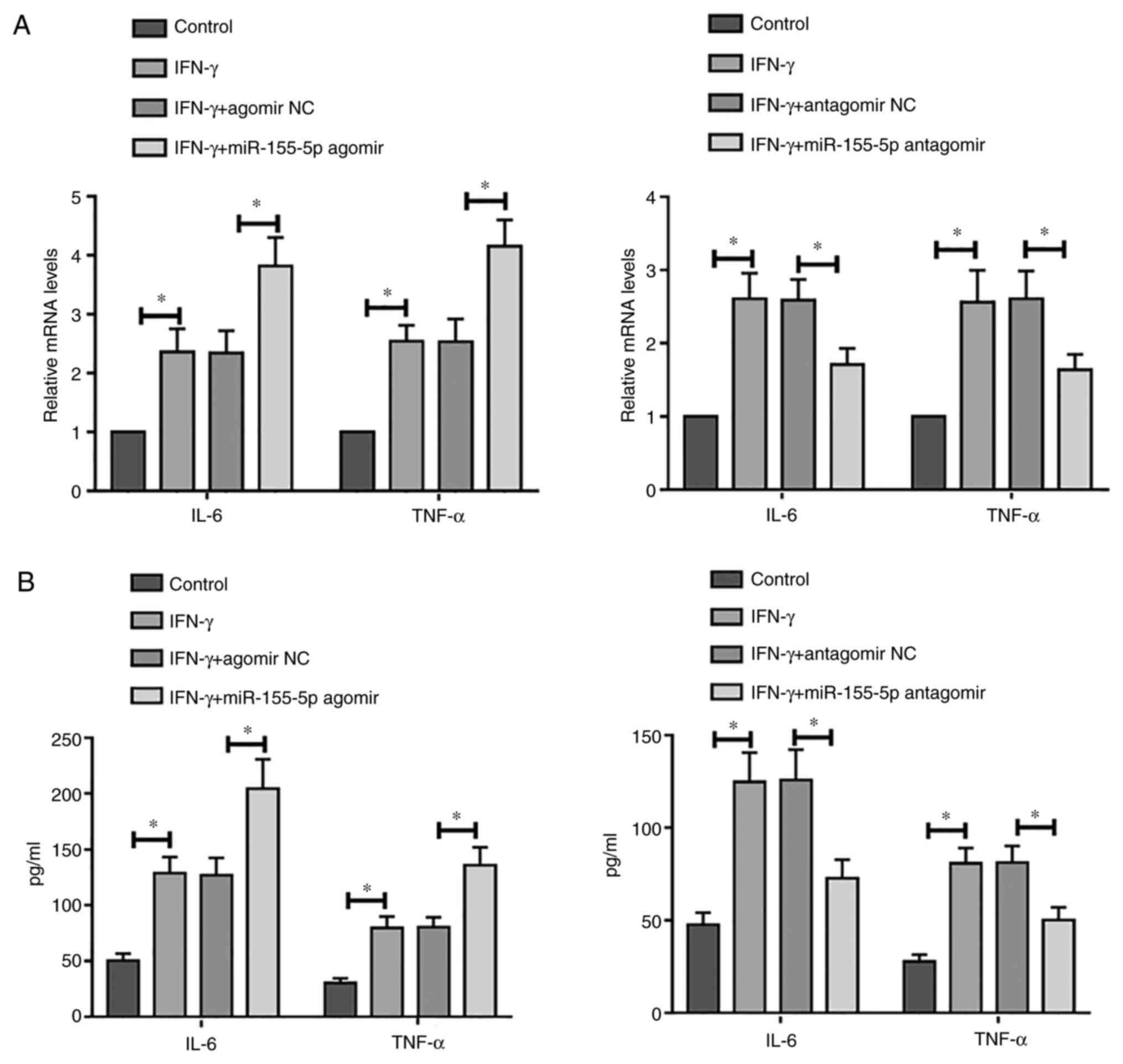
The association between miR-155-5p and inflammation
in IFN-γ-treated SGECs was assessed. Compared with those in
control, treatment with IFN-γ significantly increased the mRNA
expression levels of IL-6 and TNF-α in SGECs, which was
significantly enhanced further following overexpression of
miR-155-5p (Fig. 3A). By contrast,
this phenomenon was reversed by miR-155-5p knockdown (Fig. 3A). Similar results were obtained
according to results from ELISA (Fig.
3B). Taken together, these results suggest that overexpression
of miR-155-5p may promote IFN-γ-induced inflammation, whilst
miR-155-5p knockdown may alleviate IFN-γ-induced inflammation in
SGECs.
miR-155-5p activates the NF-κB
signaling pathway in IFN-γ-treated SGECs
The effects of miR-155-5p knockdown and
overexpression on the NF-κB signaling pathway in IFN-γ-treated
SGECs were next assessed. Western blot analysis demonstrated that
the p-I-κB/I-κB ratio and the nuclear/cytoplasmic ratio of p65 were
significantly increased following treatment with IFN-γ, which was
significantly potentiated following the transfection with
miR-155-5p agomir (Fig. 4A and
B). By contrast, they were
significantly reversed following transfection with miR-155-5p
antagomir (Fig. 4A and B). Immunofluorescence staining
demonstrated that the overexpression of miR-155-5p promoted the
nuclear translocation of NF-κB p65 (total non-phosphorylated
version) in IFN-γ-treated cells, whilst miR-155-5p knockdown
resulted in the opposite effect being observed (Fig. 4C). Following the transfection with
miR-155-5p agomir/agomir NC, SGECs were treated with IFN-γ (10
ng/ml) and PDTC (100 µM). The results demonstrated that the
blockade of NF-κB signaling by PDTC significantly decreased the
expression levels of IL-6 and TNF-α in miR-155-5p-overexpressed
SGECs (Fig. 4D). Collectively,
these results suggest that miR-155-5p overexpression aggravates
IFN-γ-induced NF-κB signaling in SGECs.
 | Figure 4miR-155-5p activates the NF-κB
signaling pathway in IFN-γ-treated SGECs. (A-C) SGECs were
transfected with miR-155-5p agomir/agomir NC or miR-155-5p
antagomir/antagomir NC for 24 h, before the transfected SGECs were
treated with IFN-γ at a concentration of 10 ng/ml for 12 h.
Expression of (A) p-I-κB, I-κB and (B) activation of NF-κB p65 was
measured by western blotting. (C) Immunofluorescence staining and
DIC images of p65 in each group were shown (magnification, x400).
(D) miR-155-5p agomir/agomir NC-transfected SGECs were treated with
IFN-γ (10 ng/ml) and PDTC (100 µM) for 12 h, before the levels of
IL-6 and TNF-α were detected by ELISA. All data were presented as
mean ± SD, n=3. *P<0.05. SGECs, salivary gland
epithelial cells; n-, nuclear; c-, cytoplasmic; NC, negative
control; IFN-γ, interferon-gamma; PDTC, pyrrolidine
dithiocarbamate; IL-6, interleukin-6; TNF-α, tumor necrosis
factor-α; p-, phosphorylated; I-κB, inhibitor of NF-κB; DIC,
differential interference contrast. |
ARRB2 is a downstream target gene of
miR-155-5p
The binding sites of miR-155-5p on ARRB2 were
predicted using TargetScan 7.2 (http://www.targetscan.org/vert_72), where miR-155-5p
was predicted to target ARRB2 directly (Fig. 5A). Dual-luciferase reporter assay
results showed that luciferase activity in miR-155-5p agomir + WT
3'UTR group was significantly decreased compared with that in the
miR-155-5p agomir + MT 3'UTR and Agomir NC + WT 3'UTR groups
(Fig. 5A). The association between
miR-155-5p expression and ARRB2 was subsequently assessed. The
results demonstrated that ARRB2 mRNA and protein expression levels
were significantly inhibited in IFN-γ-treated SGECs following
transfection with miR-155-5p agomir compared with those in
IFN-γ-treated cells transfected with NC agomir (Fig. 5B and C). Conversely, ARRB2 mRNA and protein
expression levels were significantly elevated in IFN-γ-treated
SGECs following transfection with miR-155-5p antagomir compared
with those in IFN-γ-treated cells transfected with NC antagomir
(Fig. 5B and C). Subsequently, the ARRB2 overexpression
plasmid and miR-155-5p agomir were co-transfected into SGECs before
IFN-γ (10 ng/ml) was used to treat the transfected cells for 12 h.
The transfection efficiency of the ARRB2 plasmid into SGECs was
first verified by western blotting (Fig. 5D). In the presence of both IFN-γ and
miR-155-5p mimics, overexpression of ARRB2 significantly reduced
the expression levels of IL-6 and TNF-α (Fig. 5E). In addition, the overexpression
of ARRB2 significantly suppressed the miR-155-5p
overexpression-induced apoptosis in IFN-γ-treated SGECs (Fig. 5G). Translocation of NF-κB p65 from
the cytoplasm to the nucleus, which was observed to be induced by
the overexpression of miR-155-5p, was also significantly abrogated
following the overexpression of ARRB2 in IFN-γ-treated SGECs
(Fig. 5F). Taken together, these
results suggest that ARRB2 may partially or completely mediate the
effects of miR-155-5p on inflammation and apoptosis in
IFN-γ-treated SGECs.
 | Figure 5ARRB2 is a downstream target gene of
miR-155-5p. (A) The specific binding site of miR-155-5p on ARRB2 is
shown, where the interaction between miR-155-5p and ARRB2 was
assessed using the dual-luciferase activity assay. (B) mRNA levels
of ARRB2 in IFN-γ-treated SGECs after miR-155-5p agomir or
antagomir transfection were measured using reverse
transcription-quantitative PCR. (C) Protein levels of ARRB2 in
IFN-γ-treated SGECs after miR-155-5p agomir or antagomir
transfection were determined by western blotting. (D)
Overexpression efficiency of ARRB2 in salivary gland epithelial
cells was measured by western blotting. (E-G) SGECs were
co-transfected with ARRB2 vector and miR-155-5p agomir before they
were treated with IFN-γ (10 ng/ml) for 12 h. (E) The levels of IL-6
and TNF-α were detected by ELISA. (F) The expression and activation
of NF-κB p65 was measured by western blotting. (G) Both early and
late apoptotic cells were assessed by flow cytometry. The regions
of Q2-2 + Q2-4 represent the apoptotic cells. All data were
presented as mean ± SD, n=3. *P<0.05. ARRB2,
β-arrestin 2; IFN-γ, interferon-γ; SGECs, salivary gland epithelial
cells; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; miR,
microRNA; WT, wild type; mut, mutant; UTR, untranslated region. |
Discussion
The results of the present study demonstrated that
treatment with IFN-γ increased miR-155-5p expression, such that
apoptosis and inflammation in IFN-γ-treated SGECs may be induced by
this increased miR-155-5p expression. Furthermore, it was
demonstrated that miR-155-5p may activate NF-κB signaling by
negatively regulating ARRB2, thereby promoting salivary gland
damage in SS.
SS is an autoimmune disease, particularly in the
exocrine glands, such as salivary and lacrimal glands (34). However, the pathogenesis of SS
remains unclear. Therefore, it is necessary to study the
pathogenesis of SS and its potential therapeutic targets. It has
been previously demonstrated that miRNAs can regulate in immune
responses, including infection and autoimmunity (35,36).
Previous studies have also reported that miR-155-5p exerts an
important regulatory role in the generation of humoral and cellular
immune responses during infection and autoimmunity (37,38).
Salivary gland damage is a common clinical symptom
of SS (39). Previous studies have
demonstrated that patients with SS and animal models of SS exhibit
secretory dysfunction, particularly in the salivary gland
epithelium (40,41). In addition, inflammation and
apoptosis of SGECs have also been reported to be a possible
mechanism for impaired secretory function (42). Release of proinflammatory cytokines,
including TNF-α and IFN-γ, in the exocrine glands of patients with
SS and apoptosis of SGECs significantly increases (43,44).
The present study investigated the effects of miR-155-5p on the
apoptosis and inflammation in SGECs. Previous studies have
demonstrated that miR-155-5p expression is positively associated
with primary SS (19). High levels
of miR-155-5p have also been reported in inflammatory lesion
models, such as cerebral ischemia-reperfusion injury (45). As previously reported, an
inflammatory lesion model was established in SGECs by treatment
with IFN-γ, where IFN-γ-treatment increased apoptosis and IL-6 and
TNF-α mRNA expression (30).
Results from the present study demonstrated that miR-155-5p
overexpression promoted IFN-γ-induced apoptosis in SGECs, since
cell viability was decreased and the apoptotic rate was increased,
in addition to the increased expression levels of apoptosis-related
proteins in miR-155-5p overexpressing cells. These results also
demonstrated that miR-155-5p overexpression promoted IFN-γ-induced
inflammation, which was evidenced by the increased IL-6 and TNF-α
levels in miR-155-5p overexpressing cells. Overall, these results
suggest that miR-155-5p may exert a role in salivary gland damage
during SS by promoting the inflammatory response and apoptosis of
SGECs.
NF-κB is chronically active in several inflammatory
autoimmune diseases, including inflammatory bowel disease (46), rheumatoid arthritis (47) and SS (48). Sisto et al (49) demonstrated that the NF-κB signaling
pathway is activated in human SGECs derived from active primary
patients with SS. Lisi et al (50) reported that activation of NF-κB
signaling is a potentially important mechanism for SS development.
Furthermore, it has been demonstrated that dysregulation of NF-κB
in glandular epithelial cells results in Sjogren's-like features
(51). Activation of NF-κB
signaling promotes inflammation and induces apoptosis of human
SGECs in primary SS (52).
Proinflammatory cytokines, such as IFN-γ, activate the IκB kinase
complex, which phosphorylates IκB and targets it for proteasomal
degradation (53). This releases
NF-κB which, after phosphorylation, allows it to translocate into
the nucleus (53). NF-κB either
acts alone in the nucleus or with other transcription factors to
induce target gene expression (53). The results of the present study
demonstrated that the phosphorylation levels of IκB and the nuclear
translocation of p65 were increased, suggesting that miR-155-5p
activates NF-κB signaling. Taken together, these results suggest
that miR-155-5p may promote salivary gland damage in SS by
regulating the NF-κB signaling pathway.
ARRB2 is a downstream target gene of miR-155-5p
(54). The results of the present
study verified this association. Li et al (55) demonstrated that the overexpression
of ARRB2 may inhibit the release of proinflammatory cytokines and
decrease experimental arthritis severity. In addition, ARRB2 has
exhibited antiapoptotic effects in human endometrial cancer
heterotransplants in nude mice (56,57).
The results of the present study demonstrated that overexpression
of ARRB2 reversed the effects of miR-155-5p overexpression on the
inflammatory response, apoptosis and the NF-κB signaling pathway in
this inflammatory lesion model. ARRB2 has been previously reported
to inhibit the NF-κB signaling pathway in a sepsis mouse model and
LPS-induced liver injury (23,26).
Collectively, these results suggest that miR-155-5p may promote
salivary gland damage in SS by negatively regulating ARRB2.
Notably, miR-155-5p and IFN-γ can influence each other, whereby the
overexpression of miR-155-5p increased the production of IFN-γ
(58). IFN-γ has been demonstrated
to induce miR-155-5p expression in human dermal lymphatic
endothelial cells (58-60).
Results of the present study demonstrated that the treatment with
IFN-γ induced miR-155-5p expression, indicating that IFN-γ may in
part induce the apoptosis and inflammation of SGECs by regulating
miR-155-5p expression.
In conclusion, functional studies in the present
study demonstrated that miR-155-5p overexpression can promote
IFN-γ-induced apoptosis and inflammation in SGECs. Mechanistic
studies have indicated that miR-155-5p activates NF-κB signaling by
negatively regulating ARRB2, thereby promoting salivary gland
damage of SS. The results of the present study verified the role
and the potential molecular mechanism of miR-155-5p in salivary
gland damage in SS, suggesting that miR-155-5p may serve to be a
potential target for SS treatment.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by a grant from the
Fundamental Research Business Expense of Universities in
Heilongjiang Province (grant no. 2018-KYYWFMY-0060).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JLZ and HZZ designed the study and wrote the
manuscript. LLZ and HS performed the experiments, confirmed the
authenticity of all the raw data and conducted statistical
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Hongqi Hospital Affiliated to Mudanjiang Medical
University (Mudanjiang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baldini C, Talarico R, Tzioufas AG and
Bombardieri S: Classification criteria for Sjogren's syndrome: A
critical review. J Autoimmun. 39:9–14. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Reale M, D'Angelo C, Costantini E, Laus M,
Moretti A and Croce A: MicroRNA in Sjögren's syndrome: Their
potential roles in pathogenesis and diagnosis. J Immunol Res.
2018(7510174)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ramos-Casals M, Tzioufas AG and Font J:
Primary Sjögren's syndrome: New clinical and therapeutic concepts.
Ann Rheum Dis. 64:347–354. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jimenez SA and Piera-Velazquez S:
Potential role of human-specific genes, human-specific microRNAs
and human-specific non-coding regulatory RNAs in the pathogenesis
of systemic sclerosis and Sjögren's syndrome. Autoimmun Rev.
12:1046–1051. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Saito M, Ota Y, Ohashi H, Dei Y, Shimoyama
K, Suzuki D, Hayashi H and Ogawa N: CD40-CD40 ligand signal induces
the intercellular adhesion molecule-1 expression through nuclear
factor-kappa B p50 in cultured salivary gland epithelial cells from
patients with Sjögren's syndrome. Mod Rheumatol. 17:45–53.
2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gottenberg JE, Cagnard N, Lucchesi C,
Letourneur F, Mistou S, Lazure T, Jacques S, Ba N, Ittah M,
Lepajolec C, et al: Activation of IFN pathways and plasmacytoid
dendritic cell recruitment in target organs of primary Sjögren's
syndrome. Proc Natl Acad Sci USA. 103:2770–2775. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bushati N and Cohen SM: MicroRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen CZ, Li L, Lodish HF and Bartel DP:
MicroRNAs modulate hematopoietic lineage differentiation. Science.
303:83–86. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mestdagh P, Feys T, Bernard N, Guenther S,
Chen C, Speleman F and Vandesompele J: High-throughput stem-loop
RT-qPCR miRNA expression profiling using minute amounts of input
RNA. Nucleic Acids Res. 36(e143)2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang F, Shan S, Huo Y, Xie Z, Fang Y, Qi
Z, Chen F, Li Y and Sun B: miR-155-5p inhibits PDK1 and promotes
autophagy via the mTOR pathway in cervical cancer. Int J Biochem
Cell Biol. 99:91–99. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Elton TS, Selemon H, Elton SM and
Parinandi NL: Regulation of the MIR155 host gene in physiological
and pathological processes. Gene. 532:1–12. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jiang K, Hu J, Luo G, Song D, Zhang P, Zhu
J and Sun F: miR-155-5p promotes oxalate- and calcium-induced
kidney oxidative stress injury by suppressing MGP expression. Oxid
Med Cell Longev. 2020(5863617)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Goncalves-Alves E, Saferding V, Schliehe
C, Benson R, Kurowska-Stolarska M, Brunner JS, Puchner A, Podesser
BK, Smolen JS, Redlich K, et al: MicroRNA-155 controls T helper
cell activation during viral infection. Front Immunol.
10(1367)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vigorito E, Kohlhaas S, Lu D and Leyland
R: miR-155: An ancient regulator of the immune system. Immunol Rev.
253:146–157. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tavasolian F, Abdollahi E, Rezaei R,
Momtazi-Borojeni AA, Henrotin Y and Sahebkar A: Altered expression
of microRNAs in rheumatoid arthritis. J Cell Biochem. 119:478–487.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cao W, Qian G, Luo W, Liu X, Pu Y, Hu G,
Han L, Yuan L, A X and Deng D: miR-125b is downregulated in
systemic lupus erythematosus patients and inhibits autophagy by
targeting UVRAG. Biomed Pharmacother. 99:791–797. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Johansson A, Nyberg WA, Sjöstrand M,
Moruzzi N, Bergman P, Khademi M, Andersson M, Piehl F, Berggren PO,
Covacu R, et al: miR-31 regulates energy metabolism and is
suppressed in T cells from patients with Sjögren's syndrome. Eur J
Immunol. 49:313–322. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gourzi VC, Kapsogeorgou EK, Kyriakidis NC
and Tzioufas AG: Study of microRNAs (miRNAs) that are predicted to
target the autoantigens Ro/SSA and La/SSB in primary Sjögren's
syndrome. Clin Exp Immunol. 182:14–22. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen JQ, Zilahi E, Papp G, Sipka S and
Zeher M: Simultaneously increased expression of microRNA-155 and
suppressor of cytokine signaling 1 (SOCS1) gene in the peripheral
blood mononuclear cells of patients with primary Sjögren's
syndrome. Int J Rheum Dis. 20:609–613. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang X, Huang G, Mu J, Cong Z, Chen S, Fu
D, Qi J and Li Z: Arrb2 promotes endothelial progenitor
cell-mediated postischemic neovascularization. Theranostics.
10:9899–9912. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen G, Xie RG, Gao YJ, Xu ZZ, Zhao LX,
Bang S, Berta T, Park CK, Lay M, Chen W and Ji RR: β-arrestin-2
regulates NMDA receptor function in spinal lamina II neurons and
duration of persistent pain. Nat Commun. 7(12531)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kallifatidis G, Smith DK, Morera DS, Gao
J, Hennig MJ, Hoy JJ, Pearce RF, Dabke IR, Li J, Merseburger AS, et
al: β-arrestins regulate stem cell-like phenotype and response to
chemotherapy in bladder cancer. Mol Cancer Ther. 18:801–811.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sharma D, Malik A, Lee E, Britton RA and
Parameswaran N: Gene dosage-dependent negative regulatory role of
β-arrestin-2 in polymicrobial infection-induced inflammation.
Infect Immun. 81:3035–3044. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zeng LX, Tao J, Liu HL, Tan SW, Yang YD,
Peng XJ, Liu ZH, Jiang J and Wu B: β-arrestin2 encourages
inflammation-induced epithelial apoptosis through ER stress/PUMA in
colitis. Mucosal Immunol. 8:683–695. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gaffal E, Jakobs M, Glodde N, Schröder R,
Kostenis E and Tüting T: β-arrestin 2 inhibits proinflammatory
chemokine production and attenuates contact allergic inflammation
in the skin. J Invest Dermatol. 134:2131–2137. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jiang MP, Xu C, Guo YW, Luo QJ, Li L, Liu
HL, Jiang J, Chen HX and Wei XQ: β-arrestin 2 attenuates
lipopolysaccharide-induced liver injury via inhibition of
TLR4/NF-κB signaling pathway-mediated inflammation in mice. World J
Gastroenterol. 24:216–225. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Vakrakou AG, Polyzos A, Kapsogeorgou EK,
Thanos D and Manoussakis MN: Impaired anti-inflammatory activity of
PPARγ in the salivary epithelia of Sjögren's syndrome patients
imposed by intrinsic NF-κB activation. J Autoimmun. 86:62–74.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sisto M, Barca A, Lofrumento DD and Lisi
S: Downstream activation of NF-κB in the EDA-A1/EDAR signalling in
Sjögren's syndrome and its regulation by the ubiquitin-editing
enzyme A20. Clin Exp Immunol. 184:183–196. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the care and use of laboratory animals. 8th
edition. Washington (DC), National Academies Press (US), 2011.
|
|
30
|
Xin M, Liang H, Wang H, Wen D, Wang L,
Zhao L, Sun M and Wang J: Mirt2 functions in synergy with miR-377
to participate in inflammatory pathophysiology of Sjögren's
syndrome. Artif Cells Nanomed Biotechnol. 47:2473–2480.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang C, Li Y, Zhang XY, Liu L, Tong HZ,
Han TL, Li WD, Jin XL, Yin NB, Song T, et al: Therapeutic potential
of human minor salivary gland epithelial progenitor cells in liver
regeneration. Sci Rep. 7(12707)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gao Y, Li M, Zhang X, Bai T, Chi G, Liu JY
and Li Y: Isolation, culture and phenotypic characterization of
human sweat gland epithelial cells. Int J Mol Med. 34:997–1003.
2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Brito-Zerón P, Baldini C, Bootsma H,
Bowman SJ, Jonsson R, Mariette X, Sivils K, Theander E, Tzioufas A
and Ramos-Casals M: Sjogren syndrome. Nat Rev Dis Primers.
2(16047)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mehta A and Baltimore D: MicroRNAs as
regulatory elements in immune system logic. Nat Rev Immunol.
16:279–294. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tahamtan A, Teymoori-Rad M, Nakstad B and
Salimi V: Anti-inflammatory microRNAs and their potential for
inflammatory diseases treatment. Front Immunol.
9(1377)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ceppi M, Pereira PM, Dunand-Sauthier I,
Barras E, Reith W, Santos MA and Pierre P: MicroRNA-155 modulates
the interleukin-1 signaling pathway in activated human
monocyte-derived dendritic cells. Proc Natl Acad Sci USA.
106:2735–2740. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kurowska-Stolarska M, Alivernini S,
Ballantine LE, Asquith DL, Millar NL, Gilchrist DS, Reilly J, Ierna
M, Fraser AR, Stolarski B, et al: MicroRNA-155 as a proinflammatory
regulator in clinical and experimental arthritis. Proc Natl Acad
Sci USA. 108:11193–11198. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Milic V, Colic J, Cirkovic A, Stanojlovic
S and Damjanov N: Disease activity and damage in patients with
primary Sjogren's syndrome: Prognostic value of salivary gland
ultrasonography. PLoS One. 14(e0226498)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Daniels TE, Silverman S Jr, Michalski JP,
Greenspan JS, Sylvester RA and Talal N: The oral component of
Sjögren's syndrome. Oral Surg Oral Med Oral Pathol. 39:875–885.
1975.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cha S, Nagashima H, Brown VB, Peck AB and
Humphreys-Beher MG: Two NOD Idd-associated intervals contribute
synergistically to the development of autoimmune exocrinopathy
(Sjögren's syndrome) on a healthy murine background. Arthritis
Rheum. 46:1390–1398. 2002.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li P, Yang Y, Jin Y, Zhao R, Dong C, Zheng
W, Zhang T, Li J and Gu Z: B7-H3 participates in human salivary
gland epithelial cells apoptosis through NF-κB pathway in primary
Sjögren's syndrome. J Transl Med. 17(268)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Baker OJ, Camden JM, Redman RS, Jones JE,
Seye CI, Erb L and Weisman GA: Proinflammatory cytokines tumor
necrosis factor-alpha and interferon-gamma alter tight junction
structure and function in the rat parotid gland Par-C10 cell line.
Am J Physiol Cell Physiol. 295:C1191–C1201. 2008.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Manganelli P and Fietta P: Apoptosis and
Sjögren syndrome. Semin Arthritis Rheum. 33:49–65. 2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Shi Y, Li K, Xu K and Liu QH: miR-155-5p
accelerates cerebral ischemia-reperfusion injury via targeting
DUSP14 by regulating NF-κB and MAPKs signaling pathways. Eur Rev
Med Pharmacol Sci. 24:1408–1419. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Atreya I, Atreya R and Neurath MF:
NF-kappaB in inflammatory bowel disease. J Intern Med. 263:591–596.
2008.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Roman-Blas JA and Jimenez SA: NF-kappaB as
a potential therapeutic target in osteoarthritis and rheumatoid
arthritis. Osteoarthritis Cartilage. 14:839–848. 2006.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lisi S, Sisto M, Soleti R, Saponaro C,
Scagliusi P, D'Amore M, Saccia M, Maffione AB and Mitolo V: Fcgamma
receptors mediate internalization of anti-Ro and anti-La
autoantibodies from Sjögren's syndrome and apoptosis in human
salivary gland cell line A-253. J Oral Pathol Med. 36:511–523.
2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sisto M, Lisi S, Lofrumento DD, Ingravallo
G, Maiorano E and D'Amore M: A failure of TNFAIP3 negative
regulation maintains sustained NF-κB activation in Sjögren's
syndrome. Histochem Cell Biol. 135:615–625. 2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lisi S, Sisto M, Lofrumento DD and D'Amore
M: Sjögren's syndrome autoantibodies provoke changes in gene
expression profiles of inflammatory cytokines triggering a pathway
involving TACE/NF-κB. Lab Invest. 92:615–624. 2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wang X, Shaalan A, Liefers S, Coudenys J,
Elewaut D, Proctor GB, Bootsma H, Kroese FGM and Pringle S:
Dysregulation of NF-kB in glandular epithelial cells results in
Sjögren's-like features. PLoS One. 13(e0200212)2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sisto M, Lorusso L and Lisi S: TLR2
signals via NF-κB to drive IL-15 production in salivary gland
epithelial cells derived from patients with primary Sjögren's
syndrome. Clin Exp Med. 17:341–350. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1(a001651)2009.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhou Y, Song Y, Shaikh Z, Li H, Zhang H,
Caudle Y, Zheng S, Yan H, Hu D, Stuart C and Yin D: MicroRNA-155
attenuates late sepsis-induced cardiac dysfunction through JNK and
β-arrestin 2. Oncotarget. 8:47317–47329. 2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Li P, Cook JA, Gilkeson GS, Luttrell LM,
Wang L, Borg KT, Halushka PV and Fan H: Increased expression of
beta-arrestin 1 and 2 in murine models of rheumatoid arthritis:
Isoform specific regulation of inflammation. Mol Immunol. 49:64–74.
2011.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Hong F, Zhang Y, Cheng W, Sun X and Wang
J: β-arrestin-2 up-regulates toll-like receptor 2 signaling and
inhibits apoptosis in human endometrial cancer heterotransplants in
nude mice. BMC Cancer. 19(1035)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Li Y, Sun X, Zhang Y, Huang J, Hanley G,
Ferslew KE, Peng Y and Yin D: Morphine promotes apoptosis via TLR2,
and this is negatively regulated by beta-arrestin 2. Biochem
Biophys Res Commun. 378:857–861. 2009.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Trotta R, Chen L, Ciarlariello D, Josyula
S, Mao C, Costinean S, Yu L, Butchar JP, Tridandapani S, Croce CM
and Caligiuri MA: miR-155 regulates IFN-γ production in natural
killer cells. Blood. 119:3478–3485. 2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Yee D, Shah KM, Coles MC, Sharp TV and
Lagos D: MicroRNA-155 induction via TNF-α and IFN-γ suppresses
expression of programmed death ligand-1 (PD-L1) in human primary
cells. J Biol Chem. 292:20683–20693. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Kim JH, Jou I and Joe EH: Suppression of
miR-155 expression in IFN-γ-treated astrocytes and microglia by
DJ-1: A possible mechanism for maintaining SOCS1 expression. Exp
Neurobiol. 23:148–154. 2014.PubMed/NCBI View Article : Google Scholar
|