Introduction
Lower back pain (LBP) results in disability, which
severely affects the quality of daily life of patients and exerts
economic pressure on the patients and their families.
Intervertebral disk (IVD) degeneration (IDD) is one of the major
contributors to LBP and disability (1,2). The
pathological mechanisms underlying disk degeneration are still not
fully understood, and current treatments, such as physiotherapy,
anti-inflammatory/analgesic medication and surgery, focus on
attenuating the pain symptoms rather than the underlying
pathogenesis (3). Thus,
identification of the underlying molecular mechanism of IDD is
urgently needed.
The IVD comprises three parts: Cartilaginous
endplates (CEPs), annulus fibrosus (AF) and nucleus pulposus (NP)
(4). The central NP, a gelatinous
tissue, is the primary component of the IVD that is responsible for
relieving stress and maintaining the structure and function of the
disk by distributing hydraulic pressure equally to adjacent AF and
CEPs (5). NP cells are considered
to be the major cells in the NP tissue due to their ability to
produce and sustain the gelatinous extracellular matrix (6). In its early stage, IDD primarily
occurs in the central NP and presents with decreased production of
NP cells and the extracellular matrix (7). Numerous studies have demonstrated that
an increased apoptotic rate of NP cells is a key contributor to IDD
initiation and progression; during the course of NP aging, NP cell
apoptosis can induce degenerative cascades and result in structural
instability of the IVD (8-11).
Therefore, investigating the molecular mechanisms of human NP cell
apoptosis may provide insights into potential IDD treatments.
Long non-coding RNAs (lncRNAs) are >200
nucleotides in length and modulate gene expression levels at the
transcriptional (recruitment of transcriptional factors),
post-transcriptional (sponging of microRNAs) and epigenetic (DNA
methylation and histone modification) levels by interacting with
DNA, RNA and proteins, thus participating in cell proliferation,
apoptosis, differentiation and the immune response (12-14).
A recent study reported that 137 lncRNAs were differentially
expressed between tissue samples from patients with IDD and healthy
controls (15). A previous study
has also demonstrated that high levels of lncRNA DNA polymerase ε
(lncPolE) are associated with high-grade IDD, and lncPolE
overexpression enhances apoptosis in human NP cells (16). In addition, accumulating evidence
has indicated that microRNAs (miRNAs or miRs), another group of
non-coding transcripts, are also involved in IDD by modulating NP
cell proliferation and apoptosis (17,18).
In degenerative NP cells, miR-573 inhibits apoptosis by suppressing
Bax expression (19). A number of
studies have demonstrated that lncRNAs with aberrant expression are
associated with various diseases by acting as competing endogenous
(ce)RNAs to negatively modulate miRNAs and inhibit their biological
functions (20,21). For example, LINC01133 is
downregulated in gastric cancer tissues and cell lines and
functions as a miR-106a-3p sponge (22). However, to the best of our
knowledge, few studies have investigated the crosstalk between
lncRNAs and miRNAs in IDD.
In the present study, the role of RP11-81H3.2 and
the interaction between RP11-81H3.2, miR-1539 and COL2A1 in IDD
progression were investigated, contributing to a better
understanding of the pathogenesis behind IDD.
Materials and methods
Tissue samples
A total of 30 tissue samples per group were
collected from the IDD and control groups between August 2017 and
June 2018 from the Department of orthopedics, 987 Hospital of
Peoples Liberation Army of China Joint Logistics Support Force
(Baoji, China). All included patients (12 females and 18 males;
average age 42.39±5.33, range 37-62 years) had typically clinical
symptoms and the degree of IDD was evaluated using magnetic
resonance imaging (MRI) scans according to a modified Pfirrmann
grading classification (23). All
the study procedures were approved by the institutional Ethics
Committee of 987 Hospital of PLA Joint Logistics Support Force
(approval no. 20171023). Written informed consent was obtained from
all participants.
Human NP cell isolation and
culture
Human NP cells were isolated as previously described
(24) and cultured in a commercial
NP cell medium (ScienCell Research Laboratories, Inc.) containing
15% (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml
streptomycin/penicillin at 37˚C in a humidified atmosphere with 5%
CO2.
Cell transfection
RP11-81H3.2 was overexpressed using the expression
plasmid pcDNA3.1(+) (Invitrogen; Thermo Fisher Scientific Inc.).
Empty vectors without RP11-81H3.2 cDNA were used as negative
control. sh-RP11-81H3.2, miR-1539 mimic, miR-1539 inhibitor,
si-COL2A1 and their negative controls were purchased from Shanghai
GenePharma Co., Ltd. Cells were transfected with sh-RP11-81H3.2,
miR-1539 mimic, miR-1539 inhibitor, si-COL2A1 and their negative
controls (Table I) at 50 nM
concentration using Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. Cells were collected for further analysis 48 h after
transfection.
 | Table ISequences of sh-RP11-81H3.2, miR-1539
mimic, miR-1539 inhibitor, si-COL2A1 and their negative
controls. |
Table I
Sequences of sh-RP11-81H3.2, miR-1539
mimic, miR-1539 inhibitor, si-COL2A1 and their negative
controls.
|
Oligonucleotides | Sequence
(5'→3') |
|---|
| sh-NC |
GAGACACUGUCACGAUGUUGUGUG |
| sh-RP11-81H3.2 |
GGUGUCAGAGAAGGCUGAAUUGGGU |
| si-NC |
CACUCAGUGAGUGUCUCAC |
| si-COL2A1 |
CCUGGAGACAUCAAGGAUA |
| mimic NC |
UCACAACCUCCUAGAAAGAGUAGA |
| miR-1539 mimic |
UCCUGCGCGUCCCAGAUGCCC |
| inhibitor NC |
UCACAACCUCCUAGAAAGAGUAGA |
| miR-1539
inhibitor |
UCCUGCGCGUCCCAGAUGCCC |
Reverse transcription-quantitative
(RT-q)PCR analysis
Total RNA was obtained from tissues or cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and reverse-transcribed to cDNA using a PrimeScript RT
reagent kit (Takara Biotechnology Co., Ltd.) following the
manufacturer's protocols. RT-PCR was performed using an ABI Prism
7900HT (Applied Biosystems; Thermo Fisher Scientific, Inc). The
thermocycling conditions were: 95˚C for 10 min, and 40 cycles of
95˚C for 15 sec and 60˚C for 60 sec. Quantitative measurements were
determined using the 2-ΔΔCq method (25). The primers were obtained from
Shanghai Sangon Pharmaceutical Co., Ltd. GAPDH was used as an
endogenous control. The sequences of the primers used in the
present study are listed in Table
II.
 | Table IIPrimers used in the present
study. |
Table II
Primers used in the present
study.
| Gene | Sequences |
|---|
| RP11-81H3.2 | F:
5'-CCGGATGCCAGTCTACTACG-3' |
| | R:
5'-TGATGTGCCAGGGAAGAAAGCCTA-3' |
| miR-1539 | F:
5'-GGCTCTGCGGCCTGCAGG-3' |
| | R:
5'-ATGGTGTCGTGGAGTCG-3' |
| COL2A1 | F:
5'-ACCTTGGACGCCATGAAA-3' |
| | R:
5'-GTGGACAGTAGACGGAGGAA-3' |
| GAPDH | F:
5'-AGACACCATGGGGAAGGTGAA-3' |
| | R:
5'-ATTGCTGATGATCTTGAGGCTG-3' |
Luciferase reporter assay
Potential target miRNAs of RP11-81H3.2 were
predicted using LncBase V2 (http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2/index).
miR-1539 targeted candidate genes and the binding sites were
predicted using the online tools, TargetScan V7.2 (http://www.targetscan.org/vert_72/) and miRDB
(http://www.mirdb.org/mining.html).
Luciferase reporter plasmids, including the wild-type (Wt)
RP11-81H3.2, mutant (Mut) RP11-81H3.2, Wt COL2A1 3'-untranslated
region (UTR) and Mut COL2A1 3'-UTR (Mut COL2A1), were obtained from
Guangzhou RiboBio Co., Ltd.NP cells were co-transfected with
luciferase reporter vectors, including Wt or Mut RP11-81H3.2 or
COL2A1 3'-UTR, and miR-1539 mimic or miR-negative control (NC)
using the Lipofectamine® 3000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). After 48 h, luciferase activity
was measured using a Dual-Luciferase Reporter Assay System
(Beyotime Institute of Biotechnology). The relative luciferase
activity was normalized to Renilla luciferase activity.
Flow cytometry (FCM) assay
FCM was used to analyze the apoptotic rate with an
Annexin V-FITC/PI Apoptosis Detection kit (Vazyme Biotech Co.,
Ltd.). Briefly, cells from different treatment groups were washed
three times with phosphate-buffered saline solution and incubated
with Annexin V-FITC and PI solution for 15 min at 37˚C in the dark.
Then, cells were analyzed using a flow cytometer (FACScan, BD
Biosciences) with CellQuest 3.0 software (BD Biosciences).
MTT assay
Briefly, suspended cells were plated onto 96-well
plates at a density of 4x103 cells/well and incubated at
37˚C for the indicated time periods. Next, 20 µl MTT was added and
incubated for 4 h at 37˚C. Then, 200 µl DMSO was added to each
well, and the absorbance at 450 nm was detected using an
enzyme-linked immunosorbent assay reader.
Western blot analysis
Total proteins were collected from cells using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China). After measuring the protein
concentration using the BCA Protein Assay kit (Beyotime Institute
of Biotechnology), equal amounts of protein (25 µg) were subjected
to 10% SDS-PAGE and transferred to PVDF membranes (EMD Millipore).
After blocking with 1% BSA (Sigma-Aldrich; Merck KGaA) for 1 h at
room temperature. The membranes were incubated with anti-Bcl-2
(1:2,000; cat. no. ab182858), anti-Bax (1:1,000; cat. no. ab32503),
anti-caspase-3 (1:500; cat. no. ab13847), anti-cleaved caspase-3
(1:500; cat. no. ab49822), anti-caspase-9 (1:1,000; cat. no.
ab32539), anti-Cleaved caspase-9 (1:1,000; cat. no. ab2324) and
anti-GAPDH (1:2,500; cat. no. ab9485) antibodies overnight at 4˚C,
then incubated in horseradish peroxidase-conjugated secondary
antibodies (1:2,000; cat. no. ab6721) at room temperature for 1 h.
All antibodies were purchased from Abcam. Finally, the protein
bands were visualized using an electrochemiluminescence system
(Cytiva) and analyzed using ImageJ software (version 1.49; National
Institutes of Health).
TUNEL and DAPI staining
Apoptosis was also detected using a One-step TUNEL
apoptosis detection kit (Beyotime Institute of Biotechnology).
Briefly, Cells (1x105) were washed with PBS for 5 min
three times and fixed in 4% paraformaldehyde at 4˚C for 20 min.
Subsequently, the cells were maintained in 50 µl TUNEL reaction
buffer at 37˚C for 1 h and counterstained with DAPI to stain the
cell nuclei at 37˚C for 5-10 min. Antifade mounting medium
(Beyotime Institute of Biotechnology) was used. Images (x100) from
four fields of view were captured using a fluorescence microscope
(Olympus DP72; Olympus Corporation).
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. GraphPad Prism software (version
5.0; GraphPad Software, Inc.) was used for data analysis.
Comparisons between two groups were performed using unpaired
Student's t-test. One way ANOVAs, followed by Tukey's post hoc
tests were performed to compare >2 groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
RP11-81H3.2 expression levels are
downregulated in NP tissue samples from patients with IDD
In order to elucidate the role of RP11-81H3.2 in
IDD, RT-qPCR assay was used to detect the RP11-81H3.2 expression
levels in NP cells in tissue samples from the IDD and control
groups. RP11-81H3.2 expression levels were significantly decreased
in NP cells from IDD tissues compared with those from normal
tissues (Fig. 1A). Moreover, the
RP11-81H3.2 expression levels decreased as disk degeneration grade
increased in patients with IDD (Fig.
1B). These results demonstrated that RP11-81H3.2 may be
involved in IDD progression.
RP11-81H3.2 dysregulation affects the
apoptosis and viability of NP cells from tissue samples of patients
with IDD
In order to determine the effects of RP11-81H3.2 on
cell viability, RP11-81H3.2 silencing (sh-RP11-81H3.2) or
overexpression (RP11-81H3.2) was induced in NP cells. The
efficiency was confirmed by RT-PCR (Fig. 2A). Viability was significantly
suppressed in NP cells derived from tissue samples of patients with
IDD compared with those derived from normal tissue (Fig. 2B). In addition, cell viability was
significantly promoted in cells with RP11-81H3.2 overexpression
(RP11-81H3.2) compared with that in the negative control cells NC).
By contrast, silencing RP11-81H3.2 significantly suppressed cell
viability compared with that in the sh-NC group. FCM assay and
TUNEL staining were performed to determine the apoptotic rates of
NP cells. The results demonstrated that silencing RP11-81H3.2
increased apoptosis, whereas RP11-81H3.2 overexpression decreased
apoptosis in NP cells compared with that in the corresponding
control groups (Fig. 2C and
D). Western blotting results
revealed that RP11-81H3.2 overexpression significantly increased
Bcl-2 (an anti-apoptotic gene) expression levels while decreasing
the expression levels of Bax and the active forms of caspase-3 and
caspase-9 (pro-apoptotic genes) in NP cells. By contrast, silencing
RP11-81H3.2 achieved the opposite effects (Fig. 2E).
 | Figure 2Effects of overexpression or
silencing of RP11-81H3.2 on the apoptosis of NP cells derived from
tissue samples of patients with IDD. (A) RP11-81H3.2 expression
levels were detected using reverse transcription-quantitative PCR
in NP cells transfected with the RP11-81H3.2 overexpression vector,
sh-RP11-81H3.2 and control. (B) Cell viability was determined using
the MTT method. (C) Results of Annexin V-FITC/PI combined with flow
cytometry of NP cell apoptosis. (D) TUNEL and DAPI staining of NP
cells following transfection. (E) Expression levels of caspase-3,
cleaved caspase-3, caspase-9, cleaved caspase-9, Bcl-2 and Bax were
determined by western blotting. *P<0.05 and
**P<0.01. IDD, intervertebral disk degeneration; NP,
nucleus pulposus; sh, short hairpin; OD, optical density; PI,
propidium iodide. |
RP11-81H3.2 directly interacts with
miR-1539
One of the mechanisms by which lncRNAs exerts their
functions is by acting as a molecular sponge of miRNA to liberate
mRNA transcripts targeted by miRNA (26). In order to determine whether
RP11-81H3.2 exhibited a similar mechanism, online bioinformatics
software was used to predict its target miRNAs, which revealed that
miR-1539 had a sequence complementary to that of RP11-81H3.2
(Fig. 3A). To test the direct
interaction between RP11-81H3.2 and miR-1539, luciferase reporter
vectors containing RP11-81H3.2 with Wt or Mut miR-1539 binding
sites were constructed. The miR-1539 mimic significantly decreased
Wt-RP11-81H3.2-regulated luciferase activity but did not affect the
Mut-RP11-81H3.2-regulated luciferase activity in NP cells (Fig. 3B), indicating that RP11-81H3.2
directly bound miR-1539. Subsequently, the effects of silencing
RP11-81H3.2 on miR-1539 expression levels in NP cells were
assessed. RT-qPCR analysis demonstrated that RP11-81H3.2 knockdown
significantly enhanced miR-1539 expression levels compared with
those in cells transfected with sh-NC (Fig. 3C). In order to determine whether
RP11-81H3.2 was influenced by miR-1539, NP cells were transfected
with the miR-1539 mimic or inhibitor. The results revealed that
RP11-81H3.2 mRNA expression levels significantly decreased
following transfection with miR-1539 mimic compared with those in
cells transfected with the mimic NC (Fig. 3D). However, RP11-81H3.2 mRNA
expression levels increased following transfection with miR-1539
inhibitor compared with inhibitor NC (Fig. 3E). In addition, the miR-1539
expression levels in the degenerative NP tissues were measured by
RT-qPCR; degenerative NP tissue displayed significantly higher
miR-1539 expression levels compared with those in normal NP tissues
(Fig. 3F).
RP11-81H3.2 decreases apoptosis by
inhibiting miR-1539 expression levels
Next, it was determined whether RP11-81H3.2
suppressed apoptosis by sequestering miR-1539 in NP cells.
RP11-81H3.2 overexpression promoted viability of NP cells derived
from IDD tissue samples, whereas miR-1539 mimic counteracted this
effect compared with that in the respective control groups
(Fig. 4A). NP cells were
transfected with RP11-81H3.2 in combination with miR-1539 mimic,
and the apoptotic rates were detected by TUNEL staining and FCM
analysis. The results demonstrated that RP11-81H3.2 overexpression
inhibited apoptosis compared with that in the cells transfected
with the empty vector, whereas miR-1539 mimic counteracted this
effect (Fig. 4B and C). Similarly, the western blot results
also revealed that overexpression of RP11-81H3.2 decreased the
apoptosis of NP cells as evidenced by the decreased levels of
cleaved caspase-9, cleaved caspase-3 and Bax, while increased level
of Bcl-2 (Fig. 4D). Co-transfection
with miR-1539 mimic and RP11-81H3.2 overexpression vector
ameliorated the anti-apoptotic effect of RP11-81H3.2 overexpression
in NP cells (Fig. 4D). Taken
together, these data suggested that miR-1539 is a critical
downstream target of RP11-81H3.2 during its regulation of
apoptosis.
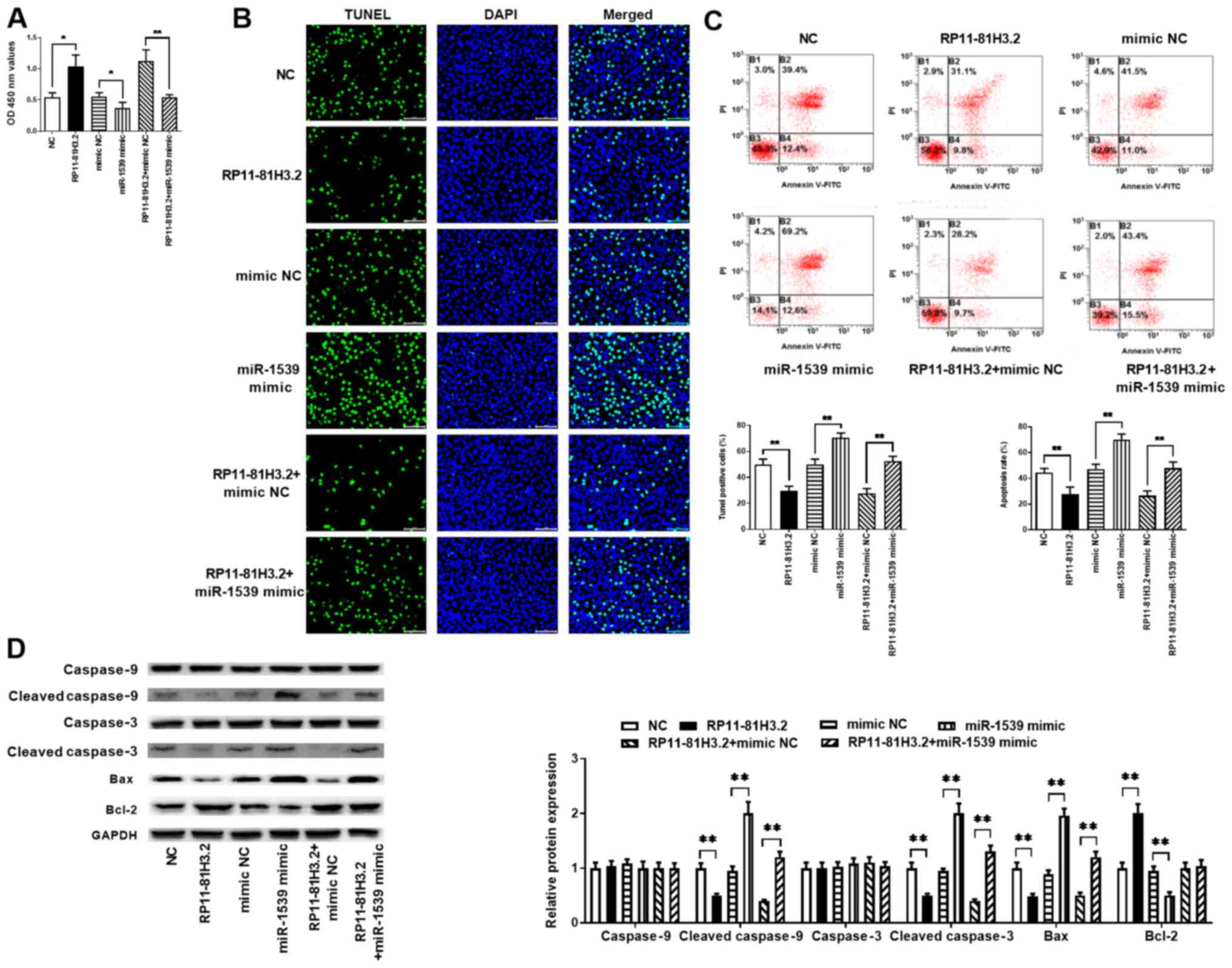 | Figure 4RP11-81H3.2 decreases apoptosis by
inhibiting miR-1539 expression. NP cells from tissue samples of
patients with intervertebral disk degeneration were transfected
with NC, RP11-81H3.2, mimic NC, miR-1539 mimic, RP11-81H3.2 + mimic
NC and RP11-81H3.2 + miR-1539 mimic. (A) Cell viability was
determined using the MTT assay. (B) TUNEL and DAPI staining of NP
cells. (C) Results of Annexin V-FITC/PI combined with flow
cytometry of NP cell apoptosis. (D) Expression levels of caspase-3,
cleaved caspase-3, caspse-9, cleaved caspase-9, Bcl-2 and Bax were
detected using western blotting. *P<0.05 and
**P<0.01. miR, microRNA; NP, nucleus pulposus; NC,
negative control; OD, optical density; PI, propidium iodide. |
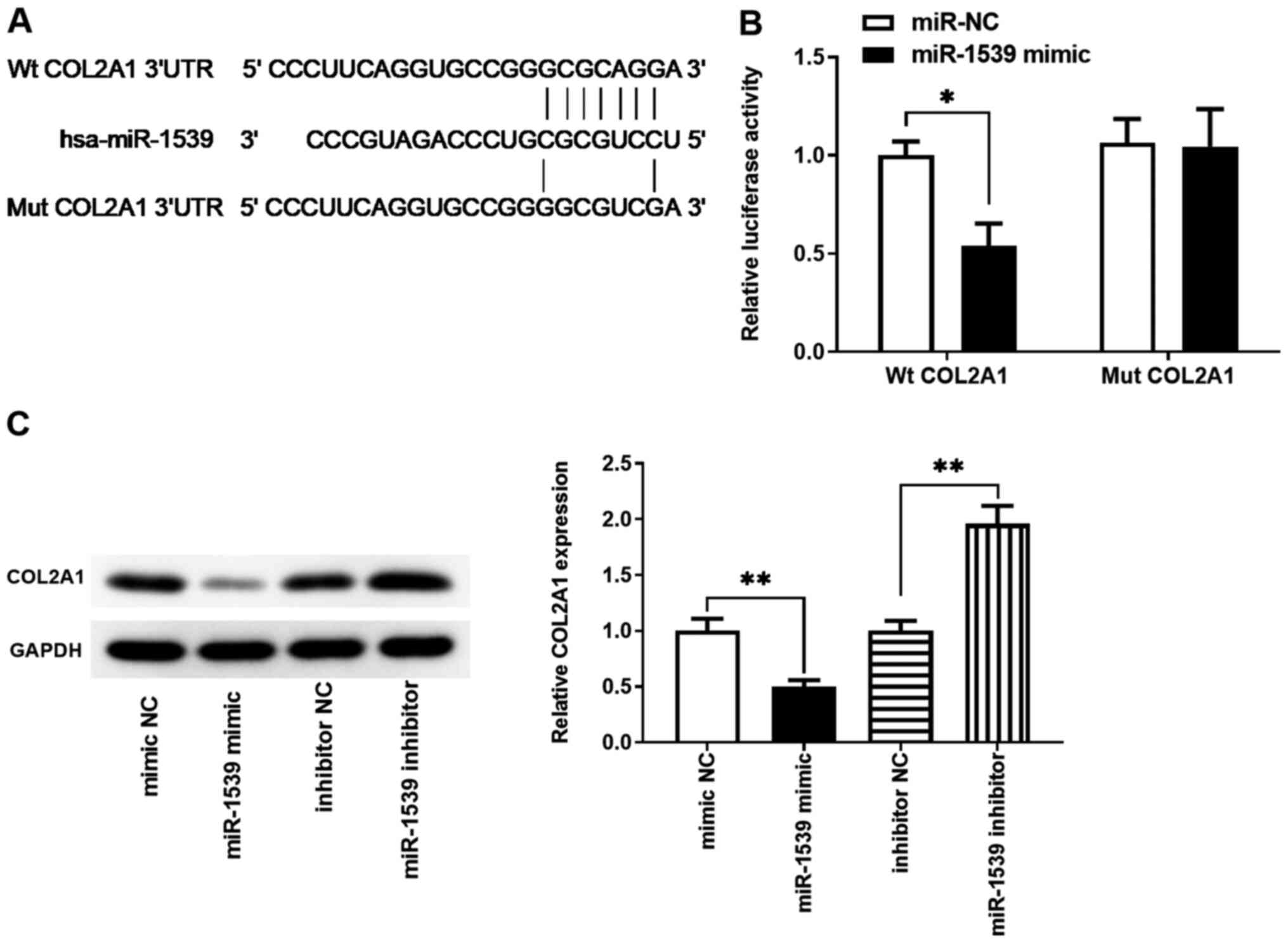
COL2A1 is a target gene of
miR-1539
In order to determine whether RP11-81H3.2 inhibited
apoptosis by affecting the targets of miR-1539, bioinformatics
analysis was performed to predict the potential target genes of
miR-1539. COL2A1 was identified as a potential target, and the
possible binding sites between miR-1539 and the COL2A1 mRNA and in
3'-UTR are displayed in Fig. 5A. To
confirm the direct interaction between miR-1539 and COL2A1, the
3'-UTR of COL2A1 was inserted downstream of the luciferase reporter
gene; the miR-1539 mimic significantly decreased the luciferase
activity (Fig. 5B). The western
blot results demonstrated that cells transfected with the miR-1539
mimic exhibited decreased COL2A1 protein expression levels compared
with those in the cells transfected with mimic NC, whereas the
miR-1539 inhibitor reversed this effect. Therefore, the results of
the present study suggested that miR-1539 modulated COL2A1
expression levels by directly binding to its 3'-UTR.
Dysregulation of RP11-81H3.2, miR-1539
and COL2A1 affects NP cell apoptosis
The regulatory effects of abnormal expression levels
of RP11-81H3.2, miR-1539 and COL2A1 on NP cell apoptosis were
further evaluated. First, COL2A1 expression levels in NP cells
following knockdown of RP11-81H3.2 (sh-RP11-81H3.2) or transfection
with miR-1539 mimic were assessed. Both RP11-81H3.2 deletion and
miR-1539 mimic significantly decreased COL2A1 protein expression
levels compared with those in the control in western blot analysis
(Fig. 6A). Transfection of NP cells
with siRNA was used to interfere with COL2A1 expression levels, and
the deletion effect was confirmed by western blotting (Fig. 6A). Next, an MTT assay was performed
to test the viability of NP cells. The results demonstrated that
the miR-1539 mimic decreased the viability of human degenerative NP
cells, which is similar to the suppressed cell viability caused by
the deletion of RP11-81H3.2 or COL2A1 (Fig. 6B). Moreover, the FCM analysis
demonstrated that inhibition of RP11-81H3.2 or COL2A1, or the
overexpression of miR-1539 promoted the apoptosis of human
degenerative NP cells (Fig. 6C). In
addition, knockdown of COL2A1 induced the apoptosis of NP cells
while overexpression of RP11-81H3.2 in COL2A1-downregulated cells
increased the cell viability and decreased apoptosis of NP cells
(Fig. 6D and E).
 | Figure 6Comparison of the effects of
downregulated RP11-81H3.2, upregulated miR-1539 and si-COL2A1 on NP
cells. (A) COL2A1 protein expression levels in the sh- RP11-81H3.2,
miR-1539 mimic, si-COL2A1 and NC groups were measured using western
blotting. (B) Cell proliferation was detected using MTT analysis.
(C) Apoptosis was detected using flow cytometry. Knockdown of
COL2A1 alleviated the effects of RP11-81H3.2 overexpression on (D)
promotion of cell viability and (E) prevention of apoptosis of NP
cells. *P<0.05 and **P<0.01. miR,
microRNA; si, small interfering; COL2A1, collagen type 2 α 1 chain;
NP, nucleus pulposus; sh, short hairpin; NC, negative control; OD,
optical density; PI, propidium iodide. |
Discussion
IDD is a common disease of the spine that results in
a degenerative musculoskeletal disorder (27). Increasing evidence has indicated
that dysregulated non-coding RNAs, including miRNAs and lncRNAs,
participate in the progression of IDD, including processes such as
abnormal proliferation, apoptosis and inflammatory cytokine
production in human degenerative NP cells (27-29).
For example, Wan et al (30)
have identified 116 lncRNAs and 260 mRNAs that are differentially
expressed in NP cells isolated from degenerative and normal samples
(obtained from cadaveric donors) using an lncRNA-mRNA microarray,
and validated the upregulation of lncRNA RP11-296A18.3 in
degenerative IVDs by RT-qPCR. To the best of our knowledge,
however, the exact regulatory network of non-coding RNAs in IDD has
not yet been fully elucidated. Therefore, investigating the
functions of non-coding RNAs in the progression of IDD and the
underlying mechanisms may contribute to the development of novel
therapeutics for IDD.
The general function of RP11-81H3.2 remains largely
unknown. Recently, a number of studies have investigated the role
of RP11-81H3.2 in cancer progression and reported that RP11-81H3.2
promotes the progression of gastric cancer via the miR-339/HNRNPA1
axis and that of hepatocellular carcinoma via the
miR-490-3p/consequential tankyrase 2 axis (31,32).
Other roles of RP11-81H3.2 have not been documented. The present
study identified RP11-81H3.2 as a novel lncRNA that contributes to
IDD progression. RP11-81H3.2 expression levels were significantly
decreased in NP tissue samples from patients with IDD compared with
those from healthy subjects, and inversely associated with
high-grade disk degeneration, which indicated that RP11-81H3.2 may
be involved in the development of IDD.
NP cells are the primary components in central NP
tissue and are responsible for modulating the synthesis of the
matrix in IVD (33,34). Gruber and Hanley (35) first observed apoptotic disk cells in
degenerative disk tissue samples. Additional investigations have
verified that NP cell apoptosis serves an important role in the
development of human IDD by contributing to the loss of
extracellular matrix content (33,34).
Therefore, targeting antiapoptotic genes has become a novel
strategy for the treatment of IDD. Certain studies have sought to
inhibit disk cell apoptosis by overexpressing Bcl-2 or using
caspase inhibitors and the methods were effective in preventing
apoptotic cell death in vitro (36-38).
In the present study, the effect of RP11-81H3.2 on apoptosis of NP
cells derived from tissue samples of patients with IDD was assessed
via FCM and TUNEL staining; the results demonstrated that
RP11-81H3.2 overexpression significantly decreased, whereas
RP11-81H3.2 deletion increased the apoptotic rate of NP cells.
Increased Bcl-2 and decreased Bax expression levels attenuate the
activation of caspase-3 and caspase-9 to inhibit apoptosis
(39). Accordingly, in the present
study, overexpression of RP11-81H3.2 significantly increased the
levels of the anti-apoptotic protein Bcl-2, but decreased the
expression levels of the pro-apoptotic protein Bax, cleaved
caspase-3 and caspase-9 in NP cells. By contrast, knockdown of
RP11-81H3.2 markedly reversed these effects. These results
demonstrated that RP11-81H3.2 inhibited NP cell apoptosis by
upregulating Bcl-2 and downregulating Bax.
Although decreased RP11-81H3.2 expression levels
promote apoptosis of degenerative NP cells, the downstream
molecular pathways mediating this effect have not yet been
identified. A previous study demonstrated that lncRNAs serve as
miRNA sponges, thereby enabling the expression of RNAs targeted by
these miRNAs (29). The results of
the present study demonstrated that miR-1539 expression levels were
increased in degenerative NP tissue compared with those in healthy
NP tissue. Therefore, it was hypothesized that RP11-81H3.2 may
function as a sink gene for miR-1539 in apoptosis. Bioinformatics
analysis identified complementary sequences between the 3'-UTR of
RP11-81H3.2 and miR-1539, and the interaction between RP11-81H3.2
and miR-1539 was verified by luciferase reporter assay. RP11-81H3.2
knockdown enhanced the levels of miR-1539. In addition, miR-1539
mimic counteracted the inhibitory effect of RP11-81H3.2
overexpression on the apoptosis of NP cells derived from IDD tissue
samples. These results indicated that RP11-81H3.2 acted as an
endogenous sponge to downregulate miR-1539, thus participating in
NP cell apoptosis. The role of miR-1539 in biological processes has
not previously been well documented; to the best of our knowledge,
the present study was the first to demonstrate the involvement of
miR-1539 in the regulation of apoptosis.
COL2A1 is a potential target gene involved in NP
cell apoptosis, was identified by bioinformatics-based target
prediction analysis in the present study. Luciferase reporter assay
demonstrated that miR-1539 directly interacted with COL2A1 mRNA and
modulated its expression levels. Moreover, RP11-81H3.2 knockdown or
miR-1539 mimic significantly decreased COL2A1 expression levels,
promoted apoptosis and inhibited proliferation of NP cells. Cheng
et al (40) reported that
knockdown COL2A1 significantly inhibited cell viability and
promoted apoptosis in human chondrocyte cell line CHON-001. The
present results were in agreement with the aforementioned study.
Taken together, these results indicated involvement of the
RP11-81H3.2-miR-1539-COL2A1 regulatory network in the development
of IDD via influencing NP cell apoptosis.
In conclusion, the results of the present study
demonstrated that low RP11-81H3.2 expression levels were associated
with high-grade IDD and a novel role of RP11-81H3.2 in the
development of IDD was identified. The present findings also
demonstrated the molecular mechanism underlying the involvement of
RP11-81H3.2 in the progression of IDD and revealed that the
RP11-81H3.2/miR-1539/COL2A1 axis accounted for NP cell apoptosis.
The present study elucidated the protective effects of RP11-81H3.2
against NP cell apoptosis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from the
Peoples Liberation Army of China Youth Training Project for Medical
Science (grant no. 15QNP014).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XDY designed the study and reviewed and revised the
manuscript. LQ and SYP wrote the manuscript. LQ, YPZ, JY and JPX
performed the experiments. SYP, BC, HZ and CZ analyzed the data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients and the study protocol was approved by the Ethics
Committee of Shaanxi Provincial People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vadalà G, Russo F, Di Martino A and Denaro
V: Intervertebral disc regeneration: From the degenerative cascade
to molecular therapy and tissue engineering. J Tissue Eng Regen
Med. 9:679–690. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cheung KM, Karppinen J, Chan D, Ho DW,
Song YQ, Sham P, Cheah KS, Leong JC and Luk KD: Prevalence and
pattern of lumbar magnetic resonance imaging changes in a
population study of one thousand forty-three individuals. Spine
(Phila Pa 1976). 34:934–940. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Freemont AJ, Watkins A, Le Maitre C,
Jeziorska M and Hoyland JA: Current understanding of cellular and
molecular events in intervertebral disc degeneration: Implications
for therapy. J Pathol. 196:374–379. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Erwin WM and Hood KE: The cellular and
molecular biology of the intervertebral disc: A clinician's primer.
J Can Chiropr Assoc. 58:246–257. 2014.PubMed/NCBI
|
|
5
|
Priyadarshani P, Li Y and Yao L: Advances
in biological therapy for nucleus pulposus regeneration.
Osteoarthritis Cartilage. 24:206–212. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Loreto C, Musumeci G, Castorina A, Loreto
C and Martinez G: Degenerative disc disease of herniated
intervertebral discs is associated with extracellular matrix
remodeling, vimentin-positive cells and cell death. Ann Anat.
193:156–162. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Antoniou J, Steffen T, Nelson F,
Winterbottom N, Hollander AP, Poole RA, Aebi M and Alini M: The
human lumbar intervertebral disc: Evidence for changes in the
biosynthesis and denaturation of the extracellular matrix with
growth, maturation, ageing, and degeneration. J Clin Invest.
98:996–1003. 1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen D, Xia D, Pan Z, Xu D, Zhou Y, Wu Y,
Cai N, Tang Q, Wang C, Yan M, et al: Metformin protects against
apoptosis and senescence in nucleus pulposus cells and ameliorates
disc degeneration in vivo. Cell Death Dis. 7(e2441)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen J, Xie JJ, Jin MY, Gu YT, Wu CC, Guo
WJ, Yan YZ, Zhang ZJ, Wang JL, Zhang XL, et al: Sirt6
overexpression suppresses senescence and apoptosis of nucleus
pulposus cells by inducing autophagy in a model of intervertebral
disc degeneration. Cell Death Dis. 9(56)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jiang L, Zhang X, Zheng X, Ru A, Ni X, Wu
Y, Tian N, Huang Y, Xue E, Wang X and Xu H: Apoptosis, senescence,
and autophagy in rat nucleus pulposus cells: Implications for
diabetic intervertebral disc degeneration. J Orthop Res.
31:692–702. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lv J, Li S, Wan T, Yang Y, Cheng Y and Xue
R: Inhibition of microRNA-30d attenuates the apoptosis and
extracellular matrix degradation of degenerative human nucleus
pulposus cells by up-regulating SOX9. Chem Biol Interact.
296:89–97. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Batista PJ and Chang HY: Long noncoding
RNAs: Cellular address codes in development and disease. Cell.
152:1298–1307. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Yang L, Froberg JE and Lee JT: Long
noncoding RNAs: Fresh perspectives into the RNA world. Trends
Biochem Sci. 39:35–43. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Qu Z, Quan Z, Zhang Q, Wang Z, Song Q,
Zhuang X, Fu C, Xu F, Liu Y, Wang Y, et al: Comprehensive
evaluation of differential lncRNA and gene expression in patients
with intervertebral disc degeneration. Mol Med Rep. 18:1504–1512.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li X, Lou Z, Liu J, Li H, Lei Y, Zhao X
and Zhang F: Upregulation of the long noncoding RNA lncPolE
contributes to intervertebral disc degeneration by negatively
regulating DNA polymerase epsilon. Am J Transl Res. 11:2843–2854.
2019.PubMed/NCBI
|
|
17
|
Zhao Z, Zheng J, Ye Y, Zhao K and Wang R
and Wang R: MicroRNA-25-3p regulates human nucleus pulposus cell
proliferation and apoptosis in intervertebral disc degeneration by
targeting Bim. Mol Med Rep. 22:3621–3628. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li Z, Yu X, Shen J, Chan MTV and Wu WK:
MicroRNA in intervertebral disc degeneration. Cell Prolif.
48:278–283. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang R, Wen B and Sun D: miR-573 regulates
cell proliferation and apoptosis by targeting Bax in nucleus
pulposus cells. Cell Mol Biol Lett. 24(2)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shao Y, Ye M, Li Q, Sun W, Ye G, Zhang X,
Yang Y, Xiao B and Guo J: LncRNA-RMRP promotes carcinogenesis by
acting as a miR-206 sponge and is used as a novel biomarker for
gastric cancer. Oncotarget. 7:37812–37824. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Min L, Garbutt C, Tu C, Hornicek F and
Duan Z: Potentials of long noncoding RNAs (LncRNAs) in Sarcoma:
From biomarkers to therapeutic targets. Int J Mol Sci.
18(731)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yang XZ, Cheng TT, He QJ, Lei ZY, Chi J,
Tang Z, Liao QX, Zhang H, Zeng LS and Cui SZ: LINC01133 as ceRNA
inhibits gastric cancer progression by sponging miR-106a-3p to
regulate APC expression and the Wnt/β-catenin pathway. Mol Cancer.
17(126)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yu HJ, Bahri S, Gardner V and Muftuler LT:
In vivo quantification of lumbar disc degeneration: Assessment of
ADC value using a degenerative scoring system based on Pfirrmann
framework. Eur Spine J. 24:2442–2448. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang S, Li J, Tian J, Yu Z, Gao K, Shao J,
Li A, Xing S, Dong Y, Li Z, et al: High amplitude and low frequency
cyclic mechanical strain promotes degeneration of human nucleus
pulposus cells via the NF-kappaB p65 pathway. J Cell Physiol.
233:7206–7216. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Qi X, Zhang DH, Wu N, Xiao JH, Wang X and
Ma W: ceRNA in cancer: Possible functions and clinical
implications. J Med Genet. 52:710–718. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li Z, Chen X, Xu D, Li S, Chan MTV and Wu
WKK: Circular RNAs in nucleus pulposus cell function and
intervertebral disc degeneration. Cell Prolif.
52(e12704)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li Z, Li X, Chen C, Li S, Shen J, Tse G,
Chan MTV and Wu WKK: Long non-coding RNAs in nucleus pulposus cell
function and intervertebral disc degeneration. Cell Prolif.
51(e12483)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chen WK, Yu XH, Yang W, Wang C, He WS, Yan
YG, Zhang J and Wang WJ: lncRNAs: Novel players in intervertebral
disc degeneration and osteoarthritis. Cell Prolif.
50(e12313)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wan ZY, Song F, Sun Z, Chen YF, Zhang WL,
Samartzis D, Ma CJ, Che L, Liu X, Ali MA, et al: Aberrantly
expressed long noncoding RNAs in human intervertebral disc
degeneration: A microarray related study. Arthritis Res Ther.
16(465)2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen FR, Sha SM, Wang SH, Shi HT, Dong L,
Liu D, Cheng Y, An M, Wang Y and Zhang J: RP11-81H3.2 promotes
gastric cancer progression through miR-339-HNRNPA1 interaction
network. Cancer Med. 9:2524–2534. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen W, Li K, Zhu K, Yan R, Cai QC, Li WH
and Dang CX: RP11-81H3.2 acts as an oncogene via microRNA-490-3p
inhibition and consequential Tankyrase 2 up-regulation in
hepatocellular carcinoma. Dig Dis Sci. 65:2949–2958.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ding F, Shao ZW and Xiong LM: Cell death
in intervertebral disc degeneration. Apoptosis. 18:777–785.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang F, Zhao X, Shen H and Zhang C:
Molecular mechanisms of cell death in intervertebral disc
degeneration (Review). Int J Mol Med. 37:1439–1448. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gruber HE and Hanley EN: Analysis of aging
and degeneration of the human intervertebral disc. Comparison of
surgical specimens with normal controls. Spine (Phila Pa 1976).
23:751–757. 1998.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sudo H and Minami A: Regulation of
apoptosis in nucleus pulposus cells by optimized exogenous Bcl-2
overexpression. J Orthop Res. 28:1608–1613. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sudo H and Minami A: Caspase 3 as a
therapeutic target for regulation of intervertebral disc
degeneration in rabbits. Arthritis Rheum. 63:1648–1657.
2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang YH, Zhao CQ, Jiang LS and Dai LY:
Lentiviral shRNA silencing of CHOP inhibits apoptosis induced by
cyclic stretch in rat annular cells and attenuates disc
degeneration in the rats. Apoptosis. 16:594–605. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dong X, Ni B, Fu J, Yin X, You L, Leng X,
Liang X and Ni J: Emodin induces apoptosis in human hepatocellular
carcinoma HepaRG cells via the mitochondrial caspasedependent
pathway. Oncol Rep. 40:1985–1993. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cheng F, Hu H, Sun K, Yan F and Geng Y:
miR-455-3p enhances chondrocytes apoptosis and inflammation by
targeting COL2A1 in the osteoarthritis model. Biosci Biotechnol
Biochem. 84:695–702. 2020.PubMed/NCBI View Article : Google Scholar
|