Introduction
Bronchial asthma is a heterogeneous disease
characterized by chronic airway inflammation and airway
responsiveness (1). The disease can
occur at any age, but is more common in children and is a common
cause of pediatric emergency worldwide (2). Approximately one-third of children
present with wheezing before the age of two, and about one-fifths
of children present with recurrent or persistent wheezing (3). The number of children with bronchial
asthma in China is reported to be about 30 million (4). Childhood asthma in the young age group
(aged <5 years) in developing countries is often misdiagnosed as
pneumonia without effective treatment, leading to increased
morbidity and mortality in this age group (5). Although the rate of bronchial asthma
in China has risen from 28.7% in 2008 to 39.2% in 2016, it remains
at a low level (6). With the
development of traditional Chinese medicine (TCM) research, the
advantages of TCM in the treatment of childhood bronchial asthma
have been clinically valued (7).
Therefore, it is of importance to find effective methods or drugs
to treat childhood bronchial asthma from the perspective of
TCM.
TCM has been widely used and demonstrated to be
effective in the treatment of bronchial asthma (8-10).
Zhike pingchuan granules (ZKPC) play a role in relieving coughing
and reducing sputum to relieve asthma. Their ingredients include
Gingko, Lumbricus, Perilla leaves, rhizome
Pinelliae preparata, Mibaibu stemona root, Radix
asteris, Folium eriobotryae, Platycodon
grandiflorus, Scutellaria baicalensis, apricot kernel,
peach kernel, Cortex mori and Digupi. Jin et
al (11) demonstrated that
Ginkgo lactone showed anti-inflammatory and anti-oxidant
effects in a rat model of Aβ1-40 induced Alzheimer's
disease, improving nerve injury and cognitive function. Thorpe
et al (12) indicated that
the Gingko biloba extract EGb 761 could inhibit inflammation
and thermal hyperalgesia for the treatment of inflammatory pain.
Wuwei dilong decoction could inhibit the infiltration and spread of
inflammatory cells in asthma (13).
In addition, the active ingredients of Aster tataricus and
Pinellia ternata were both demonstrated to have
anti-inflammatory properties (14,15).
Airway inflammation is an important factor in the
development of bronchial asthma. IL-6 is synthesized and secreted
by lymphocytes and mononuclear macrophages after activation and is
related to local inflammatory response, participates in the body's
inflammatory damage process and is also the main inflammatory
factor of bronchial asthma (16,17).
Janus kinase 2 (JAK2)/STAT3 signaling plays an important role in
various biological activities, such as tumorigenesis and
inflammation (18). JAK2 can
phosphorylate STAT3, which activates STAT3 and downstream target
genes (19). Studies have shown
that JAK2/STAT3 signaling is crucial for the pathogenesis of asthma
(20,21). Furthermore, Simon et al
(22) found that STAT3 inhibition
could suppress proliferation of airway smooth muscle cells in
asthma. Therefore, the JAK2/STAT3 signaling pathway plays crucial
roles in the development and progression of asthma. Yan et
al (23) demonstrated that JAK2
is associated with airway remodeling in bronchial asthma. Shi et
al (24) indicated that IL-6
could promote the activation of the JAK2/STAT3 pathway.
Therefore, the present study aimed to investigate
the therapeutic effects of ZKPC on bronchial asthma, as well as the
underlying mechanism related to the IL-6/JAK2/STAT3 pathway.
Materials and methods
Mouse model of bronchial asthma
A total of 60 male C57BL/6J mice
(specific-pathogen-free; age, 6-8 weeks; weight range, 16-20 g)
were purchased from Beijing Vital River Laboratory Animal
Technology Co., Ltd., and were maintained under a 12-h light/dark
cycle at room temperature (22±2˚C) with a humidity of 45% and
allowed ad libitum access to water and food. Each group
contained 10 mice. Normal saline (5 ml) was suspended with 200 mg
aluminum hydroxide to prepare a 4% aluminum hydroxide gel. A total
of 1.5 mg ovalbumin (OVA; Thermo Scientific Fisher, Inc.) was fully
dissolved in 2.5 ml normal saline, which was then suspended in 2.5
ml 4% aluminum hydroxide gel to prepare the sensitizing solution.
Mice was intraperitoneally injected with 0.2 ml/day of newly
prepared sensitizing solution for 15 days in total. ZKPC granules
(manufactured by Henan Shizhen Pharmaceutical Co., Ltd.) were
purchased from the Second Affiliated Hospital of Henan University
of Chinese Medicine. ZKPC solution was prepared by mixing ZKPC
granules with distilled water to obtain a final ZKPC solution
concentration of 0.1 g/ml.
In the first set of experiments, from days 15-30,
the mice in the Model group were placed in a transparent airtight
chamber and inhaled aerosolized 1% OVA solution naturally for 30
min/day (n=10). Mice in the control group were treated with normal
saline in the same manner (n=10).
In the ZKPC-Low (L) group, mice were administered
with equivalent doses of ZKPC (0.525 g/kg) by gavage 30 min prior
to aerosol inhalation (n=10). In the ZKPC-High (H) group, mice were
administered with ZKPC at four times the equivalent dosage (2.1
g/kg) by gavage 30 min prior to aerosol inhalation (n=10). At 24 h
after the last aerosol inhalation, mice were anesthetized via
intraperitoneal injection of 1% pentobarbital sodium (40 mg/kg),
and blood from the eyeballs (0.2-0.3 ml) and bronchoalveolar lavage
fluid (BALF) were obtained. Subsequently, anaesthetized mice were
killed by cervical dislocation and lung tissues were extracted from
mice after confirmation of cardiac arrest. The control group and
model group were given equal amounts of distilled water.
In the second set of experiments, mice in the
fedratinib (Fedr) group were administered with Fedr (60 mg/kg) by
gavage 30 min prior to aerosol inhalation (n=10). In the ZKPC +
Fedr group, mice were administered with Fedr (60 mg/kg) and ZKPC at
four times the equivalent dosage (2.1 g/kg) by gavage 30 min prior
to aerosol inhalation (n=10). The dose of Fedr was determined and
modified based on a previous study (25). Mice health and behavior were
monitored every day. All animal experiments were approved by the
Animal Experimental Ethics Committee of Henan University of
Traditional Chinese Medicine (approval no. 20190412WZ).
Dry/wet (D/W) ratio of the lungs
The lower lobe of the right lung was taken, of which
the wet weight was recorded. The lung dry weight was recorded after
baking in an oven for 72 h. The D/W ratio of lung was then
calculated.
Hematoxylin and eosin (H&E)
staining
Lung tissue was collected, fixed with 10% formalin
solution at 4˚C for >24 h and dehydrated in different
concentrations (low to high) of ethanol. Samples were then embedded
in paraffin, which were cut into 5 µm sections. Following
hematoxylin staining for 3 min and eosin staining for 3 min at room
temperature, the slices were sealed. Finally, the pathological
morphology of mouse lung tissue was observed under a full-field
pathological slice scanner.
ELISA
BALF and serum (stored at -80˚C) were thawed. The
levels of IL-1β, TNF-α and IL-6 in BALF and serum were detected by
IL-1β ELISA kit (cat. no. PI301), TNF-α ELISA kit (cat. no. PT512)
and IL-6 ELISA kit (cat. no. PI326), respectively, according to the
manufacturer's protocol (Beyotime Institute of Biotechnology).
Detection of oxidative stress
factors
Part of the lung tissue was prepared into 10% tissue
homogenate, which was centrifuged at 10,000 x g for 20 min at 4˚C.
The supernatant was collected and tested with reactive oxygen
species (ROS; cat. no. E-BC-K138-F; Elabscience Biotechnology
Inc.), malondialdehyde (MDA; cat. no. S0131S; Beyotime Institute of
Biotechnology) and superoxide dismutase (SOD; cat. no. S0109;
Beyotime Institute of Biotechnology) kits.
Western blot analysis
Lung tissue samples were homogenized using RIPA
buffer and centrifuged at 10,000 x g for 10 min at 4˚C. Protein
concentration was determined using the BCA method. Total protein
(25 µg) were loaded per lane and separated via 10% SDS-PAGE,
followed transfer to PVDF membranes. Subsequently, membranes were
blocked in 5% non-fat milk in TBS-Tween-20 (0.05%) buffer for 1 h
at 25˚C and incubated with primary antibodies overnight at 4˚C.
Membranes were then incubated with rabbit IgG horseradish
peroxidase-conjugated secondary antibody (cat. no. 7074; dilution,
1:1,000; Cell Signaling Technologies, Inc.) for 1 h at 25˚C. The
primary antibodies (all from Cell Signaling Technology, Inc.) used
were as follows: Anti-IL-6 (cat. no. 12912; dilution, 1:1,000),
anti-Bcl2 (cat. no. 3498; dilution, 1:1,000), anti-Bax (cat. no.
2772; dilution, 1:1,000), anti-cleaved (c) caspase-3 (cat. no.
9661; dilution, 1:1,000), anti-caspase-3 (cat. no. 9662; dilution,
1:1,000), anti-JAK2 (cat. no. 3230; dilution, 1:1,000),
anti-phosphorylated (p)-JAK2 (cat. no. 3771; dilution, 1:1,000),
anti-STAT3 (cat. no. 12640; dilution, 1:1,000), anti-p-STAT3 (cat.
no. 9145; dilution, 1:2,000) and anti-GAPDH (cat. no. 5174;
dilution, 1:1,000). Bands were visualized using an enhanced
chemiluminescence system (Amersham Biosciences; Cytiva) and band
gray values were semi-quantified using ImageJ software (version
1.0; National Institutes of Health).
Statistical analysis
Data were represented by the mean ± SD. SPSS 20.0
(IBM Corp.) was used to conduct one-way ANOVA and Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference. All experiments were replicated three
times.
Results
ZKPC improves lung injury induced by
bronchial asthma
The D/W ratio of model group was significantly lower
compared with the control group. In the ZKPC-H group, the D/W ratio
was significantly increased compared with the model group (Fig. 1A). In the model group, alveolar
spaces presented with obvious hyperemia, bleeding and cell
infiltration, and alveolar walls were not clearly observed. Upon
increasing concentrations of ZKPC, hyperemia, bleeding and cell
infiltration in tissue interspace and alveolar cavity were markedly
decreased, and the structure of most alveolar walls were complete
in the ZKPC-H group (Fig. 1B).
ZKPC improves inflammation and
oxidative stress in bronchial asthma mice
As shown in Fig. 2A,
the levels of IL-1β, TNF-α and IL-6 were significantly upregulated
in BALF, which was gradually suppressed by increased concentrations
of ZKPC. The levels of IL-1β, TNF-α and IL-6 in serum showed
similar changes to that in BALF (Fig.
2B). As shown in Fig. 2C-E, the
levels of ROS and MDA increased while SOD levels decreased in lung
tissues of the model group, and ZKPC effectively reversed the
levels of ROS, MDA and SOD.
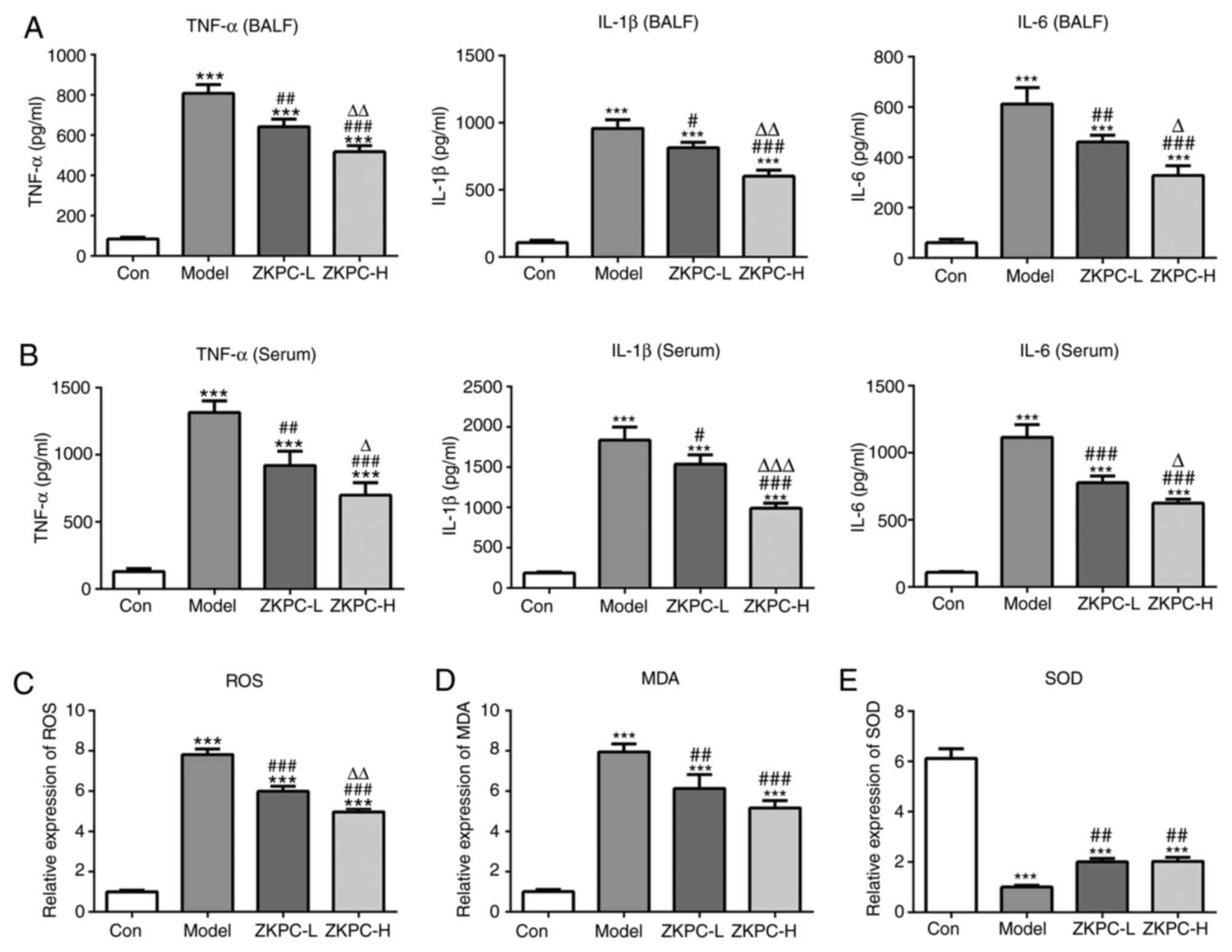 | Figure 2ZKPC improves inflammation and
oxidative stress in bronchial asthmatic mice. The levels of IL-1β,
TNF-α and IL-6 in (A) BALF and (B) serum were analyzed by ELISA.
(C) ROS levels in lung tissues was detected using a ROS kit. (D)
MDA levels in lung tissues was detected using an MDA kit. (E) SOD
levels in lung tissues was detected using a SOD kit.
***P<0.001 vs. Con group. #P<0.05,
##P<0.01 and ###P<0.001 vs. Model
group. ∆P<0.05 and ∆∆P<0.01 and
∆∆∆P<0.001 vs. ZKPC-L group. n=10. BALF,
bronchoalveolar lavage fluid; ROS, reactive oxygen species; MDA,
malondialdehyde; SOD, superoxide dismutase; ZKPC, zhike pingchuan
granules; L, low; H, high; Con, control. |
ZKPC inhibits cell apoptosis and the
IL-6/JAK2/STAT3 pathway in lung tissue
The expression levels of IL-6, Bax and c-caspase-3
increased while Bcl-2 expression levels declined in lung tissues of
model mice. Upon increasing concentrations of ZKPC, the expression
levels of IL-6, Bax and c-caspase-3 were gradually downregulated
while Bcl-2 expression levels were upregulated in lung tissues
(Fig. 3A). p-JAK2 and p-STAT3
levels in lung tissues of model mice was upregulated, which was
partially reversed by increasing concentrations of ZKPC (Fig. 3B).
 | Figure 3ZKPC inhibits cell apoptosis and the
IL-6/JAK2/STAT3 pathway in lung tissue. (A) The expression of IL-6,
Bax, Bcl-2, c-caspase-3 and pro-caspase-3 in lung and (B) levels of
t-JAK2, p-JAK2, t-STAT3 and p-STAT3 in lung tissues were determined
by Western blot analysis. **P<0.01 and
***P<0.001 vs. Con group. #P<0.05,
##P<0.01 and ###P<0.001 vs. Model
group. ∆P<0.05 vs. ZKPC-L group. n=10. c, cleaved; t,
total; p, phosphorylated; ZKPC, zhike pingchuan granules; L, low;
H, high; Con, control. |
Fedratinib enhances the improvement
effects of ZKPC on bronchial asthma-induced lung injury
Fedratinib treatment upregulated the D/W ratio
compared with the model group, and the D/W ratio was further
increased with co-treatment of ZKPC and fedratinib (Fig. 4A). Lung injury in Fedr group was
alleviated while the degree of improvement was lower compared with
the ZKPC group. Co-treatment of ZKPC and fedratinib showed the
highest improvement effects on lung tissues (Fig. 4B).
Fedratinib enhances the improvement
effects of ZKPC on inflammation and oxidative stress in bronchial
asthmatic mice
Fedratinib treatment downregulated the levels of
IL-1β, TNF-α and IL-6, and co-treatment of ZKPC and fedratinib
showed further inhibition effects on the levels of IL-1β, TNF-α and
IL-6 in both BALF and serum (Fig.
5A and B). The levels of ROS
and MDA decreased and SOD levels increased in lung tissues treated
with fedratinib, and co-treatment of ZKPC and fedratinib further
decreased levels of ROS and MDA, while SOD levels did not
significantly change (Fig.
5C-E).
 | Figure 5Fedratinib enhances the improvement
effects of ZKPC on inflammation and oxidative stress in bronchial
asthmatic mice. (A) The levels of IL-1β, TNF-α and IL-6 in BALF and
(B) serum were analyzed by ELISA. (C) ROS levels in lung tissues
were detected using a ROS kit. (D) MDA levels in lung tissues was
detected using an MDA kit. (E) SOD levels in lung tissues was
detected using a SOD kit. n=10. ***P<0.001 vs. Con
group. #P<0.05, ##P<0.01 and
###P<0.001 vs. Model group. ∆P<0.05,
∆∆P<0.01 and ∆∆∆P<0.001 vs. ZKPC group.
$P<0.05, $$P<0.01 and
$$$P<0.001 vs. Fedr group. BALF, bronchoalveolar
lavage fluid; ROS, reactive oxygen species; MDA, malondialdehyde;
SOD, superoxide dismutase; ZKPC, zhike pingchuan granules; L, low;
H, high; Con, control; Fedr, fedratinib. |
Fedratinib enhances the inhibitory
effects of ZKPC on cell apoptosis and the IL-6/JAK2/STAT3 pathway
in lung tissue
The expression levels of IL-6, Bax and c-caspase-3
declined, while Bcl-2 expression levels increased in lung tissues
of model mice treated with fedratinib. Meanwhile, the expression on
IL-6, Bax, c-caspase-3 and Bcl-2 were further declined by
co-treatment of ZKPC and fedratinib (Fig. 6A). Fedratinib treatment
downregulated p-JAK2 levels, which was further suppressed by
co-treatment of ZKPC and fedratinib. p-STAT3 levels slightly
decreased in the Fedr group and further decreased in ZKPC + Fedr
group (Fig. 6B).
 | Figure 6Fedratinib enhances the inhibitory
effects of ZKPC on cell apoptosis and the IL-6/JAK2/STAT3 pathway
in lung tissue. (A) The expression of IL-6, Bax, Bcl-2, c-caspase-3
and pro-caspase-3 and (B) levels of t-JAK2, p-JAK2, t-STAT3 and
p-STAT3 in lung tissues were determined by western blot analysis.
**P<0.01 and ***P<0.001 vs. Con group.
###P<0.001 vs. Model group. ∆∆∆P<0.001
vs. ZKPC group. $$P<0.01 vs. Fedr group. (n=10)
**P<0.01 and ***P<0.001 vs. Con group.
#P<0.05, ##P<0.01 and
###P<0.001 vs. Model group. ∆P<0.05,
∆∆P<0.01 and ∆∆∆P<0.001 vs. ZKPC group.
$$P<0.01 vs. Fedr group. c, cleaved; t, total; p,
phosphorylated; ZKPC, zhike pingchuan granules; L, low; H, high;
Con, control. |
Discussion
Asthma is presented with repeated and reversible
airway obstruction, which is often associated with airway
hyperresponsiveness and inflammation (26). Although glucocorticoid drugs are the
preferred treatment for alleviating asthma, there are many adverse
effects caused glucocorticoids, which makes a safe dose difficult
to determine. In addition, asthmatic patients are unable to inhale
glucocorticoids daily, limiting the curative effect of
glucocorticoids in asthma therapy (27,28).
Therefore, it is urgent to find newly identified drugs for the
treatment of asthma that are highly efficacious, easily managed and
with fewer adverse effects.
As a pre-inflammatory factor with multiple
biological functions, IL-6 plays an important role in the
proliferation and differentiation of immune-modulating inflammatory
response cells, and is closely related to the incidence of
inflammatory diseases, such as asthma and rheumatoid arthritis
(29). IL-6 expression was shown to
be high in the serum, lung tissues and BALF of asthmatic patients
(30,31). Kuhn et al (32) found that in mouse experiments,
significant expression of IL-6 resulted in the expansion of
alveolar cavities, followed by the infiltration of peripheral
tracheal monocytes, which gradually resulted in the thickening of
the airway wall, airway epithelial fibrosis and airway remodeling.
The present study found increased levels of IL-6, IL-1β and TNF-α
in the serum and BALF of bronchial asthmatic mice. In addition,
ZKPC treatment decreased the levels of IL-6, IL-1β and TNF-α in
serum and BALF.
A study showed that STAT3 mediated the occurrence
and development of wheezing and could reduce airway inflammation
and remodeling by inhibiting the activation of STAT3 signaling
(20). Gavino et al
(33) found that STAT3 activation
could lead to the accumulation of local inflammatory factors in the
airway, hence aggravating the original airway inflammation. This
indicated that inhibiting STAT3 expression could block the
transmission of relevant inflammatory signals, effectively treat
airway inflammation, control repeated wheezing and result in the
prevention of asthma. Endogenous STAT3 could promote the ability of
smooth muscle cells to generate blood vessels by activating
endothelial growth factors in asthmatic patients, which could be a
possible target for asthma treatment (34). JAK2 is demonstrated to be important
in the pathogenesis of asthma (35). Gong et al (36) indicated that kaempferol suppressed
eosinophil infiltration and airway inflammation in mice with
allergic asthma by inhibiting JAK2. Nakano et al (37) found that niflumic acid alleviated
IL-13-induced asthma in mice by suppressing JAK2. IL-6 can combine
with soluble IL-6R to activate glycoprotein 130 of the cell
membrane surface, thus activating JAK2/STAT3 signaling pathway
(38). The present study confirmed
that the expression levels of IL-6, p-JAK2 and p-STAT3 in lung
tissues of bronchial asthmatic mice were significantly increased,
which were downregulated by ZKPC treatment. Fedratinib, a JAK2
inhibitor, further enhanced the improvement effects of ZKPC on
bronchial asthmatic mice.
Various traditional Chinese medicines related to
pingchuan have been demonstrated to improve asthma. Liu et
al (39) indicated that
pingchuan formula alleviated asthma in mice by restoring the
balance of the T-helper cell17/regulatory T cell ratio. Pan et
al (40) found that Yanghe
Pingchuan granules could ameliorate airway remodeling in a asthma
rat model. Wang et al (41)
indicated that Wenyang Yiqi Pingchuan improved lung pathomorphology
and decreased the contents of nitric oxide and endothelin-1 in lung
tissues of rats with bronchial asthma. These compounds contain
ingredients which are also found in ZKPC. The present study found
that ZKPC could alleviate lung pathomorphology, decreased the
levels of oxidative stress and suppressed cell apoptosis in lung
tissues of bronchial asthmatic mice.
In conclusion, ZKPC partially reversed the D/W ratio
and improved the lung pathomorphology of bronchial asthmatic mice.
ZKPC inhibited inflammation, oxidative stress levels and cell
apoptosis by suppressing the IL-6/JAK2/STAT3 pathway. In addition,
the effects of ZKPC on bronchial asthma was further promoted by
fedratinib treatment.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by grants from the National
Natural Science Foundation project: Experimental study on the
regulation of TGF-1/VEGF expression in airway remodeling in
asthmatic rats based on the new theory of ‘hidden wind stagnation
and lodge phlegm’ pathogenesis (grant no. 81574020). Traditional
Chinese Medicine Scientific Research Special Project of Henan
Province (grant no. 20-21ZY2057); Henan Provincial Key Research and
Development and Promotion Special Project (Science and Technology
Targeted) (grant no. 212102311143)
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM designed the experiments and revised the
manuscript. YR and YL performed the experiments and wrote the
manuscript. YR, YL, SW, ZL, YY, XG, JH, SZ, HS, XT, QW, CC and YZ
analyzed and interpreted the data. YR, YL, SZ and HS confirmed the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Experimental Ethics Committee of Henan University of Traditional
Chinese Medicine (approval no. 20190412WZ).
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Wu J and Hong J: Highlights of bronchial
asthma in children guideline (2016 edition) for its diagnosis and
treatment. World Clinic Drugs. 39:512–517. 2018.(In Chinese).
|
|
2
|
Masoli M, Fabian D, Holt S and Beasley R:
Global Initiative for Asthma (GINA) Program. The global burden of
asthma: Executive summary of the GINA Dissemination Committee
report. Allergy. 59:469–478. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Alvarez-Alvarez I, Niu H, Guillen-Grima F
and Aguinaga-Ontoso Ι: Meta-analysis of prevalence of wheezing and
recurrent wheezing in infants. Allergol Immunopathol (Madr).
46:210–217. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu SJ, Wang TT, Cao SY, Tan YQ and Chen
LZ: A Meta analysis of risk factors for asthma in Chinese children.
Zhongguo Dang Dai Er Ke Za Zhi. 20:218–223. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
5
|
Østergaard MS, Nantanda R, Tumwine JK and
Aabenhus R: Childhood asthma in low income countries: An invisible
killer? Prim Care Respir J. 21:214–219. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lin JT, Wang WQ, Zhou X, Yin KS, Liu CT,
Wang CZ, Huang M, Chen P, Yuan YD, Cai SX, et al: Trends of asthma
control, disease management and perception in China. Zhonghua Jie
He He Hu Xi Za Zhi. 41:191–195. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
7
|
Hsieh KH: Evaluation of efficacy of
traditional Chinese medicines in the treatment of childhood
bronchial asthma: Clinical trial, immunological tests and animal
study. Taiwan Asthma Study Group. Pediatr Allergy Immunol.
7:130–140. 1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang R and Lin J: Analysis of the
mechanism of Zhichuanling oral liquid in treating bronchial asthma
based on network pharmacology. Evid Based Complement Alternat Med.
2020(1875980)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang L, Zheng X, Hui Y, Wang B, Yang Y,
Feng X, Zhang T, Ma L and Zhang X: Adjuvant treatment with
Xiaoqinglong formula for bronchial asthma: Protocol of systematic
review and meta-analysis. Medicine (Baltimore).
98(e17053)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Su YH, Yu XY, Geng LM, Su KG and Guo YY:
Oral administration versus iontophoresis of Sang-Su decoction (a
formula for dispersing lung to calm panting and resolve phlegm and
for dredging collateral) in the treatment of acute bronchial
asthma: A comparative study on their efficacy and impacts on the
prognosis. Int J Clin Exp Med. 11:1305–1311. 2018.
|
|
11
|
Jin X, Wang D, Xie J, Zhang L and Liu Q:
Effect of Ginkgo Lactone on cholinergic neurotransmitters,
oxidative stress and inflammation in AD model rats induced by
Aβ1-40. J Chin Med Mater. 11:2693–2696. 2019.(In
Chinese).
|
|
12
|
Thorpe LB, Goldie M and Dolan S: Central
and local administration of Gingko biloba extract EGb
761® inhibits thermal hyperalgesia and inflammation in
the rat carrageenan model. Anesth Analg. 112:1226–1231.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li XH, Tu XY, Zhang DX, Xu JF, Wang WY,
Zhang Y and Du YM: Effects of wuwei dilong decoction on
inflammatory cells and cytokines in asthma model guinea pigs. J
Tradit Chin Med. 29:220–223. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wu WB, Zhu C and Luo C: Research on the
protective effect of alkaloids Pinelliaf Rhizoma on
inflammatory injury of lung epithelial cells. J Inner Mong Agric
Univ. 39:1–8. 2018.
|
|
15
|
Su XD, Jang HJ, Li HX, Kim YH and Yang SY:
Identification of potential inflammatory inhibitors from Aster
tataricus. Bioorg Chem. 92:1–9. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Doganci A, Sauer K, Karwot R and Finotto
S: Pathological role of IL-6 in the experimental allergic bronchial
asthma in mice. Clin Rev Allergy Immunol. 28:257–270.
2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Martinez-Nunez RT, Bondanese VP, Louafi F,
Francisco-Garcia AS, Rupani H, Bedke N, Holgate S, Howarth PH,
Davies DE and Sanchez-Elsner T: A microRNA network dysregulated in
asthma controls IL-6 production in bronchial epithelial cells. PLoS
One. 9(e111659)2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu Z, Gan L, Zhou Z, Jin W and Sun C:
SOCS3 promotes inflammation and apoptosis via inhibiting JAK2/STAT3
signaling pathway in 3T3-L1 adipocyte. Immunobiology. 220:947–953.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Imada K and Leonard WJ: The Jak-STAT
pathway. Mol Immunol. 37:1–11. 2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang N, Liu K, Liu J, Gao X, Zeng Z,
Zhang Y and Chen J: Interleukin-37 alleviates airway inflammation
and remodeling in asthma via inhibiting the activation of NF-κB and
STAT3 signalings. Int Immunopharmacol. 55:198–204. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Simeone-Penney MC, Severgnini M, Tu P,
Homer RJ, Mariani TJ, Cohn L and Simon AR: Airway epithelial STAT3
is required for allergic inflammation in a murine model of asthma.
J Immunol. 178:6191–6199. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Simon AR, Takahashi S, Severgnini M,
Fanburg BL and Cochran BH: Role of the JAK-STAT pathway in
PDGF-stimulated proliferation of human airway smooth muscle cells.
Am J Physiol Lung Cell Mol Physiol. 282:L1296–L1304.
2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yan YR, Luo Y, Zhong M and Shao L:
miR-216a inhibits proliferation and promotes apoptosis of human
airway smooth muscle cells by targeting JAK2. J Asthma. 56:938–946.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shi J, Li J, Yang S, Hu X, Chen J, Feng J,
Shi T, He Y, Mei Z, He W, et al: LncRNA SNHG3 is activated by E2F1
and promotes proliferation and migration of non-small-cell lung
cancer cells through activating TGF-β pathway and IL-6/JAK2/STAT3
pathway. J Cell Physiol. 235:2891–2900. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tang Y: Lipid cholesterol mediates the
mechanism of arterial thrombosis by regulating the proliferation
and activation of peripheral blood cells (unpublished PhD thesis).
Jilin University, 2020.
|
|
26
|
Papi A, Brightling C, Pedersen SE and
Reddel HK: Asthma. Lancet. 391:783–800. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Brannan JD: Bronchial hyperresponsiveness
in the assessment of asthma control: Airway hyperresponsiveness in
asthma: its measurement and clinical significance. Chest. 1138
(Suppl 2):11S–17S. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sarnes E, Crofford L, Watson M, Dennis G,
Kan H and Bass D: Incidence and US costs of
corticosteroid-associated adverse events: A systematic literature
review. Clin Ther. 33:1413–1432. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Papanicolaou DA, Wilder RL, Manolagas SC
and Chrousos GP: The pathophysiologic roles of interleukin-6 in
human disease. Ann Intern Med. 128:127–137. 1998.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dimitrova D, Youroukova V,
Ivanova-Todorova E, Tumangelova-Yuzeir K and Velikova T: Serum
levels of IL-5, IL-6, IL-8, IL-13 and IL-17A in pre-defined groups
of adult patients with moderate and severe bronchial asthma. Respir
Med. 154:144–154. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kado S, Nagase T and Nagata N: Circulating
levels of interleukin-6, its soluble receptor and
interleukin-6/interleukin-6 receptor complexes in patients with
type 2 diabetes mellitus. Acta Diabetol. 36:67–72. 1999.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kuhn C III, Homer RJ, Zhu Z, Ward N,
Flavell RA, Geba GP and Elias JA: Airway hyperresponsiveness and
airway obstruction in transgenic mice. Am J Respir Cell Mol Biol.
22:289–295. 2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gavino AC, Nahmod K, Bharadwaj U,
Makedonas G and Tweardy DJ: STAT3 inhibition prevents lung
inflammation, remodeling, and accumulation of Th2 and Th17 cells in
a murine asthma model. Allergy. 71:1684–1692. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lv J, Sun B, Mai Z, Jiang M and Du J:
STAT3 potentiates the ability of airway smooth muscle cells to
promote angiogenesis by regulating VEGF signalling. Exp Physiol.
102:598–606. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Dickason RR, English JD and Huston DP:
Engineering of a functional interleukin-5 monomer: A paradigm for
redesigning helical bundle cytokines with therapeutic potential in
allergy and asthma. J Mol Med (Berl). 74:535–546. 1996.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gong JH, Shin D, Han SY, Kim JL and Kang
YH: Kaempferol suppresses eosionphil infiltration and airway
inflammation in airway epithelial cells and in mice with allergic
asthma. J Nutr. 142:47–56. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nakano T, Inoue H, Fukuyama S, Matsumoto
K, Matsumura M, Tsuda M, Matsumoto T, Aizawa H and Nakanishi Y:
Niflumic acid suppresses interleukin-13-induced asthma phenotypes.
Am J Respir Crit Care Med. 173:1216–1221. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pitman H, Innes BA, Robson SC, Bulmer JN
and Lash GE: Altered expression of interleukin-6, interleukin-8 and
their receptors in decidua of women with sporadic miscarriage. Hum
Reprod. 28:2075–2086. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Liu F, Yu J, Bai L, Xue Z and Zhang X:
Pingchuan formula improves asthma via restoration of the Th17/Treg
balance in a mouse model. BMC Complement Altern Med.
15(234)2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pan LY, Han YQ, Wang YZ, Chen QQ, Wu Y and
Sun Y: Mechanism of Yanghe Pingchuan granules treatment for airway
remodeling in asthma. Drug Des Devel Ther. 12:1941–1951.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang XH, Yang MX and Yu WT: Effect of
Wenyang Yiqi Pingchuan recipe on pathomorphology of lung and its
regulation on lung tissue contents of nitric oxide and endothelin-1
in rats with bronchial asthma. Zhongguo Zhong Xi Yi Jie He Za Zhi.
29:435–438. 2009.PubMed/NCBI(In Chinese).
|




















