Introduction
Diabetic cardiomyopathy is a common complication of
diabetes. Due to metabolic disorders and microvascular lesions,
diabetic cardiomyopathy leads to extensive focal myocardial
necrosis and subclinical cardiac dysfunction, which eventually
develops into heart failure, arrhythmia, cardiac shock and even
sudden death in severe cases (1-3)
Rutin is a flavonoid compound extracted from plants,
and has been reported to exert antioxidant, anti-inflammatory,
anti-allergic and anti-viral effects (4,5). Rutin
is thought to act on multiple tissues and organs in the body. For
example, rutin has been demonstrated to protect gastric mucosal
cells from damage (6) and has been
suggested to promote the growth and proliferation of osteoblasts
and inhibit osteoporosis (7). Rutin
may also regulate the development of rat immune organs, including
the chest, kidney and spleen (8).
Traditional Chinese medicine rutin is thought to target a variety
of signaling pathways to regulate tissues and organs (9). Previous studies have indicated that
rutin can alleviate myocardial dysfunction in diabetic rats
(10,11). Rutin also has a protective effect on
cobalt chloride-induced hypoxia injury of myocardial cells
(12). Moreover, rutin inhibits
apoptosis induced by myocardial ischemia reperfusion and protects
H9C2 cells from hydrogen peroxide-mediated damage via ERK1/2 and
PI3K/AKT signaling (13).
Endoplasmic reticulum stress (ERS) is a cellular
process induced by a variety of severe stress conditions, including
hypoxia, ischemia, heat shock, gene mutation and oxidative stress
(14,15). ERS affects the folding of proteins
in the endoplasmic reticulum and leads to the activation of the
unfolded protein response (UPR) (16). The UPR mediates ERS and serves a
role in activation of three major signaling pathways, including
PERK, insulin response element 1 (IRE1) and activating
transcription factor 6 (ATF6) (17-19).
ERS-mediated apoptosis is associated with the IRE1α-mediated C-Jun
N-terminal kinase cascade and the PERK-dependent induction of the
pro-apoptotic transcription factor C/EBP-homologous protein (CHOP)
pathway (20-22).
Therefore, it was hypothesized that prolonged or excessive ERS may
lead to apoptosis, and a decrease in the level of ERS-induced
apoptosis may alleviate diabetic cardiomyopathy. In addition, rutin
has been reported to exert a protective effect against
lipopolysaccharide-induced mastitis by inhibiting the activation of
the nuclear factor-κB signaling pathway and reducing ERS (23). However, to the best of our
knowledge, studies on the effects of rutin on hyperglycemia-induced
myocardial model cell injury from the perspective of ERS have not
been reported.
The present study induced myocardial model cell
damage at the cellular level, determined the effect of rutin on
myocardial cell damage and discussed its underlying mechanism. The
present study offers a strong theoretical basis for the use of
rutin in the treatment of diabetic cardiomyopathy.
Materials and methods
Cell culture
H9C2 myoblast cells, a model of cardiomyocytes, were
purchased from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences and were cultured in DMEM supplemented
with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.). The cells
were cultured in a humidified 37˚C incubator with 5%
CO2. In the present study, the normal glucose group (5
mM glucose; NG group) was set as a control group, while the
mannitol group (MA; 45 µM for 24 h; Sigma-Aldrich; Merck KGaA) was
established to exclude the osmotic pressure effects of high glucose
(HG) on cells. H9C2 cells were incubated in complete medium
containing 35 mM glucose (final concentration in the medium) for 24
h at 37˚C, which was referred to as the HG group (24). H9C2 cardiac cells were administered
rutin (cat. no. 19362115; Sigma-Aldrich; Merck KGaA) at final
concentrations of 2, 10 and 50 µM for 24 h at 37˚C (13). Additionally, H9C2 cells were treated
with 2 µM ERS activator thapsigargin (TG; cat. no. T7459; Thermo
Fisher Scientific, Inc.) for 4 h.
Cell Counting Kit (CCK)-8
The viability of H9C2 cells was assessed using a
CCK-8 assay (Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. Cells were seeded (1x103
cells/well) into 96-well plates and were treated with rutin before
10 µl of CCK-8 solution was added to each well and the plates
incubated for 4 h at 37˚C. The absorbance was recorded at 450 nm
using a microplate reader.
Measurement of serum lactate
dehydrogenase (LDH)
A Cytotox 96 nonradioactive cytotoxicity assay kit
(cat. no. G1780; Promega Corporation) was used according to the
manufacturer's instructions. Cells were seeded (1x103
cells/well) into 96-well plates and treated with Rutin. Then, 10 µl
cell supernatant was mixed with 100 µl LDH reaction reagent at room
temperature for 30 min. The absorbance was determined using an
enzyme-linked immunosorbent assay reader (Victor X3; PerkinElmer,
Inc.) with a 490 nm filter.
TUNEL assay
Cell apoptosis was analyzed using a
Click-iT® TUNEL Alexa Fluor® Imaging assay
(cat. no. C10245; Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Cells were seeded
(1x106 cells/well) into 6-well plates and treated with
rutin. Then, cells were collected and washed three times with PBS.
Following fixation with 4% paraformaldehyde at room temperature for
20 min, the cells were washed twice with PBS and 0.2% Triton-X-100
was added to the cells at room temperature for 5 min. Subsequently,
50 µl TUNEL assay solution (Boehringer Mannheim) was added to the
cells and incubated at 37˚C in the dark for 60 min. The detection
solution was discarded and cells were washed three times with PBS.
Images of the FITC-labeled TUNEL-positive cells were captured using
an Olympus IX70 inverted microscope (magnification, x200; Olympus
Corporation) according to the manufacturer's instructions. Cells
were stained with DAPI (Thermo Fisher Scientific, Inc.) at room
temperature for 15 mins.
Western blotting
Cells were seeded (1x106 cells/well) into
6-well plates and treated with Rutin. The H9C2 cells were collected
and lysed with RIPA lysis buffer (Beyotime Institute of
Biotechnology) for 30 min on ice. Subsequently, protein
concentration was determined using a BCA protein assay kit (Bio-Rad
Laboratories, Inc.). A total of 40 µg protein was loaded onto each
lane of 10% SDS-polyacrylamide gels to separate various proteins,
which were subsequently transferred onto PVDF membranes. The
membranes were blocked with 10% skimmed milk for 2 h at room
temperature, followed by incubation with primary antibodies
overnight at 4˚C. Subsequently, the membranes were incubated with
goat anti-rabbit horseradish peroxidase-conjugated IgG secondary
antibodies (1:5,000; cat. no. AA24142, Abcam) at room temperature
for 1 h. The signals were detected using an enhanced
chemiluminescence reagent (Cytiva) and ImageJ software (version
146; National Institutes of Health) was used to analyze the fold
changes of protein expression levels. The primary antibodies
anti-Bax (1:1,000; cat. no. 14796S), anti-caspase-3 (1:1,000; cat.
no. 9953S), anti-cleaved caspase-3 (1:1,000; cat. no. 9953S),
anti-Bcl-2 (1:1,000; cat. no. 15071S), anti-HSPA5 (1:1,000; cat.
no. 3183S), anti-IRE1α (1:1,000; cat. no. 3294T), anti-X-box
binding protein 1 (XBP1; 1:1,000; cat. no. 12782S), anti-ATF6
(1:1,000; cat. no. 65880T) anti-CHOP (1:1,000; cat. no. 2895T) and
anti-GAPDH (1:1,000; cat. no. 5174S) were obtained from Cell
Signaling Technology, Inc. In addition, anti-cleaved caspase-12
(1:1,000; cat. no. ab62484) and anti-caspase-12 (1:1,000; cat. no.
ab8177) antibodies were obtained from Abcam.
Statistical analysis
Data are presented as the mean ± SD (n≥3).
Statistical analyses were performed using SPSS statistical software
(version 22.0; IBM Corp.). Differences between multiple groups were
assessed using a one-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Rutin inhibits the apoptosis of H9C2
cells stimulated by HG
The structure of rutin is presented in Fig. 1. Different concentrations of rutin
(2, 10 and 50 µM) were applied to H9C2 cells and a CCK-8 assay was
used to detect the activity of H9C2 cells. Rutin had no significant
effect on the viability of H9C2 cells (Fig. 2A). This indicated that the
concentration of rutin used was not cytotoxic to H9C2 cells.
HG-stimulated H9C2 cells were treated with different concentrations
of rutin and the cells were sorted into the NG, MA, HG, HG + 2 µM
rutin, HG +10 µM rutin and HG + 50 µM rutin groups. The results of
the CCK-8 assay demonstrated that, compared with the MA group, cell
viability was reduced in the HG group. Moreover, after
administering increasing concentrations of rutin, cell viability
was enhanced in a concentration-dependent manner (Fig. 2B).
An LDH kit was used to detect the effect of rutin on
cytotoxicity induced by HG. The results demonstrated that
cytotoxicity was significantly increased by HG in comparison with
MA. After rutin was administered, the HG-induced cytotoxicity
gradually decreased as the concentration of rutin increased
(Fig. 2C).
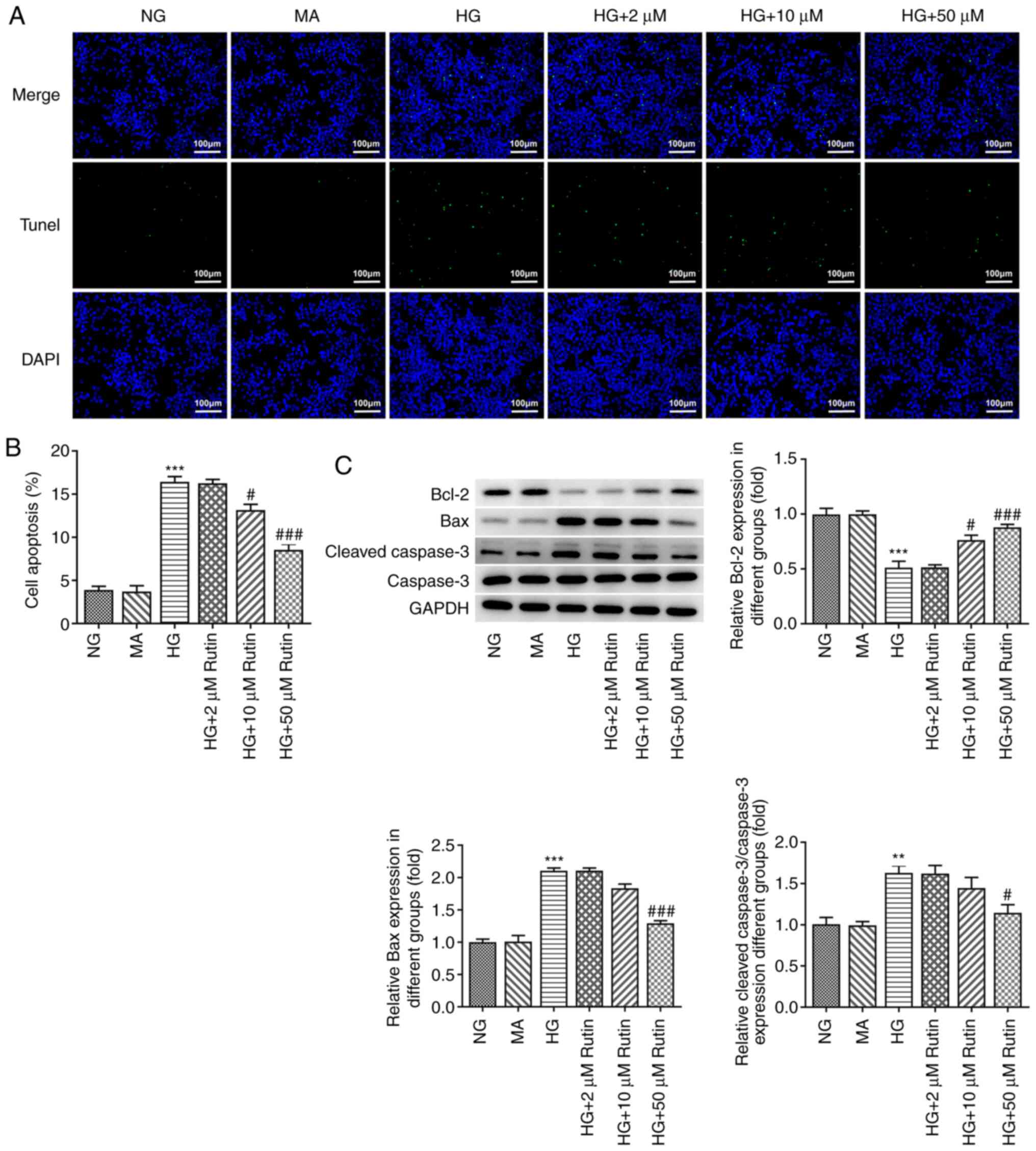
Apoptosis was assessed using a TUNEL assay, and the
results are presented in Fig. 3.
Compared with the MA group, the apoptotic rate was significantly
increased after HG incubation (Fig.
3A), accompanied by increased expression levels of Bax and
cleaved caspase-3 and the reduced expression of Bcl-2 (Fig. 3B). Compared with the HG group, the
apoptotic rate was significantly reduced in the HG + 2 µM rutin
group, the HG + 10 µM rutin group and the HG + 50 µM Rutin group,
accompanied by decreased expression levels of Bax and cleaved
caspase-3, and the increased expression of Bcl-2. These results
indicated that Rutin may inhibit the apoptosis of H9C2 cells
induced by HG.
Rutin inhibits ERS in HCc2 cells
treated with HG
Western blotting was used to determine the
expression levels of the proteins GRP78, IRE1α, XBP1, ATF6, CHOP,
cleaved caspase 12 and caspase 12, which are associated with
ERS-related pathways. It was revealed that, compared with MA
treatment, expression levels of the ERS-related proteins GRP78,
IRE1α, XBP1, ATF6, CHOP and cleaved caspase-12 were significantly
increased in the HG treatment group, indicating increased ERS
levels. Compared with the HG group, the expression levels of
ERS-related proteins in the HG + 2 µM rutin group and the HG +10 µM
rutin group were decreased, but this was not significant; however,
the expression levels of ERS-related proteins in the HG + 50 µM
rutin group were significantly decreased (Fig. 4). Therefore, 50 µM rutin was
selected for subsequent experiments. These results preliminarily
indicated that rutin may inhibit the ERS of H9C2 cells treated with
HG.
 | Figure 4Rutin inhibits ERS in H9C2 cells
treated with HG. Western blotting analysis was used to determine
the expression levels of ERS related proteins. ERS, endoplasmic
reticulum stress; HG, high glucose; NG, normal glucose; MA,
mannitol; HSPA5, heat shock protein A5; IRE1α, insulin response
element 1α; XBP1, X-box binding protein 1; CHOP, C/EBP-homologous
protein; ATF6, activating transcription factor 6.
***P<0.001 vs. MA. #P<0.05,
##P<0.01, ###P<0.001 vs. HG. |
TG reverses the anti-apoptotic effect
of Rutin on H9C2 cells induced by HG
To further verify the results, 2 µM of the ERS
activator TG was added to cells for 4 h, and the cells were divided
into the NG group, MA group, HG group, HG + rutin group and HG +
rutin + TG group. It was found that, compared with the HG + rutin
group, the viability of the HG + rutin + TG group was significantly
decreased (Fig. 5A), while the
cytotoxicity (Fig. 5B) and
apoptosis (Fig. 5C and D) levels were significantly increased.
These effects were accompanied by increased expression levels of
Bax and cleaved caspase-3, and the decreased expression of Bcl-2
(Fig. 5E). These results
demonstrated that TG reversed the anti-apoptotic effect of rutin on
H9C2 cells treated with HG.
 | Figure 5TG reverses the anti-apoptotic effect
of rutin on H9C2 cells induced by high glucose. (A) Cell viability
was determined by CCK-8. (B) LDH kit was used to determine
cytotoxicity. (C) Tunel assay determined the apoptosis level of
cells. (D) Quantitative analysis of the apoptosis level in cells.
(E) Western blot analysis was used to determine the expression
levels of apoptosis related proteins. TG, thapsigargin; CCK-8, cell
counting kit-8; LDH, lactate dehydrogenase; HG, high glucose; NG,
normal glucose; MA, mannitol. **P<0.01,
***P<0.001 vs. MA. #P<0.05,
##P<0.01, ###P<0.001 vs. HG.
ΔP<0.05, ΔΔΔP<0.001 vs. HG + Rutin. |
TG reverses the anti-ERS effect of
rutin on H9C2 cells treated with HG
ERS-related proteins were also detected in the
cells, as presented in Fig. 6.
Compared with the HG + rutin group, GRP78, IRE1α, XBP1, ATF6, CHOP
and cleaved caspase-12 expression levels were significantly
increased in the HG + rutin + TG group, indicating that TG reversed
the anti-ERS effect of Rutin in H9C2 cells treated with HG.
 | Figure 6TG reverses the anti-ERS effect of
rutin on H9C2 cells treated with HG. Western blotting analysis was
used to determine the expression levels of ERS related proteins.
TG, thapsigargin; ERS, endoplasmic reticulum stress; HG, high
glucose; NG, normal glucose; MA, mannitol; HSPA5, heat shock
protein A5; IRE1α, insulin response element 1α; XBP1, X-box binding
protein 1; CHOP, C/EBP-homologous protein; ATF6, activating
transcription factor 6 **P<0.01,
***P<0.001 vs. MA. #P<0.05,
##P<0.01, ###P<0.001 vs. HG.
ΔP<0.05, ΔΔP<0.01,
ΔΔΔP<0.001 vs. HG + Rutin. |
Discussion
Diabetic cardiomyopathy is one of the main
complications of diabetes and one of the largest causes of
mortality in patients with diabetes (25). Moreover, its pathogenesis is complex
and has not been fully defined (26). In recent years, studies have
reported that excessive ERS lead to the apoptosis of cardiomyocytes
(27-29).
Thus, how to alleviate ERS-induced injury is a hot topic of
research. The present study induced a model of myocardial damage in
H9C2 myoblasts using HG. The results demonstrated that cell
viability, was reduced while cytotoxicity, apoptosis and the ERS
level were increased after HG treatment. These experimental results
were consistent with those of Cao et al (24), indicating that the diabetic
myocardial injury model was successfully induced.
In the present study H9C2 myoblasts were incubated
with HG to form a diabetic myocardial injury model. However, a
limitation of this work is that the regulatory effect of rutin on
diabetic myocardial injury in rats was not determined in
vivo. In addition, though the present study explored the effect
of rutin on HG-induced myocardial apoptosis and injury,
inflammation, which plays an important role in the myocardial
injury induced by high glucose (24) was not addressed. In future
experiments, our research group will address these limitations.
A previous study revealed that rutin alleviated
hypoxia/reoxygenation myocardial cell injury by upregulating
sirtuin 1 expression (30). Rutin
also has a protective effect against cardiotoxicity induced by
pirobilin via the TGF-β1/p38MAPK signaling pathway (31). In addition, rutin improves metabolic
acidosis and fibrosis in rats with alloxan-induced diabetic
nephropathy and cardiomyopathy (32). However, to the best of our
knowledge, the role of rutin in HG-induced cardiomyocyte injury has
not been previously reported. The present results indicated that
rutin may inhibit the apoptosis and ERS response of HG-treated H9C2
cells and may therefore be useful in the treatment of diabetic
cardiomyopathy.
GRP78 is an important substance involved in the
folding of proteins in ERS. Moreover, its expression level
increases significantly during ERS, and it can be used as a marker
molecule for ERS (33). The
endoplasmic reticulum and a steady state of cell function are vital
to balance the proteome, and so, when cells are in a state of ERS,
a series of regulatory mechanisms are rapidly activated to combat
this stress state (34). Among
them, the UPR is the major regulatory mechanism in response to ERS
(35). The UPR controls the
expression levels of endoplasmic reticulum-associated proteins via
three protein receptors located in the endoplasmic reticulum (PERK,
IRE1α and ATF6) (36,37). In addition, activation of caspase 12
has been revealed to be mainly associated with ERS (38,39).
Therefore, the present study assessed ERS status by determining the
expression levels of GRP78, IRE1α, XBP1, ATF6, CHOP, cleaved
caspase 12 and caspase 12. The present study found that after HG
administration, the expression levels of ERS-related proteins were
significantly increased in comparison with controls. After the
administration of rutin, the HG-induced expression levels of
ERS-related proteins were significantly reduced, though the
administration of TG could significantly reverse the inhibitory
effect of rutin on HG-induced ERS in cardiomyocytes. Thus, the
present study demonstrated that rutin could inhibit the ERS
response to reduce HG-induced myocardial cell injury.
In conclusion, rutin may alleviate cardiomyocyte
injury induced by HG through inhibition of apoptosis and ERS. The
present study provides a theoretical basis for the treatment of
myocardial injury with rutin.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW made substantial contributions to the conception
and design of the study, and the acquisition of data. RW and JL
made substantial contributions to analysis and interpretation of
data. ZY confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lorenzo-Almoros A, Cepeda-Rodrigo JM and
Lorenzo O: Diabetic cardiomyopathy. Rev Clin Esp, 2020 (In English,
Spanish) (Epub ahead of print).
|
|
2
|
Xia L and Song M: Role of non-coding RNA
in diabetic cardiomyopathy. Adv Exp Med Biol. 1229:181–195.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tan Y, Zhang Z, Zheng C, Wintergerst KA,
Keller BB and Cai L: Mechanisms of diabetic cardiomyopathy and
potential therapeutic strategies: Preclinical and clinical
evidence. Nat Rev Cardiol. 17:585–607. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hosseinzadeh H and Nassiri-Asl M: Review
of the protective effects of rutin on the metabolic function as an
important dietary flavonoid. J Endocrinol Invest. 37:783–788.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ganeshpurkar A and Saluja AK: The
pharmacological potential of rutin. Saudi Pharm J. 25:149–164.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dubey S, Ganeshpurkar A, Shrivastava A,
Bansal D and Dubey N: Rutin exerts antiulcer effect by inhibiting
the gastric proton pump. Indian J Pharmacol. 45:415–417.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ruangsuriya J, Charumanee S, Jiranusornkul
S, Sirisa-Ard P, Sirithunyalug B, Sirithunyalug J, Pattananandecha
T and Saenjum C: Depletion of β-sitosterol and enrichment of
quercetin and rutin in Cissus quadrangularis Linn fraction enhanced
osteogenic but reduced osteoclastogenic marker expression. BMC
Complement Med Ther. 20(105)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Manzoni AG, Passos DF, Leitemperger JW,
Storck TR, Doleski PH, Jantsch MH, Loro VL and Leal DBR:
Hyperlipidemia-induced lipotoxicity and immune activation in rats
are prevented by curcumin and rutin. Int Immunopharmacol.
81(106217)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nouri Z, Fakhri S, Nouri K, Wallace CE,
Farzaei MH and Bishayee A: Targeting multiple signaling pathways in
cancer: The rutin therapeutic approach. Cancers (Basel).
12(2276)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Guimaraes JF, Muzio BP, Rosa CM,
Nascimento AF, Sugizaki MM, Fernandes AA, Cicogna AC, Padovani CR,
Okoshi MP and Okoshi K: Rutin administration attenuates myocardial
dysfunction in diabetic rats. Cardiovasc Diabetol.
14(90)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang R, Shi Z, Chen L, Zhang Y, Li J and
An Y: Rutin alleviates diabetic cardiomyopathy and improves cardiac
function in diabetic ApoEknockout mice. Eur J Pharmacol.
814:151–160. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sundaram RL, Sali VK and Vasanthi HR:
Protective effect of rutin isolated from Spermococe hispida against
cobalt chloride-induced hypoxic injury in H9c2 cells by inhibiting
oxidative stress and inducing apoptosis. Phytomedicine. 51:196–204.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jeong JJ, Ha YM, Jin YC, Lee EJ, Kim JS,
Kim HJ, Seo HG, Lee JH, Kang SS, Kim YS and Chang KC: Rutin from
Lonicera japonica inhibits myocardial ischemia/reperfusion-induced
apoptosis in vivo and protects H9c2 cells against hydrogen
peroxide-mediated injury via ERK1/2 and PI3K/Akt signals in vitro.
Food Chem Toxicol. 47:1569–1576. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Oakes SA: Endoplasmic reticulum stress
signaling in cancer cells. Am J Pathol. 190:934–946.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu Q, Korner H, Wu H and Wei W:
Endoplasmic reticulum stress in autoimmune diseases. Immunobiology.
225(151881)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hetz C and Saxena S: ER stress and the
unfolded protein response in neurodegeneration. Nat Rev Neurol.
13:477–491. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang Y, Cao L and Liu X: Ghrelin
alleviates endoplasmic reticulum stress and inflammation-mediated
reproductive dysfunction induced by stress. J Assist Reprod Genet.
36:2357–2366. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lima NCR, Melo TQ, Sakugawa AYS, Melo KP
and Ferrari MFR: Restoration of Rab1 levels prevents endoplasmic
reticulum stress in hippocampal cells during protein aggregation
triggered by rotenone. Neuroscience. 419:5–13. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Brenjian S, Moini A, Yamini N, Kashani L,
Faridmojtahedi M, Bahramrezaie M and Amidi F: Resveratrol treatment
in patients with polycystic ovary syndrome decreased
pro-inflammatory and endoplasmic reticulum stress markers. Am J
Reprod Immunol. 83(e13186)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Suzuki R, Fujiwara Y, Saito M, Arakawa S,
Shirakawa JI, Yamanaka M, Komohara Y, Marumo K and Nagai R:
Intracellular accumulation of advanced glycation end products
induces osteoblast apoptosis via endoplasmic reticulum stress. J
Bone Miner Res. 35:1992–2003. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Villalobos-Labra R, Subiabre M, Toledo F,
Pardo F and Sobrevia L: Endoplasmic reticulum stress and
development of insulin resistance in adipose, skeletal, liver, and
foetoplacental tissue in diabesity. Mol Aspects Med. 66:49–61.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rozpedek W, Pytel D, Mucha B, Leszczynska
H, Diehl JA and Majsterek I: The Role of the PERK/eIF2α/ATF4/CHOP
signaling pathway in tumor progression during endoplasmic reticulum
stress. Curr Mol Med. 16:533–544. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Su S, Li X, Li S, Ming P, Huang Y, Dong Y,
Ding H, Feng S, Li J, Wang X, et al: Rutin protects against
lipopolysaccharide-induced mastitis by inhibiting the activation of
the NF-κB signaling pathway and attenuating endoplasmic reticulum
stress. Inflammopharmacology. 27:77–88. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cao R, Fang D, Wang J, Yu Y, Ye H, Kang P,
Li Z, Wang H and Gao Q: ALDH2 overexpression alleviates high
glucose-induced cardiotoxicity by inhibiting NLRP3 inflammasome
activation. J Diabetes Res. 2019(4857921)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bugger H and Abel ED: Molecular mechanisms
of diabetic cardiomyopathy. Diabetologia. 57:660–671.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tadic M, Cuspidi C, Calicchio F, Grassi G
and Mancia G: Diabetic cardiomyopathy: How can cardiac magnetic
resonance help? Acta Diabetol. 57:1027–1034. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zeng Z, Huang N, Zhang Y, Wang Y, Su Y,
Zhang H and An Y: CTCF inhibits endoplasmic reticulum stress and
apoptosis in cardiomyocytes by upregulating RYR2 via inhibiting
S100A1. Life Sci. 242(117158)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li L, Peng X, Guo L, Zhao Y and Cheng Q:
Sepsis causes heart injury through endoplasmic reticulum
stress-mediated apoptosis signaling pathway. Int J Clin Exp Pathol.
13:964–971. 2020.PubMed/NCBI
|
|
29
|
Wilson AJ, Gill EK, Abudalo RA, Edgar KS,
Watson CJ and Grieve DJ: Reactive oxygen species signalling in the
diabetic heart: Emerging prospect for therapeutic targeting. Heart.
104:293–299. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yang H, Wang C, Zhang L, Lv J and Ni H:
Rutin alleviates hypoxia/reoxygenation-induced injury in myocardial
cells by up-regulating SIRT1 expression. Chem Biol Interact.
297:44–49. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang Y, Zhang Y, Sun B, Tong Q and Ren L:
Rutin protects against pirarubicin-induced cardiotoxicity through
TGF-β1-p38 MAPK signaling pathway. Evid Based Complement Alternat
Med. 2017(1759385)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ganesan D, Albert A, Paul E,
Ananthapadmanabhan K, Andiappan R and Sadasivam SG: Rutin
ameliorates metabolic acidosis and fibrosis in alloxan induced
diabetic nephropathy and cardiomyopathy in experimental rats. Mol
Cell Biochem. 471:41–50. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ardic S, Gumrukcu A, Gonenc Cekic O, Erdem
M, Reis Kose GD, Demir S, Kose B, Yulug E, Mentese A and Turedi S:
The value of endoplasmic reticulum stress markers (GRP78 and CHOP)
in the diagnosis of acute mesenteric ischemia. Am J Emerg Med.
37:596–602. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Oakes SA and Papa FR: The role of
endoplasmic reticulum stress in human pathology. Annu Rev Pathol.
10:173–194. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hetz C and Papa FR: The unfolded protein
response and cell fate control. Mol Cell. 69:169–181.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Gong J, Wang XZ, Wang T, Chen JJ, Xie XY,
Hu H, Yu F, Liu HL, Jiang XY and Fan HD: Molecular signal networks
and regulating mechanisms of the unfolded protein response. J
Zhejiang Univ Sci B. 18:1–14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wu Z, Wang H, Fang S and Xu C: Roles of
endoplasmic reticulum stress and autophagy on H2O2-induced
oxidative stress injury in HepG2 cells. Mol Med Rep. 18:4163–4174.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zuo S, Kong D, Wang C, Liu J, Wang Y, Wan
Q, Yan S, Zhang J, Tang J, Zhang Q, et al: CRTH2 promotes
endoplasmic reticulum stress-induced cardiomyocyte apoptosis
through m-calpain. EMBO Mol Med. 10(e8237)2018.PubMed/NCBI View Article : Google Scholar
|