Introduction
Diabetes mellitus, also known simply as diabetes, is
one of the most serious health-threatening diseases worldwide.
According to the Austrian Diabetes Report, in 2017, ~415 million
people worldwide lived with diabetes (1). Diabetic kidney disease (DKD) is one of
the major microvascular complications of diabetes and is the most
common cause of end-stage renal disease worldwide (2). Therefore, it is urgent to explore new
prevention and treatment strategies for DKD. The pathogenesis of
DKD has not been fully elucidated. The complex pathogenesis of DKD
mainly focuses on hyperglycaemia, lipid metabolism disorders,
abnormal haemodynamics, inflammatory cytokines, oxidative stress
and cell apoptosis (3,4). An increasing body of research has
confirmed that oxidative stress and inflammation play crucial roles
in DKD (5,6). Inflammation can lead to excessive
reactive oxygen species (ROS), which can cause lipid peroxidation
reactions and decreased cellular antioxidant capacity (7). In contrast, ROS and ROS-induced DNA
damage can promote inflammatory responses and fibrotic processes
(8). The term ‘OxInflammation’
refers to a prepathological condition; it has been well-documented
that mild-subclinical chronic inflammation is related to chronic
and systemic oxidative stress with in a vicious circle (9). Silent information regulator 1 (SIRT1)
is a nicotinamide adenosine dinucleotide-dependent protein
deacetylase with remarkable abilities to prevent diseases and even
reverse aspects of ageing. It can regulate a variety of biological
processes, including inflammatory and metabolic disorders (10,11).
Renoprotective effects of SIRT1 have been found in experimental
models of renal disorders, including DKD (12,13).
Although many interventions have been found to be
effective against DKD, including lifestyle adjustment, glycaemic
control, blood pressure control, renin-angiotensin system blockers,
sodium-dependent glucose transporter-2 inhibitors and glucagon-like
peptide-1 agonists, few have been established as optimal options
(2). The discovery of new
interventions, which overcome these limitations (such as restricted
use in chronic renal failure and non-specific effect of reducing
albuminuria) to delay the evelopment of DKD is warranted. At
present, the use of medicinal plants and their natural components
as future drugs for the treatment of diabetes and its complications
has received considerable interest from researchers worldwide. The
Chinese decoction compound centella formula (CCF) was designed by a
famous contemporary doctor of traditional Chinese medicine,
Professor Yongjun Wang (14). The
composition and daily dose for adults of this decoction are as
follows: Centella asiatica (L.) Urb. (JiXueCao), 30 g;
Astragalus Membranaceus Fish. (HuangQi), 30 g; Tripterygium
wilfordii Hook. f. (LeiGongTeng), 15 g. In a previous
study, it was observed that CCF reduced the quantity of proteins
found in the 24 h urinary protein test, improved renal function,
inhibited mesangial cell proliferation and mesangial matrix
accumulation, and prevented sclerosis of the glomerulus in stage
3-4 patients with DKD treated for 3 months. A small number of
patients (2/43) had a drug-related increase in alanine
transaminase, which was within 3 times the normal value and
returned to normal levels after symptomatic treatment (15). Although CCF has been indicated to be
an effective treatment for DKD in a clinical setting, the exact
mechanism is still unclear.
In the present study, the protective effects of CCF
against renal injuries in streptozotocin (STZ)-induced diabetic
rats were examined, along with the possible mechanisms associated
with the inhibition of crosstalk between inflammatory responses and
oxidant stress and the regulation of SIRT1.
Materials and methods
Animals and experimental design
A total of 30 male Sprague Dawley rats
(specific-pathogen-free grade; 4-5 weeks old, weighing 100±10 g)
were purchased from Shanghai SIPPR-Bk Laboratory Animal Co., Ltd.
The experiment was approved by the Zhejiang Chinese Medical
University Animal Ethics Committee. The rats were reared in a
standard experimental animal laboratory with free access to food
and water in a specific pathogen-free laboratory environment under
the following conditions: Temperature, 20-25˚C; humidity, 50-65%
and a 12 h light/dark cycle. A total of 7 rats were fed ordinary
feed to represent the normal control (NC). Considering the
mortality rate of the pathological models, 23 rats were fed
high-fat feed for 112 days (72.5% ordinary feed formula plus 10%
lard, 10% sucrose, 2.0% cholesterol, 0.5% cholic acid and 5% yolk
powder). The feed was purchased from Beijing Boaigang Biotechnology
Co., Ltd. On the 29th day of the experiment, the same 23 rats all
received a single intraperitoneal injection of STZ (MilliporeSigma
Canada Co.) at a dose of 35 mg/kg; two of the 23 rats gradually
lost weight after injection of STZ and appeared to show slower
activity, as well as eating less food and grooming less. For
ethical reasons, these two rats were eventually sacrificed. It was
speculated that the abnormal response of the two rats was due to a
faulty intraperitoneal injection procedure. The remaining 21 rats
were randomly divided into the diabetic control (DC) group (n=7),
CCF group (n=7) and losartan (LST) group (n=7). As a classic drug
for the treatment of diabetic nephropathy, LST was used as the
positive control drug for centella formula in this experiment.
Plasma fasting blood glucose (FBG) was measured from the tail vein,
and FBG levels >16.7 mmol/l (72 h after injection) were
considered diabetic rats (14).
Subsequently, the drug intervention was carried out from the 32nd
to the 112th day. The rats in the CCF group were treated with a
concentrated solution of CCF at 2 ml/day per rat. The dose of
losartan potassium was 4.5 mg/kg/day per rat (diluted to 2 ml with
normal saline) in the LST group (Merck Sharp &
Dohme-Hoddesdon). The rats in the NC and DC groups received the
same volume of normal saline. The drug intervention was
administered by gavage. All rats were sacrificed by decapitation on
the 112th day of the experiment after anaesthesia. Firstly, the
midline of the abdomens of rats were cut open following local skin
disinfection. The kidneys were then carefully dissociated, and the
kidney blood vessels were clamped with hemostatic forceps and
ligated. The kidneys were then removed, and the abdomens were
sutured.
Preparation of CCF
One daily dose of CCF for each rat included 0.8 g of
Centella asiatica (L.) Urb. (JiXueCao), 0.8 g of
Astragalus membranaceus Fish. (HuangQi), and 0.4 g of
Tripterygium wilfordii Hook. f. (LeiGongTeng). The rat CCF
dose was calculated based on the human dose according to the body
surface area formula (16): A=k
(W2/3)/10,000, where k=9.1 (A, Body surface area,
m2; K, A constant, which varies with animal species; W,
weight, g). The herbs were mixed in water, decocted for 45 min and
then concentrated. The final volume of the concentrated decoction
was 2 ml for one rat per day. The decoction was stored at 4˚C.
Chemical analysis of CCF extracts by
high-performance liquid chromatography
To prepare the asiaticoside reference solution, 6.75
mg asiaticoside reference (National Institute for the Control of
Pharmaceutical and Biological Products) was precisely weighed and
added to methanol to obtain a 0.27 mg/ml solution. To prepare the
sample solution, 5 ml of this solution was added to a test tube and
heated (100˚C) in a water bath for evaporation until dry.
Subsequently, the solution was dissolved in methanol and placed in
an ultrasonic bath for 30 min. The final volume was 2 ml in a
volumetric flask. After shaking well, the extraction solution was
filtered with a 0.45 µm filter membrane. For content
analysis, the precise absorption of the reference asiaticoside
solution (10 µl) and sample solution (10 µl) was
analysed by injecting the samples into a liquid chromatograph
(Varian ProStar 230 Solvent delivery module; Varian) with the
following conditions: Column, YMC ODS C18 (YMC Co., Ltd.) inner
diameter: 4.6 mm, length: 250 mm; particle diameter: 5 µm);
mobile phase, acetonitrile:water (30:70); atomization temperature,
40˚C; gasification temperature, 90˚C; flow rate, 1.0 ml/min;
nitrogen flow rate, 1.6 l/min.
To prepare the astragaloside reference solution,
7.50 mg astragaloside reference (National Institute for the Control
of Pharmaceutical and Biological Products) was precisely weighed,
and methanol was added (0.30 mg/ml). To prepare the sample
solution, 30 ml sample solution was extracted by adding 40 ml of
80% methanol as the solvent overnight; 20 ml methanol solvent was
added again. Subsequently, the resultant mixture was heated under
reflux for 4 h. The methanol extract was evaporated to dryness, and
the desiccated residue was dissolved in water. The solution was
then extracted four times with 40 ml of n-butanol and washed in 40
ml ammonia solution twice. After discarding the ammonia solution,
n-butanol was evaporated to dryness, and the residue was dissolved
in 5 ml of water. Then, the crude extracts were filtered through a
D101 macroporous adsorption resin column (length, 12 cm; inner
diameter. 1.5 cm). A total of 50 ml of water was used as the eluent
and discarded later, followed by ethanol elution. The solution was
eluted with 40% ethanol (30 ml), and the eluate was discarded. The
solution was then eluted again with 70% ethanol (80 ml). The eluent
solution was collected and evaporated to dryness. The residue was
dissolved in methanol and transferred to a 5 ml volumetric flask.
After the addition of methanol to the flask (to 5 ml), the sample
was shaken. For content analysis, the precision absorption of the
astragaloside reference solution (10 µl) and sample solution
(10 µl) was measured by liquid chromatography (Varian
ProStar 230 Solvent delivery module; Varian) with the following
conditions: Column, YMC ODS C18 (YMC Co., Ltd.)(inner diameter: 4.6
mm, length: 250 mm; particle diameter: 5 µm); mobile phase,
acetonitrile:water (35:65); the other details were the same as
those aforementioned.
To prepare the triptolide reference solution, 3.01
mg triptolide reference (content ≥98%; National Institute for the
Control of Pharmaceutical and Biological Products) was precisely
weighed, and methanol was added (0.03 mg/ml). To prepare the sample
solution, 5 ml of solution was added to a test tube. Water was used
to dilute the solution to 25 ml. The supernatant was collected
after centrifugation (5,702 x g, room temperature) and heated in a
water bath for evaporation until dry. Subsequently, methanol (2.5
ml) and methylene chloride (2.5 ml) were added to the residue in
neutral alumina (10 g; diameter, 1.5 cm; wet packing column). A
mixture of methanol and methylene chloride (1:3) was used for
elution. The eluent was collected at 100 ml and steamed. The
residue was dissolved in methanol and transferred to a 5 ml
volumetric flask. After shaking well, the extraction solution was
filtered with a 0.45 µm filter membrane. For content
analysis, the precise absorption of the triptolide reference
solution (10 µl) and sample solution (10 µl) were
analysed by liquid chromatography (Varian ProStar 230 Solvent
delivery module; Varian) with the following conditions: Column, YMC
ODS C18 (YMC Co., Ltd.) (inner diameter: 4.6 mm, length: 250 mm;
particle diameter: 5 µm); mobile phase, acetonitrile:water
(30:70); detection wavelength, 220 nm; the other details were the
same as those aforementioned.
Analysis of FBG, urine
protein-to-creatinine ratio (UPCR), serum creatinine (Scr) and
blood urea nitrogen (BUN)
FBG was measured from the tail vein on the 1st,
32nd, 42nd, 56th, 84th and 112th days of the study using Accu-Chek
Performa glucometers [Roche Diagnostics (Shanghai) Co., Ltd.].
Metabolic cages were used to collect urine. The UPCR was determined
by the pyrogallol red method (MilliporeSigma) using an AU5800
automatic biochemical analyser (Beckman Coulter, Inc.) on the 1st
and 112th day. The rats were anaesthetised prior to blood draws (~8
ml per rat). All the rats were anaesthetised with intraperitoneal
administration of 10% chloral hydrate (400 mg/kg) (17). Scr and BUN were measured using an
AU5800 automatic biochemical analyser (Beckman Coulter, Inc.) on
the 112th day.
Renal histology analysis by H&E
staining and periodic acid-Schiff (PAS) staining
One side of the rat kidney was fixed with 10%
neutral buffered formalin (at room temperature ≥24 h), dehydrated
with gradient alcohol (at room temperature, 75% x2, 30 min; 95% x3,
30 min; and 100% x2, 30 min), cleared with xylene, waxed, embedded
and sectioned (3 µm). Tissue sections were deparaffinized in
xylene and stained with H&E for 20 min at room temperature. For
PAS staining, histological sections were deparaffinized, oxidized
in 1% aqueous periodate solution for 15 min and washed three times
in distilled water. Sections were then soaked in Schiff solution
for 15 min. Then, the sections were rinsed under running water for
15 min. Subsequently, the nuclei were stained with hematoxylin
(room temperature for 10 min) followed by differentiation with
ethanol-hydrochloric acid. The areas of nuclei appeared blue, while
the basal membrane of the glomerulus and renal tubules, cytoplasm,
mesangial matrix of the glomerulus and collagen fibers were red
under light microscopy (magnification, x200 or x400)(BX51; Olympus
Corporation). The relative mesangial matrix index and the relative
glomerular volume were calculated (18,19).
Ultrastructure of the kidney through
transmission electron microscopy
The rat renal cortical tissue samples (1x1x1 mm)
were collected and fixed with 2.5% glutaraldehyde (60 min) and 1%
osmic (120 min) acid at 4˚C After dehydration, the tissues were
embedded in epoxy resin. The ultrathin sections (100 nm) were
stained with uranium acetate-lead citrate and then examined with a
transmission electron microscope (JEM1400; JEOL, Ltd.). The foot
process fusion rate of the podocyte in each specimen was calculated
(20,21).
Analysis of SOD and MDA activities in
rat renal tissues
SOD activity and MDA content were analysed with SOD
test kits (WST-1 method; cat.no. A001-3-1; Nanjing Jiancheng
Bioengineering Institute) and MDA test kits (TBA method; cat.no.
A003-1-1; Nanjing Jiancheng Bioengineering Institute) according to
the manufacturer's instructions.
ELISA assay for NF-κB p65 and TNF-α in
kidney tissues
The levels of TNF-α and NF-κB p65 in the rat kidney
tissues were measured using TNF-α (cat. no. ELK1396; ELK
Biotechnology, Ltd.) and NF-κB p65 ELISA kits (cat. no. ELK5691;
ELK Biotechnology, Ltd.) according to the manufacturer's
instructions.
Reverse transcription-quantitative PCR
(RT-qPCR) for SIRT1 expression level
Total RNA from the rat kidney tissues was extracted
using the TRIzol® method (Invitrogen; Thermo Fisher
Scientific, Inc.). The total RNA concentration was analysed by a UV
spectrophotometer (260 nm/280 nm). Then, cDNA was synthesized using
a PrimeScript™ RT reagent Kit with gDNA Eraser (Takara
Bio, Inc.). Subsequently, qPCR was performed using a SYBR Green
real-time PCR kit (Takara Bio, Inc.). GAPDH was used as a reference
gene. The primer sequences are presented in Table I. The expression levels of mRNA were
quantified using the 2-ΔΔCq method (22).
 | Table IPrimer sequences used for the
quantitative real-time polymerase chain reaction. |
Table I
Primer sequences used for the
quantitative real-time polymerase chain reaction.
| Gene | Forward primer
(5'-3') | Reverse primer
(5'-3') |
|---|
| SIRT1 (141 bp) |
GCTCGCCTTGCTGTGGACTTC |
GTGACACAGAGATGGCTGGAACTG |
| GAPDH (252 bp) |
ACAGCAACAGGGTGGTGGAC |
TTTGAGGGTGCAGCGAACTT |
Western blotting for SIRT1 and NOX4
protein expression level in renal tissue
Protein contents of the rat renal tissues were
quantified using the BCA method. 5% SDS-PAGE gels were prepared.
Subsequently, a sample buffer was added to the protein samples (50
µg/lane). The mixtures were denatured at 95˚C for 10 min
before electrophoresis was performed. After migration, all PVDF
membranes were blocked with 5% skim milk for 30 min at room
temperature, followed by incubation with primary antibodies against
SIRT1 (1:600; cat. no. 13161-1-AP; Wuhan Sanying Biotechnology) and
NOX4 (1:600; cat. no. 14347-1-AP; Wuhan Sanying Biotechnology)
overnight at 4˚C. After TBST washing, the membranes were incubated
with secondary antibody (anti-rabbit; 1:10,000; cat. no. DW-GAR007;
Jackson ImmunoResearch Laboratories, Inc.) for 60 min at room
temperature. An enhanced chemiluminescence reagent was used for
visualization (Applygen Technologies Inc.), and Image-Pro Plus 6.0
software (Media Cybernetics, Inc.) was employed to analyse the
results.
Immunohistochemistry assay for SIRT1
protein expression level determination and localisation in kidney
tissues
Blocks of rat kidney tissues smaller than 0.5x 0.5x
0.1 cm were taken for fixation with 10% neutral buffered formalin
(room temperature for ≥24 h), dehydrated with an alcohol gradient
(room temperature, 75% x2, 30 min; 95% x3, 30 min; and 100% x2, 30
min), cleared with xylene, waxed, embedded and sectioned (3
µm). Then, the sections were treated with citric acid
antigen repair buffer and washed with PBS three times. Sections
were blocked using 10% normal goat serum (cat. no. SL038; Beijing
Solarbio Science & Technology Co., Ltd.) for 20 min at room
temperature. The primary antibody against SIRT1 (1:50; cat. no.
13161-1-AP; Wuhan Sanying Biotechnology) was added, the slides were
incubated at 4˚C overnight and washed with PBS. Secondary
antibodies (anti-rabbit; 1:50,000; cat. no. sp-9001; Jackson
ImmunoResearch Laboratories, Inc.) were added (37˚C for 20 min).
Then, the sections were washed with PBS three times. Finally,
immunostaining was visualized using 3,3'-diaminobenzidine and
counterstained with haematoxylin (room temperature for 1 min). The
results were observed under an inverted microscope (BX43, Olympus
Corporation) at a magnification of x400. Brown staining was
considered positive and the staining intensity was calculated as
the integral optical density (IOD). Five IODs for each section were
randomly collected for semi-quantitative analysis. The average
optical density (AOD) was calculated using Image-Pro Plus 6.0.
Statistical methods
Statistics were performed using SPSS 19.0 software
(IBM Corp.). All data are presented as the mean ± standard
deviation. The significant differences among the four groups were
analysed by one-way ANOVA, followed by post hoc comparison with
Tukey's (equal variances assumed) or Dunnett's T3 test (equal
variances not assumed). P<0.05 was considered to indicate
a statistically significant difference.
Results
Concentration of representative
components in CCF
In a previous study (14), the concentration of representative
components in CCF was measured. The representative chemical
components in CCF were detected by high-performance liquid
chromatography (Table II). The
peak retention times and concentrations of asiaticoside,
astragaloside and triptolide were measured carefully (Fig. 1)
 | Table IIConcentrations of representative
components in CCF. |
Table II
Concentrations of representative
components in CCF.
| Standard
substance | Concentration in
CCF (mg/ml) |
|---|
| Asiaticoside | 0.3412 |
| Astragaloside | 0.0635 |
| Triptolide | 0.0001 |
General conditions
The rats in the DC group exhibited dull fur, weight
loss, increased appetite, thirst and frequency of urination
compared with the rats in the NC group. In the LST and CCF groups,
these symptoms were improved.
CCF reduces the UPCR in DKD rats
The results indicated that UPCR was significantly
increased in the DC group compared with the NC group on day 112 (DC
vs. NC; P<0.01; Fig. 2A). The
CCF group showed a significantly reduced UPCR compared with the DC
group (CCF vs. DC; P<0.01). This effect was independent of blood
sugar, since CCF did not notably reduce FBG (Fig. 2B). A similar response was exhibited
in the LST group (LST vs. DC; P<0.01; Fig. 2A and B). To investigate renal function in
diabetic rats, Scr and BUN levels were measured. However, there was
no significant difference between NC and DC (Fig. 2C and D).
 | Figure 2(A) UPCR, (B) FBG, (C) Scr and (D)
BUN were measured in four groups of Sprague-Dawley rats (n=7). Data
are expressed as the mean ± standard deviation.
**P<0.01 vs. the NC group, ##P<0.01 vs.
the DC group. UPCR, urine protein-to-creatinine ratio; FBG, fasting
blood glucose; Scr, serum creatinine; BUN, blood urea nitrogen;
CCF, compound centella formula; LST, losartan (4.5 mg/kg/day); DC,
diabetic control; NC, normal control. |
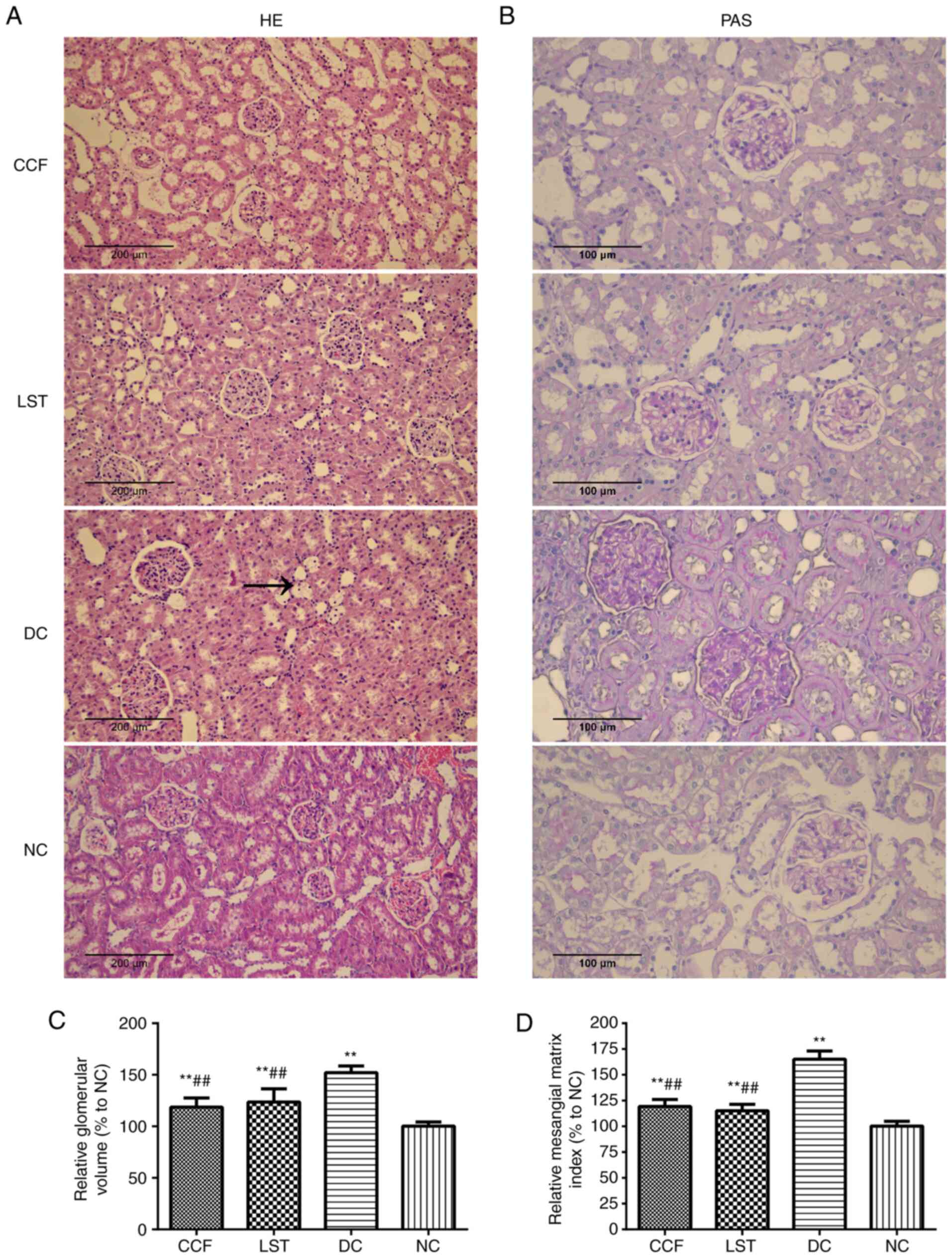
CCF alleviates renal histological
damage in DKD rats
Histopathological sections were stained with PAS
(magnification, x400) and H&E (magnification x200; Fig. 3A and B). The microscope observations indicated
clear glomerular and renal tubular structures, with opened
glomerular capillaries in the NC group rats. Conversely, the
glomerular volume was significantly increased in the DC group rats
compared with the NC group rats. The basement membrane of the
tubules and glomeruli was thickened with mesangial cell and stroma
hyperplasia in the DC group. The relative glomerular volume and
mesangial matrix index were increased in the DC group compared with
the NC group (DC vs. NC; P<0.01; Fig. 3C and D). Renal tubule vacuolization was also
observed in the DC group. Some inflammatory cells could be found in
the interstitial tissues or renal tubules. In the CCF group and LST
group, these pathological changes were markedly improved (Fig. 3A and B).
Transmission electron microscopy showed that the
foot process was complete and orderly in the NC group rats, without
obvious glomerular basement membrane lesions. However, in the DC
group, most of the foot processes were fused (Fig. 4A). The fusion degree of the process
was increased in the DC group compared with the NC group (DC vs.
NC; P<0.01). After CCF or LST treatment, podocyte lesions were
significantly improved (CCF vs. DC, P<0.01; LST vs. DC,
P<0.01) (Fig. 4B).
CCF inhibits renal oxidative stress in
DKD rats
As presented in Fig.
5A, the levels of SOD were significantly decreased in the DC
group compared with the NC group (DC vs. NC; P<0.01), while MDA
and NOX4 levels were increased (DC vs. NC; P<0.01; Fig. 5B-D). CCF treatment partially
restored the expression levels of SOD (CCF vs. DC; P<0.01) and
decreased MDA (CCF vs. DC; P<0.01) and NOX4 (CCF vs. DC;
P<0.01) to some extent. LST had a similar result (Fig. 5).
CCF attenuates renal inflammation in
DKD rats
Quantification of TNF-α and NF-κB p65 suggested that
the phosphorylation level of NF-κB p65 was significantly
upregulated in the DC group compared with the NC group (DC vs. NC;
P<0.01), while CCF treatment partially reduced the expression
level to some extent (CCF vs. DC, P<0.01; Fig. 6A). The level of TNF-α was also
increased in the DC group compared with the NC group (DC vs. NC;
P<0.01). CCF also significantly reduced the expression level of
TNF-α (CCF vs. DC; P<0.01; Fig.
6B). The expression trends of NF-κB p65 and TNF-α were similar
in the CCF and LST groups.
CCF increases the expression levels of
SIRT1 mRNA and protein in DKD rats
The mRNA levels of SIRT1 in rat renal tissues were
significantly decreased in the DC group compared with the NC group
(DC vs. NC; P<0.01; Fig. 7A).
Treatment with either CCF or LST reversed this trend (CCF vs. DC;
P<0.01; LST vs. DC; P<0.01). The protein expression level of
SIRT1 as measured by western blotting was decreased in the DC group
compared with the NC group (DC vs. NC, P<0.01; Fig. 7B). SIRT1 protein level was increased
by CCF and LST treatment (CCF vs. DC; P<0.01; LST vs. DC;
P<0.01; Fig. 7C).
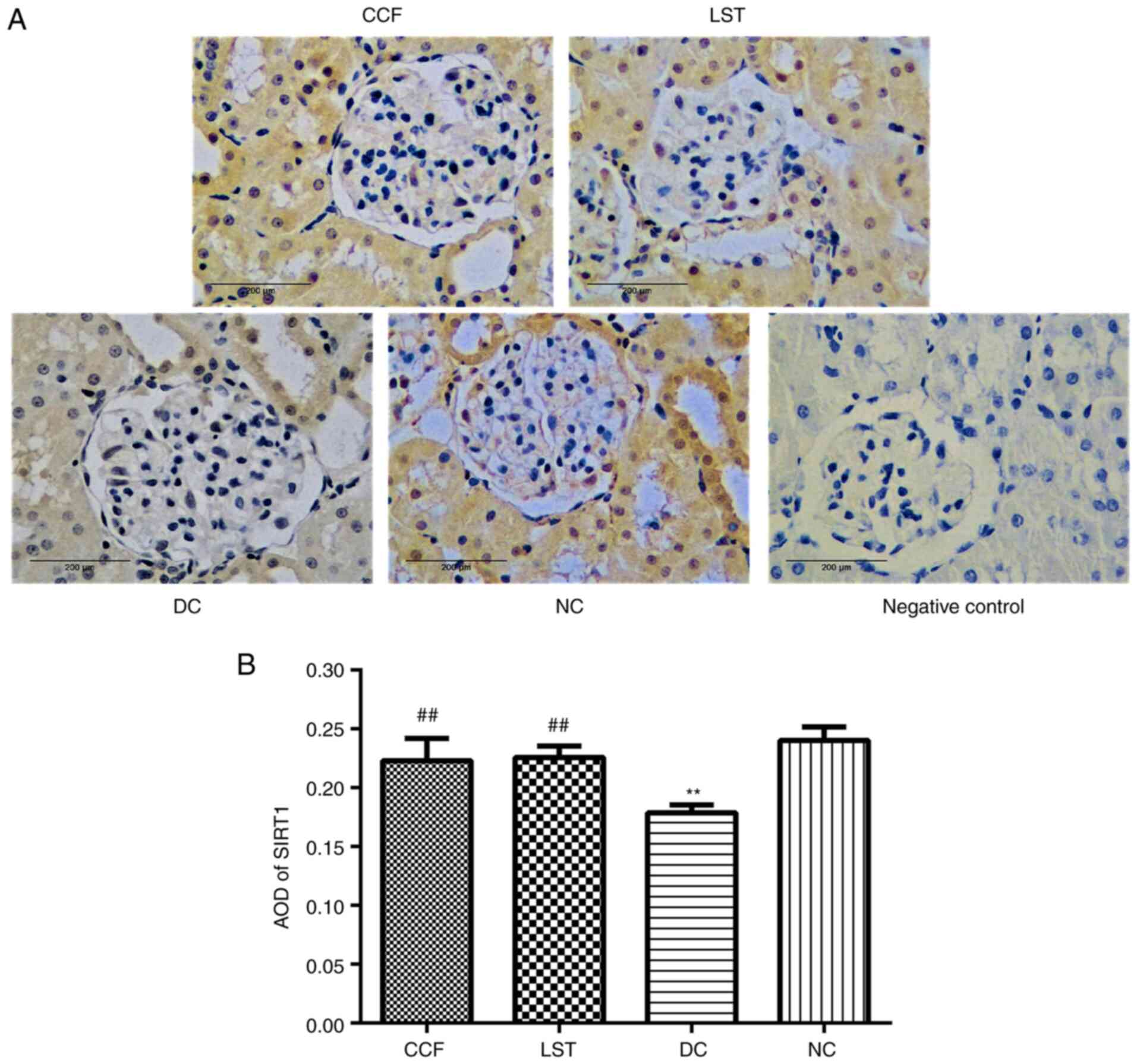
Immunohistochemical staining of SIRT1 protein
indicated that SIRT1 was mainly concentrated in the nucleus and
cytoplasm of renal tubular epithelial cells, partially in the
glomerulus, showing light or brown-yellow colour in the NC group
(Fig. 8A). Slightly stained cells
were observed in the DC group. The staining was increased in the
CCF and LST groups compared with the DC group. The AOD of SIRT1 was
the highest in the NC group and the lowest in the DC group (DC vs.
NC; P<0.01; Fig. 8B). The AOD in
the CCF and LST groups was greater than that in the DC group (CCF
vs. DC, P<0.01; LST vs. DC, P<0.01). There was no significant
difference in the AOD of SIRT1 between CCF and LST.
Discussion
DKD is one of the most important long-term
complications of diabetes and is a primary cause for dialysis
(23-25).
The classic pathological process of DKD includes glomerular
hypertrophy, basement membrane and mesangial matrix thickening,
typical nodular glomerular sclerosis and, as a final consequence,
extensive glomerular sclerosis (26). Currently, there are two
well-established types of rodent models for diabetes research. One
type is the genetic spontaneous diabetes model, and the other is
the experimentally induced diabetes models. The combination of
high-fat diet with intraperitoneal STZ is widely used for inducing
diabetes in rats (27). Previous
research has indicated that male Wistar rats that received a
high-fat diet for 4 weeks (28 days), followed by next day
administration of STZ (35 mg/kg, via intraperitoneal injection),
exhibited a fasting glucose level which was considered diabetic
(28). According to the modelling
method in previous literature, the rats in the DC, CCF and LST
groups received a single intraperitoneal injection of 35 mg/kg STZ
the day following a 28-day high-fat diet in the present study. In
previous studies, pathological changes were mainly observed 16
weeks after experimentally inducing diabetes in rats (presence of
albuminuria, kidney histological changes including glomerulomegaly,
inflammatory infiltration and tubulointerstitial fibrosis)
(29,30). Therefore, in the present study, all
rats were sacrificed after 16 weeks, on the 112th day. In the
present study, STZ-induced diabetic rats exhibited increased
proteinuria, increased glomerulus, mesangial cell and stroma
proliferation, renal tubule vacuolisation and foot process fusion.
However, CCF reduced proteinuria and alleviated renal pathology.
This renoprotection was independent of blood glucose control, as no
significant decrease in blood sugar was observed.
Among numerous contributing factors, inflammatory
and oxidative stress play crucial roles in the progression of DKD.
Evidence indicates that low-grade inflammation usually exists in
diabetic patients before the development of DKD (31). Inflammation has been observed in the
serum and renal tissues of patients with DKD (32,33).
NF-κB is a nuclear transcription factor that mainly regulates a
large number of genes associated with inflammation and the
immunological response (34). It is
normally present in the cytoplasm in an inactive form. Activated
NF-κB translocates into the nucleus and regulates the generation of
proinflammatory cytokines, such as TNF-α (35). Although all of these cytokines are
involved in the inflammatory response, TNF-α appears to be a
critical mediator of the inflammatory cascade. A previous study has
demonstrated that TNF-α reduced glomerular blood flow and
glomerular filtration rate, and increased endothelin-1 production,
inducing vasoconstriction, disrupting the glomerular filtration
barrier which lead to proteinuria, and finally exerted direct
apoptotic and cytotoxic effects on glomerular cells (36). One clinical trial indicated that
TNF-α levels in urinary tissue were associated with the presence
and severity of microalbuminuria in patients with type 2 diabetes
mellitus (33). Consistent with
clinical data, diabetic rats had a significantly higher increased
level of urinary and renal interstitial concentrations of TNF-α
before the increase in albuminuria (37). The present study demonstrated that
the levels of the inflammatory factors NF-κB and TNF-α increased in
the renal tissue of DKD rats, but significantly decreased with CCF
treatment. This indicated that CCF may have a role in controlling
the extent of the inflammatory response.
Oxidative stress plays another important role in the
pathogenesis and progression of diabetes. Increased free radicals
will interact with lipids, proteins and nucleic acids, leading to
membrane integrity loss, structural or functional changes in
proteins and gene mutation (38).
MDA is one of the final products of polyunsaturated fatty acid
peroxidation in cells, which affects ion exchange from the cell
membranes, leading to cross-linking of the compounds located in the
membrane as well as adverse consequences such as changing enzyme
activity in parallel with ion permeability (39). MDA is an independent risk factor for
DKD and reflects the level of lipid peroxidation and the severity
of free radical attack on cells (40). NOX4 is a nicotinamide adenine
dinucleotide phosphate oxidase subunit that is considered to be a
main enzyme that contributes to increased oxidative stress in DKD.
Previous studies have indicated that NOX4 expression is elevated in
diabetic kidney injury, and podocyte-specific knockout of NOX4
attenuates DKD (41,42). However, humans possess enzymatic and
nonenzymatic antioxidant defence systems to protect against the
harmful effects of free radicals. For example, SOD can eradicate
oxygen free radicals (43). Data
generated from a previous study indicated that the level of SOD
decreased in DKD animal models (44). In the present study, results
revealed that the levels of NOX4 and MDA expression were increased,
while the levels of SOD were decreased in DKD rats compared with
control rats. After treatment with CCF, these effects were
reversed, and kidney condition was improved. Hence, it is
hypothesised that the renoprotective effect of CCF may be mediated
by antioxidants.
‘OxInflammation’ is a novel operative term that
defines the deleterious crosstalk between inflammatory and redox
systemic processes, leading to systemic or local damage in the long
run (45). On the one hand, a
feature of the inflammatory response is the generation of a
pro-oxidative environment due to the production of pro-oxidant
species. Increased production of TNF-α can stimulate the activation
of nicotinamide adenine dinucleotide phosphate in mesangial cells
and induce the production of ROS in endothelial cells. On the other
hand, oxidative stress induces the release of inflammatory
cytokines, such as TNF-α (46). The
present results agree with these findings. The trend in
inflammatory markers was consistent with the trend in oxidative
stress markers and contrasted the trend in antioxidant markers.
These results supported the existence of crosstalk between
inflammatory and redox systemic processes.
SIRT1 is the most widely studied member of the SIRT
family and is responsible for the deacetylation of proteins
involved in the regulation of cell proliferation, cell
differentiation, senescence, gene expression, mitochondrial
biogenesis, fatty acid oxidation, apoptosis, autophagy and cellular
metabolic balance (10,47). The renoprotective effects of SIRT1
have been reported in various experimental models of renal
disorders, including DKD (12,13). A
previous study has reported that decreased SIRT1 expression levels
resulted in kidney dysfunction in diabetic rats, with increased
proteinuria and decreased creatinine clearance. In contrast, the
increase of SIRT1 expression levels mediated the hypertrophy of
glomerular mesangial cells under diabetic conditions and alleviated
renal damage (48). As a regulator
of OxInflammation, previous studies have revealed some interesting
outcomes regarding SIRT1. When SIRT1 levels decreased, oxidative
stress and NF-κB levels increased in STZ-induced diabetic rats
(49,50). The activation of SIRT1 leads to
NF-κB deacetylation, thus reducing the release of inflammatory
factors and decreasing the severity of diabetic nephropathy
(51). Interestingly, a similar
phenomenon was observed in the present study, in which renal SIRT1
levels decreased in the DC group and eventually increased with CCF
treatment. Furthermore, it was also observed that CCF inhibited
diabetes-induced oxidative damage and inflammatory factors and
increased the expression level of SIRT1.
In conclusion, based on the results of the present
study and data available in the literature, it is suggested that
OxInflammation is involved in the progression of DKD as a
consequence of the crosstalk between inflammatory and oxidative
stress mediators. Moreover, SIRT1 may play an important role in
regulating OxInflammation. CCF efficiently protected the kidney
from diabetes, and the mechanism could be linked to the inhibition
of OxInflammation and the upregulation of SIRT1. However, the
necessary constituents of CCF require further study, and in
vitro experiments will be performed to investigate the exact
target of CCF.
Acknowledgements
Not applicable.
Funding
Funding: This research was financially supported by the Zhejiang
Provincial Natural Science Foundation of China (grant no.
LQ19H290005), the National Natural Science Foundation of China
under (grant no. 81973760) and the Science and Technology Program
for Health and Family Planning of Hangzhou (grant no. 2017A58).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HYC, QYJ and QZ contributed to the conception and
design of the study, and confirmed the authenticity of all raw
data. XHL performed the histological analysis of the kidney. QZ was
a major contributor in writing the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving
animals were in accordance with the ethics standards of Zhejiang
Chinese Medical University. This article does not contain any
studies with human participants performed by any of the
authors.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
König A, Schwarzinger B, Stadlbauer V,
Lanzerstorfer P, Iken M, Schwarzinger C, Kolb P, Schwarzinger S,
Mörwald K, Brunner S, et al: Guava (Psidium guajava) fruit extract
prepared by supercritical CO2 extraction inhibits
intestinal glucose resorption in a double-blind, randomized
clinical study. Nutrients. 11(1512)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bakris GL, Hahr A, Khardori R, Koya D,
Molitch M, Prischl FC, Schernthaner G and Thajudeen B: Managing
diabetic nephropathies in clinical practice. Overview of diabetic
nephropathy. 10.1007/978-3-319-08873-0: 1-21, 2017.
|
|
3
|
Varga ZV, Giricz Z, Liaudet L, Haskó G,
Ferdinandy P and Pacher P: Interplay of oxidative,
nitrosative/nitrative stress, inflammation, cell death and
autophagy in diabetic cardiomyopathy. Biochim Biophys Acta.
1852:232–242. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Flyvbjerg A: The role of the complement
system in diabetic nephropathy. Nat Rev Nephrol. 13:311–318.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Choi JS, Kim J, Park J, Pyo S, Hong YK, Ku
S and Kim MR: Blood glycemia-modulating effects of melanian snail
protein hydrolysates in mice with type II diabetes. Int J Mol Med.
39:1437–1451. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mizuno Y, Yamamotoya T, Nakatsu Y, Ueda K,
Matsunaga Y, Inoue MK, Sakoda H, Fujishiro M, Ono H, Kikuchi T, et
al: Xanthine oxidase inhibitor febuxostat exerts an
anti-inflammatory action and protects against diabetic nephropathy
development in KK-Ay obese diabetic mice. Int J Mol Sci.
20(4680)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rajesh M, Mukhopadhyay P, Bátkai S, Patel
V, Saito K, Matsumoto S, Kashiwaya Y, Horváth B, Mukhopadhyay B,
Becker L, et al: Cannabidiol attenuates cardiac dysfunction,
oxidative stress, fibrosis, and inflammatory and cell death
signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol.
56:2115–2125. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Duecker R, Baer P, Eickmeier O, Strecker
M, Kurz J, Schaible A, Henrich D, Zielen S and Schubert R:
Oxidative stress-driven pulmonary inflammation and fibrosis in a
mouse model of human ataxia-telangiectasia. Redox Biol. 14:645–655.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Valacchi G, Virgili F, Cervellati C and
Pecorelli A: OxInflammation: From subclinical condition to
pathological biomarker. Front Physiol. 9(858)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bonkowski M and Sinclair D: Slowing ageing
by design: The rise of NAD+ and sirtuin-activating
compounds. Nat Rev Mol Cell Biol. 17:679–690. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Guo R, Liu W, Liu B, Zhang B, Li W and Xu
Y: SIRT1 suppresses cardiomyocyte apoptosis in diabetic
cardiomyopathy: An insight into endoplasmic reticulum stress
response mechanism. Int J Cardiol. 191:36–45. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hao CM and Haase V: Sirtuins and their
relevance to the kidney. J Am Soc Nephrol. 21:1620–1627.
2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wakino S, Hasegawa K and Itoh H: Sirtuin
and metabolic kidney disease. Kidney Int. 88:691–698.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhu Q, Zeng J, Li J, Chen X, Miao J, Jin Q
and Chen H: Effects of compound centella on oxidative stress and
Keap1-Nrf2-ARE pathway expression in diabetic kidney disease rats.
Evid Based Complement Alternat Med. 2020(9817932)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mengjie Z, Ziyang B, Liqiang Y, Danfeng G,
Lin W and Jiao Z: Clinical Study on Modified Compound Jixuecao Tang
for Chronic Glomerulonephritis and Chronic Kidney Disease at the
Third Stage. Journal of New Chinese Medicine: 2019.
|
|
16
|
Spiers DE and Candas V: Relationship of
skin surface area to body mass in the immature rat: A
reexamination. J Appl Physiol Respir Environ Exerc Physiol.
56:240–243. 1984.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ding Y, Zhang R, Zhang K, Lv X, Chen Y, Li
A, Wang L, Zhang X and Xia Q: Nischarin is differentially expressed
in rat brain and regulates neuronal migration. PloS One.
8(e54563)2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhu X, Chen Y, Chen Q, Yang H and Xie X:
Astaxanthin promotes Nrf2/ARE signaling to alleviate renal
fibronectin and collagen IV accumulation in diabetic rats. J
Diabetes Res. 2018(6730315)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Artacho-Perula E, Roldan-Villalobos R,
Salcedo-Leal I and Vaamonde-Lemos R: Stereological estimates of
volume-weighted mean glomerular volume in streptozotocin-diabetic
rats. Lab Invest. 68:56–61. 1993.PubMed/NCBI
|
|
20
|
Liu HF, Guo LQ, Huang YY, Chen K, Tao JL,
Li SM and Chen XW: Thiazolidinedione attenuate proteinuria and
glomerulosclerosis in Adriamycin-induced nephropathy rats via slit
diaphragm protection. Nephrology (Carlton). 15:75–83.
2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Loeffler I and Wolf G: Pathophysiologie
der diabetischen Nephropathie. Der Nephrologe. 12:391–399.
2017.
|
|
22
|
Karolina L, Hannes O, Risul A, Arvind P,
Regina G, Taylor RF, Moosa M, Ann C, Karolina K and Larsson TE:
Arterial klotho expression and FGF23 effects on vascular
calcification and function. Plos One. 8(e60658)2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Martynyuk L, Martynyuk L, Ruzhytska O and
Martynyuk O: Effect of the herbal combination canephron N on
diabetic nephropathy in patients with diabetes mellitus: Results of
a comparative cohort study. J Altern Complement Med. 20:472–478.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tavafi M: Diabetic nephropathy and
antioxidants. J Nephropathology. 2:20–27. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gosmanov AR, Wall BM and Gosmanova EO:
Diagnosis and treatment of diabetic kidney disease. Am J Med Sci.
347:406–413. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sego S: Pathophysiology of diabetic
nephropathy. Nephrol Nurs J. 34:631–633. 2008.PubMed/NCBI
|
|
27
|
Magalhães DA, Kume WT, Correia FS, Queiroz
TS, Allebrandt Neto EW, Santos MP, Kawashita NH and França SA:
High-fat diet and streptozotocin in the induction of type 2
diabetes mellitus: A new proposal. An Acad Bras Ciênc.
91(e20180314)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Guex CG, Reginato FZ, de Jesus PR,
Brondani JC, Lopes GH and Bauermann LF: Antidiabetic effects of
Olea europaea L. leaves in diabetic rats induced by high-fat
diet and low-dose streptozotocin. J Ethnopharmacol. 235:1–7.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ahad A, Ganai AA, Mujeeb M and Siddiqui
WA: Ellagic acid, an NF-κB inhibitor, ameliorates renal function in
experimental diabetic nephropathy. Chemico-Biological Interactions.
219:64–75. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ahad A, Ganai AA, Mujeeb M and Siddiqui
WA: Chrysin, an anti-inflammatory molecule, abrogates renal
dysfunction in type 2 diabetic rats. Toxicol Appl Pharmacol.
279:1–7. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Serdar M, Sertoglu E, Uyanik M, Tapan S,
Bilgi C and Kurt I: Comparison of 8-hydroxy-2'-deoxyguanosine
(8-OHdG) levels using mass spectrometer and urine albumin
creatinine ratio as a predictor of development of diabetic
nephropathy. Free Radic Res. 46:1291–1295. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yang M, Jun L, Zhou X, Ding H, Xu J, Yang
B, Sun B, Xiao D, Yu J and Gong Q: Correlation analysis between
serum vitamin D levels and lower extremity macrovascular
complications in individuals with type 2 diabetes mellitus. J
Diabetes Res. 2019(4251829)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lampropoulou IT, Stangou M, Papagianni A,
Didangelos T, Iliadis F and Efstratiadis G: TNF-α and
microalbuminuria in patients with type 2 diabetes mellitus. J
Diabetes Res. 2014(394206)2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Malikova J, Zdarilova A and Hlobilkova A:
Effects of sanguinarine and chelerythrine on the cell cycle and
apoptosis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub.
150:5–12. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2(17023)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xu XY and Ye SW: Efficacy assessment of
treating post-stroke shoulder-hand syndrome patients of yin
deficiency yang hyperactivity with blood stasis stagnation
collaterals syndrome by yishen tongluo decoction. Zhongguo Zhong Xi
Yi Jie He Za Zhi. 34:1069–1073. 2014.PubMed/NCBI(Article in Chinese).
|
|
37
|
Kalantarinia K, Awad AS and Siragy HM:
Urinary and renal interstitial concentrations of TNF-α increase
prior to the rise in albuminuria in diabetic rats. Kidney Int.
64:1208–1213. 2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Takebayashi K, Matsumoto S, Aso Y and
Inukai T: Aldosterone blockade attenuates urinary monocyte
chemoattractant protein-1 and oxidative stress in patients with
type 2 diabetes complicated by diabetic nephropathy. J Clin
Endocrinol Metab. 91:2214–2217. 2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kowalczuk K and Stryjecka-Zimmer M: The
influence of oxidative stress on the level of malondialdehyde (MDA)
in different areas of the rabbit brain. Ann Univ Mariae Curie
Sklodowska Med. 57:160–164. 2002.PubMed/NCBI
|
|
40
|
Kaefer M, De Carvalho JA, Piva SJ, da
Silva DB, Becker AM, Sangoi MB, Almeida TC, Hermes CL, Coelho AC,
Tonello R, et al: Plasma malondialdehyde levels and risk factors
for the development of chronic complications in type 2 diabetic
patients on insulin therapy. Clin Lab. 58:973–978. 2012.PubMed/NCBI
|
|
41
|
Li X, Cai W, Lee K, Liu B, Deng Y, Chen Y,
Zhang X, He J and Zhong Y: Puerarin attenuates diabetic kidney
injury through the suppression of NOX4 expression in podocytes. Sci
Rep. 7(14603)2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ribaldo PD, Souza DS, Biswas SK, Block K,
Faria J, Lopes de Faria JM and Lopes de Faria JB: Green tea
(Camellia sinensis) attenuates nephropathy by downregulating
Nox4 NADPH oxidase in diabetic spontaneously hypertensive rats. J
Nutr. 139:96–100. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Peng Q, Liu F and Liang X: Superoxide
dismutase and plant resistance to the environmental stress.
Heilongjiang Agricultural Science. 1:31–34. 2002.
|
|
44
|
Samarghandian S, Borji A, Delkhosh M and
Samini F: Safranal treatment improves hyperglycemia, hyperlipidemia
and oxidative stress in streptozotocin-induced diabetic rats. J
Pharm Pharm Sci. 16:352–362. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Tisato V, Gallo S, Melloni E, Celeghini C,
Passaro A, Zauli G, Secchiero P, Bergamini C, Trentini A,
Bonaccorsi G, et al: TRAIL and ceruloplasmin inverse correlation as
a representative crosstalk between inflammation and oxidative
stress. Mediators Inflamm. 2018(9629537)2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Elmarakby AA and Sullivan JC: Relationship
between oxidative stress and inflammatory cytokines in diabetic
nephropathy. Cardiovasc Ther. 30:49–59. 2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Webster BR, Lu Z, Sack MN and Scott I: The
role of sirtuins in modulating redox stressors. Free Radical Biol
Med. 52:281–290. 2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yang X, Zhang B, Lu X, Yan M, Wen Y, Zhao
T and Li P: Effects of Tangshen Formula on urinary and plasma
liver-type fatty acid binding protein levels in patients with type
2 diabetic kidney disease: Post-hoc findings from a multi-center,
randomized, double-blind, placebo-controlled trial investigating
the efficacy and safety of Tangshen Formula in patients with type 2
diabetic kidney disease. BMC Complement Altern Med.
16(246)2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Iskender H, Dokumacioglu E, Sen TM, Ince
I, Kanbay Y and Saral S: The effect of hesperidin and quercetin on
oxidative stress, NF-κB and SIRT1 levels in a STZ-induced
experimental diabetes model. Biomed Pharmacother. 90:500–508.
2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Huang K, Gao X and Wei W: The crosstalk
between Sirt1 and Keap1/Nrf2/ARE anti-oxidative pathway forms a
positive feedback loop to inhibit FN and TGF-β1 expressions in rat
glomerular mesangial cells. Exp Cell Res. 361:63–72.
2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Du YG, Zhang KN, Gao ZL, Dai F, Wu XX and
Chai KF: Tangshen formula improves inflammation in renal tissue of
diabetic nephropathy through SIRT1/NF-κB pathway. Exp Ther Med.
15:2156–2164. 2018.PubMed/NCBI View Article : Google Scholar
|