Introduction
Bronchopulmonary dysplasia (BPD) is one of the most
common complications in extremely preterm infants and is associated
with many factors, including mechanical ventilation, hyperoxia,
genetic predisposition, infections and inflammation (1). New BPD manifests as impaired lung
alveolarization and aberrant microvasculature (2). Evidence indicates that angiogenesis is
crucial for lung development, while alveolarization impairment due
to disrupted angiogenesis is critical to the development of BPD
(3-5).
A great deal of clinical and animal research has shown that there
is a relationship between infection/inflammation and BPD (6-8).
BPD was increased in preterm infants who had a history of
chorioamnionitis (6). In animal
models, intra-amniotic lipopolysaccharide (LPS) administration
impaired the growth of alveoli and vessels (7,8).
Although increasing evidence shows that intrauterine inflammation
can impair alveolarization, the exact mechanism remains unclear,
and no effective therapies are currently available to prevent
BPD.
Notch signaling is an evolutionarily conserved
intercellular signaling mechanism that has a major role in
regulating cell fate in mammals and other vertebrates. Notch
signaling is activated when Notch ligands bind to Notch receptors
that are expressed on the surface of the adjacent cell. In mammals,
there are five Notch ligands [jagged canonical Notch ligand (Jag)
1, Jag2, delta-like canonical Notch ligand (Dll) 1, Dll3 and Dll4]
and four Notch receptors (Notch1-4) (9,10).
Among these Notch receptors and ligands, Notch4 and its ligand Dll4
are primarily expressed in the vascular endothelium. Notch4 has
been reported to be associated with arteriovenous malformations.
Notch4 or Dll4 overexpression in the vasculature results in
defective embryonic angiogenesis, while Dll4 or Notch receptor
deficiency leads to embryonic lethality with profound vascular
defects (11). These results
suggest that balanced levels of Notch4/Dll4 signaling are necessary
for normal vascular development. However, the role of Notch4/Dll4
signaling during vascular remodeling in BPD has not been well
studied.
The current study focused on the expression of
Notch4, Dll4 and related factors in vivo, in a rat model,
and in vitro, in rat pulmonary microvascular endothelial
cells (PMVECs), to verify the effect of Notch4/Dll4 signaling in
intrauterine infection on lung development.
Materials and methods
Animal model
Twenty pregnant Sprague-Dawley rats were purchased
from the Laboratory Animal Center of Zhejiang Academy of Medicine
in China and randomized into two groups: The Escherichia
coli-infected group (n=10) and the control group (n=10). All
animals were housed under standard laboratory conditions at 22±2˚C
with a constant 12-h light/dark cycle. On embryonic day E15,
pregnant rats were anesthetized with 2% sodium pentobarbital (40
mg/kg, intraperitoneal injection). Then, 0.4 ml of either an E.
coli suspension (2.5-4x108 colony-forming units per
ml) or saline was injected into the uterine cervix of the rats
(12,13). All animal procedures and protocols
were reviewed and approved by the Animal Care Committee of Zhejiang
University.
All pregnant rats were allowed to give birth
naturally. They were checked daily to confirm whether they gave
birth, and the day of birth was considered as postnatal day 0 (P0)
for the pups. Most of the rats were delivered at 21-22 days.
Furthermore, there was no significant difference between the
control group and the E. coli-treated group regarding the
duration of pregnancy and day of delivery. The period of the human
or rat lung development has been divided into five stages:
Embryonic stage, pseudoglandular stage, canalicular stage, saccular
stage and alveolar stage. BPD is mainly observed at the saccular
stage. One pup per litter from each group was sacrificed at P3
(saccular stage), P7 and P14 (alveolar stage). The rats were
anesthetized with 2% sodium pentobarbital (40 mg/kg,
intraperitoneal injection). These rats were euthanized by
exsanguination via excision of the inferior vena cava before the
lung tissues were collected. Thus, lung tissue samples from P3 to
P14 were harvested in both groups (n=10 per time point). The right
upper lobe was fixed for 24 h at room temperature in 10% neutral
formaldehyde for histopathological examination. Other specimens
were frozen in liquid nitrogen and stored at -80˚C for further
analysis. Intrauterine infection was confirmed by the presence of
inflammation in histological sections of the uterus and placenta,
as described in a previous study (12).
Histology and
immunohistochemistry
Lung samples were fixed overnight in 10% formalin at
a pressure of 20 cm H2O. Then, the tissues were
dehydrated in graded ethanol and embedded in paraffin. Sections of
5 µm thickness were cut and stained with hematoxylin and eosin
(H&E). Radial alveolar counts (RACs) were measured by drawing a
perpendicular line from the respiratory bronchiole to either the
pleura or the nearest connective tissue septum, and the number of
alveoli transected by this line was counted. Three sections per
time point were randomly selected for analysis. Five fields from
each section were used to perform morphometric analysis and RACs
using a light microscope (magnification, x100).
Furthermore, the sections were used for
immunohistochemical (IHC) staining. Briefly, tissue sections were
subjected to antigen retrieval using citrate buffer (pH=6.0).
Hydrogen peroxide (3%) was used for 10 min at room temperature for
blocking of endogenous peroxidases. The sections were then
incubated with primary antibodies targeting CD34 (1:2,500; cat. no.
ab81289; Abcam) overnight at 4˚C, followed by incubation with
secondary antibodies (HRP-conjugated goat anti-rabbit IgG; 1:200;
cat. no. ab6721; Abcam) for 1 h at room temperature. The IHC
reaction color was visualized with 3,3 diaminobenzidine
(Sigma-Aldrich; Merck KGaA) as the substrate using a light
microscope (magnification, x200). Image-Pro Plus version 6.0
software (Media Cybernetics, Inc.) was used for analysis.
Cell culture
Rat PMVECs were obtained from Qingqi (Shanghai)
Biotechnology Development Co., Ltd. and were incubated in
serum-free DMEM (Invitrogen; Thermo Fisher Scientific, Inc.) for 24
h. PMVECs were pretreated with 10 µmol/l DAPT (cat. no. ab120633;
Abcam) or equal volume of vehicle PBS for 24 h according to
previous studies (14,15). Subsequently, the cells were
stimulated with or without 10 µg/ml LPS in serum-free DMEM for 48
h, resulting in the following three experimental groups: Control
group, PMVECs treated with PBS vehicle control only; LPS group,
PMVECs treated with LPS only; and DAPT+LPS group, PMVECs treated
with DAPT and LPS (16-18).
MTT assay
Cell proliferation and viability were measured with
an MTT assay. Briefly, exponentially growing cells
(4x104/well) were seeded in a 96-well plate and cultured
for 72 h. For each group, the wells were randomly sampled every 24
h, and MTT solution (5 mg/ml, 20 µl/well; Sigma-Aldrich; Merck
KGaA) was added to the selected wells. After the wells were
incubated for an additional 2 h at 37˚C, 200 µl of DMSO was added
to the wells, and the cell viabilities were determined by measuring
the optical density values at 490 nm with a microplate reader
(ELX-800; BioTek Instruments, Inc.).
Apoptosis assay
The effect of Notch4/Dll4 signaling on PMVEC
apoptosis was evaluated by Annexin V staining. PMVECs were
collected, washed 3 times with PBS buffer and suspended in binding
buffer at a density of 106 cells/ml. Then, the cells
were stained with Annexin V-fluorescein isothiocyanate (FITC) and
propidium iodide using an Annexin V FITC apoptosis detection kit
(JingMei Biotechnology Co., Ltd.), according to the manufacturer's
protocol. Apoptotic cells were subsequently analyzed by flow
cytometry (LSRFortessa SORP; BD Biosciences) and FlowJo v7.6
software (FlowJo LLC).
RNA preparation and reverse
transcription-quantitative PCR
Total RNA was extracted from small pieces of frozen
lung tissue or from cells by using a RNeasy Mini kit (Qiagen GmbH).
The RNA was then reverse transcribed into cDNA using High-Capacity
cDNA Reverse Transcription Kit (Invitrogen; Thermo Fisher
Scientific, Inc.), which was used for quantitative PCR on an ABI
PRISM 7500 Real-Time PCR System (Thermo Fisher Scientific, Inc.) as
previously described (12). A
SYBR-Green PCR kit (Takara Biotechnology Co., Ltd.) was used for
the qPCR analysis using the 2-ΔΔCq method (19). The primers (Table I) were designed using Primer Express
3.0 software (Applied Biosystems; Thermo Fisher Scientific, Inc.)
and synthesized by Takara Biotechnology Co., Ltd. GAPDH was used as
an endogenous control gene to normalize the amount of cDNA. The
amplification procedure was performed in two steps: Initial
denaturation at 95˚C for 3 min and 40 cycles of 95˚C for 15 sec and
60˚C for 45 sec.
 | Table ISequences of primers used for reverse
transcription-quantitative PCR. |
Table I
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Primer | Sequence
(5'-3') | PCR product length
(bp) |
|---|
| Dll4 | Forward |
AGAGGAGAAGGAGGAGGAATG | 130 |
| | Reverse |
CCCTTGACTCTTCCCTTGATG | |
| Notch4 | Forward |
ATTCCCAGTGCTGGCTTCTC | 177 |
| | Reverse |
GAGCGTTGTTGCAGCCTTTC | |
| NF-κB | Forward |
AGCTGCTATTGGATTACAC | 109 |
| | Reverse |
AGATGGCTAGAAAGAACAC | |
| Flk-1 | Forward |
TTACTGTCCAGCCTGCTAC | 173 |
| | Reverse |
CCAAAGAGCGTCCAAGTTC | |
| Flt-1 | Forward |
ATAGCGTGGGACAGTAGG | 164 |
| | Reverse |
CTCGGTGGGCTTATTTGG | |
| VEGF | Forward |
GAGTCTGTGCTCTGGGATTTG | 188 |
| | Reverse |
TCCTGCTACCTCTTTCCTCTG | |
| GAPDH | Forward |
GTCGGTGTGAACGGATTTG | 181 |
| | Reverse |
TCCCATTCTCAGCCTTGAC | |
Western blot analysis
Total protein was extracted from frozen lung tissue
samples (40 mg) or cells using RIPA buffer (Abcam). Equal amounts
of protein determined using the BCA method (50 µg) were separated
by 10% SDS-PAGE and transferred onto nitrocellulose membranes by
electroblotting. Then, the membranes were blocked with 5% nonfat
dried milk for 2 h at room temperature and incubated with primary
antibodies overnight at 4˚C. The following primary antibodies were
used: Anti-Dll4 (1:1,000; cat. no. ab183532; Abcam), anti-Notch4
(1:1,000; cat. no. ab184742; Abcam), anti-VEGF (1:1,000; cat. no.
ab72807; Abcam), anti-fetal liver kinase 1 (Flk-1; 1:2,000; cat.
no. ab221679; Abcam), anti-FMS-like tyrosine kinase 1 (Flt-1;
1:2,000; cat. no. ab184784; Abcam), anti-NF-κB (1:1,000; cat. no.
8242; Cell Signaling Technology, Inc.) and anti-GAPDH (1:2,000;
cat. no. 5174; Cell Signaling Technology, Inc.). The next day, the
membranes were washed with TBST (containing 0.05% Tween 20 in TBS
buffer) three times for 10 min, followed by incubation with
anti-rabbit (cat. no. ab6721), anti-mouse (cat. no. ab6728) and
anti-goat (cat. no. ab6741) HRP-conjugated secondary antibodies
(all 1:5,000; all from Abcam) for 2 h at room temperature. Finally,
the proteins were detected by chemiluminescence (ECL Plus Detection
kit; Beyotime Institute of Biotechnology). Then, the membranes were
exposed to X-ray film to visualize the protein bands. Protein bands
were scanned and quantified using a computer-based image analysis
system (Gel-Pro analyzer software 4.0; Media Cybernetics, Inc.).
GAPDH was used as the endogenous control.
Statistical analysis
All data are presented as the mean ± standard
deviation (SD). A t-test was used to measure the statistical
significance of the difference between the means of two groups
(E. coli-infected group and control group). The differences
among multiple groups of samples (PMVEC treatment groups) were
evaluated using analysis of variance with Tukey's post hoc test.
Data analyses were performed using the SPSS software package
version 18 for Windows (SPSS Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Intrauterine infection impairs lung
development
During the present study, no dams died after
intrauterine E. coli administration. There were few
intrauterine fetal deaths and pregnancy losses in the two groups.
Ninety-eight live pups from the control group and ninety-four live
pups from the E. coli-infected group were eligible for this
study (Table II). Compared with
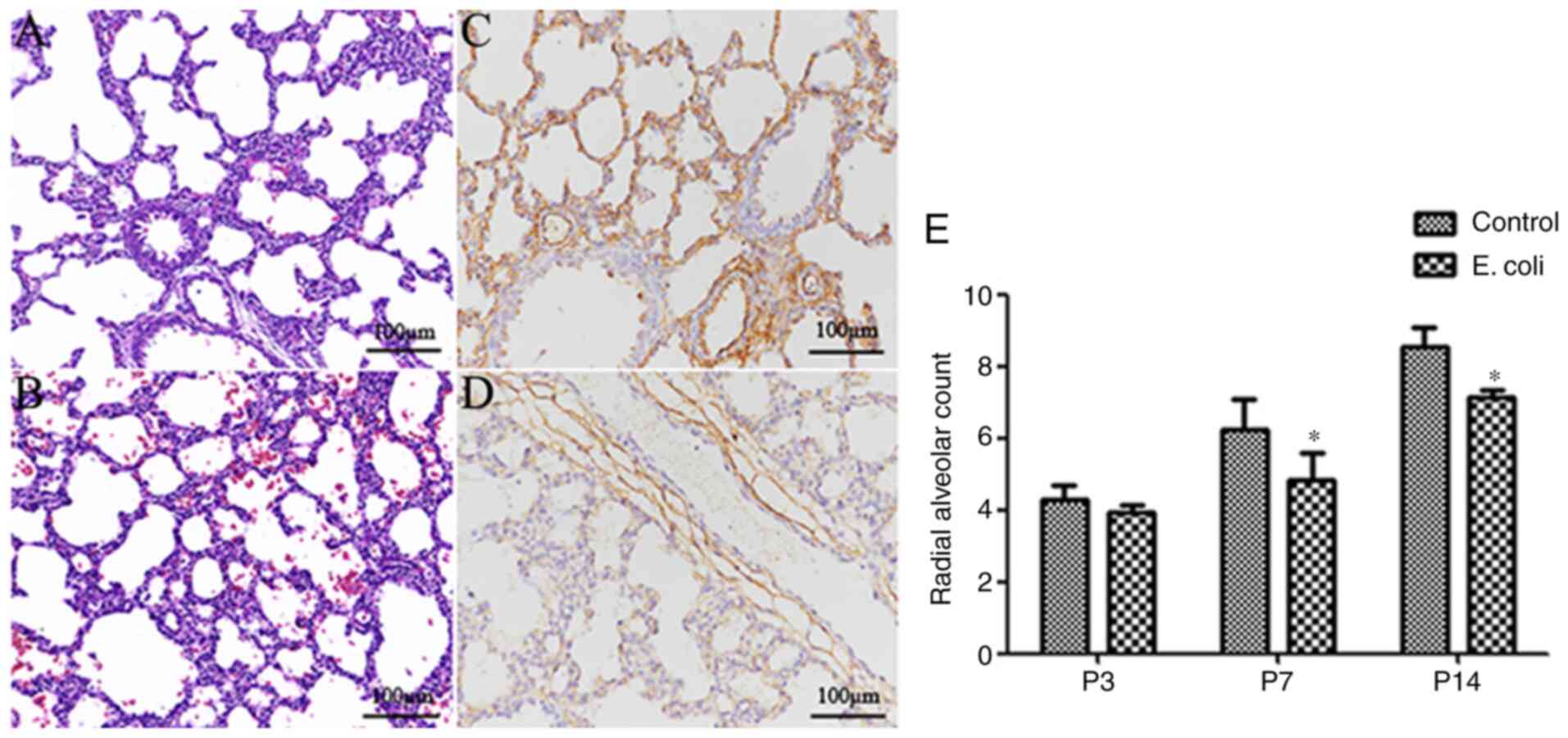
that of pups in the control group (Fig.
1A), the lung morphology of the pups in the E.
coli-infected group displayed fewer alveolar numbers, larger
alveoli, fewer secondary septa and thickened alveolar septa
(Fig. 1B). Furthermore, a higher
microvessel density (CD34 expression in lung tissue) was observed
in the control group (Fig. 1C)
compared with the E. coli-infected group (Fig. 1D). There was no marked difference in
the histological evidence of alveolar structure destruction or
fibrosis between the two groups. The RAC, as an important index of
lung development, increased gradually from P3 to P14. Notably, the
RAC of the control group at P7 and P14 was significantly higher
compared with that of the E. coli-infected group (both
P<0.05; Fig. 1E).
 | Table IIInduction of pregnancy losses by
intrauterine infection. |
Table II
Induction of pregnancy losses by
intrauterine infection.
| Time points |
Litters/fetuses | Escherichia
coli-treated group (n=10) | Control group
(n=10) |
|---|
| P3,7,14 | Pregnant
rats/fetuses | 10/94 | 10/98 |
| | Stillborn
litters | 1 | 0 |
| | Stillborn pups | 5 | 3 |
Notch4, Dll4, NF-κB, VEGF, Flt-1 and
Flk-1 are aberrantly expressed in the rat lung after intrauterine
infection
To investigate whether intrauterine infection
altered the expression of Notch4, Dll4, NF-κB, VEGF, Flt-1 (also
known as VEGFR1) and Flk-1 (also known as VEGFR2) in the rat lung,
PCR and western blot analysis were performed. Compared with those
of the control group, the mRNA expression levels of Notch4
(Fig. 2A) and Dll4 (Fig. 2B) after intrauterine infection were
significantly upregulated at P3 (P<0.05). A similar trend in
Notch4 and Dll4 protein expression was observed by western blotting
(Fig. 2G). However, there was no
significant difference in expression between the two groups at the
other time points. There was a significant difference in the Flk-1
mRNA expression levels at P3 and P7 between the two groups (both
P<0.05; Fig. 2E), while the mRNA
expression levels of Flt-1 were significantly increased at P14
compared with those of the control group (P<0.05; Fig. 2F). Additionally, the results
demonstrated that the protein and mRNA expression levels of NF-κB
were significantly increased in the E. coli-infected group
at P3 compared with the control group (P<0.05; Fig. 2C and G). The protein expression levels of Flk-1
in lung tissues from the E. coli-infected group were
significantly higher compared with those of the control group at P3
(Fig. 2H); however, there was no
difference in VEGF or Flt-1 protein expression levels between the
two groups at P3 (Fig. 2H).
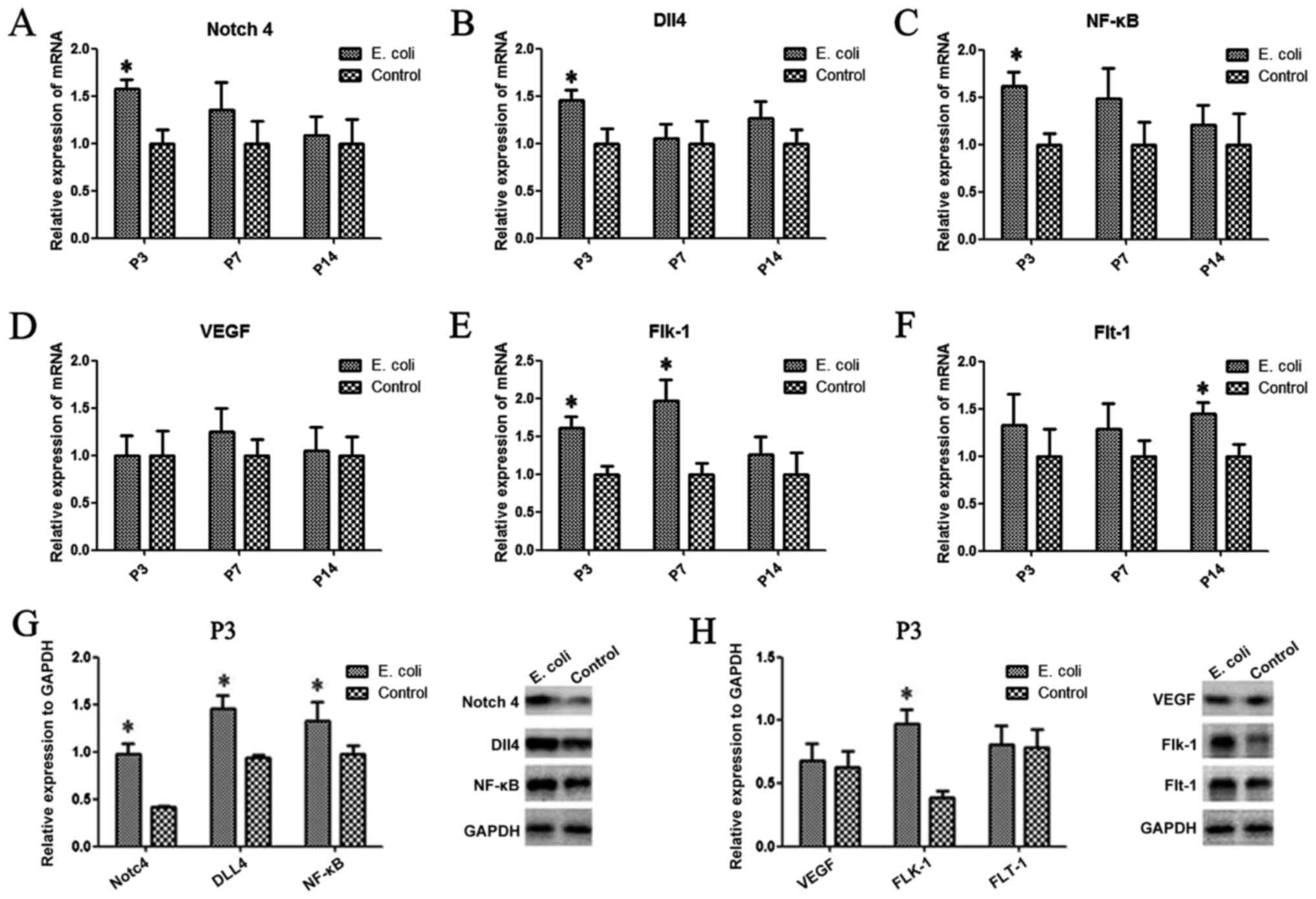 | Figure 2Aberrant expression of Notch4, Dll4,
NF-κB, VEGF, Flt-1 and Flk-1 in the rat lung after intrauterine
infection. (A) The mRNA expression levels of Notch4, (B) Dll4, (C)
NF-κB, (D) VEGF, (E) Flk-1 and (F) Flt-1 were evaluated in the two
groups by reverse transcription-quantitative PCR. (G) The protein
expression levels of Notch4, Dll4 and NF-κB, and (H) VEGF, Flt-1
and Flk-1 were determined in the two groups at P3 by western
blotting. Data are presented as the mean ± SD (n=10 per group).
*P<0.05. Dll4, delta-like canonical Notch ligand 4;
Flt-1, FMS-like tyrosine kinase 1; Flk-1, fetal liver kinase 1; P,
postnatal day. |
LPS induces the expression of Notch4,
Dll4, VEGF, Flt-1 and Flk-1 in PMVECs
PMVECs were exposed to different concentrations of
LPS to determine the optimal concentration for further experiments.
The dose of 10 µg/ml LPS was selected because it induced the
greatest mRNA upregulation of Notch4 and Dll4 (Fig. 3A). Subsequently, RT-qPCR analysis
revealed that the mRNA expression levels of Notch4 and Dll4 in the
LPS-treated PMVEC group were significantly increased compared with
those of control cells (both P<0.05; Fig. 3B and C). The Notch4 and Dll4 mRNA levels in the
LPS group were increased by 3.3 and 4.6-fold, respectively. In
addition, the mRNA expression levels of VEGF and its receptors
Flt-1 and Flk-1 in PMVECs showed a similar increase as that of
Notch4/Dll4 (all P<0.05; Fig.
3D-F). Compared with those of the control group, the mRNA
expression levels of VEGF, Flt-1 and Flk-1 in the LPS group were
elevated by 6.5, 6.7 and 5.3-fold, respectively. Similar results
were also observed for the protein levels by western blot analysis
(Fig. 3G). The present findings
suggest that LPS treatment can induce the expression of Notch4,
Dll4, VEGF, Flt-1 and Flk-1 in PMVECs.
Notch4/Dll4 inhibition reverses the
LPS-induced upregulation of VEGF, Flt-1 and Flk-1 in PMVECs
To determine whether the Notch4/Dll4 signaling
pathway is involved in the LPS-induced upregulation of angiogenic
markers in PMVECs, the present study determined the levels of VEGF,
Flt-1 and Flk-1 following inhibition of Notch4/Dll4 signaling.
Notch4/Dll4 inhibition by DAPT pretreatment suppressed LPS-induced
VEGF, Flt-1 and Flk-1 mRNA expression by 73, 78 and 70%,
respectively, compared with that of the LPS group (Fig. 3D-F). Western blotting also confirmed
that VEGF, Flt-1 and Flk-1 protein expression in the DAPT+LPS group
was significantly decreased compared with that in the LPS group
(P<0.05; Fig. 3G). These data
demonstrate that the Notch4/Dll4 pathway may regulate LPS-induced
VEGF, Flt-1 and Flk-1 expression in PMVECs.
LPS-induced NF-κB expression is
reversed by inhibiting Notch4/Dll4 in PMVECs
The promoters of VEGF, Flt-1 and Flk-1 contain
binding sites for the transcription factor NF-κB. To investigate
the role of Notch4/Dll4 in the regulation of angiogenic gene
expression in PMVECs, the present study determined the expression
levels of NF-κB following LPS treatment. The protein expression
levels of NF-κB in the LPS group were significantly increased
compared with those in the control group (P<0.05; Fig. 4A). Of note, this upregulation was
significantly suppressed by DAPT treatment (Fig. 4A). Additionally, LPS induced a
5.9-fold increase in NF-κB mRNA levels compared with those of the
control cells (Fig. 4B), while
these were decreased by 67% following DAPT treatment (Fig. 4B). These data suggest that the
effect of LPS on NF-κB expression in PMVECs may be modulated by
Notch4/Dll4 signaling.
 | Figure 4Inhibition of Notch4/Dll4 signaling
decreased the expression of NF-κB, inhibited cell apoptosis and
promoted cell proliferation in PMVECs. (A) Protein and (B) mRNA
expression levels of NF-κB in PMVECs in the control, LPS and
DAPT+LPS groups. (C) Cell viability in the control, LPS and
DAPT+LPS groups was measured using an MTT assay. (D) Representative
plots and quantitative flow cytometric analysis of the apoptotic
rates of PMVECs in the control, LPS and DAPT+LPS groups. In the
plots, quadrant a shows the proportion of dead/necrotic cells
(Annexin V-FITC-/PI+), quadrant b shows the
late apoptotic cells (Annexin V-FITC+/PI+),
quadrant c shows the early apoptotic cells (Annexin
V-FITC+/PI-) and quadrant d shows the live
cells (Annexin V-FITC-/PI-). The sum of
quadrants b and c was calculated as the apoptotic rate. Data are
presented as the mean ± SD (n=6 per group). *P<0.05
with comparisons shown by lines. Dll4, delta-like canonical Notch
ligand 4; PMVECs, pulmonary microvascular endothelial cells; LPS,
lipopolysaccharide; PI, propidium iodide. |
Notch4/Dll4 inhibition increases cell
proliferation and decreases the apoptosis rate of LPS-stimulated
PMVECs
Compared with that of the control group, LPS
suppressed PMVEC proliferation (Fig.
4C). In specific, as the culture time increased, the numbers of
viable cells were gradually decreased in the LPS group compared
with the control group. Pretreatment with DAPT attenuated this
suppression compared with the LPS alone group. The cell viability
in the DAPT+LPS group was higher compared with that in the LPS
group at each time point, and the differences between the two
groups were significant (P<0.05; Fig. 4C).
Next, flow cytometry analysis revealed that the
inhibition of Notch4/Dll4 signaling abrogated the LPS-induced
apoptosis in PMVECs. The average apoptotic rate of PMVECs in the
LPS group (exposed to LPS for 48 h; 49.5% apoptotic cells) was
significantly higher compared with that of cells in the control
group (4.2% apoptotic cells; P<0.05). In the DAPT+LPS group, the
average rate of apoptosis was 20%, which was significantly lower
compared with that of the LPS group (P<0.05; Fig. 4D). These results indicate a key role
of Notch4/Dll4 signaling in PMVEC proliferation and apoptosis.
Discussion
The present study revealed that intrauterine E.
coli infection impaired alveolarization and vascular
development in the rat lung, which was marked by decreased
microvessel density, fewer alveoli, fewer secondary septa, and
larger alveoli compared with the control group. Notch4 and Dll4
expression was significantly increased in the E.
coli-infected group at P3 compared with the control group.
Furthermore, the inhibition of Notch4 signaling in PMVECs
contributed to decreases in LPS-induced VEGF, Flt-1, Flk-1 and
NF-κB expression. The inhibition of Notch4/Dll4 signaling
accelerated cell proliferation and decreased the apoptosis rate of
LPS-stimulated PMVECs.
Numerous studies have demonstrated the relationship
between intrauterine infection/inflammation and BPD. It has become
increasingly evident that intrauterine infection/inflammation
impairs pulmonary vascular development in premature infants
(6,20). The pulmonary vasculature serves an
essential role in normal lung development. Dysregulated
angiogenesis during fetal growth may impair alveolarization and
contribute to the development of BPD. In the present study,
intrauterine infection in a rat model resulted in the arrest of
pulmonary alveolarization similar to that in human BPD, and
impairments in normal lung vascular development were marked by
reduced microvessel density in the lung.
However, the molecular mechanisms of vascular
development in BPD remain unclear. Notch receptors and their
ligands have important roles in vascular development. The combined
loss of Notch4 and Notch1 in mice results in defects in vascular
remodeling (21). Among the Notch
family members, Notch4 and Dll4 are primarily expressed in vascular
endothelial cells. The overexpression of activated Notch4 results
in vascular patterning defects (22), while Dll4 haploinsufficiency induces
major defects in vascular development, resulting in embryonic
lethality (23,24). Qiao et al (25) also showed that inhibiting Notch
signaling with a γ-secretase inhibitor protected against
angiotensin II-induced pulmonary vascular remodeling. These
findings emphasize the critical role of Notch4/Dll4 in vascular
development. Notably, the present results demonstrated that Notch4
and Dll4 expression was significantly increased in the E.
coli-infected group at P3. In vitro, Notch4/Dll4 mRNA
expression levels in LPS-stimulated PMVECs were also significantly
higher compared with those in the controls. These data reveal that
Notch4/Dll4 signaling may be critically involved in intrauterine
infection-induced impairments in lung development.
VEGF and VEGFR have key roles in stimulating
angiogenesis and vasculogenesis (26,27).
The appropriate expression of VEGF not only contributes to the
formation of capillaries but also controls alveolar and bronchial
growth (28). Changes in VEGF and
its receptors result in angiogenesis disorders, which contribute to
detrimental remodeling of small arteries (7). In the current study, there was a
significant difference in the Flk-1 mRNA levels at P3 and P7
between the two groups, while the mRNA expression levels of Flt-1
were significantly increased at P14 compared with those of the
control group. The present data demonstrated that intrauterine
infection disturbed VEGF signaling. Furthermore, inhibiting
Notch4/Dll4 signaling decreased apoptosis, promoted cell
proliferation, and decreased the expression of VEGF and its
receptors Flk-1 and Flt-1 in LPS-induced PMVECs. These results
indicate that Notch4/Dll4 and VEGF signaling may be associated with
intrauterine infection-induced BPD.
Currently, the role of Notch4/Dll4 signaling in the
process of BPD is not well understood. To explore the molecular
mechanisms underlying Notch4-mediated regulation of lung
development, the present study evaluated the expression of NF-κB.
As a pivotal regulator of inflammation, the NF-κB pathway has been
shown to promote lung injury in many models (29). In addition, it was observed that the
activation of NF-κB promoted alveolarization and pulmonary
angiogenesis (29). Alvira
(30) suggested that NF-κB might
play an essential, unique and beneficial role in lung development.
In the present study, LPS induced a large increase in NF-κB
expression in PMVECs compared with that of the control cells.
Furthermore, the inhibition of Notch4 signaling by a γ-secretase
inhibitor significantly decreased NF-κB expression in PMVECs
compared with that of the LPS group. Taken together, these results
demonstrated that Notch4/Dll4 signaling may have an important role
in the development of BPD induced by intrauterine infection,
possibly through the NF-κB pathway and downstream target genes,
such as VEGF, Flk-1 and Flt-1. However, the present study has
certain limitations, such as the lack of Notch4 knockout or
overexpression experiments, which would further clarify the effect
of Notch4/Dll4 on lung development.
In conclusion, the current study demonstrated that
early gestational intrauterine infection arrested alveolarization
and vascular development in the lung and disrupted Notch4/Dll4
signaling. Furthermore, Notch4/Dll4 signaling may have an important
role in lung development by regulating the expression of VEGF,
Flk-1 and Flt-1. Thus, targeting the Notch4/Dll4 pathway might
provide a novel therapeutic strategy for the treatment of BPD in
the future.
Acknowledgements
Not applicable.
Funding
Funding: This work was financially supported by the National
Natural Science Foundation of China (grant no. 81401235; recipient,
Canyang Zhan).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CZ and LC conceived and designed the study,
collected and analyzed the data and wrote the manuscript. CZ, YS
and JP performed experiments. YS performed the data analysis and
interpretation. TY designed the study, analyzed the data and
supervised the entire project. CZ and YS confirm the authenticity
of the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal procedures and protocols were reviewed
and approved by the Animal Care Committee of Zhejiang University
(Hangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zysman-Colman Z, Tremblay GM, Bandeali S
and Landry JS: Bronchopulmonary dysplasia-trends over three
decades. Paediatr Child Health. 18:86–90. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jobe AH: What is BPD in 2012 and what will
BPD become? Early Hum Dev. 88:S27–S28. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Thébaud B and Abman SH: Bronchopulmonary
dysplasia: Where have all the vessels gone? Roles of angiogenic
growth factors in chronic lung disease. Am J Respir Crit Care Med.
175:978–985. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
De Paepe ME, Greco D and Mao Q:
Angiogenesis-related gene expression profiling in ventilated
preterm human lungs. Exp Lung Res. 36:399–410. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stenmark KR and Abman SH: Lung vascular
development: Implications for the pathogenesis of bronchopulmonary
dysplasia. Annu Rev Physiol. 67:623–661. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hartling L, Liang Y and Lacaze-Masmonteil
T: Chorioamnionitis as a risk factor for bronchopulmonary
dysplasia: A systematic review and meta-analysis. Arch Dis Child
Fetal Neonatal Ed. 97:F8–F17. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cao L, Wang J, Tseu I, Luo D and Post M:
Maternal exposure to endotoxin delays alveolarization during
postnatal rat lung development. Am J Physiol Lung Cell Mol Physiol.
296:L726–L737. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim DH, Choi CW, Kim EK, Kim HS, Kim BI,
Choi JH, Lee MJ and Yang EG: Association of increased pulmonary
interleukin-6 with the priming effect of intra-amniotic
lipopolysaccharide on hyperoxic lung injury in a rat model of
bronchopulmonary dysplasia. Neonatology. 98:23–32. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kume T: Ligand-Dependent notch signaling
in vascular formation. Adv Exp Med Biol. 727:210–222.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hori K, Sen A and Artavanis-Tsakonas S:
Notch signaling at a glance. J Cell Sci. 126:2135–2140.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Caolo V, Molin DG and Post MJ: Notch
regulation of hematopoiesis, endothelial precursor cells, and blood
vessel formation: Orchestrating the vasculature. Stem Cells Int.
2012(805602)2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhan CY, Yuan TM, Sun Y and Yu HM: Early
gestational intrauterine infection induces postnatal lung
inflammation and arrests lung development in a rat model. J Matern
Fetal Neonatal Med. 24:213–222. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pan J, Zhan C, Yuan T, Wang W, Shen Y, Sun
Y, Wu T, Gu W, Chen L and Yu H: Effects and molecular mechanisms of
intrauterine infection/inflammation on lung development. Respir
Res. 19(93)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Uenishi GI, Jung HS, Kumar A, Park MA,
Hadland BK, McLeod E, Raymond M, Moskvin O, Zimmerman CE, Theisen
DJ, et al: NOTCH signaling specifies arterial-type definitive
hemogenic endotheliumfrom human pluripotent stem cells. Nat Commun.
9(1828)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhao H, Xu CN, Huang C, Jiang J and Li L:
Notch1 signaling participates in the release of inflammatory
mediators in mouse RAW264.7 cells via activating NF-κB pathway. Xi
Bao Yu Fen Zi Mian Yi Xue Za Zhi. 33:1310–1315. 2017.PubMed/NCBI(In Chinese).
|
|
16
|
Wang Y, Chen H, Li H, Zhang J and Gao Y:
Effect of angiopoietin-like protein 4 on rat pulmonary
microvascular endothelial cells exposed to LPS. Int J Mol Med.
32:568–576. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhou HS, Li M, Sui BD, Wei L, Hou R, Chen
WS, Li Q, Bi SH, Zhang JZ and Yi DH: Lipopolysaccharide impairs
permeability of pulmonary microvascular endothelial cells via
connexin40. Microvasc Res. 115:58–67. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang K, Wang P, Huang S, Wang X, Li T,
Jin Y, Hehir M and Xu C: Different mechanism of LPS-induced calcium
increase in human lung epithelial cell and microvascular
endothelial cell: A cell culture study in a model for ARDS. Mol
Biol Rep. 41:4253–4259. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Landry JS, Chan T, Lands L and Menzies D:
Long-Term impact of bronchopulmonary dysplasia on pulmonary
function. Can Respir J. 18:265–270. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Krebs LT, Xue Y, Norton CR, Shutter JR,
Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J,
Callahan R, et al: Notch signaling is essential for vascular
morphogenesis in mice. Genes Dev. 14:1343–1352. 2000.PubMed/NCBI
|
|
22
|
Uyttendaele H, Ho J, Rossant J and
Kitajewski J: Vascular patterning defects associated with
expression of activated notch4 in embryonic endothelium. Proc Natl
Acad Sci USA. 98:5643–5648. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gale NW, Dominguez MG, Noguera I, Pan L,
Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, et
al: Haploinsufficiency of delta-like 4 ligand results in embryonic
lethality due to major defects in arterial and vascular
development. Proc Natl Acad Sci USA. 101:15949–15954.
2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Trindade A, Kumar SR, Scehnet JS,
Lopes-da-Costa L, Becker J, Jiang W, Liu R, Gill PS and Duarte A:
Overexpression of delta-like 4 induces arterialization and
attenuates vessel formation in developing mouse embryos. Blood.
112:1720–1729. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Qiao LN, Xu HB, Shi K, Zhou TF, Hua YM and
Liu HM: Role of notch signal in angiotensin II induced pulmonary
vascular remodeling. Transl Pediatr. 2:5–13. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Muratore CS, Luks FI, Zhou Y, Harty M,
Reichner J and Tracy TF: Endotoxin alters early fetal lung
morphogenesis. J Surg Res. 155:225–230. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kallapur SG, Bachurski CJ, Le Cras TD,
Joshi SN, Ikegami M and Jobe AH: Vascular changes after
intra-amniotic endotoxin in preterm lamb lungs. Am J Physiol Lung
Cell Mol Physiol. 287:L1178–L1185. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bhatt AJ, Pryhuber GS, Huyck H, Watkins
RH, Metlay LA and Maniscalco WM: Disrupted pulmonary vasculature
and decreased vascular endothelial growth factor, Flt-1, and TIE-2
in human infants dying with bronchopulmonary dysplasia. Am J Respir
Crit Care Med. 164:1971–1980. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hou Y, Liu M, Husted C, Chen C,
Thiagarajan K, Johns JL, Rao SP and Alvira CM: Activation of the
nuclear factor-κB pathway during postnatal lung inflammation
preserves alveolarization by suppressing macrophage inflammatory
protein-2. Am J Physiol Lung Cell Mol Physiol. 309:L593–L604.
2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Alvira CM: Nuclear factor-kappa-B
signaling in lung development and disease: One pathway,
numerousfunctions. Birth Defects Res A Clin Mol Teratol.
100:202–216. 2014.PubMed/NCBI View Article : Google Scholar
|