Introduction
Endometriosis (EMs) is a painful disorder in which
the uterine lining (endometrium) grows abnormally outside the
uterus, and it frequently occurs in the lower abdomen or pelvic
area near the ovaries and fallopian tubes (1). Patients with endometriosis often
suffer from dysmenorrhea, pain during sexual intercourse, lower
abdominal pain and infertility (1).
Endometriosis is generally considered to be a
disease associated with chronic inflammation (1). The tissues associated with
endometriosis, especially in abdominal wall and ovarian
endometriosis, are infiltrated by a large number of inflammatory
cells, including CD3+ T cells, CD4+ T helper
(Th) cells, CD8+ cytotoxic T cells, CD450+
memory T cells, CD68+ macrophages, CD20+ B
cells, Th17 cells and regulatory T cells (2). M2 macrophages exist in greater numbers
in endometriotic tissues compared with the surrounding tissues
(3). In addition, Th17 cells and
macrophages are abundant in the peritoneal fluid of patients with
endometriosis, and the Th17 cell number has been indicated to
positively correlate with the disease severity (4-6).
Although peritoneal macrophages in patients with endometriosis
produce and secrete large amounts of inflammatory mediators, such
as TNF-α, IL-1β, IL-6 and vascular endothelial growth factor (VEGF)
(7), the phagocytic ability of the
macrophages is reduced. Therefore, the macrophages fail to
effectively remove the uterine endometrial debris in the context of
an inflammatory environment, thereby promoting the implantation of
the endometrial cells and subsequent endometriosis (8).
Currently, major therapeutic strategies for
endometriosis include surgical and medical treatment (9). However, surgical treatment for the
removal of endometrioma is associated with a high risk of
recurrence of endometriosis (10),
and medical treatment causes a decrease in estrogen levels, leading
to premature menopause (11).
Therefore, there is increasing interest in the biological treatment
of endometriosis through which the production of inflammatory
cytokines may be inhibited by regulating the differentiation of
macrophages and T cells.
Protein arginine methyltransferases (PRMTs) serve a
role in protein methylation. PRMT5 catalyzes the formation of
monomethylarginine and symmetric dimethylarginine in proteins,
regulates a variety of target genes such as CDKN2A and multiple
signaling pathways, such as the SNAIL/cadherin-1 pathway and the
leukemia inhibitory factor/signal transducer and activator of
transcription 3 signaling pathway (12), and participates in the
post-transcriptional splicing and processing of RNA, cell
proliferation, differentiation, apoptosis and tumorigenesis
(12). A previous study
demonstrated that PRMT5 is involved in the inflammatory response in
endothelial cells, contributing to the pathogenesis of various
diseases such as atherosclerosis (13). In addition, overexpression of PRMT5
has been indicated to promote the proliferation of memory T cells,
thereby enhancing IL-2 expression in multiple sclerosis (14). It has been demonstrated that
inhibition of PRMT5 expression can reduce the risk of colitis by
promoting the regulatory T cell differentiation of Th cells and
subsequently suppressing the production of TNF-α, IL-6 and
IL-13(15).
As the production of cytokines by activated
macrophages serves an important role in the pathogenesis of
endometriosis, it was hypothesized that PRMT5 contributes to
macrophage activation, thereby promoting endometriosis development.
The present study examined the effects of serum and extracts of
eutopic endometrium from patients with endometriosis on PRMT5
expression in THP-1-derived macrophages, as well as the underlying
signaling pathways. Furthermore, the role of PRMT5 in macrophage
activation in vitro and in vivo was also
investigated.
Materials and methods
Reagents
Phorbol 12-myristate 13-acetate (PMA; cat. no.
P8139) and lipopolysaccharide (LPS; cat. no. L2630) were purchased
from Sigma-Aldrich (Merck KGaA). The Luminex cytokine panel kit
(cat. no. LXSAHM) was purchased from R&D Systems, Inc. Rabbit
PRMT5 antibody (cat. no. 18436-1-AP) was purchased from ProteinTech
Group, Inc. IL-6 (cat. no. BMS213-2) and IFN-γ-induced protein 10
(IP-10) ELISA kits (cat. no. KAC2361) were purchased from Thermo
Fisher Scientific, Inc. SB431542 (cat. no. S1067), which is used as
an inhibitor of Smad 2/3(16), was
purchased from Selleck Chemicals. NF-κB inhibitor SN50 (cat. no.
SML1471) and PRMT5 inhibitor EPZ015666 were obtained from
MedChemExpress.
Sample collection
A total of 25 female patients with ovarian cysts or
infertility were enrolled from the Department of Obstetrics and
Gynecology of The Second Xiangya Hospital of Central South
University (Changsha, China) between December 2018 and October
2019. These patients were diagnosed with endometriosis by
laparoscopic and histopathological examination. According to the
revised American Fertility Society endometriosis classification,
the study population included 2 stage I-II cases, 7 stage III cases
and 16 stage IV cases (17). Of
these, 20 cases were in the proliferative phase and 5 cases were in
the secretory phase. The control group consisted of 12 patients who
were diagnosed with cervical neoplasia or uterus septum and
underwent laparoscopy, during which no endometrial lesions were
observed in the pelvic cavity. All patients (aged 20-45 years) had
regular periods and did not receive hormone therapy within 3 months
prior to surgery. There were no significant differences in age,
pregnancy, gravidity or proliferative vs. secretory phase between
the two groups (Table I).
 | Table IGeneral characteristics of the
females in the two groups. |
Table I
General characteristics of the
females in the two groups.
| Characteristic | EMS (n=25) | Control (n=12) | t-, u- or
χ2-value | P-value |
|---|
| Age, years | 28.0±4.99 | 31.75±5.05 | -1.84 | 0.07 |
| Age range,
years | 20-37 | 22-45 | | |
| Parity, n | 1.44±1.16 | 2.00±1.28 | -1.30 | 0.19 |
| Gravidity, n | 0.60±0.50 | 1.00±0.60 | -1.90 | 0.06 |
| Menstrual cycle,
number (P/S) | 20/5 | 8/4 | | 0.43 |
During surgery, endometrial biopsies were collected
via excision under sterile conditions from the control and research
groups, and they were promptly transported to the laboratory on ice
in PBS. A portion of each tissue sample was fixed in 10% formalin
solution for 12 h at room temperature. and then processed for
histological examination to exclude pathological abnormalities.
Hematoxylin-eosin staining was performed for histological
examination, and the staining was completed according to the a
previously described protocol (18).
The present study was approved by the Ethics
Committee of The Second Xiangya Hospital of Central South
University (Changsha, China). Written informed consent was obtained
from all participants.
Isolation of peritoneal monocytes
During the laparoscopy, the peritoneal cavity was
rinsed with 50 ml normal saline solution, and cell pellets were
collected via centrifugation at 376 x g for 5 min at 4˚C. Monocytes
were isolated following a standard protocol of Ficoll density
gradient centrifugation (19-21).
Briefly, Ficoll-loaded samples were centrifuged at 455 x g or 25
min at 20˚C. The buffy coat layer containing cells was then
harvested and washed three times with PBS followed by
centrifugation at 35 x g for 10 min at 20˚C to obtain monocytes.
The monocytes were then stored at -80˚C until multiple samples were
collected before performing subsequent reverse
transcription-quantitative PCR (RT-qPCR) and western blot
analyses.
Serum collection
Samples of 5 ml peripheral blood were collected from
each patient into a coagulation tube. The serum was separated by
centrifugation at 845 x g for 5 min at 4˚C and then stored at -80˚C
until further use.
Preparation of endometrial
homogenates
Endometrial homogenates were prepared as described
previously (22). Briefly,
endometrial tissues were excised during laparoscopy. A total of
~200 mg tissue was minced and homogenized in 5 ml RPMI-1640 (Gibco;
Thermo Fisher Scientific, Inc.), followed by centrifugation at
9,391 x g for 10 min at 4˚C. The supernatant was collected, and
protein concentration was determined using the BCA method. The
samples were then stored at -80˚C until further use.
Cell culture and treatment
THP-1 cells were generously provided by Dr Joseph
Huang (University of South Florida). The cells were grown in
RPMI-1640 supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 1% penicillin and 1% streptomycin at 37˚C in a
humidified atmosphere with 5% CO2. THP-1 cells were
differentiated into macrophage-like cells through treatment with 25
ng/ml PMA for 3 days at 37˚C in a humidified incubator with 5%
CO2. THP-1-derived macrophage-like cells were pretreated
with 100 ng/ml LPS for 24 h at 37˚C in a humidified incubator with
5% CO2 and then cultured in RPMI-1640 containing 10%
serum from controls and patients with endometriosis for 6 days or
cultured in homogenized solution of endometrium from controls and
eutopic endometrium from patients with endometriosis (100 µl
homogenized solution/ml medium) for 3 days. The inhibitors SN50 (50
µg/ml; treated for 1 h), SB431542 (10 µM; treated for 24 h) and
EPZ015666 (10 µM; treated for 24 h) were added to the medium before
treatment with the homogenized solution.
Western blot assay
THP-1 cells and the isolated peritoneal monocytes
were washed with cold PBS and lysed with RIPA lysis buffer
(Beyotime Institute of Biotechnology; cat. no. P0013B) on ice. Cell
lysates were collected by centrifugation at 13,523 x g for 10 min
at 4˚C, after which the supernatant was obtained and the protein
concentration was determined using a BCA assay kit (Beyotime
Institute of Biotechnology; cat. no. P0010S). Samples were then
heated at 95˚C for 5 min in loading buffer. Protein samples (10 µg)
were separated via 10% SDS-PAGE, transferred to a 0.2-µm
nitrocellulose membrane, blocked with 5% nonfat milk in TBS
containing 0.1% Tween-20 (TBST) for 2 h at room temperature, and
incubated overnight with primary antibodies against PRMT5 (1:1,000)
and β-actin (ProteinTech Group, Inc.; cat. no. 20536-1-AP;
1:10,000; internal control) at 4˚C. The membranes were then washed
with TBST three times, incubated with HRP-Goat Anti-Rabbit IgG
(H+L) (ProteinTech Group, Inc.; cat.no. SA00001-2; 1:5,000) for 1 h
at room temperature, and then washed again with TBST three times.
The chemiluminescence signal, visualized using SuperSignal West
Pico PLUS (Thermo Fisher Scientific, Inc.; cat. no. 34580) was
detected using the ChemiScope 5300 chemiluminescence system (Clinx
Science Instruments Co. Ltd.) and quantified using Quantity One
software (version 4.6.6; Bio-Rad Laboratories, Inc.).
RT-qPCR
Total RNA was extracted from THP-1 cells and the
isolated peritoneal monocytes using TRIzol® reagent
(Thermo Fisher Scientific, Inc.; cat. no. 15596026). cDNA was
synthesized using the PrimeScript RT reagent kit with gDNA Eraser
(Perfect Real Time; Takara, cat. no. RR047A) from 1 µg total RNA in
accordance with the manufacturer's protocol. Relative gene
expression levels were determined using the SYBR green PCR Master
Mix (Applied Biosystems; Thermo Fisher Scientific; cat. no.
4309155) using the 2-ΔΔCq method (23) with normalization to β-actin
expression. The thermocycling conditions were as follows: Initial
denaturation at 95°C for 30 sec, followed by 40 cycles
of 95°C for 5 sec, 60°C for 20 sec,
95°C for 1 sec, 65°C for 15 sec and
95°C for 1 sec for the dissociation curve. The primer
sequences were as follows: PRMT5 forward, 5'-GGTGAACGCTTCCCTG-3'
and reverse, 5'-TGAGACTACGGTCACTTGG-3'; β-actin forward,
5'-GAGCGCGGCTACAGCTT-3' and reverse,
5'-TCCTTAATGTCACGCACGCACGATTT-3'.
ELISA
After being treated as aforementioned, THP-1 cells
with eutopic endometrial tissue extract from patients with
endometriosis and the PRMT5 inhibitor EPZ015666, IL-6 and IP-10
levels in these cells were measured using ELISA kits (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Luminex cytokine assay
TNF-α, IL-6, IL-10 and C-C motif chemokine 20
(CCL20) levels in endometrial extracts from patients with
endometriosis and control individuals were determined using the
Luminex cytokine kit (R&D Systems, Inc.) according to the
manufacturer's instructions. Briefly, frozen endometrial extracts
were homogenized in RIPA lysis buffer [Beyotime Institute of
Biotechnology; cat. no. P0013B; 50 mM Tris (pH 7.4), 150 mM NaCl,
1% Triton X-100, 0.1% SDS, 1% sodium deoxycholate] with protease
inhibitor cocktail (Sigma-Aldrich; Merck KGaA). Total protein
levels were measured using the BCA method. TNF-α, IL-6, IL-10 and
CCL20 levels were measured using a Luminex 200 analyzer (Luminex
Corporation). The ratio of cytokine concentration to total protein
concentration (pg/ml/mg protein) was used to determine differences
between the two groups.
Statistical analysis
All quantitative data are presented as the mean ±
SD. Statistical analysis was performed using SPSS software (version
17.0; SPSS, Inc.) and visualized using GraphPad Prism software
(version 8.0; GraphPad Software, Inc.). In vitro cell
experiments were repeated three times per sample, and the average
values were used. Quantitative data were firstly examined for
normal distribution using the Kolmogorov-Smirnov test, and data
with equal variances were compared using one-way ANOVA followed by
the Least Significant Difference test for comparisons among three
groups, while unpaired t-test was used for comparisons between two
groups. Alternatively, Mann-Whitney U tests were used for
comparisons. Categorical variables were compared using the
χ2 test. The Fisher's exact test was used to analyze the
menstrual cycle variable in Table
I. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cytokine levels in endometrial
extracts from patients with endometriosis and control
individuals
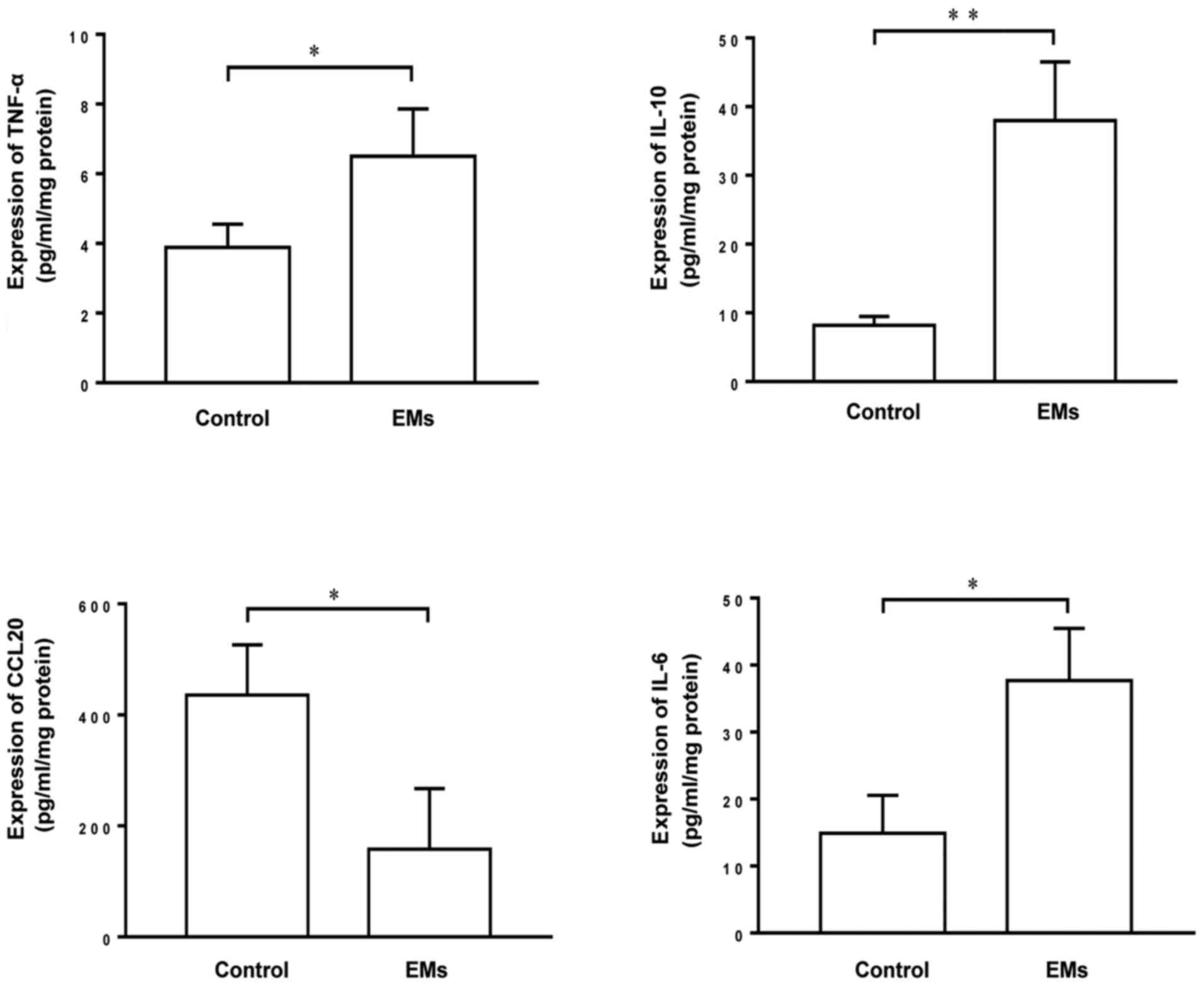
To investigate the differential expression of
cytokines in patients with endometriosis and control individuals,
the Luminex assay was used to compare the levels of TNF-α, IL-6,
IL-10 and CCL20 in endometrial tissue extracts from these patients.
The results demonstrated that the endometrial extracts of patients
with endometriosis contained significantly higher levels of TNF-α,
IL-6 and IL-10 and a decreased level of CCL20 compared with the
endometrial extracts of control individuals (Fig. 1), suggesting that there are
differences in the expression of TNF-α, IL-6, IL-10 and CCL20 that
may contribute to the inflammatory response under pathological
conditions.
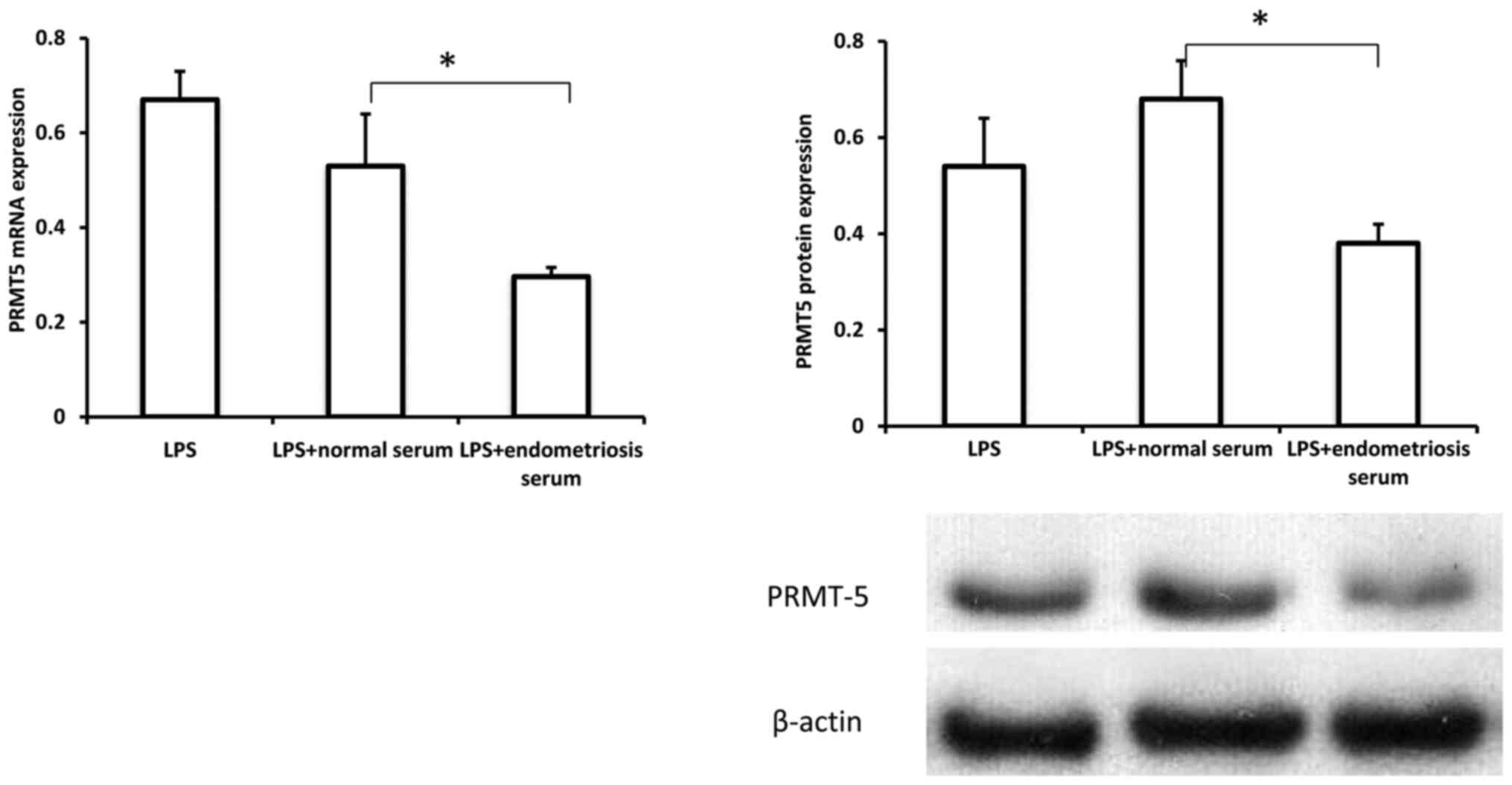
Serum from patients with endometriosis
inhibits PRMT5 expression in macrophages
To investigate the possible role of
macrophage-derived PRMT5 in endometriosis development, the
expression pattern of PRMT5 in THP-1 cells treated with serum from
controls and patients with endometriosis in the presence of LPS was
examined. As indicated in Fig. 2,
both the mRNA and protein levels of PRMT5 were significantly
decreased in THP-1 cells treated with serum from patients with
endometriosis, compared with the levels in cells treated with serum
from controls. These results revealed that PRMT5 expression in
macrophages could be downregulated in the context of endometriosis,
suggesting a potential role for macrophage-derived PRMT5 in the
pathogenesis of endometriosis.
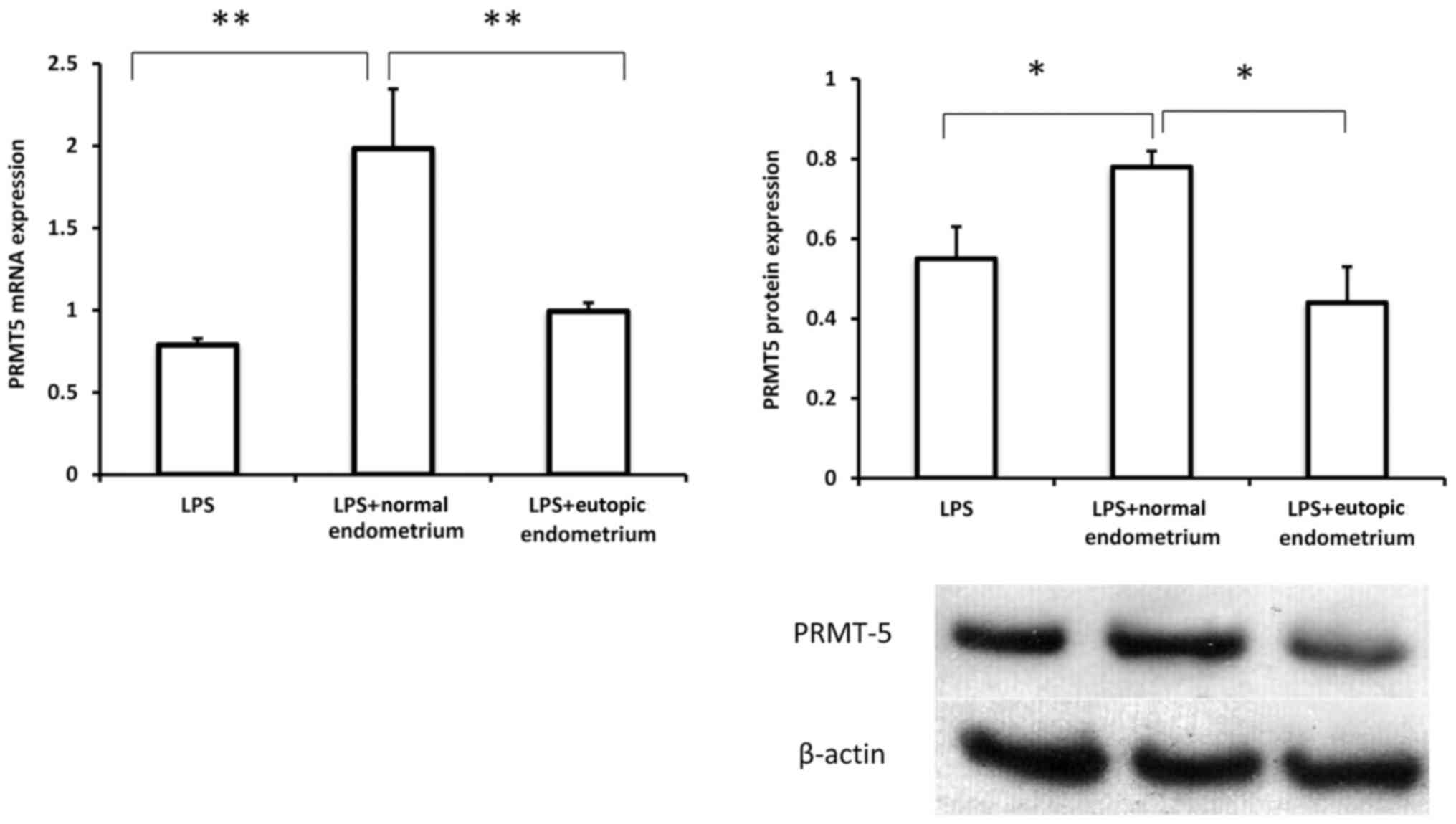
Extracts of eutopic endometrium
suppress PRMT5 expression in macrophages
To further confirm the possible involvement of
macrophage-expressed PRMT5 in endometriosis, PRMT5 expression was
examined in THP-1 cells treated with extracts of normal and eutopic
endometrium in the presence of LPS. As presented in Fig. 3, the extracts of normal endometrium
induced a substantial increase in PRMT5 expression in THP-1 cells.
However, treatment with extracts of eutopic endometrium
significantly inhibited PRMT5 mRNA and protein expression in
macrophages compared with extracts of normal endometrium,
suggesting that macrophage-derived PRMT5 may serve a role in the
interaction between macrophages and the eutopic endometrial
microenvironment, which likely contributes to the inflammatory
response under pathological conditions.
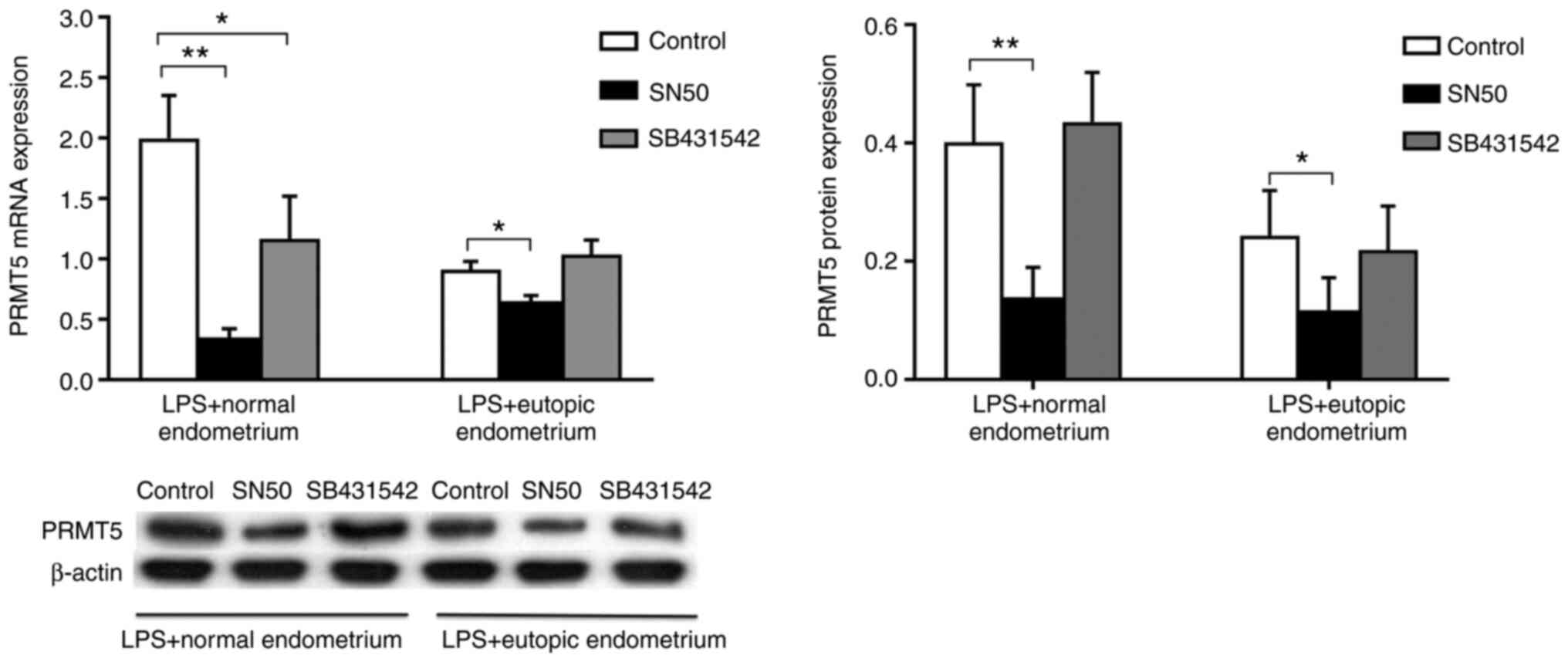
Macrophage expression of PRMT5 is
regulated in a NF-κB signaling-dependent manner
To investigate the mechanisms underlying macrophage
PRMT5 expression in endometriosis, the NF-κB inhibitor SN50 or the
Smad2/3 inhibitor SB431542 was added to cells together with
homogenized endometrial tissue to examine PRMT5 expression in THP-1
cells in the presence of LPS. The results revealed that SB431542
significantly inhibited the mRNA expression of PRMT5 in cells
treated with extracts of normal endometrium. However, SB431542
treatment demonstrated no inhibition of PRMT5 protein expression in
cells treated with extracts of normal endometrium. Additionally, no
inhibition was demonstrated in cells treated with extracts of
eutopic endometrium at both the mRNA and protein level. The results
demonstrated that SN50, but not SB431542, markedly inhibited PRMT5
mRNA and protein expression in THP-1 cells treated with extracts of
both normal and eutopic endometrium (Fig. 4). The results indicated that the
endometrial microenvironment regulated macrophage PRMT5 expression
through NF-κB signaling, but not Smad2/3 signaling.
PRMT5 mediates endometrial tissue
extract-induced macrophage activation
To examine the function of PRMT5 in the interaction
between macrophages and the endometrial microenvironment, THP-1
cells were treated with eutopic endometrial tissue extract from
patients with endometriosis containing the PRMT5 inhibitor
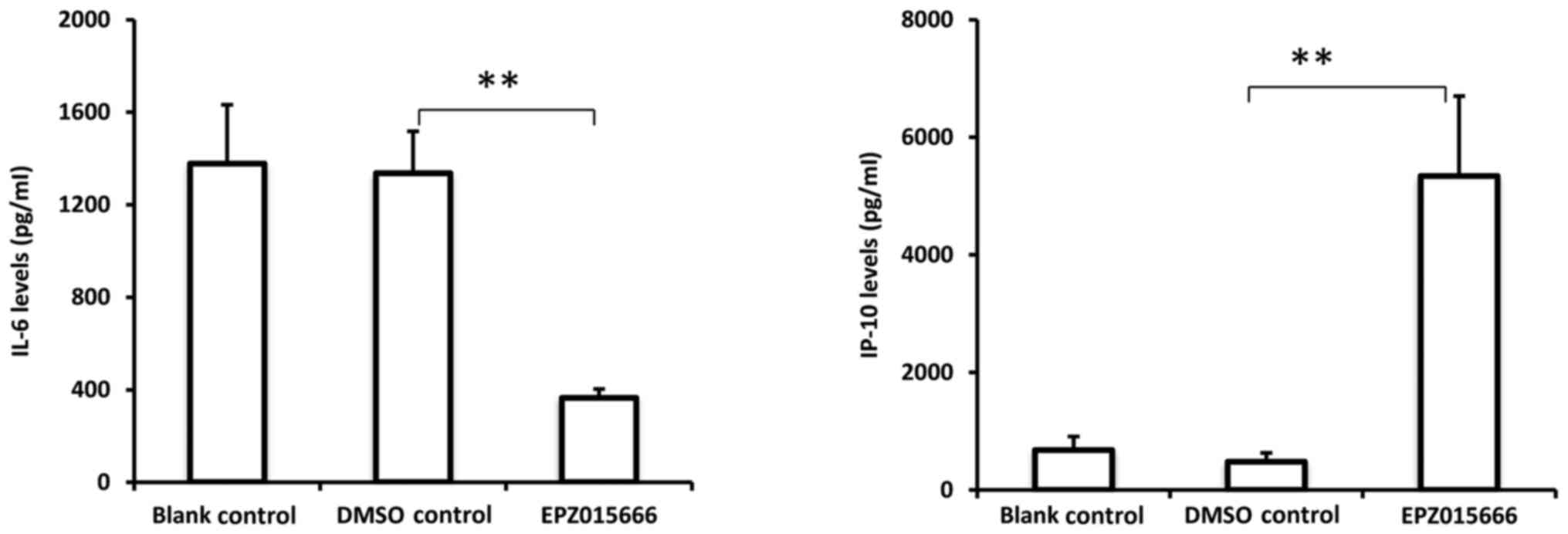
EPZ015666. As presented in Fig. 5,
EPZ015666 significantly inhibited IL-6 secretion and promoted IP-10
secretion from THP-1 cells activated by eutopic endometrial tissue
extract. These data indicated that PRMT5 is essential for
endometrial tissue extract-induced macrophage activation,
suggesting an important role for PRMT5 in mediating the interaction
between macrophages and the endometrial microenvironment.
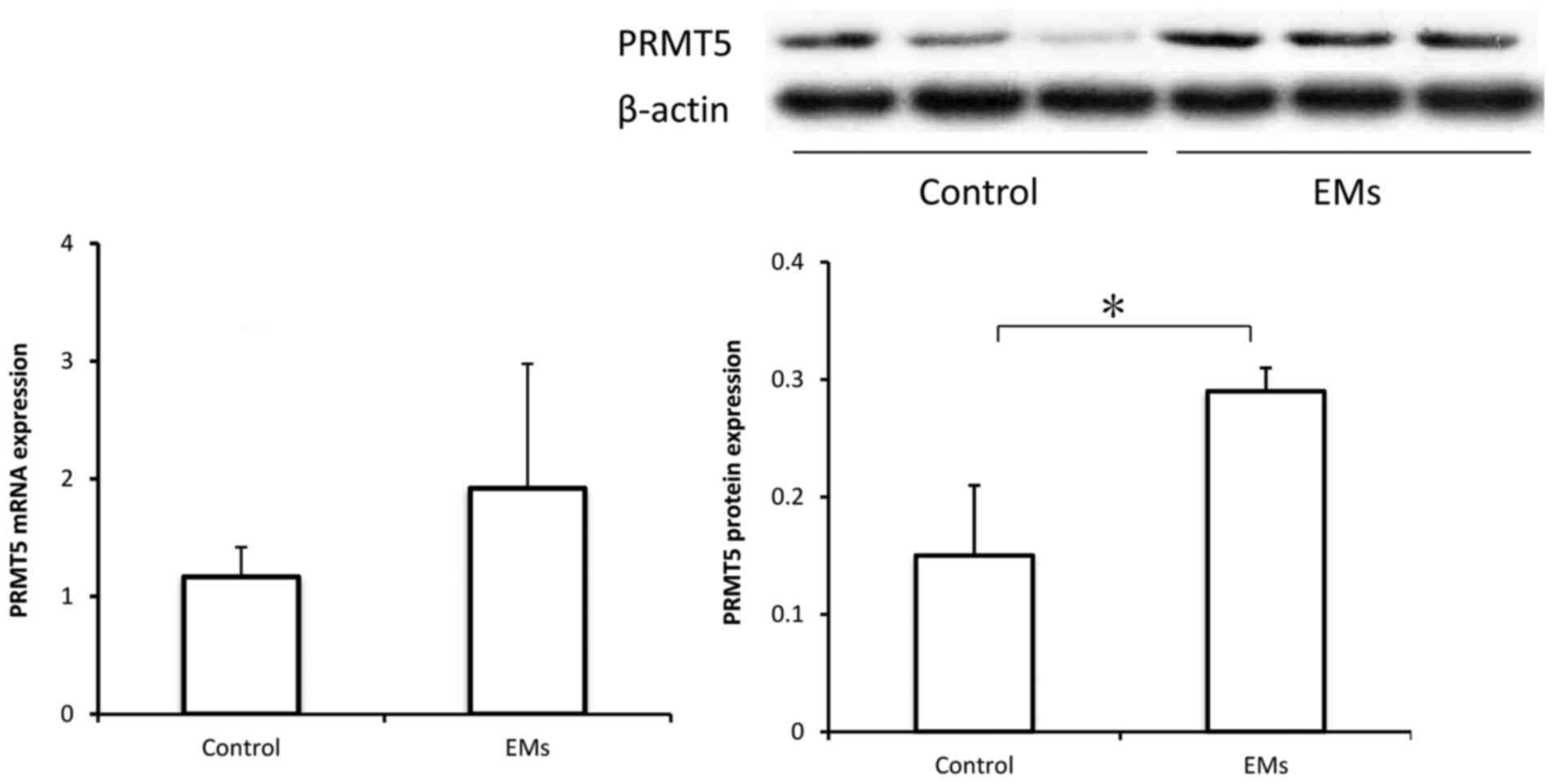
PRMT5 expression is upregulated in the
peritoneal fluid monocytes of patients with endometriosis
To further investigate the possible role of PRMT5
expression in macrophages in endometriosis, peritoneal monocytes
were collected from controls and patients with endometriosis for
measuring PRMT5 expression. Previous studies have indicated that
among the isolated CD68+ monocytes in peritoneal fluid,
85-95% are macrophages (20,21).
As demonstrated in Fig. 6, the
protein levels of PRMT5 were significantly increased in the
peritoneal monocytes from patients with endometriosis, compared
with those in the peritoneal monocytes from controls. However,
while the mRNA levels of PRMT5 appeared to also be increased, the
difference was not statistically significant.
Discussion
Previous studies have demonstrated that PRMT5 serves
a critical role in inflammatory and autoimmune diseases, such as
rheumatoid arthritis (14,15,22,24).
However, the function of PRMT5 in macrophage activation has not yet
been investigated. The present study demonstrated that the levels
of cytokines in the endometrial extracts of patients with
endometriosis were different from those in the endometrial extracts
of control individuals. NF-κB-dependent PRMT5 may contribute to
macrophage activation, resulting in the production of the
pro-inflammatory mediators IL-6 and IP-10, which likely contributes
to the pathogenesis of endometriosis (25).
Currently, the pathogenesis of endometriosis is
incompletely understood. A generally accepted concept is the theory
of retrograde menstruation, in which regurgitated endometrial cells
during menstruation recruit blood monocytes into the peritoneal
fluid and secrete large amounts of inflammatory mediators, such as
TNF-α, IL-1β, IL-6, IL-8 and VEGF, thereby promoting invasion and
angiogenesis of ectopic endometrial implants and subsequent lesion
formation (26). It has been
indicated that establishment and development of ectopic endometrial
lesions are significantly suppressed if peritoneal macrophages are
depleted in a mouse model of endometriosis (27), suggesting that interactions between
macrophages and the ectopic endometrium may serve a key role in the
development of endometriosis.
In the present study, the expression levels of
cytokines TNF-α, IL-6 and IL-10 were increased and that of CCL20
was decreased in the endometrial extracts of patients with
endometriosis compared with the endometrial extracts of control
patients. The mechanisms via which cytokines regulate macrophage
activation are relatively complex, as it has been reported that
macrophages can be activated and differentiated into distinct
subtypes under different cytokine-mediated microenvironments
(28). A recent study demonstrated
that in response to TNF-α, human endometrial stromal cell-derived
IL-6 and monocyte chemoattractant protein-1 stimulated peritoneal
macrophages toward M2-polarization, which could modulate
endometriosis (29). It was also
reported that osteoblast-derived CCL20 stimulates the recruitment
of macrophages and T cells (30).
In the current study, PRMT5 expression in THP-1-derived macrophages
was markedly downregulated following treatment with either serum or
extracts of eutopic endometrium from patients with endometriosis
compared with the serum or extracts of control endometrium samples,
indicating that PRMT5 may be a regulator of macrophage activation
in the development of endometriosis. Consistently, overexpression
of PRMT5 has been indicated to enhance the expression of major
histocompatibility complex class II (MHC II) in macrophages
(31). MHC II-dependent antigen
presentation to CD4+ T cells orchestrates the interplay
among a variety of immune cell types and regulates the humoral and
cell-mediated immune responses (32). Therefore, MHC II expression may be
inhibited via the downregulation of macrophage PRMT5 in the context
of the microenvironment of eutopic endometrial lesions, resulting
in a suppressed macrophage-dependent antigen-presenting capacity
and an accelerated development of ectopic endometrial lesions.
NF-κB, a major transcriptional factor of the
inflammatory response in immunity, has been revealed to be involved
in the pathophysiology of endometriosis (33). PRMT5 is responsible for the
methylation of Arg30 on the NF-κB subunit p65, and may thereby
regulate the expression of NF-κB target genes. In addition, PRMT5
activates NF-κB by binding to TNF-related apoptosis-inducing ligand
(34). On the other hand, NF-κB may
promote PRMT5 expression in Th cells as an upstream regulator of
PRMT5(14), which is consistent
with the present finding that the NF-κB inhibitor SN50 suppressed
PRMT5 expression in THP-1-derived macrophages treated with extracts
of either normal or eutopic endometrium. Further investigation is
required to elucidate the molecular mechanisms underlying the
interplay between PRMT5 and NF-κB.
EPZ015666 is a selective inhibitor of PRMT5 that can
specifically block PRMT5 activity (35). A previous study has revealed that
EPZ015666 inhibited IL-6 and IL-8 production by fibroblast-like
synoviocytes (24), which supports
the present finding that EPZ015666 reduced the secretion of IL-6
while increasing the secretion of IP-10 by THP-1 cells treated with
extracts of eutopic endometrium. Both studies verified that PRMT5
possesses proinflammatory properties. Interestingly, the effect of
PRMT5 on IP-10 production appears to depend on the cell type
involved in the inflammatory response. For example, PRMT5 has been
indicated to promote TNF-α-induced IP-10 production by endothelial
cells (36).
Previous studies confirmed that 85-95% of the
isolated monocytes in peritoneal fluid are macrophages (20,21).
To further investigate the possible role of PRMT5 expression in
peritoneal macrophages in endometriosis, peritoneal monocytes were
obtained from patients and it was indicated that PRMT5 protein
expression in peritoneal monocytes was significantly upregulated in
patients with endometriosis compared with controls. Possible
explanations for this discrepancy are as follows: i) T cells and
natural killer (NK) cells are present in peritoneal fluid, although
peritoneal macrophages are highly abundant; ii) the cytokines
released by ectopic endometrium influence PRMT5 expression in
peritoneal monocytes; and iii) the majority of samples were
collected from patients with stage III-IV endometriosis. Due to the
different immune profiles of eutopic endometrium between
endometriosis stages I-II and III-IV, more samples are required
from patients with stage I-II endometriosis to further examine the
association between PRMT5 expression and the development of
endometriosis (37).
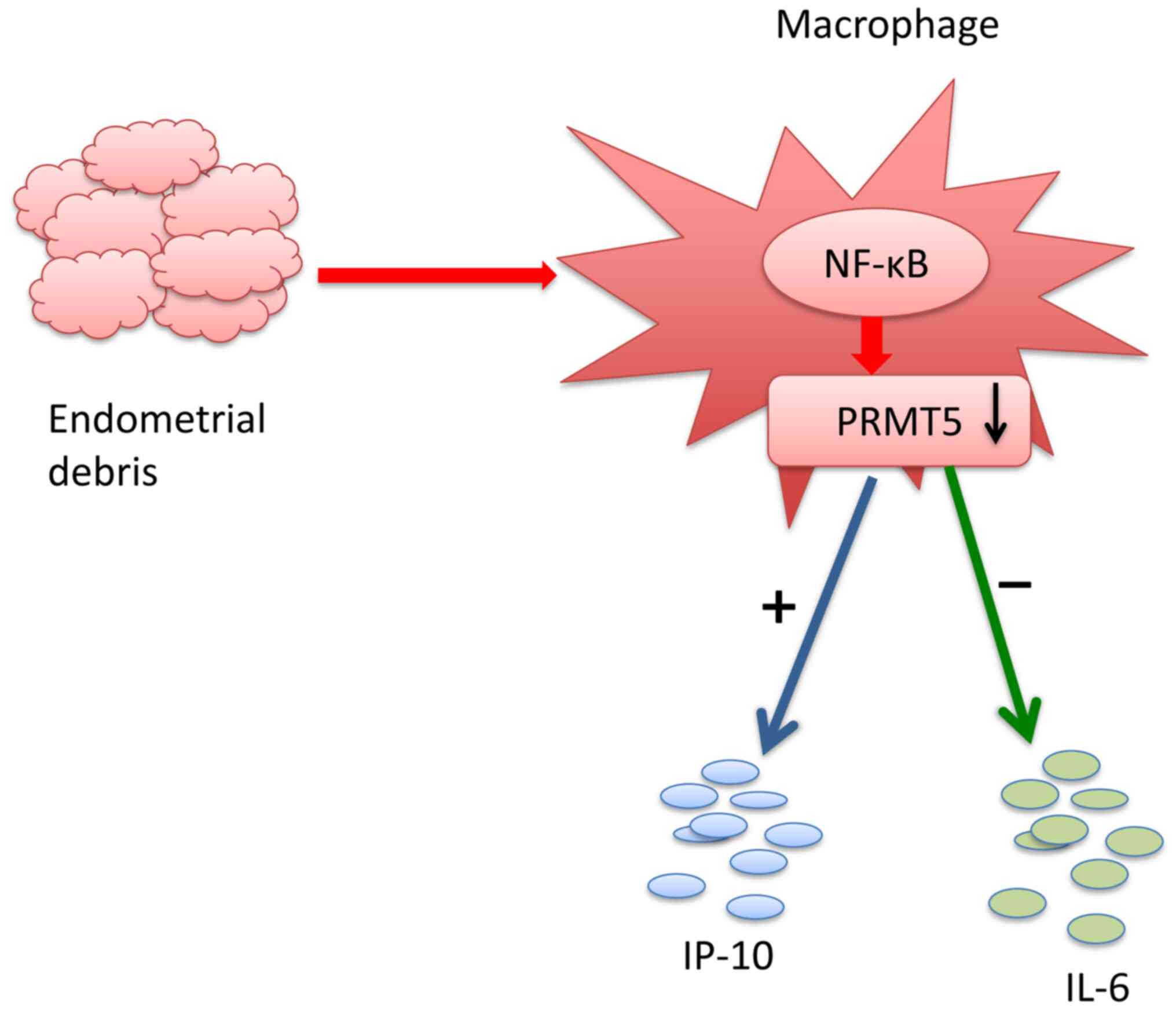
Taken together, the results of the present study
indicated that eutopic endometrium in patients with endometriosis
induced a reduction in PRMT5 expression in THP-1-derived
macrophages, resulting in the inhibition of IL-6 production and the
increased production of IP-10 (Fig.
7). The mechanism of PRMT5 in endometriosis remains unknown.
IP-10 is a key chemokine of Th1 and NK cells and the increase in
IP-10 production reduces the number of Th1 and NK cells recruited
to the peritoneal fluid (38). The
inability of these cells to effectively clear peritoneal
endometrial cells results in the successful establishment of
endometrial implants (26). In this
context, PRMT5 appears to be a novel regulator of macrophage
activation.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and analyzed during the
current study are not publicly available due to restricting patient
privacy regulations by the different countries but are available
from the corresponding author on reasonable request.
Authors' contributions
XW and XC collected and interpreted the patient
samples and data. XC and LH performed the experiments of the study.
XC and HD analyzed the data. HD designed the study was a major
contributor in writing of the manuscript. All authors read and
approved the final manuscript. HD and XC confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Second Xiangya Hospital of Central South
University (Changsha, China). Written informed consent was obtained
from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mehedintu C, Plotogea MN, Ionescu S and
Antonovici M: Endometriosis still a challenge. J Med Life.
7:349–357. 2014.PubMed/NCBI
|
|
2
|
Scheerer C, Bauer P, Chiantera V, Sehouli
J, Kaufmann A and Mechsner S: Characterization of
endometriosis-associated immune cell infiltrates (EMaICI). Arch
Gynecol Obstet. 294:657–664. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bacci M, Capobianco A, Monno A, Cottone L,
Di Puppo F, Camisa B, Mariani M, Brignole C, Ponzoni M, Ferrari S,
et al: Macrophages are alternatively activated in patients with
endometriosis and required for growth and vascularization of
lesions in a mouse model of disease. Am J Pathol. 175:547–556.
2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ahn SH, Edwards AK, Singh SS, Young SL,
Lessey BA and Tayade C: IL-17A contributes to the pathogenesis of
endometriosis by triggering proinflammatory cytokines and
angiogenic growth factors. J Immunol. 195:2591–2600.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Antsiferova YS, Sotnikova NY, Posiseeva LV
and Shor AL: Changes in the T-helper cytokine profile and in
lymphocyte activation at the systemic and local levels in women
with endometriosis. Fertil Steril. 84:1705–1711. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gogacz M, Winkler I, Bojarska-Junak A,
Tabarkiewicz J, Semczuk A, Rechberger T and Adamiak A: Increased
percentage of Th17 cells in peritoneal fluid is associated with
severity of endometriosis. J Reprod Immunol. 117:39–44.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Izumi G, Koga K, Takamura M, Makabe T,
Satake E, Takeuchi A, Taguchi A, Urata Y, Fujii T and Osuga Y:
Involvement of immune cells in the pathogenesis of endometriosis. J
Obstet Gynaecol Res. 44:191–198. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Berbic M, Schulke L, Markham R, Tokushige
N, Russell P and Fraser IS: Macrophage expression in endometrium of
women with and without endometriosis. Hum Reprod. 24:325–332.
2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim TH, Yoo JY, Choi KC, Shin JH, Leach
RE, Fazleabas AT, Young SL, Lessey BA, Yoon HG and Jeong JW and
Jeong JW: Loss of HDAC3 results in nonreceptive endometrium and
female infertility. Sci Transl Med. 11(eaaf7533)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bozdag G: Recurrence of endometriosis:
Risk factors, mechanisms and biomarkers. Womens Health (Lond).
11:693–699. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gheorghisan-Galateanu AA and Gheorghiu ML:
Hormonal therapy in women of reproductive age with endometriosis:
An update. Acta Endocrinol (Buchar). 15:276–281. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Karkhanis V, Hu YJ, Baiocchi RA, Imbalzano
AN and Sif S: Versatility of PRMT5-induced methylation in growth
control and development. Trends Biochem Sci. 36:633–641.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bandyopadhyay S, Harris DP, Adams GN,
Lause GE, McHugh A, Tillmaand EG, Money A, Willard B, Fox PL and
Dicorleto PE: HOXA9 methylation by PRMT5 is essential for
endothelial cell expression of leukocyte adhesion molecules. Mol
Cell Biol. 32:1202–1213. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Webb LM, Amici SA, Jablonski KA,
Savardekar H, Panfil AR, Li L, Zhou W, Peine K, Karkhanis V,
Bachelder EM, et al: PRMT5-selective inhibitors suppress
inflammatory T cell responses and experimental autoimmune
encephalomyelitis. J Immunol. 198:1439–1451. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zheng Y, Huang L, Ge W, Yang M, Ma Y, Xie
G, Wang W, Bian B, Li L, Nie H and Shen L: Protein arginine
methyltransferase 5 inhibition upregulates Foxp3(+) regulatory T
cells frequency and function during the ulcerative colitis. Front
Immunol. 8(596)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bian X, Li B, Yang J, Ma K, Sun M, Zhang C
and Fu X: Regenerative and protective effects of dMSC-sEVs on
high-glucose-induced senescent fibroblasts by suppressing RAGE
pathway and activating Smad pathway. Stem Cell Res Ther.
11(166)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Johnson NP, Hummelshoj L, Adamson GD,
Keckstein J, Taylor HS, Abrao MS, Bush D, Kiesel L, Tamimi R,
Sharpe-Timms KL, et al: World endometriosis society consensus on
the classification of endometriosis. Hum Reprod. 32:315–324.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fischer AH, Jacobson KA, Rose J and Zeller
R: Hematoxylin and eosin staining of tissue and cell sections. CSH
Protoc. 2008(pdb.prot4986)2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xie Q, He H, Wu YH, Zou LJ, She XL, Xia XM
and Wu XQ: Eutopic endometrium from patients with endometriosis
modulates the expression of CD36 and SIRP-α in peritoneal
macrophages. J Obstet Gynaecol Res. 45:1045–1057. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu XQ, Fang XL, Lin QH, Huang FY and Xia
XM: VEGF production and its receptor expression of MPhis in
peritoneal fluid. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 19:462–465.
2003.PubMed/NCBI(In Chinese).
|
|
21
|
McLaren J, Prentice A, Charnock-Jones DS,
Millican SA, Müller KH, Sharkey AM and Smith SK: Vascular
endothelial growth factor is produced by peritoneal fluid
macrophages in endometriosis and is regulated by ovarian steroids.
J Clin Invest. 98:482–489. 1996.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dasgupta S, Bhattacharya-Chatterjee M,
O'Malley BW Jr and Chatterjee SK: Inhibition of NK cell activity
through TGF-beta 1 by down-regulation of NKG2D in a murine model of
head and neck cancer. J Immunol. 175:5541–5550. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen D, Zeng S, Huang M, Xu H, Liang L and
Yang X: Role of protein arginine methyltransferase 5 in
inflammation and migration of fibroblast-like synoviocytes in
rheumatoid arthritis. J Cell Mol Med. 21:781–790. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li S, Fu X, Wu T, Yang L, Hu C and Wu R:
Role of interleukin-6 and its receptor in endometriosis. Med Sci
Monit. 23:3801–3807. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ulukus M and Arici A: Immunology of
endometriosis. Minerva Ginecol. 57:237–248. 2005.PubMed/NCBI
|
|
27
|
Haber E, Danenberg HD, Koroukhov N, Ron-El
R, Golomb G and Schachter M: Peritoneal macrophage depletion by
liposomal bisphosphonate attenuates endometriosis in the rat model.
Hum Reprod. 24:398–407. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu G and Yang H: Modulation of macrophage
activation and programming in immunity. J Cell Physiol.
228:502–512. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li MZ, Wu YH, Ali M, Wu XQ and Nie MF:
Endometrial stromal cells treated by tumor necrosis factor-α
stimulate macrophages polarized toward M2 via interleukin-6 and
monocyte chemoattractant protein-1. J Obstet Gynaecol Res.
46:293–301. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Doucet M, Jayaraman S, Swenson E, Tusing
B, Weber KL and Kominsky SL: CCL20/CCR6 signaling regulates bone
mass accrual in mice. J Bone Miner Res. 31:1381–1390.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fan Z, Kong X, Xia J, Wu X, Li H, Xu H,
Fang M and Xu Y: The arginine methyltransferase PRMT5 regulates
CIITA-dependent MHC II transcription. Biochim Biophys Acta.
1859:687–696. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ding Q, Chen J, Wei X, Sun W, Mai J, Yang
Y and Xu Y: RAFTsomes containing epitope-MHC-II complexes mediated
CD4+ T cell activation and antigen-specific immune
responses. Pharm Res. 30:60–69. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
González-Ramos R, Defrère S and Devoto L:
Nuclear factor-kappaB: A main regulator of inflammation and cell
survival in endometriosis pathophysiology. Fertil Steril.
98:520–528. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wei H, Wang B, Miyagi M, She Y, Gopalan B,
Huang DB, Ghosh G, Stark GR and Lu T: PRMT5 dimethylates R30 of the
p65 subunit to activate NF-κB. Proc Natl Acad Sci USA.
110:13516–13521. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chan-Penebre E, Kuplast KG, Majer CR,
Boriack-Sjodin PA, Wigle TJ, Johnston LD, Rioux N, Munchhof MJ, Jin
L, Jacques SL, et al: A selective inhibitor of PRMT5 with in vivo
and in vitro potency in MCL models. Nat Chem Biol. 11:432–437.
2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Harris DP, Bandyopadhyay S, Maxwell TJ,
Willard B and DiCorleto PE: Tumor necrosis factor (TNF)-α induction
of CXCL10 in endothelial cells requires protein arginine
methyltransferase 5 (PRMT5)-mediated nuclear factor (NF)-κB p65
methylation. J Biol Chem. 289:15328–15339. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Poli-Neto OB, Meola J, Rosa-E-Silva JC and
Tiezzi D: Transcriptome meta-analysis reveals differences of immune
profile between eutopic endometrium from stage I-II and III-IV
endometriosis independently of hormonal milieu. Sci Rep.
10(313)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Nagarsheth N, Wicha MS and Zou W:
Chemokines in the cancer microenvironment and their relevance in
cancer immunotherapy. Nat Rev Immunol. 17:559–572. 2017.PubMed/NCBI View Article : Google Scholar
|