Introduction
Although no unanimous definition of insulin
resistance (IR) exists, this condition can be described as the
decreased capacity of hepatic, muscular and adipose tissue to make
use of glucose in the presence of insulin levels that would
normally elicit this process in healthy subjects (1). This phenomenon has been described in
anabolic states such as obesity (2), inflammation (3), cancer (4), and is a core element of the metabolic
syndrome (5), or type 2 diabetes
mellitus (6). In an epidemiological
study including a large Romanian population, there were higher
levels of IR in metabolically unhealthy lean, and metabolically
healthy obese patients vs. controls (7). In addition, longitudinal studies have
indicated an association between thyroid function and IR (8,9). Both
the lowest tertile of free thyroxine (fT4) (8) and the highest tertile of free
triiodothyronine (fT3) (9) have
been associated with IR in cross-sectional studies.
The loss of insulin sensitivity could be determined
by a decreased insulin-dependent glucose utilization in all tissues
(10), a reduction in muscle and
liver glycogenolysis and gluconeogenesis (11,12),
or interference with adipocyte-myocyte cross-talk (13). Another mechanism possibly involved
in the indirect action on lipid metabolism fraction and subsequent
lipotoxicity, leads to increased IR (14).
Moreover, the early administration of levothyroxine
or metformin in subclinical hypothyroidism was associated with
improved IR in some (15,16), but not all (17,18)
studies. Consequently, the aim of the present study was to analyze
the association between subclinical hypothyroidism (SHO) and IR in
a female adult population from Bucharest, Romania.
Case study
A pilot, retrospective, case-control study was
designed that included female patients examined by two
endocrinologists and a diabetologist in an outpatient clinic in
Bucharest, Romania. The patients presented for a routine evaluation
of obesity, thyroid disease or osteoporosis. Patients that were
analyzed by the same laboratory and had the complete hormonal and
biochemical blood parameters were included. The variables
considered were: glycemia (mg/dl), glycosylated hemoglobin (HbA1c)
(%), total cholesterol (mg/dl), high-density (HDLc) and low-density
lipoprotein cholesterol (LDLc) (mg/dl), triglycerides (TG) (mg/dl),
thyroid-stimulating hormone TSH (µUI/ml), free thyroxine-fT4
(ng/dl), fasting insulin (µUI/ml) and fasting C-peptide (ng/ml).
Previous values (within one year) for these blood tests were
searched retrospectively for each patient.
The study followed the Helsinki Declaration.
Approval was obtained from the Ethics Committee of the Diabetes
Department of ‘Carol Davila’ University of Medicine and Pharmacy
(National Institute of Diabetes, Nutrition and Metabolic Diseases
N. C. Paulescu), Bucharest, Romania.
Patient data
Data regarding age, weight, height, comorbidities
and concomitant treatment were collected. Body mass index (BMI) was
estimated as weight (kg)/height (m)2. IR evaluation was
carried out as follows. The homeostatic model assessment HOMA-IR1
equation (insulin)=fasting glycemia (mg/dl) x fasting insulin
(µUI/ml)/405; HOMA-IR1 (C-peptide)=1.5 + fasting glycemia (mmol/l)
x [fasting C-peptide (nmol/l)/2800(19), and HOMA-IR2 (insulin), HOMA-IR2
(C-peptide)] was calculated with HOMA-IR software version 2.2.3,
available online (20). The cut-off
value for defining IR was at 2.5(19).
Microsoft Office Excel® file (Microsoft
Corp.) was used to create the database. Inferential statistical
analyses were carried out with the R computing and programming
environment v. 4.0.3 [R Core Team (2021). R: A language and
environment for statistical computing. R Foundation for Statistical
Computing, Vienna, Austria. URL https://www.R-project.org/.], under an RStudio local
terminal, v. 1.1.456.
Assuming that the probability of the exposure
variable (hypothyroidism) in women from the general population is
~8% (21), with a 1:1 ratio for
cases and controls, a prevalence of 25% of IR (22), and 80% power, a minimum (total)
sample size of 150 was necessary for a univariate logistic
regression model using a score test and a minimum (total) sample
size of 187 for the same model using a Wald test, to be able to
detect an odds ratio of 4 (log OR, 1.39). For the multivariate
analysis, the sample size needed was much larger, but the pilot
study data allowed for the assessment of such a study and more
precise calculations for such a study. Statistical power was
computed using the ‘sample size logistic case-control’ R package
V.0.0.6(23). A P-value
(two-tailed) <0.05 was considered significant.
Following univariate analysis (IR as a function of
the presence or absence of hypothyroidism), a pre-specified
multivariate model including BMI, age, and parameters of the lipid
profile (total cholesterol, LDLc, HDLc, and TG) as covariates
(including relevant interactions) was developed. Assessment of the
model fit was performed using specific functions from the
‘LogisticDx’ R package [Osius and Rojek's tests, Stukels tests, the
area under the receiver-operating curve (AUC) and others] (24). Interaction plots were generated
using the ‘interplot’ R package (25).
Results
In total, 176 women, of whom 91 had subclinical
hypothyroidism (SHO) with a median TSH of 5.6±2.66 vs. 1.63±1.06
uUI/ml compared with controls were included. The median age in our
population was 60±17 years, and the mean BMI was 27.79±4.76
kg/m2. The majority of our population (50%) was
diagnosed with autoimmune thyroiditis, and 17.05% with goiter,
based on previous antibody measurements and thyroid echography. In
the entire population, 8.52% underwent a thyroidectomy, and 52.84%
were treated with a levothyroxine substitute. Although >50% of
the women had concomitant treatment, the thyroid hormone levels
were not in target. The general characteristics of the population
are presented in Table I.
 | Table IGeneral characteristics of the study
population. |
Table I
General characteristics of the study
population.
| Variable | IR (n=91) | Non-IR (n=85) |
|---|
| Age
(years)a | 64±13.5 | 58±16 |
| Glycemia
(mg/dl)a | 104.98±19.48 | 98.13±12.2 |
| HbA1c
(%)a | 5.4±0.8 | 5.1±0.6 |
| Total cholesterol
(mg/dl) | 185.68±58.07 | 199.61±53.79 |
| HDLc (mg/dl) | 63±29.5 | 68±25 |
| LDLc (mg/dl) | 96.3±58.6 | 110.6±52.34 |
| TG (mg/dl)
a | 121.86±52.39 | 83.37±43.48 |
| TSH (µUI/ml) | 4.11±4.53 | 2.99±3.6 |
| FT4 (ng/dl) | 1.1±0.19 | 1.08±0.18 |
| Fasting insulin
(µUI/ml) a | 9.21±9.6 | 5.32±4.87 |
| Fasting C-peptide
(ng/ml)a | 1.55±1.9 | 1.38±0.73 |
| HOMA-IR1
(insulin)a | 2.62±2.81 | 1.29±1.2 |
| HOMA-IR2
(insulin)a | 1.32±1.32 | 0.71±0.67 |
| HOMA-IR1
(C-peptide)a | 2.96±1.32 | 2.40±0.53 |
| HOMA-IR2
(C-peptide)a | 1.60±1.5 | 1.05±0.55 |
The univariate logistic regression using
hypothyroidism as the clarifying variable found no evidence of a
significant relationship between a decreased thyroid function and
IR (OR, 1.32; P=0.36).
The second level of our analysis was to adjust for
variables most likely to be associated with IR: The lipid profile
(HDLc, LDLc, TG), BMI, and age; total cholesterol was not included
in the model as it was highly correlated with the LDLc (r=0.89, 95%
CI 0.85-0.91). The unadjusted and adjusted OR for the different
covariates are provided in Table
II and Fig. 1.
 | Table IIComparison of the hypothyroidism and
control groups, according to IR risk factors. |
Table II
Comparison of the hypothyroidism and
control groups, according to IR risk factors.
| Risk factor | Cases (n=91) [mean
(SD) or %] | Controls (n=85)
[mean (SD) or %] | Unadjusted OR (95%
CI) | Adjusted OR (95%
CI) | P-value
(Wald-test) |
|---|
| HDLc | 67.8 (18.0) | 71.4 (19.8) | 0.99
(0.97-1.00) | 1.05
(1.00-1.11) | 0.064 |
| LDLc | 110.0 (41.5) | 103.0 (37.5) | 1.00
(0.99-1.00) | 1.03
(0.99-1.07) | 0.088 |
| BMI | 28.3 (4.40) | 27.4 (5.07) | 1.24
(1.14-1.34) | 1.52
(1.22-1.89) | <0.001 |
| TG | 119.0 (62.4) | 102.0 (37.8) | 1.01
(1.01-1.02) | 1.08
(1.02-1.14) | 0.004 |
| Age | 59.8 (10.6) | 60.4 (14.4) | 1.04
(1.01-1.06) | 1.03
(1.00-1.06) | 0.075 |
| Hypoth | 100% | 0% | 1.32
(0.73-2.38) | 1.09
(0.54-2.21) | 0.803 |
|
HDLc*LDLc | NA | NA | 1 (1.00-1.00) | 1.00
(1.00-1.00) | 0.049 |
|
BMI*TG | NA | NA | 1 (1.00-1.00) | 1.00
(1.00-1.00) | 0.011 |
Leaving out the six most influential observations
resulted in some changes in the model. The ORs for the BMI and TG
were slightly increased (1.74 and 1.10), and the effect of age
became significant (OR, 1.06; P=0.005) after eliminating the
outliers in another model different from that in Table II. Instead, the P-values for the
HDL:LDL interaction, as well as the individual effect of LDL and
HDL, increased considerably beyond the conventional threshold of
significance (P=0.15, 0.22 and 0.36, respectively). Although the
influence of IR on LDLc was moderate, there was a small difference
between groups, leading to the inclusion of this variable in the
analysis. The interaction effect between HDL and LDL is described
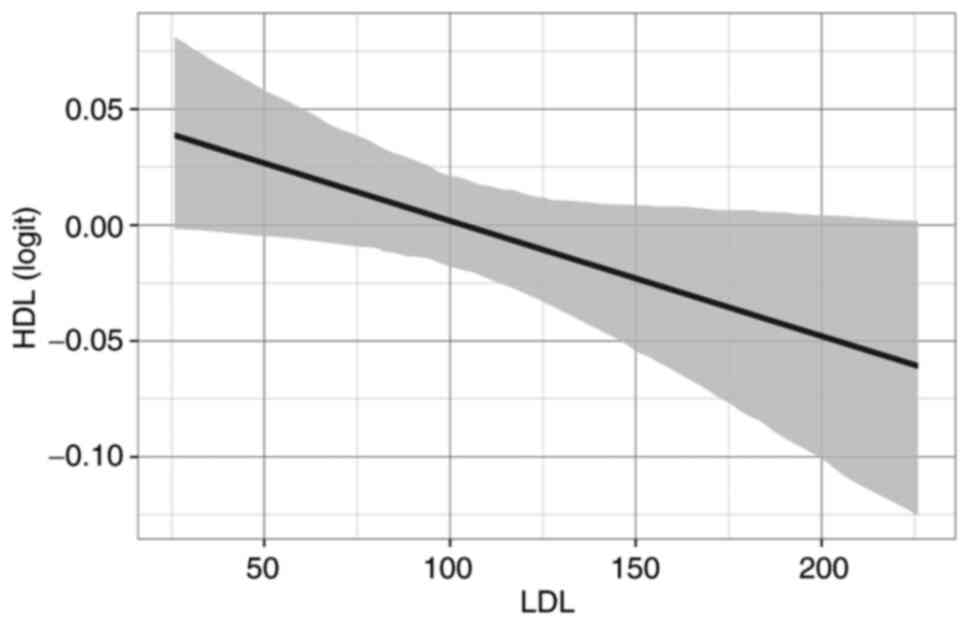
in Fig. 2.
The later evolution (over a period of one year)
towards clinically manifested hypothyroidism and diabetes,
respectively, was assessed, but no significant effect was found for
the two variables (P=0.62 and 0.83, respectively).
Discussion
The data regarding insulin sensitivity and
resistance in the course of thyroid dysfunctions remain
controversial (11,22,26-28).
In a previous cross-sectional study in the Romanian population,
Homeostatic Model Assessment of Insulin Resistance (HOMA-IR)
predicted a thyroid-stimulating hormone (TSH) increase in obese
patients with newly diagnosed thyroid pathology (29). This led to the design of a
case-control study in order to assess the relationship between the
two parameters in time (two determinations at a one-year
interval).
The absence of a significant correlation between IR
and thyroid function may be related to the relatively small sample
size as opposed to other studies (11,26-29).
The sample size considerations assumed a relatively large effect
(an OR of ~4.0). To detect an OR of 1.32 in a case-control study
such as the current one, as suggested by the data, a sample size of
over 5,000 patients would be needed. Another explanation is the
level of thyroid hormone itself, i.e.; the median TSH was smaller
than that in other studies that included patients with subclinical
hypothyroidism (SHO) (5.6 vs. 7.37 or 8.9 uUI/ml) (11,27).
The interference of hormones or metabolites leading to IR that were
previously described in some populations including cortisol
(29), prolactin (17), leptin (28), adiponectin (28,30),
homocysteine (31), or autonomic
nervous system stimulation (17)
cannot be excluded. These additional hormones were not evaluated in
the present study.
Metabolic syndrome is probably the most important
determinant of IR in our population. In women with SHO, some
components of metabolic syndrome [central obesity, triglycerides
(TG)] are more prevalent (32).
Accordingly, TG and BMI were different between the SHO and controls
in the present study, compared with the HDL-LDL interaction which
became insignificant after eliminating outliers. Thus, not the
thyroid function per se, but the coexistence of other elements of
this syndrome prevail in determining IR. Other authors have reached
the same conclusion (30).
Age is another confounder that was included in our
multivariate analysis. In pubertal children, IR may be related to
increased TSH (33), but in
adolescents with risk factors for diabetes, there was no
correlation between the two factors (34).
Although the overall balance tends to favor the
existence of a relationship between hypothyroidism and IR, this
hypothesis is based on small observational studies. The advantages
to the present study are the design that permitted us to evaluate
the IR and the thyroid function at different time periods. The same
laboratory analyzed the uniformity of the blood tests. The
susceptibility for recall and information bias was reduced because
of direct access to the laboratory database. Multivariate analyses
for age, lipid profile and treatment were adjusted; however, one of
the limits was the absence of other hormonal blood tests (the
control of other extraneous variables is incomplete, as for all
case-control studies). In addition, the relatively small sample
size may influence the results.
In summary, no association between thyroid function
tests (TSH, fT4) and IR indices were found in adult Romanian women
in a case-control study with one-year retrospective follow-up. This
relationship may be influenced by the TSH level, being evident only
in values over a certain cut-off (7 uUI/ml).
Acknowledgements
We would like to thank Roman Alina Carmen, Chirita
Maria Magdalena, and Stoica Mariana for support given with the
patient evaluation.
Funding
Funding: The authors are grateful for the support provided by
UEFISCDI, PNCDI III framework, and PN-III-P4-ID-PCE-2016-0120.
Availability of data and materials
Restrictions apply to the availability of these
data. The database is available with author's permission.
Authors' contributions
RAS, CIT, and CG realized the concept of the study.
RA, RAS are responsible for methodology. RA, RAS, and CS performed
the formal analysis. RAS and SDȘ performed data acquisition and
curation and are responsible for confirming the authenticity. RAS,
AC, APS, and RA prepared the original draft. CG, RAS, RA, RIȘ.vS,
IPT, SDȘ, and CS reviewed and edited the draft. All authors have
read and agreed to the published version of the manuscript.
Ethics approval and consent to
participate
The study was conducted according to the guidelines
of the Declaration of Helsinki. Approval was obtained from the
Ethics Committee of the Diabetes Department of ‘Carol Davila’
University of Medicine and Pharmacy (National Institute of
Diabetes, Nutrition and Metabolic Diseases N. C. Paulescu; approval
no. 2/20.03.2017), Bucharest, Romania.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schwartsburd P: Insulin resistance is a
two-sided mechanism acting under opposite catabolic and anabolic
conditions. Med Hypotheses. 89:8–10. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yazıcı D and Sezer H: Insulin resistance,
obesity and lipotoxicity. Adv Exp Med Biol. 960:277–304.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Suceveanu AI, Mazilu L, Katsiki N, Parepa
I, Voinea F, Pantea-Stoian A, Rizzo M, Botea F, Herlea V, Serban D
and Suceveanu AP: NLRP3 inflammasome biomarker-could be the new
tool for improved cardiometabolic syndrome outcome. Metabolites.
10(448)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Di Sebastiano KM, Pinthus JH,
Duivenvoorden WCM and Mourtzakis M: Glucose impairments and insulin
resistance in prostate cancer: The role of obesity, nutrition and
exercise. Obes Rev. 19:1008–1016. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pu D, Tan R, Yu Q and Wu J: Metabolic
syndrome in menopause and associated factors: A meta-analysis.
Climacteric. 20:583–591. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Abdul-Ghani MA and DeFronzo RA:
Pathogenesis of insulin resistance in skeletal muscle. J Biomed
Biotechnol. 2010(476279)2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Popa S, Mota M, Popa A, Mota E, Timar R,
Serafinceanu C, Cheta D, Graur M and Hancu N: Prevalence of
dyslipidemia and its association with cardiometabolic factors and
kidney function in the adult Romanian population: The PREDATORR
study. Diabetes Metab Syndr. 13:596–602. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Roos A, Bakker SJ, Links TP, Gans RO and
Wolffenbuttel BH: Thyroid function is associated with components of
the metabolic syndrome in euthyroid subjects. J Clin Endocrinol
Metab. 92:491–496. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ferrannini E, Iervasi G, Cobb J, Ndreu R
and Nannipieri M: Insulin resistance and normal thyroid hormone
levels: Prospective study and metabolomic analysis. Am J Physiol
Endocrinol Metab. 312:E429–E436. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rochon C, Tauveron I, Dejax C, Benoit P,
Capitan P, Fabricio A, Berry C, Champredon C, Thieblot P and
Grizard J: Response of glucose disposal to hyperinsulinaemia in
human hypothyroidism and hyperthyroidism. Clin Sci (Lond).
104:7–15. 2003.PubMed/NCBI
|
|
11
|
Maratou E, Hadjidakis DJ, Kollias A,
Tsegka K, Peppa M, Alevizaki M, Mitrou P, Lambadiari V, Boutati E,
Nikzas D, et al: Studies of insulin resistance in patients with
clinical and subclinical hypothyroidism. Eur J Endocrinol.
160:785–790. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dimitriadis G, Mitrou P, Lambadiari V,
Boutati E, Maratou E, Panagiotakos DB, Koukkou E, Tzanela M,
Thalassinos N and Raptis SA: Insulin action in adipose tissue and
muscle in hypothyroidism. J Clin Endocrinol and Metab.
91:4930–4937. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Havekes B and Sauerwein HP:
Adipocyte-myocyte crosstalk in skeletal muscle insulin resistance;
is there a role for thyroid hormone? Curr Opin Clin Nutr Metab
Care. 13:641–646. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pearce EN: Hypothyroidism and
dyslipidemia: Modern concepts and approaches. Curr Cardiol Rep.
6:451–456. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pandrc MS, Ristić A, Kostovski V,
Stanković M, Antić V, Milin-Lazović J and Ćirić J: The effect of
early substitution of subclinical hypothyroidism on biochemical
blood parameters and the quality of life. J Med Biochem.
36:127–136. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Răcătăianu N, Bolboacă SD, Sitar-Tăut AV,
Mârza S, Moga D, Valea A and Ghervan C: The effect of Metformin
treatment in obese insulin-resistant patients with euthyroid
goiter. Acta Clin Belg. 73:317–323. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Owecki M, Nikisch E and Sowiński J:
Hypothyroidism has no impact on insulin sensitivity assessed with
HOMA-IR in totally thyroidectomized patients. Acta Clin Belg.
61:69–73. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Brenta G, Berg G, Arias P, Zago V,
Schnitman M, Muzzio ML, Sinay I and Schreier L: Lipoprotein
alterations, hepatic lipase activity, and insulin sensitivity in
subclinical hypothyroidism: Response to L-T(4) treatment. Thyroid.
17:453–460. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Khan HA, Sobki SH, Ekhzaimy A, Khan I and
Almusawi MA: Biomarker potential of C-peptide for screening of
insulin resistance in diabetic and non-diabetic individuals. Saudi
J Biol Sci. 25:1729–1732. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Diabetes Trials Unit, The Oxford Center
for Diabetes, Endocrinology and Metabolism, University of Oxford.
https://www.dtu.ox.ac.uk/ToolsSoftware/. Accessed Nov
28, 2020.
|
|
21
|
Hennessey JV and Espaillat R: Subclinical
hypothyroidism: A historical view and shifting prevalence. Int J
Clin Pract. 69:771–782. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Stoica RA, Guja C, Pantea-Stoian A, Staden
RIȘ, Popa-Tudor I, Ștefan SD, Ancuceanu R, Serafinceanu C and
Ionescu Tîrgoviște C: No Association between 25-Hydroxyvitamin D
and insulin resistance or thyroid hormone concentrations in a
Romanian observational study. Medicina (Kaunas).
57(25)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mitchell HG: Sample size logistic case
control: Sample Size Calculations for Case-Control Studies. R
package version 0.0.6., 2017. https://CRAN.R-project.org/package=samplesizelogisticcasecontro.
Accessed Feb 19, 2021.
|
|
24
|
Dardis C: LogisticDx: Diagnostic Tests for
Models with a Binomial Response. R package version 0.2., 2015.
https://CRAN.R-project.org/package=LogisticDx.
Accessed Feb 19, 2021.
|
|
25
|
Solt F and Hu Y: interplot: Plot the
effects of variables in interaction terms. R package version
0.2.3., 2021. https://CRAN.R-project.org/package=interplot. Accessed
Feb 19, 2021.
|
|
26
|
Vyakaranam S, Vanaparthy S, Nori S,
Palarapu S and Bhongir AV: Study of insulin resistance in
subclinical hypothyroidism. Int J Health Sci Res. 4:147–153.
2014.PubMed/NCBI
|
|
27
|
Al Sayed A, Al Ali N, Bo Abbas Y and
Alfadhli E: Subclinical hypothyroidism is associated with insulin
resistance in Kuwaiti women. Endocr J. 53:653–657. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Guzel S, Seven A, Guzel EC, Buyuk B,
Celebi A and Aydemir B: Visfatin, leptin, and TNF-α: Interrelated
adipokines in insulin-resistant clinical and subclinical
hypothyroidism. Endocr Res. 38:184–194. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Răcătăianu N, Leach N, Bondor CI, Mârza S,
Moga D, Valea A and Ghervan C: Thyroid disorders in obese patients.
Does insulin resistance make a difference? Arch Endocrinol Metab.
61:575–583. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ahirwar AK, Singh A, Jain A, Patra SK,
Goswami B, Bhatnagar MK and Bhattacharjee J: Role of subclinical
hypothyroidism in association with adiponectin levels causing
insulin resistance in metabolic syndrome: A case control study.
Tokai J Exp Clin Med. 42:96–103. 2017.PubMed/NCBI
|
|
31
|
Ebrahimpour A, Vaghari-Tabari M, Qujeq D,
Moein S and Moazezi Z: Direct correlation between serum
homocysteine level and insulin resistance index in patients with
subclinical hypothyroidism: Does subclinical hypothyroidism
increase the risk of diabetes and cardio vascular disease together?
Diabetes Metab Syndr. 12:863–867. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Eftekharzadeh A, Khamseh ME, Farshchi A
and Malek M: The association between subclinical hypothyroidism and
metabolic syndrome as defined by the ATP III criteria. Metab Syndr
Relat Disord. 14:137–144. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Răcătăianu N, Leach NV, Bolboacă SD, Soran
ML, Opriş O, Dronca E, Valea A and Ghervan C: Interplay between
metabolic and thyroid parameters in obese pubertal children. Does
visceral adipose tissue make the first move? Acta Clin Belg.
76:40–48. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Garduño-Garcia Jde J, Camarillo Romero E,
Loe Ochoa A, Romero-Figueroa S, Huitron Bravo G, Torres García R,
Montenegro-Morales P and Mendieta-Zerón H: Thyroid function is
associated with insulin resistance markers in healthy adolescents
with risk factors to develop diabetes. Diabetol Metab Syndr.
7(16)2015.PubMed/NCBI View Article : Google Scholar
|