Introduction
Although gastrointestinal stromal tumors (GISTs) are
rare entities, they represent the most common mesenchymal tumors
arising in the digestive tract. They show variable malignant
potential and are thought to derive from the interstitial cells of
Cajal. Their most frequent anatomical location is the stomach,
followed by the jejunum, ileum, duodenum, colon and rectum
(1). Extradigestive tumors have
also been reported. However, most of them are found in close
proximity to the gastrointestinal (GI) tract, such as in the
omentum, mesentery, retroperitoneum or pleura (2). GISTs can occur at any age, but they
are more prevalent in patients between 60 and 65 years of age. On
the other hand, a subtype of GISTs usually affects younger patients
and mostly women; these tumors are frequently succinate
dehydrogenase (SDH)-deficient and preferentially involve the distal
stomach and antrum. This type of GIST is called ‘wild’-type GIST
(3). GISTs are either sporadic or
synchronous. The former represents a majority of cases. The latter
are usually associated with SDH deficiency (as in Carney triad or
Carney-Stratakis syndrome) (4).
Carney triad is caused by non-hereditary succinate dehydrogenase
complex subunit C (SDHC) hypermethylation and comprises
gastric GISTs, extra-adrenal paragangliomas and pulmonary
chondromas. Carney-Stratakis syndrome is characterized by gastric
GISTs and paragangliomas. It is a rare heritable,
autosomal-dominant condition, caused by a germline mutation of the
SDH complex. Abnormalities may be located in the SDHB, C or D
subunits (5). SDH-deficient GISTs
usually occur in young patients, are associated with more frequent
lymph vessel invasion and frequently metastasize to lymph nodes, in
addition to the liver and peritoneum. They are associated with an
indolent course, despite their metastatic spread (6).
Whenever possible, surgical resection is
recommended. Histopathological examination (resection specimen or
biopsy), along with immunohistochemistry, are mandatory for the
positive and differential diagnosis of GISTs. Targeted drug therapy
with imatinib for a long term is recommended postoperatively in
almost all cases (7).
Materials and methods
Case selection for human tissue
specimens of the study batch
A retrospective study was performed on a study batch
composed of 57 cases with GISTs, selected during an interval of 10
years (January 1, 2011 to December 31, 2020). The patients were
admitted to the Department of Surgery, ‘Sf. Pantelimon’ Emergency
Clinical Hospital, Bucharest, Romania, where surgical procedures
were performed. The study batch consisted of 37 male and 20 female
patients (sex ratio male:female=1.85/1), with ages ranging between
46 and 82 years (mean age: 68.31 years, standard deviation SD
±7.62) (Table I).
 | Table IGeneral characteristics of the
patients with GISTs included in the study. |
Table I
General characteristics of the
patients with GISTs included in the study.
| Total cases | N=57 |
|---|
| Sex | |
|
Male | 37 |
|
Female | 20 |
| Age (years) (mean ±
SD) | 68.31±7.62 |
|
Minimum | 46 |
|
Maximum | 82 |
| Tumoral dimension
(cm) (mean ± SD) | 4.64±2.02 |
|
Minimum | 2 |
|
Maximum | 15 |
| Tumoral location, n
(%) | |
|
Inferior
esophagus | 1 (1.75) |
|
Gastric,
anterior wall | 4 (7.01) |
|
Gastric,
posterior wall | 8 (14.03) |
|
Gastric,
lesser curvature | 5 (8.77) |
|
Gastric,
greater curvature | 5 (8.77) |
|
Gastric,
antrum or prepyloric | 6 (10.52) |
|
Duodenum | 1 (1.75) |
|
Jejunum | 11 (19.29) |
|
Ileum | 12 (21.05) |
|
Appendix | 1 (1.75) |
|
Descending
colon | 1 (1.75) |
|
Sigmoid | 2 (3.5) |
The study was performed according to the 1975 World
Medical Association Declaration of Helsinki ethical guidelines, as
amended in Brazil, in 2013. The tissue specimens were collected
according to national legislation, using a protocol approved by the
local bioethics committees. All the patients included in the study
previously signed the hospital's standard informed consent at their
admission, regarding medical procedures, tissue sampling and
possible future publication of their data. The local ethical
committee of ‘Sf. Pantelimon’ Emergency Clinical Hospital,
Bucharest review the protocol and provide formal approval (IRB no.
7/05.01.2021).
The inclusion criteria were as follows: i) All
patients were adults (>18 years of age); ii) all patients were
admitted to the surgical department in the designated period of
time; iii) all patients had a surgical procedure with tissue
sampling; and iv) all patients had a histopathology confirmation of
GIST, including immunohistochemistry.
The exclusion criteria were, as follows. We excluded
all the patient with digestive tumors, although suggestive for
GIST, but who had not undergone a surgical procedure and who had no
histopathological confirmation of GIST, including
immunohistochemistry. Thus, we excluded all the cases for whom the
tumors could not be confirmed as GISTs with no doubts, as the
differential diagnosis was not very clear even after
immunohistochemistry investigations were conducted.
All data were retrieved from a single surgical
center.
According to the latest World Health Organization
(WHO) classification (2019) (8,9), we
took into account the essential diagnostic criteria along with
desirable diagnostic criteria documented for GISTs. The essential
criteria included: An intramural, submucosal or subserosal mass,
spindle-cell, epithelioid or mixed cell morphology, protein product
of c-KIT (KIT) gene and/or discovered on GIST (DOG1)
immunopositivity and SDHB loss in SDH-deficient GISTs. The
desirable criteria are KIT or platelet-derived growth factor
receptor α (PDGFRA) gene mutations in approximately 85% of
tumors. In addition, the prognostic parameters for GISTs are
mitotic activity, tumor size and anatomical site (8,9).
The objective of the study was to assess, for the
patients included, the fulfillment of the diagnostic criteria
according to WHO 2019 classification.
Clinical and imagistic
investigation
Table II documents
the preoperative investigations performed for the patients included
in the study: Abdominal ultrasound, contrast computed tomography
(CT) scan, upper endoscopy, and lower endoscopy
(recto-colonoscopy). We considered as positive the results that
detected a tumor mass located on the digestive tract
(sensitivity).
 | Table IIRate of positive findings in various
investigations. |
Table II
Rate of positive findings in various
investigations.
| Investigation | No. of patients
(percent from total, n=57) (%) | Sensitivity
(%) |
|---|
| Abdominal
ultrasound | 55 (96.49) | 23 (41.81) |
| CT scan | 39 (68.42) | 33 (84.61) |
| Upper
endoscopy | 44 (77.19) | 30 (68.18) |
| Lower
endoscopy | 7 (12.28) | 2 (28.57) |
It can be noted that the upper digestive endoscopy
was useful in diagnosing all submucosal tumors with esophageal,
gastric and duodenal location. Therefore, we concluded that for
these locations, the sensitivity was 100%. Overall, considering all
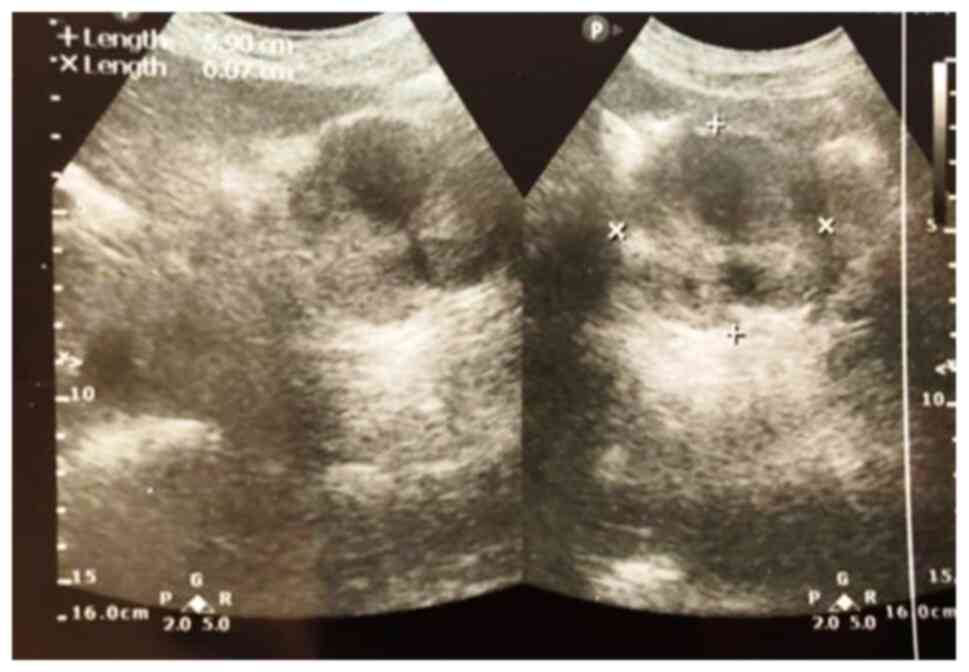
investigations, regardless of localization, CT scan (Fig. 1) was found to be significantly more
sensitive than the others (P=0.025). Abdominal ultrasound, even
with a lower sensitivity, has definite cost advantages, lack of
irradiation and reproducibility (Fig.
2).
All patients underwent surgery, benefiting from
resection of the tumor, or a segment of the digestive tract that
included the tumor. The operation was performed under conditions of
immediate emergency in 5 cases (hemorrhage, peritonitis), delayed
emergency in 27 cases and electively in 25 cases, by classical (46
cases) or laparoscopic approach (11 cases). Sometimes, digestive
GISTs can have impressive dimensions (Fig. 3).
Histopathologic investigation (tissue
sampling and staining)
Tissue specimens from surgically excised GISTs were
taken for histopathologic investigations. The fragments were
harvested from the esophagus, stomach, jejunum, ileum and sigmoid.
The selected tissue samples were fixed in 10% neutral buffered
formalin (pH 7.0) and paraffin embedded. Sections were cut at 5 µm
and stained (room temperature, 4-6 h) with standard hematoxylin and
eosin (H&E) and elastic van Gieson.
Immunohistochemical analysis (IHC) was performed for
a panel of 7 antibodies, using sections displayed on slides treated
first with poly-L-lysine. The panel consisted of the following
antibodies: CD117 (clone: T 595, RTU, Novocastra), CD34 (clone:
QBend, RTU, Novocastra), vimentin (clone: V9, RTU, Novocastra),
smooth muscle actin (clone: 1a4, RTU, Abcam), S-100 (poly, RTU,
Novocastra), DOG1 (clone: SP31, 1:100, Spring Bioscience), PDGFR-α
(clone: C-20, 1:100, Santa Cruz Biotechnology, Inc.). IHC was
performed on 3-µm thick sections from formalin-fixed
paraffin-embedded specimens.
The method used was an indirect tristadial
Avidin-Biotin-Complex technique, with a NovoLink Polymer detection
system which utilizes a novel control polymerization technology to
prepare polymeric HRP-linker antibody conjugates, according to the
manufacturer's specifications (Novocastra). Antigen retrieval
technique (enzymatic pre-treatment) was performed for some of the
aforementioned antibodies, according to the producer's
specifications.
The slides were examined and photographed on a Zeiss
Axio Imager microscope (Zeiss) and the digital images acquired with
Axio Vision program were processed and analyzed with an
incorporated software program, running under Windows 10.
Statistical analysis
Statistical analysis was conducted using the
Student's t-test, for mean, median and standard deviation. A value
of P<0.05 was considered statistically significant.
Results
We noted a quite uniform distribution of the cases
over the 10 years of our study, without statistically significant
differences, except for the year 2020, with only 3 cases, in the
context of the COVID-19 pandemic (Fig.
4).
Gastric GISTs were recorded in 28 cases (49.12%),
jejunal GISTs in 11 cases (19.3%) and ileal GISTs in 12 cases
(21%). Other locations were the sigmoid (2 cases), esophagus (1
case), duodenum (1 case), appendix (1 case) and colon (1 case). The
mean tumor size was 4.64 cm (SD ±2.02), ranging from 2 to 15 cm
(Table I). The sizes of GISTs can
be extremely variable, from tiny incidental cases to huge masses
(10). Large GISTs almost always
outgrow their vascular supply, leading to extensive areas of
necrosis and hemorrhage (11,12).
The largest tumors encountered (15 cm in the longest axis) had
gastric location. The average size of the tumors with gastric
localization was significantly larger than those for other
localizations (5.21 vs. 4.08 cm; P=0.047).
Regarding the clinical presentation of patients, we
noted the emergency presentation of over half of the patients (32
out of 57, which represents 56.14%, a high proportion). We
explained this large proportion of emergencies through the
department's profile.
As shown in Table
III, the most common emergency presentation in our study was
digestive hemorrhage [12 cases (21.05% of all patients)],
concordant with data from literature (13), followed by intestinal obstruction
[10 cases (17.54%)], the data being in accordance with our previous
observations (14).
 | Table IIIAcute presentations of patients at
admission. |
Table III
Acute presentations of patients at
admission.
| Causes of
complications at admission | No. of
patients | Percent from total
(n=57) |
|---|
| Digestive
hemorrhage | 12 | 21.05 |
| Intestinal
obstruction | 10 | 17.54 |
| Spontaneous tumor
perforation with acute peritonitis | 3 | 5.26 |
| Pyloric
stenosis | 2 | 3.50 |
| Cardial
stenosis | 1 | 1.75 |
|
Intussusception | 1 | 1.75 |
| Jaundice | 1 | 1.75 |
| Retroperitoneal
invasion | 1 | 1.75 |
| Acute
appendicitis | 1 | 1.75 |
There was also one case (male, 55 years of age) who
presented with acute appendicitis, which is a rare observation, to
date, only 9 cases being previously mentioned in the literature
(15). Two other types of acute
presentations, rarely mentioned previously in the literature, were
3 cases (5.26%) of spontaneous rupture of GIST with subsequent
peritonitis (16), an ileal
intussusception (female, 59 years) (17) and a case with retroperitoneal
invasion (18,19). Other peculiar complications included
jaundice and pyloric or cardial stenosis (Table III).
Analyzing the data from the statistical point of
view, we found a strong positive association between hemorrhage at
presentation and gastric localization (75%, P=0.025) and,
respectively, between intestinal occlusion and ileal localization
(60%, P=0.045).
From the microscopic point of view, both
spindle-shape cells and epithelioid cells were noted in the
investigated GISTs. Spindle cells were arranged in foci of
fascicles or short whorls, composed of cells with eosinophilic
cytoplasm, bland elongated nuclei and rarely with paranuclear
artifactual vacuoles (Fig. 5A).
Epithelioid cells showed nests or sheets of polygonal to plump
round cells with abundant eosinophilic to clear cytoplasm (Fig. 5B). A variable desmoplastic reaction,
due to interstitial collagen deposition was noted both in GISTs
with spindle-cells and GISTs with epithelioid cells.
CD117 stained positive, diffusely or focally, in
tumor cells showing cytoplasmic or perinuclear dot reaction. The
IHC reaction was observed in all cases, with various intensities,
both in the spindle cell and epithelioid type of tumors (Fig. 6A and C). Mast cells were used as a positive
internal control reaction. CD34 was positive in half of the cases,
with moderate or strong cytoplasmic reaction in the tumor cells, in
both types of tumors (with spindle-shape and epithelioid cells)
(Fig. 6B and D).
Capillary vessels were used as positive internal
control. DOG1 was found positive in the studied cases, in the
cytoplasm of the tumor cells (Fig.
7A). PDGFR-α was positive in all cases, in the cytoplasm of the
tumor cells, with variable intensity (Fig. 7B).
In some cases, tumor cells showed smooth muscle
differentiation, staining positive for α-smooth muscle actin (α-SMA
) or neural differentiation, staining positive for S-100 (Fig. 8A and B). The reaction was cytoplasmic with
variable intensity. IHC for vimentin was mild or moderate in the
cytoplasm of tumor cells.
Discussion
More than half of the GISTs in our study presented
as surgical emergencies. The most common emergency was digestive
hemorrhage (positively associated with gastric location), followed
by intestinal obstruction (particularly for ileal localization).
The largest dimensions were found for gastric GISTs. This fact was
also observed by other authors (20). According to the latest WHO
prognostic classification, dimension is an independent prognostic
factor for anatomic location (8).
In other research, malignant GISTs were associated with location in
organs other than the stomach (21).
The standard paraclinical examination is based on
abdominal CT scan, which is considered the gold standard in the
initial imaging of GISTs and in monitoring their therapeutic
response (22), as it allows
precise detection of the primary tumor, its local extent and
metastases. In our study, 39 patients underwent preoperative
contrast CT scan, which showed a sensitivity of 84.61%. Standard
ultrasonography is commonly used in the setting of liver metastases
(23,24). For the primary mass, ultrasound
showed a low sensitivity (41.81%). Magnetic resonance imaging (MRI)
is useful not only in rectal and duodenal primary GISTs, but also
in cases of liver metastases (25).
In our study, there were only 5 preoperative cases who underwent
MRI, so that we could not take them into consideration for
analysis. Other useful investigation techniques include endoscopy
and positron emission tomography (PET). Upper endoscopy showed a
high sensitivity for tumors located in the upper digestive tract
(esophagus, stomach, duodenum); 100% detection for submucosal
masses in our study. PET is considered superior to CT alone in the
imaging of liver metastases (26).
We had no preoperative PET scan for the patients included. A
preoperative biopsy is not generally recommended when there is a
high index of suspicion for GIST and the lesion is resectable
(27). We counted 11 mucosal
biopsies for submucosal masses, endoscopically taken, that showed
no malignant lesions. In cases of high suspicion of malignant GIST,
biopsy is even prohibitive. The prognosis is worse for ruptured
tumors and tumor biopsy can produce dissemination.
For selected indications (upper digestive sites),
upper digestive endoscopy approached 100% sensitivity in our study,
detecting submucosal masses, but biopsy is not useful since the
mucosa remains normal. Overall, regardless of the location, CT scan
was found to be the most sensitive investigation. The gastric
tumors presented as intramural masses in most of the cases, while
GISTs from the small bowel presented as subserosal extrinsic
masses. Up to three quarters were spindle-cell GISTs and the tumor
cells stained positive to KIT, DOG1 and PDGFR-α in all investigated
cases, followed by CD34, which was positive in 50% of the cases.
Molecular analysis was not performed, therefore gene mutations was
not assessed.
In the present study, all included patients
fulfilled diagnostic criteria according to the recent WHO
classification, from 2019, except that molecular analysis was not
conducted (8).
Pathology examination had a major role, both in
preoperative confirmation of diagnosis and after complete surgical
excision. The diagnosis of GISTs relies on morphology and
immunohistochemistry, and in certain cases on molecular analysis as
complementary diagnosis (28).
Gastric GISTs usually have an intramural and a luminal component.
GISTs located in the small bowel are more frequently extrinsic
masses. The cut surface is frequently whorled, fleshy, whitish-tan
to pink. Areas of necrosis, hemorrhage and cyst formation can be
seen in larger lesions (29).
Gastric GISTs are composed of spindle cells in a majority of cases.
Small and large intestine GISTs usually consist of spindle cells
arranged in sheets or storiform-like patterns, but sometimes may
have epithelioid cells. SDH-deficient tumors are often epithelioid
and patients with SDH-deficient GISTs are younger than those with
tyrosine kinase receptor gene mutant tumors (30). Extracellular acidophilic globules
composed of collagen [skenoid periodic acid-Schiff (PAS)-positive
fibers] may be observed and are usually associated with
non-aggressive intestinal tumors (9).
Regarding immunohistochemistry, approximately 95% of
the GISTs showed KIT positivity, which is considered a sensitive
and specific marker for GISTs (31). DOG1 might contribute to a definitive
diagnosis (up to half of all KIT-negative tumors show DOG1
positivity). This is frequently the case with PDGFRA-mutated
tumors, which are usually positive for DOG1 and PDGFRA (32). CD34 is an early GIST marker, but it
is less sensitive and specific than KIT and DOG1. The staining is
variable (50-90% of cases) and depends on anatomical location
(33). GISTs may also show variable
cytoplasmic staining to vimentin, SMA or desmin. Small intestine
GISTs express more often S100 and SMA. SMA is also a negative
prognostic factor in localized tumors (there is a direct
correlation between its positivity and disease relapse) (34,35).
The differential diagnosis is broad and includes
both spindle cell and epithelioid mesenchymal tumors in origin
(36). Considering this, the
differential diagnosis should be made with leiomyomas and
leiomyosarcomas [desmin is frequently positive in smooth muscle
neoplasms, but SMA and caldesmon can be positive in all the three
types (37)], intraabdominal
desmoid fibromatoses [which have a characteristic nuclear
immunopositivity for β-catenin and a weak expression for KIT
(38)], inflammatory
myofibroblastic tumors [which show desmin and SMA reactivity, while
ALK-1 can be positive in a subset of cases (39)] and schwannomas of the GI tract
[which show S100 and GFAP positivity (40)]. Other tumors that should be
considered for the differential diagnosis are solitary fibrous
tumors [which can stain positive to CD34, but they show STAT6
nuclear positivity and are negative for KIT and PDGFRA (41)], glomus tumors (which are almost
always positive for SMA, but they are negative for KIT, DOG1 and
CD34) and perivascular epithelioid cell tumors (PEComas) [which
show no DOG1 staining, but they show simultaneous positivity for
SMA, HMB45, Melan A (42)].
The main limitation of this study is represented by
its retrospective nature, which may imply selection and indication
biases, although that did not influence patient selection or
management. The second limitation is the relatively small study
population, partially related to the loss of several cases for
non-operative procedures; despite that, we should keep in mind that
our study focused on a highly selected subclass of patients from a
single surgical center. Even if there was a limited number of
cases, these cases were gathered in a period of 10 years. The third
limitation is represented by the lack of molecular analysis.
In patients with clinical symptoms and imagistic
data suggestive of a digestive tumor, the diagnosis of GIST should
be considered, even though it is not a common tumor. A correct
diagnosis is essential for appropriate treatment, with subsequent
improvement of life quality. According to a correct and complete
diagnostic, adjuvant prolonged treatment with imatinib (3 years
after surgery or even longer) improves the outcome and prognosis
(43). Risk stratification schemes
are based on tumor size, mitosis count and site and assessment of
rupture (43).
In conclusion, in our study, more than 50% of the
GISTs presented as surgical emergencies. The most common emergency
was digestive hemorrhage (associated with gastric location),
followed by intestinal obstruction (particularly for ileal
localization). The largest dimensions were found for gastric GISTs.
For selected indications (upper digestive sites), upper digestive
endoscopy approached 100% sensitivity. Overall, regardless of
location, CT scan was found to be the most sensitive
investigation.
As a common ground for discussion, there are also
divergent points of view. The understanding of GIST diagnosis and
biology has improved significantly after the introduction of new
imagistic methods and identification of new molecular markers. Yet,
GISTs represent a peculiar type of tumor that challenges a positive
and differential diagnosis with other mesenchymal tumors, both for
the clinician and the pathologist.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MC, SE, VE, AB and ZC performed the histological
examinations and IHC, and had major contributions in writing the
manuscript. BS, VPC, VDC, LIS, DS, DP and CGS analyzed and
interpreted the patient data. MC, BS and LIS searched the
literature for similar research and articles and contributed to
writing the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was conducted according to the World
Medical Association Declaration of Helsinki, using a protocol
approved by the local Bioethics Committee from ‘Sf. Pantelimon’
Emergency Clinical Hospital (Bucharest, Romania). All patients
previously signed an informed written consent concerning
hospitalization, treatment and a possible future publication of
data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no conflict or competing
interests.
References
|
1
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors: Review on morphology, molecular pathology,
prognosis, and differential diagnosis. Arch Pathol Lab Med.
130:1466–1478. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Foo WC, Liegl-Atzwanger B and Lazar AJ:
Pathology of gastrointestinal stromal tumors. Clin Med Insights
Pathol. 5:23–33. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wada R, Arai H, Kure S, Peng WX and Naito
Z: ‘Wild type’ GIST: Clinicopathological features and clinical
practice. Pathol Int. 66:431–437. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Miettinen M, Killian JK, Wang ZF, Lasota
J, Lau C, Jones L, Walker R, Pineda M, Zhu YJ, Kim SY, et al:
Immunohistochemical loss of succinate dehydrogenase subunit A
(SDHA) in gastrointestinal stromal tumors (GISTS) signals SDHA
germline mutation. The Am J Surg Pathol. 37:234–240.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gaal J, Stratakis CA, Carney JA, Ball ER,
Korpershoek E, Lodish MB, Levy I, Xekouki P, van Nederveen HF, den
Bakker MA, et al: SDHB immunohistochemistry: A useful tool in the
diagnosis of carney-stratakis and Carney triad gastrointestinal
stromal tumors. Mod Pathol. 24:147–151. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tirumani SH, Tirumani H, Jagannathan JP,
Shinagare AB, Hornick JL, George S, Wagner AJ and Ramaiya NH: MDCT
features of succinate dehydrogenase (SDH)-deficient
gastrointestinal stromal tumours. Br J Radiol.
87(20140476)2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Reichardt P: The story of imatinib in
GIST-a journey through the development of a targeted therapy. Oncol
Res Treat. 41:472–477. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang X, Bai L, Wang D, Huang X, Wei J,
Zhang W, Zhang Z and Zhou J: Gastrointestinal stromal tumor risk
classification: Spectral CT quantitative parameters. Abdom Radiol
(NY). 44:2329–2336. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dei Tos AP, Hornick JL and Miettinen M:
Gastrointestinal stromal tumour. In: WHO Classification of Tumours:
Digestive system tumours. Fukayama M, Goldblum JR, Miettinen M and
Lazar AJ (eds). 5th edition. World Health Organization (WHO),
Geneva, pp439-443, 2019.
|
|
10
|
Cavaliere D, Griseri G, Venturino E,
Schirru A, Cosce U, Caristo I, Caliendo L, Pastorino A and
Cavaliere P: Management of patients with gastrointestinal stromal
tumors: Experience from an Italian group. Tumori. 91:467–471.
2005.PubMed/NCBI
|
|
11
|
Lin SC, Huang MJ, Zeng CY, Wang TI, Liu ZL
and Shiay RK: Clinical manifestations and prognostic factors in
patients with gastrointestinal stromal tumors. World J
Gastroenterol. 9:2809–2812. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Korodi AD, Furau C, Furau G, Dimitriu M,
Socea B, Ada C, Botezatu D, Dumnici A, Totolici B, Barbu I, et al:
The types of tumoral vessels associated to GISTs are conditioning
the effectiveness of anti-vascular therapy with tyrosine kinase
receptor inhibitors. Rev Chim (Bucharest). 70:3250–3253. 2019.
|
|
13
|
Sorour MA, Kassem MI, Ghazal AE, El-Riwini
MT and Nasr AA: Gastrointestinal stromal tumors (GIST) related
emergencies. Int J Surg. 12:269–80. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Constantin VD, Socea B, Popa F, Carâp AC,
Popescu G, Vlădescu T, Ceauşu Z, Bertesteanu SVG and Ceauşu MC: A
histopathological and immunohistochemical approach of surgical
emergencies of GIST. An interdisciplinary study. Rom J Morphol
Embryol. 55 (Suppl 2):S619–S627. 2014.PubMed/NCBI
|
|
15
|
Bouassida M, Chtourou MF, Chalbi E, Chebbi
F, Hamzaoui L, Sassi S, Charfi L, Mighri MM, Touinsi H and Sassi A:
Appendiceal GIST: Report of an exceptional case and review of the
literature. Pan Afr Med J. 15(85)2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ajduk M, Mikulić D, Sebecić B, Gasparov S,
Patrlj L, Erdelez L, Skopljanac A, Staresinić M, Desković S, Sosa T
and Sitić S: Spontaneously ruptured gastrointestinal stromal tumor
(GIST) of the jejunum mimicking acute appendicitis. Coll Antropol.
28:937–941. 2004.PubMed/NCBI
|
|
17
|
Giestas S, Almeida N, Martins R, Canhoto
A, Oliveira P, Figueiredo P and Sofia C: Small bowel GIST: Clinical
presentation as intussusception and obscure bleeding. GE Port J
Gastroenterol. 23:279–281. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Miettinen M, Felisiak-Golabek A, Wang Z,
Inaguma S and Lasota J: GIST manifesting as a retroperitoneal
tumor: Clinicopathologic immunohistochemical, and molecular genetic
study of 112 cases. Am J Surg Pathol. 41:577–585. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bratu OG, Marcu RD, Socea B, Neagu TP,
Diaconu C, Scarneciu I, Turcu FL, Radavoi GD, Bratila E, Berceanu C
and Spinu AD: Immunohistochemistry particularities of
retroperitoneal tumors. Rev Chim (Bucharest). 69:1813–1816.
2018.
|
|
20
|
Sista F, Pessia B, Abruzzese V, Cecilia
EM, Schietroma M, Carlei F and Amicucci G: Twelve years of gastric
GIST. A retrospective study of laparoscopic and open approach. Ann
Ital Chir. 86:349–356. 2015.PubMed/NCBI
|
|
21
|
Park JH, Kang BK, Lee HL, Yoon JH, Lee KN,
Jun DW, Lee OY, Han DS, Yoon BC and Choi HS: Correlation between
three-dimensional volume and malignant potential of
gastrointestinal stromal tumors (GISTs). J Clin Med.
9(2763)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Parab TM, DeRogatis MJ, Boaz AM, Grasso
SA, Issack PS, Duarte DA, Urayeneza O, Vahdat S, Qiao JH and Hinika
GS: Gastrointestinal stromal tumors: A comprehensive review. J
Gastrointest Oncol. 10:144–154. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Landi B: Gastrointestinal stromal tumors:
Clinical features and diagnosis. Bull Acad Natl Med. 196:845–852.
2012.PubMed/NCBI(In French).
|
|
24
|
von Mehren M and Joensuu H:
Gastrointestinal stromal tumors. J Clin Oncol. 36:136–143.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dimitrakopoulou-Strauss A, Ronellenfitsch
U, Cheng C, Pan L, Sachpekidis C, Hohenberger P and Henzler T:
Imaging therapy response of gastrointestinal stromal tumors (GIST)
with FDG PET, CT and MRI: A systematic review. Clin Transl Imaging.
5:183–197. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sanchez-Hidalgo JM, Duran-Martinez M,
Molero-Payan R, Rufian-Peña S, Arjona-Sanchez A, Casado-Adam A,
Cosano-Alvarez A and Briceño-Delgado J: Gastrointestinal stromal
tumors: A multidisciplinary challenge. World J Gastroenterol.
24:1925–1941. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lim KT and Tan KY: Current research and
treatment for gastrointestinal stromal tumors. World J
Gastroenterol. 23:4856–4866. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Landi B, Blay JY, Bonvalot S, Brasseur M,
Coindre JM, Emile JF, Hautefeuille V, Honore C, Lartigau E, Mantion
G, et al: Gastrointestinal stromal tumours (GISTs): French
intergroup clinical practice guidelines for diagnosis, treatments
and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO).
Dig Liver Dis. 51:1223–1231. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Agaimy A and Wünsch PH: Gastrointestinal
stromal tumours: A regular origin in the muscularis propria, but an
extremely diverse gross presentation. A review of 200 cases to
critically re-evaluate the concept of so-called
extra-gastrointestinal stromal tumours. Langenbecks Arch Surg.
391:322–329. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Brcic I, Kashofer K, Skone D and
Liegl-Atzwanger B: KIT mutation in a naïve succinate dehydrogenase
deficient gastric GIST. Genes Chromosomes Cancer. 58:798–803.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gaopande VL, Joshi AR, Bhayekar PD and
Khandeparkar SG: Clinicopathologic and immunohistochemical study of
gastrointestinal stromal tumor (ten cases) and
extragastrointestinal stromal tumor (six cases) with review of
literature. J Curr Res Sci Med. 2:84–91. 2016.
|
|
32
|
Akahoshi K, Oya M, Koga T and Shiratsuchi
Y: Current clinical management of gastrointestinal stromal tumor.
World J Gastroenterol. 24:2806–2817. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hirota S: Differential diagnosis of
gastrointestinal stromal tumor by histopathology and
immunohistochemistry. Transl Gastroenterol Hepatol.
3(27)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lai J, Kresak JL, Cao D, Zhang D, Zhang S,
Leon ME, Shenoy A, Liu W, Trevino J, Starostik P, et al: Gastric
plexiform fibromyxoma: A great mimic of gastrointestinal stromal
tumor (GIST) and diagnostic pitfalls. J Surg Res. 239:76–82.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Alghamdi HM, Amr SS, Shawarby MA, Sheikh
SS, Alsayyah AA, Alamri AM, Ismail MH, Almarhabi A, Alrefaee MA and
Ahmed MI: Gastrointestinal stromal tumors, A clinicopathological
study. Saudi Med J. 40:126–130. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kang HC, Menias CO, Gaballah AH, Shroff S,
Taggart MW, Garg N and Elsayes KM: Beyond the GIST: Mesenchymal
tumors of the stomach. Radiographics. 33:1673–1690. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Turner MS and Goldsmith JD: Best practices
in diagnostic immunohistochemistry: Spindle cell neoplasms of the
gastrointestinal tract. Arch Pathol Lab Med. 133:1370–1374.
2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Penel N, Chibon F and Salas S: Adult
desmoid tumors: Biology, management and ongoing trials. Curr Opin
Oncol. 29:268–274. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lawrence B, Perez-Atayde A, Hibbard MK,
Rubin BP, Cin PD, Pinkus JL, Pinkus GS, Xiao S, Yi ES, Fletcher CD
and Fletcher JA: TPM3- ALK and TPM4-ALK oncogenes in inflammatory
myofibroblastic tumors. Am J Pathol. 157:377–384. 2000.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Voltaggio L, Murray R, Lasota J and
Miettinen M: Gastric schwannoma: A clinicopathologic study of 51
cases and critical review of the literature. Hum Pathol.
43:650–659. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chmielecki J, Crago AM, Rosenberg M,
O'Connor R, Walker SR, Ambrogio L, Auclair D, McKenna A, Heinrich
MC, Frank DA and Meyerson M: Whole-exome sequencing identifies a
recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet.
45:131–132. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Argani P, Aulmann S, Illei PB, Netto GJ,
Ro J, Cho HY, Dogan S, Landanyi M, Martignoni G, Goldblum JR and
Weiss SW: A distinctive subset of PE Comas harbors TFE3 gene
fusions. Am J Surg Pathol. 34:1395–1406. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Joensuu H: Adjuvant treatment of GIST:
Patient selection and treatment strategies. Nat Rev Clin Oncol.
l9:351–358. 2012.PubMed/NCBI View Article : Google Scholar
|