1. Introduction
Horton's arteritis is found in the literature under
various names, such as temporal arteritis, Horton's disease senile
arteritis, granulomatous arteritis or giant cell arteritis (GCA).
The term GCA refers to the type of inflammatory cells present in
the wall of the affected vessel, and the term of ‘temporal
arteritis’ refers to the frequent involvement of the temporal
artery. GCA is a vasculitis that affects large vessels. Vasculitis
is defined by the inflammation of the vascular wall (arteries,
veins, and capillaries) with consecutive vascular thrombosis,
causing ischemia and necrosis in the related tissue territories.
The cause is unknown, with changes in the immune response, such as
autoimmunity, trigger infections, and genetic susceptibility.
Smoking is a negative prognostic factor. The classification of
vasculitis is made according to several criteria with clinical
implications: The type of vessel affected, etiology, and location.
Takayasu arteritis, Horton temporal arteritis, rheumatic
polymyalgia are described as large vessel vasculitis. Horton's
arteritis is often associated with rheumatic polymyalgia (50% of
cases); in essence, it seems that they are different manifestations
of the same disease (1,2).
The underlying mechanism is the inflammation of the
vasa vasorum in the wall of the large arteries. The most affected
are the arteries in the head and neck, but large thoracic arteries
can also be affected. GCA can affect the aorta and cause an
aneurysm or dissection. Up to 67% of the patients with GCA may
experience an inflammation of the aorta that can lead over time to
an aneurysm or a dissection of this large artery. Varicella-zoster
virus antigens have been identified in 74% of the temporal artery
biopsies that have identified giant cells, suggesting that this
type of viral infection can trigger the inflammatory cascade
(3,4).
GCA affects 1 in 15,000 individuals over the age of
50 years. Although this type of arteritis most commonly affects
patients over the age of 50, it is much more common over the age of
70 and it predominantly affects women (5,6).
In this article, we aim to review the role of
temporal artery resection in the diagnosis of Horton's arteritis,
but we also discuss the hypothesis of a potential therapeutic
benefit of this procedure.
2. Pathogenesis of Horton's arteritis
The pathogenic mechanism of Horton's arteritis is
the result of an inflammatory cascade triggered by a still unknown
factor that causes dendritic cells in vessels to recruit T cells
and macrophages that form granulomatous infiltrates. The activation
of T helper 17 (Th17) lymphocytes causes the release of IL-6,
IL-17, IL-21 and IL-23 interleukins. The activation of Th17
lymphocytes leads to their continuous activation in positive
feedback via IL-6. This vicious circle can be disrupted by
glucocorticoids and, more recently, by IL-6 inhibitors (7-10).
3. Clinical picture of temporal GCA
The clinical picture in temporal GCA consists of a
daily, intermittent or continuous headache with temporal
localization, with moderate to severe intensity, as a burn or as a
pulsation, unilateral or bilateral with a history of months, years.
Other charges may include pain in the cheek or tongue during
chewing (claudication), weight loss, generalized fatigue, low-grade
fever, and frequent pain in the limbs in the context of coexisting
rheumatic polymyalgia (11,12).
Visual symptoms represent a special category of
symptoms, involving blurred vision, scotomas, and even sudden
blindness. This symptomatology is explained by the progression of
GCA-type vascular lesions in the ophthalmic artery. Sometimes, the
impaired vision is the reason for the first presentation to the
doctor. The eye can be involved in 76% of cases, by involving the
ophthalmic artery that causes anterior ischemic optic neuropathy.
Other ophthalmological symptoms in GCA may be diplopia, acute
tinnitus (13,14).
The physical examination reveals thickened temporal
arteries, sensitive to palpation, with absent or low pulse. The
palpation of the temporalis muscle does not reveal trigger points
for the painful crisis, and the consistency of the muscle is
normal. However, the temporalis muscle may be sensitive to
palpation. Vascular murmurs in the subclavian and axillary arteries
may be auscultated. A complete or incomplete inflammatory
biological syndrome may be present, but ESR is constantly increased
over 50-60 mm/1 h, as is C-reactive protein (CRP). We also observe
thrombocytosis, but also hepatic cytolysis syndrome and other
cutaneous manifestations are noted (15-17).
4. Positive diagnosis in GCA
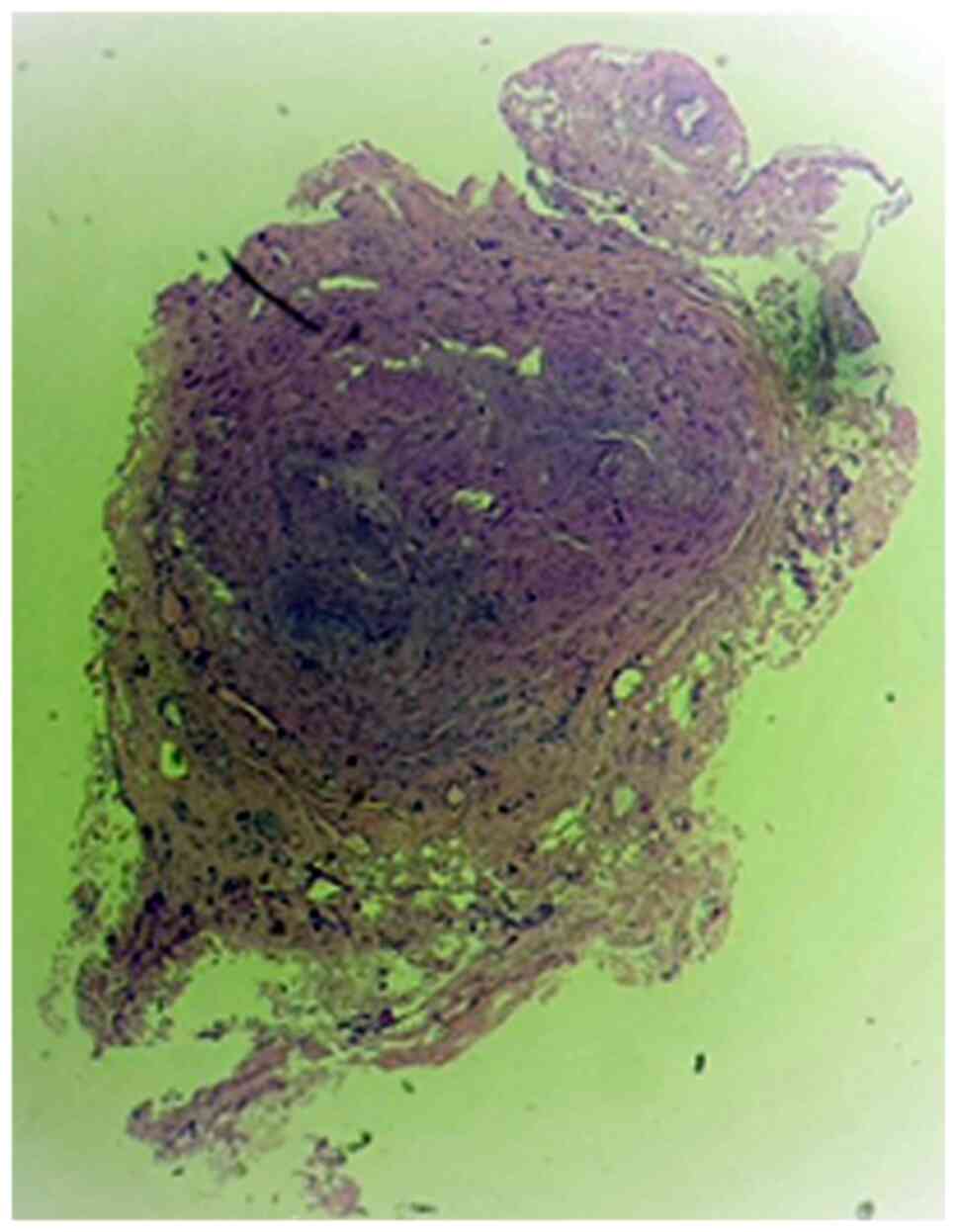
If the clinical suspicion of temporal arteritis is
raised, a temporal artery biopsy is required for confirmation
(Fig. 1). Arterial inflammation is
most commonly segmental, so in our experience, it is necessary to
excise a segment approximately 5 cm long in the artery, in order to
avoid a false-negative result (Fig.
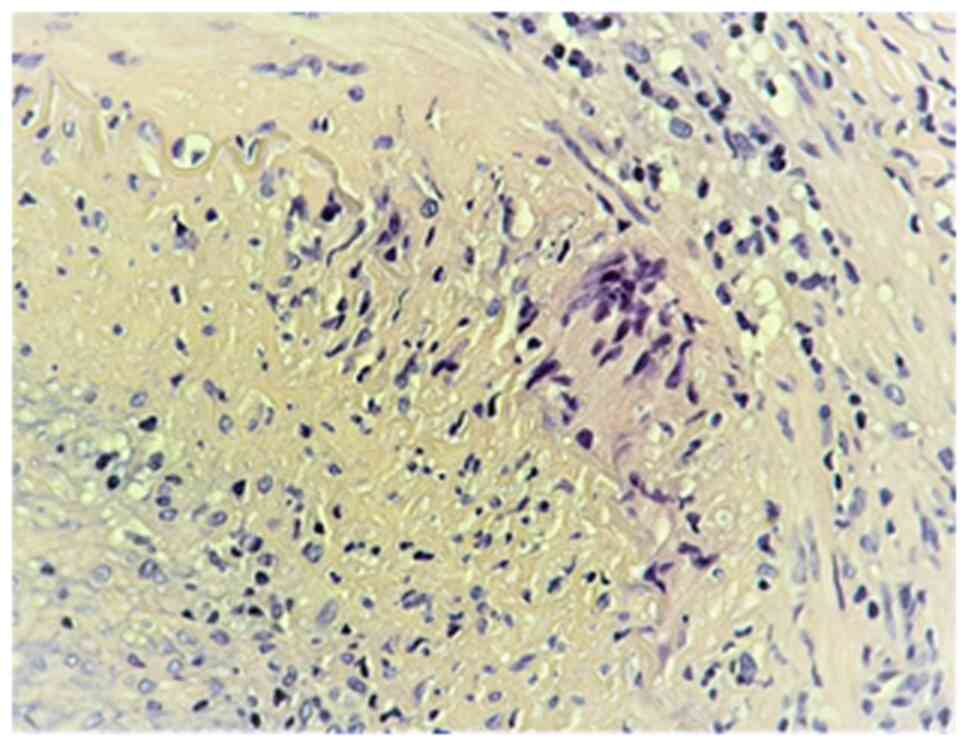
2). Histopathological examination reveals jumping microscopic
lesions, with variations in histological aspects, and focal
thickening of the intima can be observed, with interruption of the
lamina propria, with transmural inflammatory infiltrates, sometimes
with giant multinucleated cells-representative, but not mandatory
(Figs. 3 and 4) or with panvasculitis at the vasa
vasorum level, the latter with mixed inflammatory infiltrates,
including lymphoplasmocytic cells and neutrophils (Fig. 5). However, a negative result does
not exclude the diagnosis. Given that the vascular wall is
infiltrated into plaques, the biopsy may be taken from arterial
segments, where the lesions are missing. Unilateral biopsy of a
1.5-3 cm long segment has a sensitivity of 85-90%, the excision of
a temporal artery segment of at least 1 cm being mandatory.
Temporal artery biopsy may confirm a clinical diagnosis or is a
diagnostic criterion (18-20).
Color Doppler ultrasound is a non-invasive
diagnostic method with the potential to replace biopsy by
identifying the halo sign. Brain magnetic resonance imaging (MRI)
examination is mandatory to rule out a brain pathology that causes
symptoms such as headache or sudden visual loss (Fig. 6) (21,22).
The differential diagnosis includes other causes of
headache with common clinical features such as cluster headache,
migraine, carotid artery pain secondary to spontaneous carotid
artery dissection or after endarterectomy, ischemic stroke.
Idiopathic carotidodynia is also described, a self-limiting
inflammation of the carotid sheath or adventitia possibly virally
induced. Other differential diagnoses, such as chronic inflammatory
diseases, include Takayasu's arteritis and primary amyloidosis
(23-25).
5. Principles of treatment in Horton's
arteritis
Treatment for Horton's arteritis should be initiated
early after diagnosis in order to avoid sudden blindness, a
complication found in 30% of the untreated patients, due to the
involvement of the ophthalmic artery. Considering this particular
aspect, GCA is considered a medical emergency (26-28).
Prednisone or prednisolone is the treatment of
choice and should be given in high daily doses of 60 mg. It should
be started from clinical suspicion, even before confirmation by
biopsy, although initiation of steroid treatment may change the
histopathological appearance. Pain is dramatically reduced in a few
days. Once the pain subsides and the ESR value is corrected,
prednisone can be reduced in a few weeks to a dose of 5-10 mg with
continuous monitoring of ESR for 12-18 months. The administration
of IV corticosteroids becomes necessary for the acute occurrence of
acute visual loss (29-31).
In addition, tocilizumab and other immunological
modifiers seem a promising therapeutic option in refractory cases
or patients developing long term complications of corticoid
treatment (32,33).
6. Resection of the temporal artery in
Horton's arteritis
In general, patients with clinical suspicion of GCA
should have a temporal artery biopsy to confirm the diagnosis,
especially if corticosteroid therapy is to be instituted. The
decision to start corticosteroid therapy without a biopsy seems
easy, but difficulties may occur a few months after treatment, when
the side effects of corticosteroids become prominent, and it is
difficult to reduce the dose. In addition, performing the biopsy
after the initiation of cortisone therapy can influence the lesions
in the vascular wall, as previously mentioned (34).
A small number of patients with GCA (perhaps 8-10%)
have clinically obvious inflammation of the large vessels;
therefore, it is easy to choose the site of the vascular biopsy.
The biopsy should be conducted on the most symptomatic side first.
In most cases, a single biopsy is required. When the temporal
artery involved has classic physical features of inflammation with
sensitivity, swelling, and erythema above, a small part of the
abnormal segment should be sufficient to confirm the diagnosis, but
this favorable situation is quite rare. In patients with less
pronounced abnormalities, a larger sample, such as 4 to 6 cm,
should be obtained and examined microscopically at several levels
with the extemporaneous histological examination if possible, for
and subsequently with the use of paraffin-embedded sections
(Fig. 7). In order to diagnose as
many cases as possible, a specimen from the second temporal artery
should be taken in the same session if the frozen sections of the
first specimen are normal (35,36).
Undoubtedly, temporal artery resection has
diagnostic benefits in Horton's arteritis. However, there are also
some particular clinical situations in which we could see an
improvement in clinical symptoms, especially in vision deficit,
with the improvement of the visual field after surgery performed
for biopsy. In particular, we observed a considerable improvement
of the visual field in the right eye 24 h after performing the
right temporal artery biopsy in a 57-year-old female patient with a
clinical picture of GCA with severe vision impairment in the right
eye, only partially improved after corticosteroids; intraocular
pressure was normal in both eyes at the time of visual field
determination. It is difficult to estimate the impact of temporal
artery resection alone, considering most patients also have
concomitant cortisone treatment, but the rapid improvement of
symptoms immediately after surgery with the improvement of visual
acuity and visual field, along with the disappearance of headache,
can create the premises for future studies on the therapeutic
contribution of temporal artery resection in GCA. The clinical
results of the resection are more obvious if the intervention is
performed quickly after the appearance of ocular symptoms, before
the appearance of irreversible lesions on the optic nerve by distal
lesions on the vasa nervorum.
7. Conclusions
The segmental excision of the temporal artery has
definite diagnostic benefits as a confirmatory element of a
suggestive clinical picture or a diagnostic element in Horton's
arteritis; nonetheless, there are also clinical data that may argue
for a therapeutic contribution of this intervention. Thus,
segmental arterial resection sometimes results in the disappearance
of temporal pain and, in some cases, even the improvement of
vision, if practiced quickly after the onset of visual
disturbances. Future studies are needed in order to support this
clinical hypothesis. Even in cases where temporal artery resection
is followed by an improvement in the clinical picture, cortisone
treatment remains mandatory, given the systemic autoimmune
pathogenesis.
Acknowledgements
Professional editing, linguistic and technical
assistance performed by Irina Radu, Individual Service
Provider.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DV, MD and MPC contributed substantially to the
conception and design of the review, the acquisition, analysis, and
interpretation of the literature findings, and were involved in the
drafting of the manuscript. BB, OP and AC contributed substantially
to the acquisition, analysis and interpretation of the literature
findings and were involved in the drafting of the manuscript. ISE
and MGG contributed substantially to the acquisition of the
literature findings and were involved in the critical revisions of
the manuscript for important intellectual content. All authors
agreed to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved. All the
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Patient informed consent for publication of the
data/images associated with the review was obtained. The authors
followed the international regulations in accordance with the
Declaration of Helsinki and all identifying information was
removed.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dinkin M and Johnson E: One giant step for
giant cell arteritis: Updates in diagnosis and treatment. Curr
Treat Options Neurol. 23(6)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Robinette ML, Rao DA and Monach PA: The
immunopathology of giant cell arteritis across disease spectra.
Front Immunol. 12(623716)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Serling-Boyd N and Stone JH: Recent
advances in the diagnosis and management of giant cell arteritis.
Curr Opin Rheumatol. 32:201–207. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ciccia F, Rizzo A, Ferrante A, Guggino G,
Croci S, Cavazza A, Salvarani C and Triolo G: New insights into the
pathogenesis of giant cell arteritis. Autoimmun Rev. 16:675–683.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sharma A, Mohammad AJ and Turesson C:
Incidence and prevalence of giant cell arteritis and polymyalgia
rheumatica: A systematic literature review. Semin Arthritis Rheum.
50:1040–1048. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Breuer GS, Poltorak V and Nesher G:
Survival of patients with giant cell arteritis: A controversial
issue. Clin Exp Rheumatol. 38 (Suppl 124):S210–S213.
2020.PubMed/NCBI
|
|
7
|
Ralli M, Campo F, Angeletti D, Minni A,
Artico M, Greco A, Polimeni A and de Vincentiis M: Pathophysiology
and therapy of systemic vasculitides. EXCLI J. 19:817–854.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Choy EH, De Benedetti F, Takeuchi T,
Hashizume M, John MR and Kishimoto T: Translating IL-6 biology into
effective treatments. Nat Rev Rheumatol. 16:335–345.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Berghi NO, Dumitru M, Vrinceanu D,
Ciuluvica RC, Simioniuc-Petrescu A, Caragheorgheopol R, Tucureanu
C, Cornateanu RS and Giurcaneanu C: Relationship between chemokines
and T lymphocytes in the context of respiratory allergies (Review).
Exp Ther Med. 20:2352–2360. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Arias M, Heydari-Kamjani M and Kesselman
MM: Giant cell arteritis and cardiac comorbidity. Cureus.
13(e13391)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
González-Gay MÁ, Ortego-Jurado M, Ercole L
and Ortego-Centeno N: Giant cell arteritis: Is the clinical
spectrum of the disease changing? BMC Geriatr.
19(200)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gualtierotti R, Marzano AV, Spadari F and
Cugno M: Main oral manifestations in immune-mediated and
inflammatory rheumatic diseases. J Clin Med. 8(21)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hayreh SS: Giant cell arteritis: Its
ophthalmic manifestations. Indian J Ophthalmol. 69:227–235.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Albarrak AM, Mohammad Y, Hussain S, Husain
S and Muayqil T: Simultaneous bilateral posterior ischemic optic
neuropathy secondary to giant cell arteritis: A case presentation
and review of the literature. BMC Ophthalmol.
18(317)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rinden T, Miller E and Nasr R: Giant cell
arteritis: An updated review of an old disease. Cleve Clin J Med.
86:465–472. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rotaru M, Iancu GM, Gheucă Solovăstru L,
Glaja RF, Grosu F, Bold A and Costache A: A rare case of multiple
clear cell acanthoma with a relatively rapid development of the
lower legs. Rom J Morphol Embryol. 55 (Suppl 3):S1171–S1179.
2014.PubMed/NCBI
|
|
17
|
van der Geest KSM, Sandovici M, van Sleen
Y, Sanders JS, Bos NA, Abdulahad WH, Stegeman CA, Heeringa P,
Rutgers A, Kallenberg CGM, et al: Review: What is the current
evidence for disease subsets in giant cell arteritis? Arthritis
Rheumatol. 70:1366–1376. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Aghdam KA, Sanjari MS, Manafi N,
Khorramdel S, Alemzadeh SA and Navahi RAA: Temporal artery biopsy
for diagnosing giant cell arteritis: A ten-year review. J
Ophthalmic Vis Res. 15:201–209. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Brooks PJ, Glogauer M and McCulloch CA: An
overview of the derivation and function of multinucleated giant
cells and their role in pathologic processes. Am J Pathol.
189:1145–1158. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Michailidou D, Mustelin T and Lood C: Role
of neutrophils in systemic vasculitides. Front Immunol.
11(619705)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Guggenberger K and Bley T: Imaging in
large vessel vasculitides. Rofo. 191:1083–1090. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Schäfer VS, Jin L and Schmidt WA: Imaging
for diagnosis, monitoring, and outcome prediction of large vessel
vasculitides. Curr Rheumatol Rep. 22(76)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Harky A, Fok M, Howard C and Bashir M:
Current controversies in large-vessel inflammatory vasculitis and
thoracic aortic aneurysm disease. Int J Angiol. 28:215–225.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Baig IF, Pascoe AR, Kini A and Lee AG:
Giant cell arteritis: Early diagnosis is key. Eye Brain. 11:1–12.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lensen KD, Voskuyl AE, Comans EF, van der
Laken CJ and Smulders YM: Extracranial giant cell arteritis: A
narrative review. Neth J Med. 74:182–192. 2016.PubMed/NCBI
|
|
26
|
Lyons HS, Quick V, Sinclair AJ, Nagaraju S
and Mollan SP: A new era for giant cell arteritis. Eye (Lond).
34:1013–1026. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mackie SL, Brouwer E, Conway R, van der
Geest KSM, Mehta P, Mollan SP, Neill L, Putman M, Robinson PC and
Sattui SE: Clinical pathways for patients with giant cell arteritis
during the COVID-19 pandemic: An international perspective. Lancet
Rheumatol. 3:e71–e82. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
De Smit E, O'Sullivan E, Mackey DA and
Hewitt AW: Giant cell arteritis: Ophthalmic manifestations of a
systemic disease. Graefes Arch Clin Exp Ophthalmol. 254:2291–2306.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pfeil A, Oelzner P and Hellmann P: The
treatment of giant cell arteritis in different clinical settings.
Front Immunol. 9(3129)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Strehl C, Ehlers L, Gaber T and Buttgereit
F: Glucocorticoids-all-rounders tackling the versatile players of
the immune system. Front Immunol. 10(1744)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Uribe JA, Aggarwal I, Witthayaweerasak J,
Liao YJ, Berry GJ, Sab UK and Weyand CM: Refractory giant cell
arteritis complicated by vision loss from optic atrophy and
maculopathy associated with pachymeningitis. J Neuroophthalmol.
38:17–23. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mollan SP, Horsburgh J and Dasgupta B:
Profile of tocilizumab and its potential in the treatment of giant
cell arteritis. Eye Brain. 10:1–11. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Harrington R, Al Nokhatha SA and Conway R:
Biologic therapies for giant cell arteritis. Biologics. 15:17–29.
2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ing EB, Wang DN, Kirubarajan A,
Benard-Seguin E, Ma J, Farmer JP, Belliveau MJ, Sholohov G and
Torun N: Systematic review of the yield of temporal artery biopsy
for suspected giant cell arteritis. Neuroophthalmology. 43:18–25.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Berger CT, Sommer G, Aschwanden M, Staub
D, Rottenburger C and Daikeler T: The clinical benefit of imaging
in the diagnosis and treatment of giant cell arteritis. Swiss Med
Wkly. 148(W14661)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ghinai RA, Mahmood S, Mukonoweshuro P,
Webber S, Wechalekar AD and Moore SE: Diagnosing light chain
amyloidosis on temporal artery biopsies for suspected giant cell
arteritis. J Neuroophthalmol. 37:34–39. 2017.PubMed/NCBI View Article : Google Scholar
|