Introduction
Wear particles, particularly titanium (Ti) alloy
particles, are a major cause of the occurrence of aseptic loosening
of joint prostheses, which eventually results in high healthcare
costs and complex revision procedures (1). Following joint replacement,
inflammation around the surface of the prosthesis induced by Ti
alloy particles leads to bone resorption and aseptic loosening of
the implants (2,3). At present, various measures are taken
for the prevention and treatment of aseptic loosening of joint
prostheses. In addition, the use of Ti alloy particles has been
markedly reduced with the development of biomaterials and
prosthetic design. However, the issue of aseptic loosening remains
unresolved and Ti alloy particles remain the main cause of aseptic
loosening (4).
In recent years, strong evidence has emerged
indicating that the viability of osteoblasts decreases in the
presence of Ti alloy particles (~4.5x107/ml), with
ensuing inhibition of osteogenic differentiation (5). Therefore, identifying a novel
effective treatment strategy for promoting osteogenesis and
inhibiting osteolysis in the presence of Ti alloy particles appears
to be a promising approach to preventing the occurrence of aseptic
loosening (6,7).
As multipotent stem cells, bone marrow mesenchymal
stem cells (BMSCs) can differentiate into various cell lineages,
such as osteocytes, chondrocytes, adipocytes, neurocytes and
muscular cells, via the activation of specific factors (8). Relatively recent studies have reported
that BMSCs serve a crucial role during the occurrence of
osteoporosis and aseptic loosening. Various anti-osteoporosis
drugs, such as alendronate and strontium ranelate, have been
demonstrated to effectively enhance osteogenesis and inhibit
osteolysis in postmenopausal women via the Wnt/β-catenin signaling
pathway (9,10). However, reliable and effective drugs
that promote osteogenic differentiation of BMSCs and increase bone
formation and metabolism in the presence of Ti alloy particles have
yet to be developed.
As an activator of sirtuin (Sirt)1, resveratrol is
an extensively reviewed anti-inflammatory and anti-aging drug,
which has been widely investigated for the regulation of energy
expenditure and treatment of endocrine diseases (11,12).
In addition, resveratrol is an important regulator of metabolism,
it regulates gene coordination sites, telomeres and ribose, and
silences related signals to enhance cell viability and longevity
(13). In addition, accumulating
evidence has indicated that resveratrol also has bone-protective
properties. Shakibaei et al (14) reported that resveratrol promotes
osteogenic differentiation of MSCs via Sirt1/Runt-related
transcription factor 2 (Runx2) activation. Furthermore, resveratrol
may promote osteogenic differentiation and reduce adipogenic
differentiation in BMSCs via upregulation of Sirt1 and inhibition
of peroxisome proliferator-activated receptor γ (15). Therefore, resveratrol may represent
a novel therapeutic approach to promoting osteogenesis in the
presence of Ti alloy particles and preventing aseptic loosening. In
addition, several studies have demonstrated that the Wnt/β-catenin
signaling pathway is a key regulator of several cellular
activities, including differentiation, proliferation, migration and
polarity (16). However, little is
known regarding the association between resveratrol and the
Wnt/β-catenin signaling pathway in the context of aseptic loosening
of joint prostheses.
In BMSCs, the Wnt/β-catenin signaling pathway
directly regulates osteogenic differentiation (17,18).
Jing et al (19) reported
that the Wnt/β-catenin signaling pathway and osteogenic
differentiation are inhibited in osteoporosis. Following
upregulation of the canonical Wnt/β-catenin signaling pathway, the
levels of β-catenin are increased and β-catenin is translocated
into the nucleus, where it binds to T cell factor/lymphoid
enhancer-binding factor 1 (Lef-1) and regulates downstream target
gene expression (20). Furthermore,
Runx2, also known as core-binding factor 1, is a key member of the
Runx family of transcription factors, and serves an important role
in the regulation of cell proliferation (21). Several studies have reported that
Runx2 is an essential master gene in the process of osteogenic
differentiation and the expression of certain β-catenin targets
(22,23). An interaction between Ti alloy
particles and periprosthetic tissues has also been reported
(24).
The aim of the present study was to investigate
whether treatment with resveratrol can inhibit osteolysis induced
by Ti alloy particles in BMSCs. Additionally, an experimental mouse
model of aseptic loosening was used to simulate artificial joint
replacement, in order to investigate the interaction between Ti
alloy particles and periprosthetic tissues, and examine the
potential biochemical mechanisms underlying the action of
resveratrol.
Materials and methods
BMSC culture and osteogenic
differentiation
BMSCs from C57BL/6J mice and osteogenic
differentiation medium were purchased from Cyagen Biosciences, Inc.
The cells were positive for the surface markers CD29, CD44 and stem
cell antigen-1 (>70%), and negative for CD117 (<5%).
Subsequently, ~3x105 cells were cultured in α-minimum
essential medium (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin-streptomycin in a humidified atmosphere with 5%
CO2 at 37˚C in 25-cm2 flasks and 12-well
plates (Wuxi NEST Biotechnology Co., Ltd.). The culture medium was
renewed every 2 days for 2 weeks to obtain a confluent
monolayer.
The medium was changed to osteogenic differentiation
medium supplemented with 10% FBS, 1% penicillin-streptomycin, 1%
glutamine, 0.2% ascorbate, 1% β-glycerophosphate and 0.01%
dexamethasone. The cells were incubated for a further 21 days and
the medium was renewed every 3 days.
Ti alloy particle preparation
Ti alloy particles (~4.5 µm; 0.5 m2/mg;
Zimmer Biomet) were used in the present study. The particles were
sterilized at 180˚C for 45 min, soaked in 95% ethanol for 48 h and
washed five times in PBS. The Limulus Amebocyte Lysate assay was
performed to ensure the particles were free from endotoxin
(QCL-1000 Chromogenic LAL; BioWhittaker; Lonza Group, Ltd.)
(25). In vitro, the Ti
alloy particles (0.1 mg/ml) were added to α-minimum essential
medium (Invitrogen; Thermo Fisher Scientific, Inc.) for 24 h, after
which the Ti alloy particles appeared to be similar to the wear
debris retrieved from peri-prosthetic tissues (23,26-28).
For in vivo experiments, 10 µl Ti suspension containing
4x104 particles of Ti in normal saline was used, which
was equivalent to ~4x106 particles (200 µg/ml) (27).
Mice and model of aseptic
loosening
All animal experiments were approved by the Animal
Ethics Committee of Ningxia Medical University (Yinchuan, China)
and were performed at the Experimental Animal Center of Ningxia
Medical University. Female C57BL/6J mice (n=45; age, 10 weeks;
weight, 20±2 g, Experimental Animal Center of Ningxia Medical
University, Yinchuan, China) were housed in pressure-controlled
ventilation cages at a constant temperature of 25˚C with 40-70%
humidity and a 12/12 h light/dark cycle, and were fed a normal
rodent diet ad libitum.
To establish the model of aseptic loosening, the
mice were intraperitoneally anesthetized with Nembutal containing
0.6% pentobarbital sodium (40 mg/kg). Then, the tibial plateau was
exposed and a canal (diameter, 3 mm) was created with a hand drill.
Each canal was injected with 10 µl Ti alloy particle suspension and
implanted with a Ti pin, the head of which was maintained at the
same level as the tibial plateau. After the surgery, 20 µl Ti
particles were injected into the joint capsule every 2 weeks.
Experimental design
Cytotoxicity was first assessed in vitro, and
25 µM resveratrol (Sigma-Aldrich; Merck KGaA) was deemed as optimal
for maintaining cell viability. Subsequently, the cells were
treated with or without Ti alloy particles and resveratrol. After
21 days of osteogenic differentiation, the effects on osteogenesis
and the Wnt/β-catenin signaling pathway were detected by Alizarin
red S staining, western blotting and reverse
transcription-quantitative PCR (RT-qPCR). In the in vivo
experiments, 15 C57BL/6J mice were used and randomly divided into
three groups as follows: Control group (5 mice; 0.1% DMSO); low
dose group (5 mice; 10 mg/kg resveratrol) and high dose group (5
mice; 30 mg/kg resveratrol) (29).
All the mice were implanted with a Ti pin and drugs were injected
intraperitoneally twice per week for 4 weeks (28,30).
All the mice were sacrificed with 100% CO2 (10 l/min;
~20% volume displaced/min); the CO2 flow was maintained
until at least 1 min after the cessation of the animal's breathing
and heartbeat, as well as the fading of eye color, in order to
ensure animal death. Then, a pullout test of the Ti pin,
immunohistochemistry and micro-CT (µCT) were used to analyze the
bone formation around the prosthesis.
Cell Counting Kit-8 (CCK-8) assay
Cells (5x103) were cultured in a 96-well
plate overnight, followed by treatment with 12.5, 25, 50, 75 and
100 µM resveratrol for 24, 48 and 72 h. Subsequently, 10 µl CCK-8
solution (BestBio Co., Ltd.) was added and incubated for 2 h at
37˚C. For quantitative analysis, the absorbance at 450 nm was
measured using a spectrophotometer (Bio-Rad Laboratories,
Inc.).
Caspase-3 assay
To analyze the effect of various concentrations of
Ti alloy particles on the apoptosis of BMSCs, 4x106
cells were cultured in 60-mm petri dishes with 0.1, 0.5 and 1%
concentrations of Ti alloy particles. Based on a previous study
(31), the activity of caspase-3
was measured to assess the levels of apoptosis at 24, 48 and 72 h
after the addition of Ti alloy particles and Ti alloy particles
with resveratrol. Briefly, cells were washed twice in PBS, lysed in
150 µl lysis buffer from the caspase-3 colorimetric assay kit
(Nanjing KeyGen Biotech Co., Ltd., for 60 min on ice and then
centrifuged at 560 x g for 1 min at 4˚C. A supernatant sample (50
µl) was mixed with 50 µl reaction buffer and incubated for 4 h at
37˚C. The absorbance at 405 nm was measured using a
spectrophotometer (Bio-Rad Laboratories, Inc.).
Alizarin red S staining and
quantification
BMSCs were cultured in osteogenic differentiation
medium for 21 days. The matrix mineralization level was assessed by
Alizarin red S staining. The cells were fixed with 10% (v/v)
formalin at 37˚C for 15 min. Then, the cells were washed twice with
PBS and stained with 1 ml Alizarin red S (pH 4.2; Cyagen
Biosciences, Inc.) for 15 min. For quantitation, the stained cells
were incubated with 10% (w/v) cetylpyridinium chloride in 10 mM
sodium phosphate buffer (pH 7.0) for 15 min and the absorbance at
562 nm was measured.
Western blot analysis
BMSCs were washed with cold PBS and proteins were
extracted with RIPA buffer (Sigma-Aldrich; Merck KGaA). Protein
concentration was determined using a bicinchoninic acid protein
assay kit (Nanjing KeyGen Biotech Co., Ltd.). Protein (40 µg) was
separated by 10% SDS-PAGE and transferred to PVDF membranes.
Subsequently, the membranes were blocked in 5% dry skimmed milk at
room temperature for 2 h, and incubated overnight at 4˚C with the
following primary antibodies: Anti-Runx2 (cat. no. ab92336; 1:500)
and anti-osteocalcin (OCN; cat. no. ab76690; 1:1,000; all Abcam).
Secondary antibody (1:2,000; Goat Anti-Rabbit IgG; cat. no. 4970;
Cell Signaling Technology, Inc.) was then added to the membranes
for 1 h at room temperature. The membranes were exposed to enhanced
chemiluminescence reagent (Nanjing KeyGen Biotech Co., Ltd.).
Semi-quantitative analysis of protein bands was performed by a
detection system (PerkinElmer, Inc.) and ImageJ software (LI-COR
Biosciences, version 1.8.0.112).
RT-qPCR
BMSCs (1x105 cells/well) were seeded in
60-mm plates. Total RNA was extracted using TRIzol®
reagent (Thermo Fisher Scientific, Inc.) and RNA purity was
calculated using the 260/280 absorbance ratio (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.). First-strand cDNA
was synthesized using PrimeScript (Takara Bio, Inc.). qPCR was
performed using SYBR® Premix Ex Taq™ II (Takara Bio
Inc.) and the thermocycling conditions were as follows: 95˚C for 15
min, followed by 40 amplification cycles at 95˚C for 10 sec and
58˚C for 30 sec, and a final extension at 72˚C for 30 sec. Relative
mRNA expression was calculated using the 2-ΔΔCq method
(32).
The PCR primers used in the present study were as
follows: Runx2 forward, 5'-GACTGTGGTTACCGTCATGGC-3' and reverse,
5'-ACTTGGTTTTTCATAACAGCGGA-3'; OCN forward,
5'-CTTGGGTTCTGACTGGGTGT-3' and reverse, 5'-TCTAGCCCTCTGCAGGTCAT-3';
β-catenin forward, 5'-ACAGGGTGCTATTCCACGAC-3' and reverse,
5'-CTGCACAAACAATGGAATGG-3'; Lef-1 forward,
5'-GCCACCGATGAGATGATCCC-3' and reverse, 5'-TTGATGTCGGCTAAGTCGCC-3';
transcription factor (Tcf)-4 forward, 5'-ATGGCCCAAGTAGTGATGTCT-3'
and reverse, 5'-CAAACACGTCGGTCTCATACA-3'; and GAPDH forward,
5'-TGATGACATCAAGAAGGTGGTGAA-3' and reverse,
5'-TCCTTGGAGGCCATGTAGGCCAT-3'.
Immunohistochemistry examination
The tibia samples were fixed in 4% paraformaldehyde
and EDTA for 24 h at 4°C. After dehydration in ethanol,
the samples were embedded in paraffin at 60˚C and cut into 5-µm
sections using the RM2235 Rotary Microtome Basic instrument (Leica
Microsystems, Inc.). To observe the expression levels of Runx2, the
glass slides were added to an autoclave for 2 min, cooled for 20
min, and then incubated at 23˚C for 10 min with 3% hydrogen
peroxide. Following washing with PBS, primary antibody against
Runx2 (cat. no. ab92336; 1:300; Abcam) was added and incubated
overnight at 4°C. Subsequently, the slides were
incubated with secondary antibodies (1:1,000; Enzyme-labeled goat
anti-rabbit IgG polymer; cat. no. SSA018; Sino Biological). The
results were obtained using a microscopic imaging system (DM2000
LED; Leica Microsystems, Inc.) and Image-Pro Plus 6.0 software
(Media Cybernetics, Inc.).
Pullout test to assess Ti prosthesis
steadiness
After sacrificing the mice, the soft tissues around
the tibia were carefully removed and the head of the Ti pin was
exposed. Each tibia was fixed to a special clamp, aligning the long
axis of the pin with the long axis of the test machine (HP-100
Control Electronic Universal Testing machine; Yueqing Zhejiang
Instrument Scientific Co., Ltd.). Then, the HP-100 device was used
to pull the Ti pin out of the tibia at a rate of 2 mm/min. The load
values were automatically measured by software (Edburg software
Version 1.0; Yueqing ALIYIQI Instrument Co., Ltd.).
µCT scanning
The mouse tibia samples were scanned by µCT (SkyScan
1176; Bruker Corporation). Each tibia sample (without soft tissues)
was fixed in 4% paraformaldehyde at 4˚C for 4 weeks and the
tomographic scans were acquired at 900 ms exposure time, 45 kW
voltage and 550 mA current. The bone volume (BV), bone volume
fraction (BV/TV), trabecular thickness (Tb.Th), trabecular number
(Tb.N) and specific bone surface (BS/BV) of the shin bone
surrounding the prosthesis were automatically detected by NRecon
software (version 1.1.11; Bruker Corporation).
Statistical analysis
The results are presented as the mean ± SD (n=3).
One-way ANOVA and Dunnett was used to compare multiple groups. SPSS
v19.0 (IBM Corp.) was used for statistical analyses. Graphs were
prepared using GraphPad Prism (version 6.0 for Windows; GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of various doses of resveratrol
on the viability of BMSCs in vitro
First, the viability of BMSCs treated with
resveratrol was investigated in vitro. Following treatment
with 12.5, 25, 50, 75 and 100 µM resveratrol, cell viability was
detected using a CCK-8 assay (Fig.
1A). The results demonstrated that treatment with 25 µM
resveratrol effectively enhanced cell viability at 24 h
(1.188±0.049 in the control group, 1.645±0.136 in the 25 µM
resveratrol group and 1.376±0.047 in the 50 µM resveratrol group;
P<0.05; Fig. 1A). However, no
difference was observed among the groups at 48 h, and the groups
treated with 25 and 50 µM resveratrol exhibited a statistically
significant decrease in cell viability at 72 h (1.270±0.015 in the
control group, 1.184±0.031 in the 25 µM resveratrol group and
1.177±0.029 in the 50 µM resveratrol group; P<0.05; Fig. 1A). Therefore, 25 µM resveratrol was
used in subsequent experiments to avoid cytotoxicity.
Resveratrol increases osteogenesis and
stimulates the Wnt/β-catenin signaling pathway
Osteogenesis was evaluated by Alizarin red S
staining and quantification. The osteogenic differentiation of
BMSCs was significantly decreased in the presence of Ti alloy
particles (3.18±0.09 in the control group vs. 1.47±0.08 in the Ti
alloy particles group; P<0.01; Fig.
1B and C). However, following
treatment with resveratrol, bone formation inhibition by Ti
particles was reversed (3.18±0.09 in the control group vs.
3.33±0.05 in the resveratrol with Ti alloy particles group; P=0.24;
Fig. 1B and C).
The results of western blotting (Fig. 2A-C) revealed that Runx2 expression
was significantly increased after treatment with Ti alloy particles
(0.072±0.006 in the control group vs. 0.128±0.002 in the Ti alloy
particles group; P<0.01; Fig.
2B). Compared with the control group, the expression levels of
Runx2 were markedly increased in the resveratrol with Ti alloy
particles group. Similarly, the expression levels of OCN were
increased in the resveratrol with Ti alloy particles group
(0.102±0.014 in the control group vs. 0.276±0.030 in the Ti alloy
particles group; P<0.01; Fig.
2C).
 | Figure 2Resveratrol regulates osteogenic
differentiation in BMSCs treated with Ti alloy-particles via the
Wnt/β-catenin pathway. (A-C) Western blotting was used to determine
the Runx2 and OCN protein levels in BMSCs by treated with
resveratrol and Ti alloy-particles after 21 days of osteoinduction.
(D) Reverse transcription-PCR was used to analyze the mRNA levels
of Runx2, OCN, β-catenin, Lef-1 and Tcf-4 in BMSCs after 21 days of
osteogenic differentiation. (E) Caspase-3 activity assay was
performed to determine the level of apoptosis in BMSCs treated with
Ti alloy-particles. (F) After treatment with Ti alloy particles and
Resveratrol, the level of apoptosis in BMSCs was determined by
Caspase-3 activity. **P<0.01,
***P<0.001 and ****P<0.0001 vs. the
control group. n=3. BMSCs, bone marrow stem cells; Lef-1, lymphoid
enhancer binding factor 1; OCN, osteocalcin; Res, resveratrol;
Runx2, runt-related transcription factor 2; Tcf-4, transcription
factor 4; Ti, Titanium alloy-particles. |
RT-PCR analysis revealed similar results (Fig. 2D). Compared with the control group,
the expression levels of Runx2 (1.03±0.09 in the control group vs.
1.51±0.12 in the Ti alloy particles group; P<0.05) and OCN
(1.03±0.17 in the control group vs. 2.17±0.09 in the Ti alloy
particles group; P<0.01) were increased in the Ti alloy
particles group (Fig. 2D) and in
the resveratrol with Ti alloy particles group (Runx2, 1.03±0.09 in
the control group vs. 9.34±0.23 in the resveratrol with Ti alloy
particles group, P<0.01; OCN, 1.03±0.17 in the control group vs.
4.06±0.06 in the resveratrol with Ti alloy particles group,
P<0.01). However, these results did not completely match the
results of the staining analysis.
To determine the effect of resveratrol with Ti alloy
particles on the Wnt/β-catenin signaling pathway, the present study
further examined the mRNA expression levels of Lef-1, Tcf-4 and
β-catenin (Fig. 2D). The results
demonstrated that the expression levels of Lef-1 and Tcf-4 were
significantly increased in the Ti alloy particles group (1.03±0.04
vs. 1.72±0.07 and 0.97±0.06 vs. 2.14±0.10, respectively; both
P<0.01) and in the resveratrol with Ti alloy particles group
(1.03±0.04 vs. 8.14±0.27 and 0.97±0.06 vs. 10.42±0.25,
respectively; both P<0.01). However, β-catenin expression was
only increased in the resveratrol with Ti alloy particles group
(1.03±0.19 vs. 6.76±0.09; P<0.01). These results demonstrated
that resveratrol may inhibit the osteolysis induced by Ti alloy
particles via activation of the Wnt/β-catenin signaling
pathway.
In addition, since the RT-qPCR results revealed that
the expression levels of Runx2 and OCN were increased in the
Ti-alloy particles group, apoptosis was examined by assessing
caspase-3 activity at 24, 48 and 72 h after the addition of Ti
alloy particles. Compared with the control group, apoptosis was
markedly increased, particularly with 0.5 and 1% Ti alloy particles
(Fig. 2E). Subsequently, the levels
of caspase-3 were measured after treatment with resveratrol, and
the results demonstrated that resveratrol markedly decreased the
caspase-3 levels compared with the Ti alloy particles group
(Fig. 2F).
Resveratrol enhances bone
microstructure around the prosthesis
To investigate the effects of resveratrol on bone
formation in vivo, a mouse model of aseptic loosening was
established (Fig. 3A and B). Subsequently, pullout tests of the Ti
pins, immunohistochemistry and µCT scans were used to analyze the
differences in the bone microstructure among the control, low-dose
and high-dose groups. The results of the pullout test of Ti pins
(Fig. 3C) demonstrated that,
compared with the control group (1.57±0.16 N), the pulling load was
significantly increased after treatment with resveratrol,
particularly in the high-dose group (low dose resveratrol group,
9.87±0.39 N, P<0.01; high dose resveratrol group, 14.33±0.68 N;
P<0.01). In addition, the difference in pulling load between the
low- and high-dose resveratrol groups was statistically significant
(P<0.01).
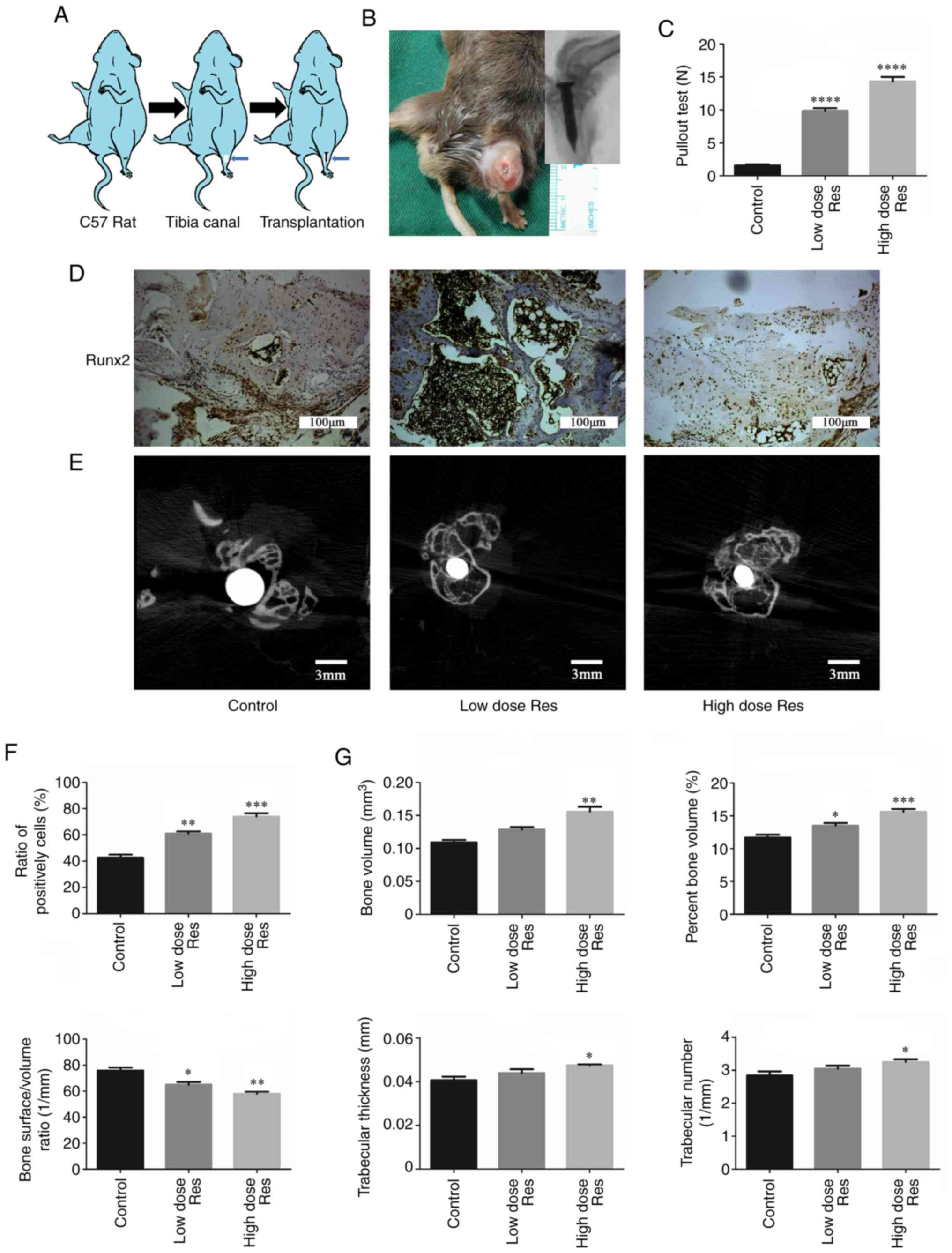 | Figure 3Resveratrol enhances bone formation
around the prosthesis in vivo. (A) Schematic drawing of the
transplantation procedure. (B) Establishment of the model of
aseptic loosening. (C) Pulling force required to remove the
titanium pin implant from the tibia with or without resveratrol.
(D-G) Immunohistochemistry and µCT were used to determine the
effects of resveratrol on bone formation. (D and F)
Immunohistochemical staining and semi-quantification of Runx2
protein expression levels in periprosthetic tissues. The ratios of
the number of cells positively stained for Runx2 to the total
number of cells were plotted. Scale bar, 100 µm. (E and G)
Cross-sectional images of titanium implants and bone microstructure
analyzed by µCT scans (Scale bar, 3 mm); the bone volume,
percentage bone volume, bone surface density, trabecular thickness
and trabecular number were calculated. *P<0.05,
**P<0.01, ***P<0.001 and
****P<0.001 vs. the control group (n=3). µCT,
micro-CT; Res, resveratrol; Runx2, runt-related transcription
factor 2. |
The expression levels of Runx2 in the bone around
the prosthesis are shown in Fig. 3D
and F. Compared with the control
group (0.427±0.023), the expression levels of Runx2 were
significantly increased in the low- and high-dose resveratrol
groups (low-dose resveratrol, 0.610±0.017, P<0.01; high-dose
resveratrol, 0.740±0.025; P<0.01). This effect was more
pronounced in the high-dose resveratrol group compared with in the
low-dose group (0.740±0.025 vs. 0.610±0.017, respectively;
P<0.05).
The µCT scan and quantification analysis
demonstrated analogous results (Fig.
3E and G). Compared with the
control group (0.109±0.004 mm3), BV was significantly
increased following treatment with a low dose (0.129±0.004
mm3; P<0.01) and a high dose of resveratrol
(0.156±0.008 mm3; P<0.01) in a dose-dependent manner
(P=0.032). Similarly, compared with the control group
(11.71±0.416%), BV/TV was increased in the low-dose (13.52±0.393%;
P<0.05) and the high-dose (15.62±0.430%; P<0.01) resveratrol
groups in a dose-dependent manner (P=0.023). By contrast, compared
with the control group (76.32±2.201/mm), BS/BV was
significantly decreased in the resveratrol groups (low-dose
resveratrol, 64.98±2.181/mm, P<0.05; low-dose
resveratrol, 58.03±1.621/mm; P<0.01). Additionally,
Tb.Th and Tb.N were increased following treatment with resveratrol,
but this increase was only significant in the high-dose group
(Tb.Th, 0.041±0.002 vs. 0.047±0.001 mm, respectively; P<0.05;
Tb.N, 2.841±0.126 vs. 3.253±0.0781/mm, respectively;
P<0.05).
Discussion
Joint arthroplasty is an effective and reliable
treatment method for osteoarthritis, and this surgery may
effectively restore joint function and control pain. However,
following surgery, aseptic loosening of prosthetic joints remains
an inevitable long-term complication and affects the success rate
of joint replacement (9). Although
previous studies have reported that an imbalance between
osteogenesis and osteolysis around the prosthesis is the key factor
in aseptic loosening, the pathogenesis of osteolysis remains
unclear and effective therapeutic drugs have not yet been developed
(33).
Resveratrol has been demonstrated to have various
biological functions, including inhibition of inflammation
(34), attenuation of apoptosis in
cardiomyocytes (35) and promotion
of cell longevity (36). In the
present study, 25 and 50 µM resveratrol effectively increased the
cell proliferation capacity at 24 h; however, at 72 h, the cell
proliferation capacity decreased in a dose-related manner. Rubiolo
et al reported that resveratrol induced cell arrest in the
G0-G1 phase but not in the S-phase in rat hepatocytes. In addition,
following treatment with 50 and 100 µM resveratrol for 24, 48 and
72 h, the number of cells in the S-phase was increased, but in
contrast, the cells in G0-G1 and G2-M phases was decreased
(37). Moreover, in the current
study, Ti alloy particles markedly inhibited the osteogenic
differentiation of BMSCs; however, following treatment with
resveratrol, osteolysis caused by Ti alloy particles was inhibited.
Similarly, Matsuda et al (38) reported that resveratrol prevents
trauma from occlusion-induced alveolar bone loss by suppressing
osteoclast differentiation. Therefore, it may be inferred that
resveratrol may prevent osteoclast formation and promote osteogenic
differentiation. However, the results of western blotting and
RT-PCR indicated that Ti-alloy particles increased the expression
of osteogenesis-related factors, such as Runx2, which is not the
same as the results obtained from Alizarin red S staining.
Therefore, the level of apoptosis of BMSCs was further examined,
and the results demonstrated that, with the increase in the
concentration of Ti-alloy particles, their apoptosis-inducing
effect became more prominent, particularly at concentrations of 0.5
and 1%. In addition, resveratrol effectively reduced the
pro-apoptotic effect of Ti-alloy particles on cells. These results
revealed that, although Ti-alloy particles can stimulate the
expression of osteogenic factors in BMSCs, their effect on cell
apoptosis is more prominent. In addition, resveratrol was shown to
effectively reduce the effect of Ti-alloy particles on apoptosis,
indicating that it may be of value in preventing Ti-alloy
particle-induced osteolysis.
Following treatment with resveratrol, the expression
levels of Runx2 and OCN were upregulated. In addition, the results
of RT-qPCR revealed that the mRNA expression levels of β-catenin,
Lef-1 and Tcf-4 were increased, suggesting that resveratrol may
enhance the osteogenic differentiation of BMSCs via the
Wnt/β-catenin signaling pathway. Of note, Ti alloy particles have
already been reported to exert an inhibitory effect on osteogenic
differentiation (39). The results
of the present study also demonstrated that osteogenesis was
inhibited by Ti alloy particles. However, the expression levels of
Runx2 and OCN were upregulated in the presence of Ti alloy
particles. These results suggested that the particles enhanced
osteogenesis, which was inconsistent with the observations
following Alizarin red S staining. In agreement with a previous
study, the Ti particles may stimulate osteogenesis in human BMSCs
(40), suggesting that Ti particles
do not directly inhibit the process of osteogenic differentiation,
but may rather affect cell viability (41).
Another aim of the present study was to determine
the effect of resveratrol on bone formation in mice with
periprosthetic osteolysis. Following treatment with resveratrol,
the pulling force of the Ti pin removed from the tibia was
increased in a dose-dependent manner. This finding supports the
hypothesis that resveratrol may decrease bone resorption.
Similarly, others have reported that Sirt1, which is activated by
resveratrol, may inhibit aseptic loosening via NF-κB deacetylation
(42). In addition, µCT scanning
demonstrated that BV and BV/TV in the periprosthetic tissue were
markedly enhanced in a dose-dependent manner. BS/BV also indicated
an anti-osteolytic effect of resveratrol in mice; however, this was
not dose-dependent. Additionally, there were marked differences in
Tb.Th and Tb.N between the high-dose resveratrol and the control
groups, although no significant differences were observed between
the low-dose resveratrol and control groups. These findings support
the hypothesis that resveratrol is able to enhance the BV, BS/BV,
BV/TV, Tb.N and Tb.Th in cases with aseptic loosening induced by
wear particles, which suggests that resveratrol may inhibit bone
resorption. In accordance with the aforementioned findings, Lee
et al (43) demonstrated
that BV was markedly increased following treatment with
resveratrol. Furthermore, the results of immunohistochemistry
demonstrated that resveratrol increased the expression levels of
Runx2 in a dose-dependent manner. These findings suggest a key role
of resveratrol in inhibiting osteolysis by upregulating Runx2
expression in an model of aseptic loosening (44). Furthermore, it should be noted that
osteoclasts must also be investigated in subsequent studies.
Specifically, co-culture of osteoblast with RAW 264.7 cells may
explain why the expression levels of Runx2 and OCN were increased
in the presence of Ti alloy particles, while osteogenesis was
inhibited (45). In addition, the
Wnt/β-catenin signaling pathway was not directly targeted in order
to demonstrate its role in the protective effects of resveratrol
against particle-associated osteolysis, which is another limitation
of the present study.
In conclusion, resveratrol effectively inhibited
wear particle-associated osteolysis in vivo and in
vitro. In addition, resveratrol upregulated Runx2 and OCN
expression, suggesting that resveratrol may be a potential
therapeutic agent for the treatment of aseptic loosening of joint
prostheses.
Acknowledgements
The authors would like to thank Dr Mao Mao (General
Hospital of Ningxia Medical University) for his technological
help.
Funding
Funding: The present study was supported by the Ningxia Natural
Science Foundation of China (grant no. NZ17137).
Availability of materials and data
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC and QJ conceived the experiments, XC and TG
conducted the experiments and wrote the revised manuscript, SS and
XF conducted the experiments, and ShZ, SiZ and YG analyzed the
results. XC and QJ confirm the authenticity of all the raw data.
All the authors reviewed the manuscript. All the authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocol in the present study was
conducted in accordance with the National Institutes of Health
guidelines for the care and use of laboratory animals and was
approved by the Ethics Committee of the General Hospital of Ningxia
Medical University (approval no. NXYKDX.2019.722-35, Yinchuan,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang ML, Sharkey PF and Tuan RS: Particle
bioreactivity and wear-mediated osteolysis. J Arthroplasty.
19:1028–1038. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lee SS, Sharma AR, Choi BS, Jung JS, Chang
JD, Park S, Salvati EA, Purdue EP, Song DK and Nam S: The effect of
TNFa secreted from macrophages activated by titanium particles on
osteogenic activity regulated by WNT/BMP signaling in
osteoprogenitor cells. Biomaterials. 33:4251–4263. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yang H, Xu Y, Zhu M, Gu Y, Zhang W, Shao
H, Wang Y, Ping Z, Hu X, Wang L and Geng D: Inhibition of
titanium-particle-induced inflammatory osteolysis after local
administration of dopamine and suppression of osteoclastogenesis
via D2-like receptor signaling pathway. Biomaterials. 80:1–10.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rao AJ, Gibon E, Ma T, Yao Z, Smith RL and
Goodman SB: Revision joint replacement, wear particles, and
macrophage polarization. Acta Biomater. 8:2815–2823.
2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vermes C, Chandrasekaran R, Jacobs JJ,
Galante JO, Roebuck KA and Glant TT: The effects of particulate
wear debris, cytokines, and growth factors on the functions of
MG-63 osteoblasts. J Bone Joint Surg Am. 83:201–211.
2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu X, Zhu S, Cui J, Shao H, Zhang W, Yang
H, Xu Y, Geng D and Yu L: Strontium ranelate inhibits
titanium-particle-induced osteolysis by restraining inflammatory
osteoclastogenesis in vivo. Acta Biomaterialia. 10:4912–4918.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Guo H, Zhang J, Hao S and Jin Q:
Adenovirus-mediated small interfering RNA targeting tumor necrosis
factor-α inhibits titanium particle-induced osteoclastogenesis and
bone resorption. Int J Mol Med. 32:296–306. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Squillaro T, Peluso G and Galderisi U:
Clinical trials with mesenchymal stem cells: An update. Cell
Transplant. 25:829–848. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Reginster JY, Brandi ML, Cannata-Andia J,
Cooper C, Cortet B, Feron JM, Genant H, Palacios S, Ringe JD and
Rizzoli R: The position of strontium ranelate in today's management
of osteoporosis. Osteoporos Int. 26:1667–1671. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Karakan NC, Akpinar A, Göze F and Poyraz
Ö: Investigating the effects of systemically administered strontium
ranelate on alveolar bone loss histomorphometrically and
histopathologically on experimental periodontitis in rats. J
Periodontol. 88:e24–e31. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Baur JA, Pearson KJ, Price NL, Jamieson
HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K,
et al: Resveratrol improves health and survival of mice on a
high-calorie diet. Nature. 444:337–342. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: The in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Liu B, Ghosh S, Yang X, Zheng H, Liu X,
Wang Z, Jin G, Zheng B, Kennedy BK, Suh Y, et al: Resveratrol
rescues SIRT1-dependent adult stem cell decline and alleviates
progeroid features in laminopathy-based progeria. Cell Metab.
16:738–750. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shakibaei M, Shayan P, Busch F, et al:
Resveratrol mediated modulation of Sirt-1/Runx2 promotes osteogenic
differentiation of mesenchymal stem cells: potential role of Runx2
deacetylation. PLoS One. 7(e35712)2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhang H, Zhang H, Zhang Y, Ng SS, Ren F,
Wang Y, Duan Y, Chen L, Zhai Y, Guo Q and Chang Z: Dishevelled-DEP
domain interacting protein (DDIP) inhibits Wnt signaling by
promoting TCF4 degradation and disrupting the TCF4/beta-catenin
complex. Cell Signal. 22:1753–1760. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gao X, Ge J, Li W, Zhou W and Xu L: Lncrna
kcnq1ot1 promotes osteogenic differentiation to relieve osteolysis
via wnt/β-catenin activation. Cell Biosci. 8(19)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Goessling W, North TE, Loewer S, Lord AM,
Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moon RT
and Zon LI: Genetic interaction of PGE2 and Wnt signaling regulates
developmental specification of stem cells and regeneration. Cell.
136:1136–1147. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Komiya Y and Habas R: Wnt signal
transduction pathways. Organogenesis. 4:68–75. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jing H, Su X, Gao B, et al: Epigenetic
inhibition of Wnt pathway suppresses osteogenic differentiation of
BMSCs during osteoporosis. Cell Death Dis. 9(176)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lucero CM, Vega OA, Osorio MM, Tapia JC,
Antonelli M, Stein GS, van Wijnen AJ and Galindo MA: The
cancer-related transcription factor Runx2 modulates cell
proliferation in human osteosarcoma cell lines. J Cell Physiol.
228:714–723. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Komori T, Yagi H, Nomura S, Yamaguchi A,
Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, et al:
Targeted disruption of Cbfa1 results in a complete lack of bone
formation owing to maturational arrest of osteoblasts. Cell.
89:755–764. 1997.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Simic P, Zainabadi K, Bell E, Sykes DB,
Saez B, Lotinun S, Baron R, Scadden D, Schipani E and Guarente L:
SIRT1 regulates differentiation of mesenchymal stem cells by
deacetylating beta-catenin. EMBO Mol Med. 5:430–440.
2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang S, Yu H, Gong W, Wu B, Mayton L,
Costello R and Wooley PH: Murine model of prosthesis failure for
the long term study of aseptic loosening. J Orthop Res. 25:603–611.
2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Geng D, Xu Y, Yang H, Wang J, Zhu X, Zhu G
and Wang X: Protection against titanium particle induced osteolysis
by cannabinoid receptor 2 selective antagonist. Biomaterials.
31:1996–2000. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Clohisy JC, Hirayama T, Frazier E, Han SK
and Abu-Amer Y: NF-kB signaling blockade abolishes implant
particle-induced osteoclastogenesis. J Orthop Res. 22:13–20.
2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
von Knoch M, Jewison DE, Sibonga JD,
Sprecher C, Morrey BF, Loer F, Berry DJ and Scully SP: The
effectiveness of polyethylene versus titanium particles in inducing
osteolysis in vivo. J Orthop Res. 22:237–243. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gu Z, Chu L and Han Y: Therapeutic effect
of resveratrol on mice with depression. Exp Ther Med. 17:3061–3064.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen HY, Lin PH, Shih YH, Wang KL, Hong
YH, Shieh TM, Huang TC and Hsia SM: Natural antioxidant resveratrol
suppresses uterine fibroid cell growth and extracellular matrix
formation in vitro and in vivo. Antioxidants (Basel).
8(99)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Grewal AK, Singh N and Singh TG: Effects
of resveratrol postconditioning on cerebral ischemia in mice: Role
of the sirtuin-1 pathway. Can J Physiol Pharmacol. 97:1094–1101.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bourne RB, Laskin RS and Guerin JS:
Ten-year results of the first 100 genesis II total knee replacement
procedures. Orthopedics. 30 (Suppl 8):S83–S85. 2007.PubMed/NCBI
|
|
31
|
Zhu YY, Wang ZJ, Ma N and Zhou JW:
Proliferation and apoptosis of lung cancer cells regulated by
gultaredoxin 3. Zhonghua Zhong Liu Za Zhi. 40:325–329.
2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rybchyn MS, Slater M, Conigrave AD and
Mason RS: An Akt-dependent increase in canonical Wnt signaling and
a decrease in sclerostin protein levels are involved in strontium
ranelate-induced osteogenic effects in human osteoblasts. J Biol
Chem. 286:23771–23779. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xie J, Zhang X and Zhang L: Negative
regulation of inflammation by SIRT1. Pharmacol Res. 67:60–67.
2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang C, Feng Y, Qu S, Wei X, Zhu H, Luo
Q, Liu M, Chen G and Xiao X: Resveratrol attenuates
doxorubicin-induced cardiomyocyte apoptosis in mice through
SIRT1-mediated deacetylation of p53. Cardiocasc Res. 90:538–545.
2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kaeberlein M, McVey M and Guarente L: The
SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces
cerevisiae by two different mechanisms. Genes Dev. 13:2570–2580.
1999.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rubiolo JA, López-Alonso H, Martín-Vázquez
V, Fol-Rodríguéz N, Vieytes MR and Vega FV: Resveratrol inhibits
proliferation of primary rat hepatocytes in G0/G1 by inhibiting DNA
synthesis. Folia Biol (Praha). 58:166–172. 2012.PubMed/NCBI
|
|
38
|
Matsuda Y, Minagawa T, Okui T and Yamazaki
K: Resveratrol suppresses the alveolar bone resorption induced by
artificial trauma from occlusion in mice. Oral Dis. 24:412–421.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Atienzar AN, Camacho-Alonso F and
Lopez-Jornet P: Effects of resveratrol and irradiation upon oral
squamous cell carcinoma cells. Acta Odontol Scand. 72:481–488.
2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kim MO, Jung H, Kim SC, Park JK and Seo
YK: Electromagnetic fields and nanomagnetic particles increase the
osteogenic differentiation of human bone marrow-derived mesenchymal
stem cells. Int J Mol Med. 35:153–160. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Erdem A, Metzler D, Cha DK and Huang CP:
The short-term toxic effects of TiO2 nanoparticles
toward bacteria through viability, cellular respiration, and lipid
peroxidation. Environ Sci Pollut Res Int. 22:17917–17924.
2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Deng Z, Jin J, Wang Z, Wang Y, Gao Q and
Zhao J: The metal nanoparticle-induced inflammatory response is
regulated by SIRT1 through NF-κB deacetylation in aseptic
loosening. Int J Nanomedicine. 12:3617–3636. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lee AM, Shandala T, Nguyen L, Muhlhausler
BS, Chen KM, Howe PR and Xian CJ: Effects of resveratrol
supplementation on bone growth in young rats and microarchitecture
and remodeling in ageing rats. Nutrients. 6:5871–5887.
2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zainabadi K, Liu CJ and Guarente L: SIRT1
is a positive regulator of the master osteoblast transcription
factor, RUNX2. PLoS One. 12(e0178520)2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Pirapaharan DC, Olesen JB, Andersen TL,
Christensen SB, Kjærsgaard-Andersen P, Delaisse JM and Søe K:
Catabolic activity of osteoblast lineage cells contributes to
osteoclastic bone resorption in vitro. J Cell Sci.
132(jcs229351)2019.PubMed/NCBI View Article : Google Scholar
|

















