Introduction
Sepsis is a clinical syndrome in which the host has
an uncontrolled response to infection and develops life-threatening
organ dysfunction (1). Sepsis and
septic shock are progressive and multifactorial diseases with high
morbidity and mortality. Each year, millions of people worldwide
suffer from sepsis and >25% of these individuals die from the
syndrome, making sepsis a major global health challenge (2).
The early systemic inflammatory response and
intestinal barrier dysfunction seen in sepsis are closely
associated with progression of the condition and the occurrence of
its most severe form, multiple organ dysfunction syndrome (3-5).
The release of a large number of pro-inflammatory cytokines in the
early stages of inflammation is considered to be an important
pathological mechanism in the development of sepsis (6,7).
Increasing concentrations of inflammatory cytokines are produced by
an excessive inflammatory response, which can cause systemic and
intestinal inflammation and the activation of the NF-κB signaling
pathway in intestinal epithelial cells (8). Inflammatory cytokines, including tumor
necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6, have
damaging effects on the tight junction structure and barrier
function of intestinal epithelial cells (9,10). The
tight junction is composed of cytoplasmic transmembrane proteins,
including occludin and junctional proteins, such as tight
junctional protein ZO-1 (ZO-1) (11). Research has indicated that tight
junctions are regulated by myosin light chain kinase (MLCK)
(12). Studies have also
demonstrated that NF-κB activity serves a crucial role in promotion
of MLCK expression (13). However,
it is not clear whether the impairment of intestinal barrier
function due to intestinal inflammatory factors is associated with
the regulation of MLCK expression by NF-κB, and the resulting
reduction in the expression of tight junctional proteins in
intestinal epithelial cells. In the present study, it is
hypothesized that the inhibition of the systemic and intestinal
inflammatory responses may be an effective means of protecting the
intestinal barrier from damage in sepsis.
Gadolinium chloride (GdCl3) is a
lanthanide compound that is commonly used to assess the function of
Kupffer cells (14,15). As GdCl3 can inhibit the
phagocytosis and secretion of Kupffer cells in the liver, it is
often used as a tool for studying the functions of
monocytes/macrophages and the pathogenesis of disease (15). GdCl3 can induce changes
in the phenotype of Kupffer cells and competes to bind to Kuppfer
cell calcium receptors, inhibiting the transcription and synthesis
of TNF-α (16). GdCl3
has not been indicated to trigger an immune response, so it has
been used in animal models of a variety of experimental diseases,
including hepatic ischemia-reperfusion injury models, obstructive
jaundice models induced by bile duct ligation, lipopolysaccharide
(LPS)-induced endotoxemia models and cecal ligation and puncture
(CLP)-induced sepsis models (17).
Previous studies have revealed that sepsis-induced acute lung
injury can be alleviated by the GdCl3-mediated
inhibition of inflammatory mediators release, including the release
of TNF-α by macrophages (18).
TNF-α and IL-6, which is released by Kupffer cells in the early
stages of endotoxemia, may serve an important role in the
initiation and progression of ileal mucosal damage (19). It has been suggested that
GdCl3 inhibits the secretion of pro-inflammatory
cytokines from macrophages by inhibiting the activity of the NF-κB
signaling pathway, thereby inhibiting colonic mucosal inflammation
and alleviating the severity of intestinal inflammation in mice
(20). However, there has been
little research into the effects of GdCl3 on intestinal
function. GdCl3 has been reported to reduce pulmonary
apoptosis in acute lung injury, myocardial apoptosis during
myocardial reperfusion and hepatocyte apoptosis in acute liver
injury, through the inhibition of caspase-3 expression (15,18,21).
However, to the best of our knowledge, there has been limited study
into whether GdCl3 can inhibit the expression of
caspase-3 in intestinal cells and reduce the apoptosis of
intestinal tissue cells in sepsis model rats, thereby protecting
the function of the intestinal barrier.
The present study aimed to investigate the effects
of GdCl3 on the systemic and intestinal release of
cytokines (including TNF-α, IL-1β and IL-6) and the protective
effects of GdCl3 on intestinal barrier function in a
CLP-model of sepsis. Additionally, whether GdCl3 reduced
the expression of NF-κB protein in intestinal tissue and whether
GdCl3 could promote the expression of tight junction
proteins in intestinal cells to protect the function of the
intestinal barrier was investigated. The effect of GdCl3
on intestinal cell apoptosis was also explored to determine whether
apoptosis is associated with the expression of caspase-3.
Materials and methods
Animal model
A total of 144 male Sprague-Dawley (SD) rats
(weight, 200-250 g; age, 8-10 weeks) were purchased from Xinjiang
Medical University (experimental animal production license no.
XJYK0011, 2011). Animals were housed at a temperature of 20±1˚C,
relative humidity of 45%, noise below 85 decibels and ventilated 8
to 12 times/h on a 12 h light/dark cycle and had free access to
standard laboratory feed and tap water. All procedures were
approved by the Animal Protection and Use Committee of Shihezi
University (no. A20187-174) and were implemented in accordance with
the Animal Management Regulations of the Ministry of Health of
China (22).
Sepsis was induced using CLP. Under intraperitoneal
anesthesia induced by 1% pentobarbital (30 mg/kg; Merck KGaA), a
midline incision of ~2 cm was made on the anterior abdomen. The
cecum was carefully isolated, and ~2/3 of the cecum was ligated
using a 4-0 silk suture. The cecum was punctured in two different
places using 21-G needles and was squeezed to extrude fecal
material. The cecum was then replaced, and the abdomen was sutured.
Sham group animals were treated in an identical manner, but no
cecal ligation or puncture was performed. Each rat received a
subcutaneous injection of 1 ml normal saline for fluid
resuscitation after surgery.
SD rats were fasted and given free access to water
for 12 h prior to the experiment. They were randomly divided into 4
groups: Sham operation (sham group; n=36), GdCl3
pre-treatment with sham operation (sham + GdCl3 group;
n=36), CLP (CLP group; n=36) and GdCl3 pre-treatment
with CLP (CLP + GdCl3 group; n=36). The sham +
GdCl3 and CLP + GdCl3 groups received 20
mg/kg GdCl3 (no. 203289-1G; Sigma-Aldrich; Merck KGaA)
via tail vein injection at 1 and 2 days prior to the operation,
while the Sham and CLP groups were given the equivalent amount of
normal saline in an identical manner. After successful model
establishment (after 2-4 h of modeling, the success of the sepsis
model was judged by observing whether the rats had curled up,
vertical hair, reduced activity, fecal incontinence, increased
secretion from the corner of the eyes and decreased body
temperature), the animals were sacrificed after 6, 12 or 24 h. In
the western blot experiments, the protein expression level at 12 h
of the sham group was used and represented that of each time point
of the sham group and sham+GdCl3 group. Blood samples
were then collected from the abdominal aorta and intestinal tissue
(ileum near the cecum) samples were preserved for subsequent
experiments.
ELISA
ELISA kits from Elabscience Biotechnology Inc. were
used to assess the concentrations of TNF-α (cat. no. E-EL-R0019),
IL-6 (cat. no. E-EL-R0015) and IL-1β (cat. no. E-EL-R0012) in rat
serum or supernatant from intestinal tissue homogenization. The
serum samples were obtained by centrifugation of blood samples at
3,000 x g for 15 min at 4˚C. The tissue homogenate which was
obtained by grinding intestinal tissue, which was then centrifuged
at 5,000 x g for 10 min at 4˚C to obtain a tissue supernatant. The
serum concentration of diamine oxidase (DAO) was also measured
using a DAO ELISA kit (cat. no. E-EL-R0331; Elabscience
Biotechnology Inc.). All kits were used in accordance with the
manufacturer's protocol.
Western blot analysis
Total protein was extracted from each group of the
ileum about 5 cm above the cecum. The protein was extracted using
radioimmunoprecipitation assay buffer (cat. no. D1010; Beijing
Solarbio Science & Technology, Inc.) at a ratio of 10 mg tissue
to 100 µl buffer. The extracted turbid liquid was placed in an
ultra-high-speed centrifuge with at 12,000 x g for 20 min at 4˚C
and protein content of the resulting solution was determined using
the bicinchoninic acid method (cat. no. P0012, Beyotime Institute
of Biotechnology). An equal amount of protein (30 µg/lane) from
each sample was separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis. Proteins were then
transferred onto PVDF membranes. After blocking with 5% skim milk
for 2 h at room temperature, the membrane was incubated with the
primary antibodies of interest or an anti-β-actin antibody
(1:1,000; cat. no. TA-09; ZSGB-BIO; OriGene Technologies Inc.)
overnight at 4˚C. The primary antibodies were anti-occludin
(1:1,000; cat. no. ab216327; Abcam), anti-ZO-1 (1:500; cat. no.
sc-33725; Santa Cruz Biotechnology, Inc.), anti-MLCK (1:5,000; cat.
no. ab76092; Abcam), anti-NF-κB (1:1,000; cat. no. 8242; Cell
Signaling Technology Inc.) and anti-caspase-3 (1:500; cat. no.
ab13847; Abcam). After washing, the membrane was incubated with
horseradish peroxidase-conjugated secondary antibody (1:2,000; goat
anti-rabbit; cat. no. ZF-0311; ZSGB-BIO or goat anti-mouse; cat.
no. ZF-0312, OriGene Technologies, Inc.) at 37˚C for 90 min.
Proteins were detected using a chemiluminescence system and
visualized using a gel imaging system (ChemiDoc™ Touch; Bio-Rad
Laboratories, Inc.). The results were analyzed using intensity
quantification software (ImageLab 5.2; Bio-Rad Laboratories,
Inc.).
Intestinal permeability assay
An intragastric injection of 600 mg/kg (125 mg/ml) 4
kDa fluorescein isothiocyanate-dextran (FD4; Sigma-Aldrich; Merck
KGaA) was administered ~6 h prior to sacrifice. Blood samples were
centrifuged at 12,000 x g for 4 min at 4˚C, and the resulting
plasma was diluted with an equal volume of PBS; pH 7.4). An
excitation wavelength of 480 nm and emission wavelength of 520 nm
were used to analyze fluorescence with a full wavelength scanning
multifunction reader (Varioskan Flash; Thermo Fisher Scientific
Inc.). Standard curves of FITC-dextran concentrations were obtained
by serial dilution of the FD4 solution with PBS (0-12.5 mg/ml).
Intestinal epithelial apoptosis
Intestinal tissue was fixed in 4% paraformaldehyde
for 48 h at room temperature (~20˚C), embedded in paraffin, and cut
into 5-µm sections. A TUNEL apoptosis assay kit (Sigma-Aldrich;
Merck KGaA) was used according to the manufacturer's protocol.
After dewaxing, hydration and cell permeabilization using 0.2%
Triton X-100 (ZSGB-BI; cat. no. ZLI-9308), TUNEL reaction solution,
converter-peroxidase, and 3,3'-diaminobenzidine (DAB; ZSGB-BIO;
cat. no. ZLI-9018) were added dropwise in sequence. At room
temperature, 100 µl DAB substrate was added dropwise to the tissue
on the glass coverslip for color development. After dropping, the
samples were observed under the microscope, and the color
development was stopped when the appropriate amount of
yellowish-brown appeared. The stained cells appeared as if the
chromatin was condensed, marginalized and divided into blocks
(apoptotic bodies), and the nuclear membrane was cracked. After
sealing with neutral balsam, the samples were mounted under glass
coverslip with glycerol and analyzed under light microscope
(magnification, x200). Five fields of view were randomly selected
from each tissue and analyzed separately by three professional
pathology teachers.
Intestinal histopathology and damage
index
Tissues were fixed with 4% paraformaldehyde at 4˚C
for >24 h, embedded in paraffin and serially sectioned (5 µm).
Slides were stained with hematoxylin and eosin (H&E, 20% Harris
for 10 min and 0.5% eosin for 1 min) at room temperature. The
sections were examined under a DP microscope (Olympus Corporation)
at x200 magnification. Intestinal injuries were assessed using the
Chiu scoring system (23,24). Three senior pathology professors,
who were blinded to the study, randomly selected 5 visual fields in
each tissue section to score, and finally took the average
value.
Statistical analysis
Data analysis was performed using SPSS 21.0
statistical software (IBM Corp.). Normally distributed measurement
data are presented as the mean ± standard deviation and were
analyzed using a one-way ANOVA. An LSD post-hoc test was used if
equal variances were assumed and a Tamhane' T2 post-hoc test was
used if equal variances were not assumed. Non-normally distributed
data are presented as the median ± interquartile range and were
analyzed using Kruskal-Wallis non-parametric test. The Dunn's
all-pairwise test was used to analyze differences between two
groups following Kruskal-Wallis test. Each analysis was repeated
three times. Differences with P<0.05 were considered
statistically significant.
Results
GdCl3 reduces serum and
intestinal inflammatory markers in CLP rats
To verify the effect of GdCl3 on systemic
inflammation and the intestinal inflammatory response in sepsis
model rats, an ELISA was used to determine TNF-α, IL-6 and IL-1β
levels in rat serum and intestinal tissues. The results indicated
that serum levels of TNF-α, IL-6 and IL-1β were reduced in the
CLP+GdCl3 group compared with those in the CLP group at
both 6 and 12 h (P<0.05, Fig.
1A, C and E), but that there was no difference
between these groups at 24 h (Fig.
1A, C and E). However, TNF-α, IL-6 and IL-1β levels
in intestinal tissues were significantly reduced in the
CLP+GdCl3 group compared with those in the CLP group at
all time points (Fig. 1B, D and F).
 | Figure 1Effect of GdCl3 treatment
on systemic and intestinal inflammatory responses following
CLP-induced sepsis. Levels of (A) TNF-α in sera, (B) TNF-α in
intestinal tissue, (C) IL-1β in sera, (D) IL-1β in intestinal
tissue (E) IL-6 in serum and (F) IL-6 in intestinal tissue of
CLP-treated rats with or without GdCl3 pretreatment.
Data were measured at 6, 12 and 24 h after CLP operation and are
presented as the mean ± SD (n=6). *P<0.05 vs. sham
group at the same time point; #P<0.05 vs. CLP group
at the same time point; ns, not significant vs. sham group at the
same time point; NS, not significant vs. CLP group at 24 h.
GdCl3, gadolinium chloride; CLP, cecal ligation and
puncture; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β;
IL-6, interleukin-6. |
GdCl3 reduces intestinal
permeability and intestinal injury in CLP rats
ELISA was used to determine levels of DAO, and
therefore intestinal barrier integrity, in rat serum. The results
indicated that the level of DAO was significantly higher in the CLP
group compared with the sham group at 6, 12 and 24 h (P<0.05;
Fig. 2A). However, the level of DAO
in the CLP + GdCl3 group was lower than that in the CLP
group at 6, 12 and 24 h (P<0.05; Fig. 2A). To evaluate the degree of
intestinal injury more directly, H&E staining of intestinal
tissues was performed and the degree of intestinal injury scored
according to Chiu's criteria. The results revealed that at 6, 12
and 24 h, the CLP + GdCl3 group exhibited less
intestinal tissue damage than the CLP group (Fig. 3A), and the intestinal injury score
was lower than that in the CLP group (P<0.05; Fig. 3B). To evaluate the permeability of
the intestinal tract, serum levels of FD4 were assessed. The
experimental results indicated that the intestinal permeability of
the CLP+GdCl3 group was lower compared with the CLP
group at each time point (P<0.05; Fig. 2B).
GdCl3 promotes the
expression of tight junction proteins occludin and ZO-1 and reduces
MLCK expression in CLP rats
The intestinal occludin and ZO-1 proteins reflect
the integrity of the intestinal mechanical barrier. MLCK regulates
the permeability of intestinal epithelial cells and the expression
of occludin and ZO-1(25). The
results indicated that the expression of occludin and ZO-1 proteins
were significantly reduced in the CLP group compared with that in
the sham group (P<0.05; Fig.
4A-C). However, the expression levels of occludin and ZO-1 were
increased in the CLP + GdCl3 group compared with the CLP
group at 6, 12 and 24 h (P<0.05, Fig. 4A-C). The expression of MLCK was
reduced in the CLP + GdCl3 group compared with the CLP
group (P<0.05, Fig. 4A and
D).
GdCl3 reduces expression of
NF-κB in the intestines of CLP rats
To verify whether GdCl3 regulates
intestinal inflammation in septic rats via the NF-κB pathway,
western blot analysis was used to determine the expression of NF-κB
p65 protein in rat intestines. The results demonstrated that the
expression of NF-κB was significantly increased in the CLP group
compared with the sham group, but was reduced in the CLP +
GdCl3 group compared with the CLP group at 6, 12 and 24
h (P<0.05, Fig. 5A and B).
GdCl3 alleviates apoptosis
of intestinal tissue cells in CLP rats
Intestinal tissue cell death is also an important
indicator of the integrity of the intestinal mechanical barrier
(26). Western blot analysis was
used to determine the expression of caspase-3 in rat intestinal
tissue. The results indicated that the expression of caspase-3
(P<0.05; Fig. 6A and B) were significantly increased in the CLP
group compared with the sham group. However, compared with the CLP
group, the expression of caspase-3 (P<0.05; Fig. 6A and B) were lower in the CLP + GdCl3
group at 6, 12 and 24 h. TUNEL assays were used to determine the
apoptosis level of intestinal cells and apoptotic cells were
stained brown and analyzed under a light microscope. A very small
amount of brown stained cells was observed in the sham groups and
sham GdCl3+ groups (Fig.
7A). In the CLP group, the number of apoptotic cells increased
significantly, and the number of expressions gradually increased
over time (Fig. 7A). Pretreatment
of septic rats with GdCl3 could reduce the number of
intestinal apoptotic epithelial cells of three different time
points (Fig. 7A). The results
indicated that the rate of apoptosis of intestinal cells
(P<0.05; Fig. 7B) were
significantly increased in the CLP group compared with the sham
group. However, compared with the CLP group, the apoptotic rate of
intestinal cells (P<0.05; Fig.
7B) were lower in the CLP + GdCl3 group at 6, 12 and
24 h.
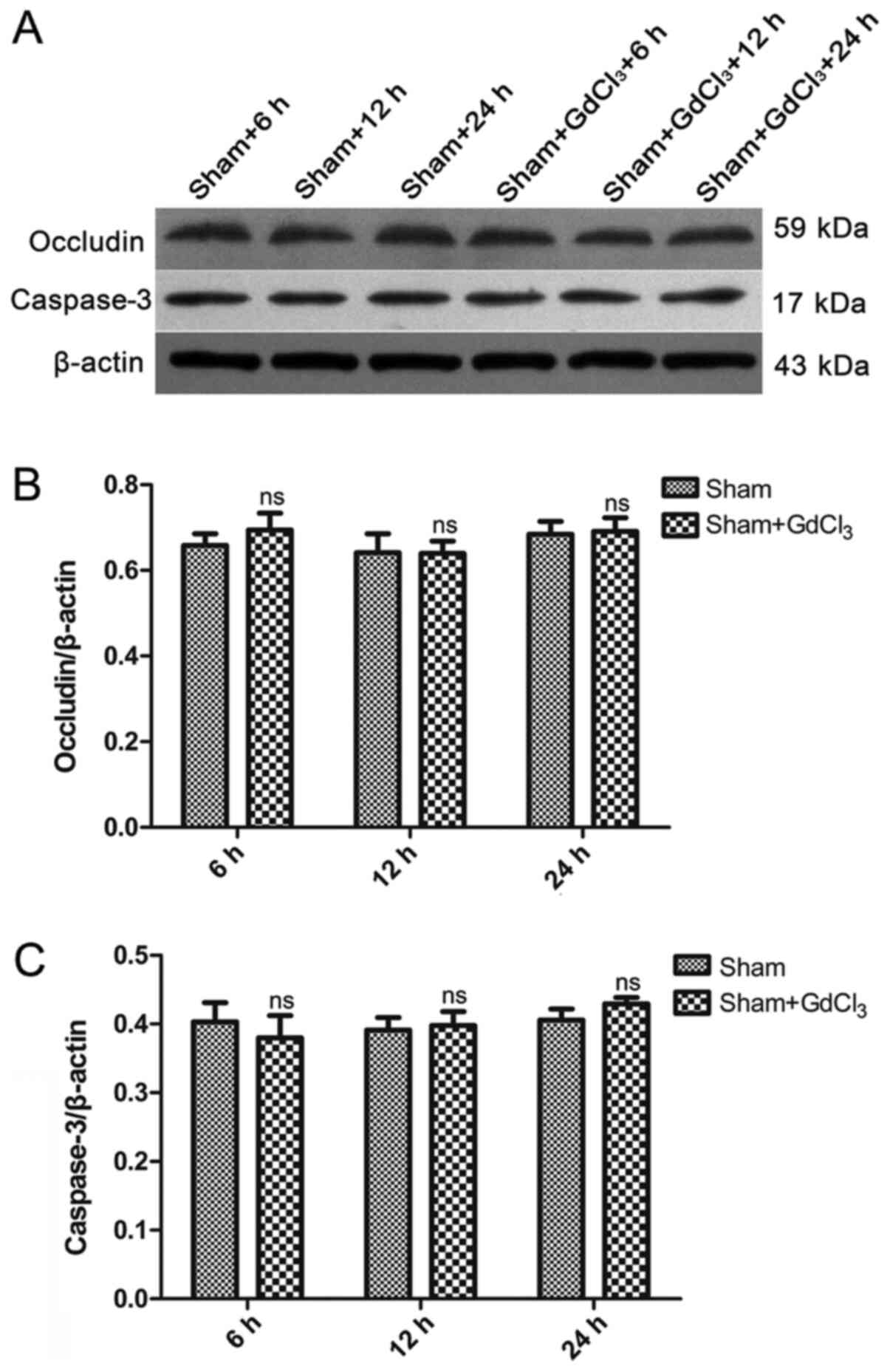
GdCl3 has no effect on
inflammation, intestinal mechanical barrier, or intestinal injury
in non-CLP rats
An ELISA was used to determine the levels of serum
and intestinal pro-inflammatory factors in rats. Levels of TNF-α,
IL-6 and IL-1β in the serum and intestines of the sham +
GdCl3 group were similar to those in the sham group at
6, 12 and 24 h (Fig. 1). Western
blot analysis was used to detect the expression of occludin and
caspase-3 protein in the intestines. The results demonstrated that
there was no difference in the expression of occludin or caspase-3
between the sham and the sham + GdCl3 at any of the
three time points (Fig. 8).
Intestinal tissue apoptosis levels were determined using a TUNEL
assay, and the results indicated no significant difference between
the sham and the sham + GdCl3 groups (Fig. 7). The results of a DAO ELISA, an
indicator of intestinal damage and use of FD4, and an indicator of
intestinal permeability, indicated that there were no differences
in intestinal damage or permeability between the sham and the sham
+ GdCl3 group at any of the three time-points (Fig. 2). H&E staining was used to
verify that the sham + GdCl3 treatment did not cause
changes in the intestinal tissues of rats compared those in the
with sham group (Fig. 3A). Based on
Chiu's scoring standard for the degree of intestinal injury, the
difference between the sham and the sham + GdCl3 group
at any of the three time-points was not statistically significant
(Fig. 3B).
Discussion
The release of a large number of pro-inflammatory
cytokines at an early stage of the inflammatory response is
considered to be an important pathological mechanism for the
development of sepsis, and the resulting intestinal tissue
inflammation can cause destruction of intestinal barrier function
(27). Inflammatory cytokines,
including TNF-α and IL-1β, have been demonstrated to serve a role
in this dysfunction (28).
Intestinal barrier function damage in sepsis leads to an increase
in intestinal permeability (29).
This allows multiple antigens, bacteria and other toxic metabolites
in the intestinal lumen to invade the intestinal tissue, causing
further damage to the intestinal tract, aggravating the
inflammatory response of the intestinal tissue and destroying the
integrity of the intestinal epithelial barrier. This may progress
to invasion of the lymphatic tissue and circulating blood,
resulting in systemic inflammation (30). This creates a cycle that causes the
eventual outcome of increased distal organ damage and risk of death
(31,32). It is therefore hypothesized that the
inhibition of intestinal inflammation may be an effective method
for preventing intestinal barrier dysfunction in sepsis.
GdCl3 acts to inhibit the phagocytosis
and secretion of Kupffer cells, thereby alleviating the
inflammatory response (33).
Studies have also demonstrated that endotoxemia and excessive
activation of Kupffer cells in numerous severe disease states
(34). Inhibition of Kupffer cell
function can ameliorate systemic inflammatory response syndrome
(SIRS), while activation of Kupffer function can aggravate SIRS,
thereby increasing the likelihood of multiple organ damage,
including intestinal damage (35).
Studies have confirmed that GdCl3 pretreatment can
reduce the apoptosis of lung parenchymal cells and lung
inflammation, thereby reducing lung injury in LPS-induced sepsis
(18). However, the effects of
GdCl3 pretreatment on the intestinal tract have rarely
been reported.
The results of the present study indicated that in
healthy rats, GdCl3 had no effect on the inflammatory
response, intestinal tight junction protein expression or
intestinal cell apoptosis. In contrast, in the CLP-induced septic
rats, expression of intestinal pro-inflammatory cytokines was
reduced at 6 and 12 h by treatment with GdCl3. At 24 h,
the expression of TNF-α, IL-6 and IL-1β in the circulating blood of
rats was not significantly different in CLP + GdCl3 rats compared
to CLP rats, but levels in the intestinal tract were reduced in CLP
+ GdCl3 rats compared with the CLP group at 24 h. This finding
indicated that localized inflammation is likely to have progressed
into a systemic inflammatory response as the duration of sepsis was
prolonged, at which point it could not be suppressed by the
inhibition of Kupffer cells alone. These findings have some
similarities with previous research (14). This study suggests that inactivation
of Kupffer cells by GdCl3 had no effect on inflammation
and systemic inflammatory response following CLP-induced sepsis.
However, there were some differences compared with the previous
research. The previous experimental research was based on the
experimental data obtained from blood sample of mice collected 8 h
after the successful establishment of the CLP model, but we
obtained the data from blood sample of rats collected at the 24 h
time point (14). These differences
may be associated with the rat species used. In sepsis, a large
number of inflammatory cytokines, including TNF-α and IL-1β, can
cause systemic and intestinal inflammatory reactions and activate
NF-κB signaling pathways in intestinal tissues (36). Following the activation of NF-κB in
the intestinal mucosa, and NF-κB can bind to inflammatory cytokine
gene promoter sequences in immune cells to promote their expression
(10). Western blot analysis was
used to determine the expression of NF-κB p65. The results
indicated that, at 6, 12 and 24 h, GdCl3 treatment could
inhibit the expression of NF-κB in intestinal tissues of septic
rats. Taken together, the results of ELISAs and western blot
analysis indicated that GdCl3 could alleviate intestinal
tissue inflammation in sepsis model rats and that this may be due
to inhibition of NF-κB pathway activation.
FD4 is an indicator that is used to evaluate the
function of the intestinal epithelial barrier. It cannot be
absorbed in bowel lumen or degraded in the blood (4). In healthy animals, it is rarely able
to enter the circulation through gaps between intestinal epithelial
cells (37). Studies have confirmed
that DAO in plasma is mainly derived from intestinal mucosal
epithelial cells (38). DAO is
released into the blood after intestinal mucosal cells are damaged
or necrotic, which leads to an increase of DAO concentration in the
circulation. DAO activity in peripheral blood is relatively stable
(39). Accordingly, the degree of
damage and integrity of the intestinal mucosal mechanical barrier
can be indirectly determined by assessing the changes in DAO in
peripheral blood (40). The results
of the present study indicated that the levels of DAO and FD4 in
CLP + GdCl3 rats were reduced at each time point (6, 12,
and 24 h) when compared with CLP model rats. This indicated an
improvement in the intestinal barrier function of sepsis model rats
treated with GdCl3. Similar results were obtained using
H&E staining of intestinal tissue and Chiu's score to evaluate
the severity of intestinal injury.
The intestinal barrier is a selective barrier. The
material in the intestinal lumen has two potential pathways through
the intestinal mucosa: The transcellular pathway and the
paracellular pathway (41,42). The intestinal paracellular pathway
is largely regulated by tight junction proteins (43). Tight junctions are composed of
occludin, claudins, ZO proteins and linked mature molecules. Among
them, occludin and ZO-1 proteins are the most important. Studies
have shown that sepsis can reduce the expression of ZO-1 and
occludin in the intestinal epithelium (44). MLCK is a
Ca2+/calmodulin-dependent protein kinase that is part of
an important signaling pathway in regulation of the function of
tight junction proteins (42).
Experiments have demonstrated that MLCK can also regulate the
structure of tight junction proteins and affect the permeability of
the intestinal mucosa by regulating the expression of occludin,
claudins and Zos (42). The
expression of MLCK is associated with the activation of NF-κB.
After activation of NF-κB in the intestinal mucosa, it can bind to
the MLCK gene promoter sequence in intestinal epithelial cells to
promote the expression of MLCK (45). Previous studies have also indicated
that inflammatory cytokines can disrupt tight junctions between
epithelial cells by activating the NF-κB and MLCK pathways
(46,47). The results of the present study
suggested that the expression of ZO-1 and occludin was
significantly upregulated in the intestinal tissues of septic rats
treated with GdCl3, while expression of MLCK was
significantly downregulated. Taken together, with the result that
expression of NF-κB in intestinal tissue is reduced by
GdCl3, the results indicated that GdCl3
reduced the expression of MLCK through inhibition of the activation
of NF-kB, which increased the expression of occludin and ZO-1,
which served a role in protecting intestinal barrier function.
Intestinal mucosal barrier dysfunction is thought to
be associated with excessive intestinal epithelial cell apoptosis,
and apoptosis serves an important role in maintaining intestinal
mucosal epithelial homeostasis (48). Apoptosis is a process of active cell
death under the control of genes, which plays an important role in
regulating the development of the body, maintaining the stability
of the internal environment and ensuring normal physiological
functions (49). If apoptosis is
abnormal, that is, and the normal order of apoptosis is disrupted,
it can cause a series of diseases. In recent years, it has been
demonstrated that intestinal cell apoptosis serves an important
role in diseases with impaired intestinal mucosal barrier (50). If cell apoptosis is dysregulated, it
can cause intestinal mucosal atrophy, which leads to intestinal
dysfunction (51). In animal models
of sepsis, intestinal epithelial cell apoptosis is significantly
elevated, and inhibition of this intestinal epithelial cell
apoptosis can improve the survival rate of septic mice (52). Studies have demonstrated that the
key to a series of cellular apoptosis-related reactions is the
activation of caspase protease (53). Caspase-3 is the key to regulate
apoptosis and serves a decisive role in the final stage of
apoptosis, if caspase-3, which is also known as the ̔death
protease’ is activated, apoptosis is inevitable (54,55).
In the present study, apoptosis of intestinal cells was evaluated
using a TUNEL assay and western blot analysis of caspase-3. The
results indicated that the apoptotic rate of intestinal cells and
expression of caspase-3 was decreased in CLP + GdCl3
rats compared with CLP rats suggesting that GdCl3
treatment reduces the apoptosis of intestinal tissue cells in
septic rats, and that this effect may be associated with the
inhibition of the caspase-3 expression.
In conclusion, the results of the present study
suggested that GdCl3 may alleviate the systemic and
intestinal inflammatory response. However, there were no
differences in cytokine or chemokine levels between
GdCl3-treated and GdCl3-untreated septic rats
at 24 h, suggesting that levels of pro-inflammatory factors in the
circulation may not reflect the cytokine secretion levels of
Kupffer cells. Studies have indicated that a protective effect of
GdCl3 on intestinal inflammatory injury may be achieved
by inhibiting the production of pro-inflammatory cytokines in
Kupffer cells or by inhibiting intestinal macrophages (20). The results of the present study
indicated that GdCl3 injection into the tail vein can
ameliorate intestinal inflammation in rats. However, it is
necessary to further clarify whether GdCl3 functions by
downregulating the release of pro-inflammatory cytokines from
intestinal mucosal macrophages or from liver Kupffer cells. The
results of the present study demonstrated that, the expression of
tight junction proteins in the intestines was increased in CLP +
GdCl3 rats compared with CLP rats, and the apoptosis of
intestinal cells was also decreased, thereby reducing the degree of
intestinal damage. It is therefore hypothesized that the protective
effect of GdCl3 on intestinal barrier function in sepsis
model rats may be due to a reduced intestinal inflammatory response
and reduced expression of NF-κB. This may induce reduced expression
of MLCK, which increases the expression of occludin and ZO-1 in the
intestine. It is also hypothesized that the protective effect of
GdCl3 on intestinal barrier function in septic rats may
be associated with the inhibition of caspase-3 overexpression.
Acknowledgements
Not applicable.
Funding
Funding: The present study were supported by grants from the
National Natural Science Foundation Project (grant no. U1803127),
Key Science and Technology Research Projects in Key Areas of the
Corps 2018 (grant no. 2018AB019) and Xinjiang Uygur Autonomous
Region Graduate Student Innovation Project (grant no.
XJGR12016042).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YHZ performed all animal experiments and revised the
manuscript. SWZ and YHZ were major contributors in writing the
manuscript and performed the statistical analysis. WJZ, JTD and YHZ
jointly designed the study. SWZ, HJZ, HYQ, YQZ, XLL, SL, HZ, JDW,
ZYZ, HZW, MS and JL participated in and completed animal
experiments. JZ and FW participated in and guided the statistical
analysis. SWZ, FW and YHZ confirmed the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Shihezi University (Shihezi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Angus DC, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang C, Chi C, Guo L, Wang X, Guo L, Sun
J, Sun B, Liu S, Chang X and Li E: Heparin therapy reduces 28-day
mortality in adult severe sepsis patients: A systematic review and
meta-analysis. Crit Care. 18(563)2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Andersen K, Kesper MS, Marschner JA,
Konrad L, Ryu M, Kumar Vr S, Kulkarni OP, Mulay SR, Romoli S,
Demleitner J, et al: Intestinal dysbiosis, barrier dysfunction, and
bacterial translocation account for CKD-related systemic
inflammation. J Am Soc Nephrol. 28:76–83. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li Z, Zhang X, Zhou H, Liu W and Li J:
Exogenous s-nitrosoglutathione attenuates inflammatory response and
intestinal epithelial barrier injury in endotoxemic rats. J Trauma
Acute Care Surg. 80:977–984. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xiong R: Effect of ecological immune
enteral nutrition intervention on intestinal barrier function and
systemic inflammatory response in rat models with severe
pancreatitis. J Hainan Med Univ. (22)2016.
|
|
6
|
Schulte W, Bernhagen J and Bucala R:
Cytokines in sepsis: Potent immunoregulators and potential
therapeutic targets-an updated view. Mediators Inflamm.
2013(165974)2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Singh G, Singh G, Bhatti R, Gupta M, Kumar
A, Sharma A and Singh Ishar MP: Indolyl-isoxazolidines attenuates
LPS-stimulated pro-inflammatory cytokines and increases survival in
a mouse model of sepsis: Identification of potent lead. Eur J Med
Chem. 153:56–64. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lai JL, Liu YH, Liu C, Qi MP, Liu RN, Zhu
XF, Zhou QG, Chen YY, Guo AZ and Hu CM: Indirubin inhibits
LPS-induced inflammation via TLR4 abrogation mediated by the NF-kB
and MAPK signaling pathways. Inflammation. 40:1–12. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chee ME, Majumder K and Mine Y:
Intervention of dietary dipeptide Gamma-l-Glutamyl-l-Valine (γ-EV)
ameliorates inflammatory response in a mouse model of LPS-induced
sepsis. J Agric Food Chem. 65:5953–5960. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yu M, Shao D, Liu J, Zhu J, Zhang Z and Xu
J: Effects of ketamine on levels of cytokines, NF-κB and TLRs in
rat intestine during CLP-induced sepsis. Int Immunopharmacol.
7:1076–1082. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fang M, Zhong WH, Song WL, Deng YY, Yang
DM, Xiong B, Zeng HK and Wang HD: Ulinastatin ameliorates pulmonary
capillary endothelial permeability induced by sepsis through
protection of tight junctions via inhibition of TNF-α and related
pathways. Front Pharmacol. 9(823)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Marchiando AM, Shen L, Graham WV, Weber
CR, Schwarz BT, Austin JR II, Raleigh DR, Guan Y, Watson AJ,
Montrose MH and Turner JR: Caveolin-1-dependent occludin
endocytosis is required for TNF-induced tight junction regulation
in vivo. J Cell Biol. 189:111–126. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhao H, Zhao M, Wang Y, Li F and Zhang Z:
Glycyrrhizic acid attenuates sepsis-induced acute kidney injury by
inhibiting NF-κB signaling pathway. Evid Based Complement Alternat
Med. 2016(8219287)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gaddam RR, Fraser R, Badiei A, Chambers S,
Cogger VC, Le Couteur DG and Bhatia M: Differential effects of
kupffer cell inactivation on inflammation and the liver sieve
following caecal-ligation and puncture induced sepsis in mice.
Shock. 47:480–490. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhu R, Guo W, Fang H, Cao S, Yan B, Chen
S, Zhang K and Zhang S: Kupffer cell depletion by gadolinium
chloride aggravates liver injury after brain death in rats. Mol Med
Rep. 17:6357–6362. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Selvaraj V, Nepal N, Rogers S, Manne ND,
Arvapalli R, Rice KM, Asano S, Fankhanel E, Ma JJ, Shokuhfar T, et
al: Inhibition of MAP kinase/NF-kB mediated signaling and
attenuation of lipopolysaccharide induced severe sepsis by cerium
oxide nanoparticles. Biomaterials. 59:160–171. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tae-Hoon K, Sang-Ho L and Sun-Mee L: Role
of Kupffer cells in pathogenesis of sepsis-induced drug
metabolizing dysfunction. FEBS J. 278:2307–2317. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kishta OA, Goldberg P and Husain SN:
Gadolinium chloride attenuates sepsis-induced pulmonary apoptosis
and acute lung injury. ISRN Inflamm. 2012(393481)2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gong JP, Wu CX, Liu CA, Li SW, Shi YJ,
Yang K, Li Y and Li XH: Intestinal damage mediated by Kupffer cells
in rats with endotoxemia. World J Gastroenterol. 8:923–927.
2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chao D, Peng W, Yanbo Y, Feixue C, Jun L
and Yanqing L: Gadolinium chloride improves the course of TNBS and
DSS-induced colitis through protecting against colonic mucosal
inflammation. Sci Rep. 4(6096)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen M, Zheng YY, Song YT, Xue JY, Liang
ZY, Yan XX and Luo DL: Pretreatment with low-dose gadolinium
chloride attenuates myocardial ischemia/reperfusion injury in rats.
Acta Pharmacol Sin. 37:453–462. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Guo P, Zhang SW, Zhang J, Dong JT, Wu JD,
Tang ST, Yang JT, Zhang WJ and Wu F: Effects of imipenem combined
with low-dose cyclophosphamide on the intestinal barrier in septic
rats. Exp Ther Med. 16:1919–1927. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen J, Zhou W, Zhou Z, Yuan T, Li B and
Zheng Y: Protective effect of salvianolic acid B against intestinal
ischemia reperfusion-induced injury in a rat model. Tropical J
Pharmaceutical Res: Nov 15, 2017 (Epub ahead of print). doi:
10.4314/tjpr.v16i10.17.
|
|
24
|
Zi-Qing H, Gan XL, Huang PJ, Wei J, Shen N
and Gao WL: Influence of ketotifen, cromolyn sodium, and compound
48/80 on the survival rates after intestinal ischemia reperfusion
injury in rats. BMC Gastroenterol. 8(42)2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yu D, Marchiando AM, Weber CR, Raleigh DR,
Wang Y, Shen L and Turner JR: MLCK-dependent exchange and actin
binding region-dependent anchoring of ZO-1 regulate tight junction
barrier function. Proc Natl Acad Sci USA. 107:8237–8241.
2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang W, Gan D, Jian J, Huang C, Luo F,
Wan S, Jiang M, Wan Y, Wang A, Li B and Zhu X: Protective effect of
ursolic acid on the intestinal mucosal barrier in a rat model of
liver fibrosis. Front Physiol. 10(956)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zabrodskii PF, Gromov MS and Maslyakov VV:
The effect of anabasine on mortality and concentration of
proinflammatory cytokines in blood of mice at early stage of
sepsis. Eksp Klin Farmakol. 77:20–22. 2014.PubMed/NCBI(In Russian).
|
|
28
|
Rana AS, Dongmei Y, Karol D and Ma TY:
Mechanism of IL-1beta-induced increase in intestinal epithelial
tight junction permeability. J Immunol. 180:5653–5661.
2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang J, Zhang S, Wu J, Zhang J, Dong J,
Guo P, Tang S, Zhang W and Wu F: Imipenem and normal saline with
cyclophosphamide have positive effects on the intestinal barrier in
rats with sepsis. Biomed Pap Med Fac Univ Palacky Olomouc Czech
Repub. 162:90–98. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tang SY, Zhang SW, Zhang J, Dong JT, Wu
JD, Guo P, Yang JT, Zhang WJ and Wu F: Effect of early fluid
resuscitation combined with low dose cyclophosphamide on intestinal
barrier function in severe sepsis rats. Drug Deliv Transl Res.
8:1254–1264. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang H, Song Z, Jin H, Cui Y, Hou M and
Gao Y: Protective effect of rhBNP on intestinal injury in the
canine models of sepsis. Int Immunopharmacol. 19:262–266.
2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yoseph BP, Klingensmith NJ, Liang Z, Breed
ER, Burd EM, Mittal R, Dominguez JA, Petrie B, Ford ML and
Coopersmith CM: Mechanisms of intestinal barrier dysfunction in
sepsis. Shock. 46:52–59. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rai RM, Zhang JX, Clemens MG and Diehl AM:
Gadolinium chloride alters the acinar distribution of phagocytosis
and balance between pro- and anti-inflammatory cytokines. Shock.
6:243–247. 1996.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kim TH and Lee SM: Role of Kupffer cells
in vasoregulatory gene expression during endotoxemia. Biomolecules
Ther. 16:306–311. 2008.
|
|
35
|
Adams DH, Eksteen B and Curbishley SM:
Immunology of the gut and liver: A love/hate relationship. Gut.
57:838–848. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen S, He Y, Hu Z, Lu S, Yin X, Ma X, Lv
C and Jin G: Heparanase mediates intestinal inflammation and injury
in a mouse model of sepsis. J Histochem Cytochem. 65:241–249.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fu J, Li G, Wu X and Zang BJ: Sodium
butyrate ameliorates intestinal injury and improves survival in a
rat model of cecal ligation and puncture-induced sepsis.
Inflammation. 42:1276–1286. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jung E, Perrone EE, Liang Z, Breed ER,
Dominguez JA, Clark AT, Fox AC, Dunne WM, Burd EM, Farris AB, et
al: Cecal ligation and puncture followed by MRSA pneumonia
increases mortality in mice and blunts production of local and
systemic cytokines. Shock. 37:85–94. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xin X, Dai W, Wu J, Fang L, Zhao M, Zhang
P and Chen M: Mechanism of intestinal mucosal barrier dysfunction
in a rat model of chronic obstructive pulmonary disease: An
observational study. Exp Ther Med. 12:1331–1336. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhu S, Feng S, Liang S and Zhao W:
Protective effect and mechanism of erythropoietin on intestinal
function in septic rats, 2016.
|
|
41
|
Rosenthal R, Günzel D, Finger C, Krug SM,
Richter JF, Schulzke JD, Fromm M and Amasheh S: The effect of
chitosan on transcellular and paracellular mechanisms in the
intestinal epithelial barrier. Biomaterials. 33:2791–2800.
2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lorentz CA, Liang Z, Meng M, Chen CW,
Yoseph BP, Breed ER, Mittal R, Klingensmith NJ, Farris AB, Burd EM,
et al: Myosin light chain kinase knockout improves gut barrier
function and confers a survival advantage in polymicrobial sepsis.
Mol Med. 23:155–165. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Anderson JM and Van Itallie CM: Physiology
and function of the tight junction. Cold Spring Harb Perspect Biol.
1(a002584)2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Fredenburgh LE, Velandia MM, Jun M, Olszak
T, Cernadas M, Englert JA, Chung SW, Liu X, Begay C, Padera RF, et
al: Cyclooxygenase-2 deficiency leads to intestinal barrier
dysfunction and increased mortality during polymicrobial sepsis. J
Immunol. 187:5255–5267. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gao YL, Wang YN, Guo YJ, Sun Y, Wang YR,
Zhou J, Zhao JM, Wu HG and Shi Y: Effect of herb-partitioned
moxibustion in improving tight junctions of intestinal epithelium
in Crohn disease mediated by TNF-α-NF-κB-MLCK pathway. J
Acupuncture Tuina Sci. 19:19–29. 2021.
|
|
46
|
Al-Sadi R, Guo S, Ye D, Rawat M and Ma T:
TNF-α modulation of intestinal tight junction permeability is
mediated by NIK/IKK-α axis activation of the canonical NF-κB
pathway. Am J Pathol. 186:1151–1165. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Feng L, Li SQ, Jiang WD, Liu Y, Jiang J,
Wu P, Zhao J, Kuang SY, Tang L, Tang WN, et al: Deficiency of
dietary niacin impaired intestinal mucosal immune function via
regulating intestinal NF-κB, Nrf2 and MLCK signaling pathways in
young grass carp (Ctenopharyngodon idella). Fish Shellfish
Immunol. 49:177–193. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhu W, Lu Q, Chen H, Feng J, Wan L and
Zhou DK: Protective effect of sodium tanshinone IIA sulfonate on
injury of small intestine in rats with sepsis and its mechanism.
Chin J Integr Med. 18:496–501. 2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Liu H, Liu Z, Zhao S, Sun C and Yang M:
Effect of BML111 on the intestinal mucosal barrier in sepsis and
its mechanism of action. Mol Med Rep. 12:3101–3106. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lin Z, Cai F, Lin N, Ye J, Zheng Q and
Ding G: Effects of glutamine on oxidative stress and nuclear
factor-κB expression in the livers of rats with nonalcoholic fatty
liver disease. Exp Ther Med. 7:365–370. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Dominguez JA, Xie Y, Dunne WM, Yoseph BP,
Burd EM, Coopersmith CM and Davidson NO: Intestine-specific Mttp
deletion decreases mortality and prevents sepsis-induced intestinal
injury in a murine model of Pseudomonas aeruginosa pneumonia. PLoS
One. 7(e49159)2012.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Yin HY, Wei JR, Zhang R, Ye XL, Zhu YF and
Li WJ: Effect of glutamine on caspase-3 mRNA and protein expression
in the myocardium of rats with sepsis. Am J Med Sci. 348:315–318.
2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Rosado JA, Lopez JJ, Gomez-Arteta E,
Redondo PC, Salido GM and Pariente JA: Early caspase-3 activation
independent of apoptosis is required for cellular function. J Cell
Physiol. 209:142–152. 2010.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Fiandalo MV and Kyprianou N: Caspase
control: Protagonists of cancer cell apoptosis. Exp Oncol.
34:165–175. 2012.PubMed/NCBI
|
|
55
|
Juraver-Geslin HA and Durand BC: Early
development of the neural plate: New roles for apoptosis and for
one of its main effectors caspase-3. Genesis. 53:203–224.
2015.PubMed/NCBI View Article : Google Scholar
|