Introduction
Liver cancer, which includes primary and secondary
liver cancer, is one of the most common malignancies (1-3).
Over the past few decades, the incidence of liver cancer has
significantly increased due to a lack of effective therapeutic
strategies, and the incidence and mortality of liver cancer are the
fifth and third highest, respectively, of all cancers (4). Hepatocellular carcinoma (HCC), a type
of primary liver cancer, has a mortality rate of 51%, making it one
of the deadliest malignancies worldwide. Chemotherapy is currently
the most common treatment for HCC, apart from surgical resection.
However, most chemotherapy drugs have high toxicities and poor
specificities to cancer cells, leading to immune system damage
(5). Therefore, novel HCC treatment
strategies are urgently required. Nanoparticle (NP)-based targeted
drug delivery has been rapidly developed as a novel therapeutic
strategy for diagnosing and treating tumors. Such NP-based systems
selectively deliver chemotherapy drugs to tumor sites, increase the
concentration of drugs at tumor sites and prolong drug half-lives
(6). They also mitigate side
effects by reducing dosages of chemotherapy drugs to achieve the
same therapeutic goals. Numerous receptors mediate active
liver-targeted drug delivery for the treatment of liver cancer,
including the asialoglycoprotein receptor (7), glycyrrhetinic acid (GA) receptor
(GA-R) (8-10),
hyaluronan (HA) receptor (11-13)
and folate receptor (14,15).
GA is a pentacyclic triterpenoid obtained from the
roots of Glycyrrhiza glabra L. (16). Numerous studies have indicated that
GA specifically combines with the GA-R widely expressed on the
surface of liver parenchymal cells. Furthermore, liver tumor
tissues possess 1.5-5-fold more GA-R than adjacent normal liver
tissues (17). Thus, a
GA-functionalized NP system possesses strong liver cell targeting
and liver distribution characteristics. HA is a natural hydrophilic
acid mucopolysaccharide that consists of repeating disaccharides of
D-glucuronic acid and N-acetyl-D-glucosamine and may specifically
combine with CD44, which is highly expressed on certain cells,
including tumor cells, dendritic cells and certain epithelial cells
(18). In addition, HA has
excellent biological properties, such as biological compatibility,
biodegradability and low toxicity (19). Therefore, HA is an ideal carrier
polymer in the construction of NPs for the targeted delivery of
drugs. Numerous studies have indicated that GA-functionalized
hyaluronic acid NPs selectively target liver tumor tissue and liver
cancer cells and reduce adverse reactions when loaded with
antitumor drugs, such as doxorubicin (DOX), paclitaxel (PTX) and
5-fluorouracil (20-22).
Docetaxel (DTX), a member of the taxane family, is a
semi-synthetic analogue of PTX and a microtubule depolymerization
inhibitor (23). It inhibits tumor
cell proliferation and exerts its antitumor effects by preventing
mitosis. DTX is a front-line, standard-of-care chemotherapeutic
drug for the treatment of several cancer types, including liver,
ovarian, breast (24), prostate,
bladder, gastric and non-small-cell lung cancers (25,26).
Furthermore, previous studies have suggested that DTX reduces
hepatocellular tumor size in nude mice and inhibits the
proliferation of HepG2 cells. However, DTX has various
disadvantages, such as low water solubility, poor stability,
hypersensitivity, hemolysis and toxic side effects (27). These disadvantages limit its
application to a certain extent.
The purpose of the present study was to assemble a
DTX-loaded carrier based on GA-modified HA (GA-HA) NPs
(DTX/GA-HA-NPs), examine the physicochemical properties of the NP
system and assess its ability to deliver DTX to HepG2 cells, a
liver cancer cell line commonly used in liver cancer research. The
present study lays a foundation for novel, effective HCC treatment
strategies.
Materials and methods
Preparation of DTX/GA-HA NPs
GA-HA was prepared as described in the previous
literature (28). In brief, GA
(1.41 g) and
4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride
(DMT-MM) 0.967 g were stirred in 30 ml methanol overnight at 25˚C
and thin layer chromatography was used to monitor the formation of
the product GA-ES. After rotary evaporation, 30 ml ethylenediamine
was added and the mixture was stirred overnight at 25˚C to obtain
the product GA-NH2. HA (60 mg) was dissolved in 10 ml
distilled water, and GA-NH2 (28.43 mg) and an
appropriate amount of absolute ethanol (~0.3-0.4 ml) were added to
dissolve the components. Subsequently, DMT-MM condensing agent was
added and the mixture was filled into a pre-treated dialysis bag
for dialysis. After freeze-drying, a theoretical degree of
substitution of 10% GA-HA NPs was obtained in the freeze-dried
product.
DTX/GA-HA-NPs were prepared by an ultrasonic
dispersion method in three steps. In brief, GA-HA-NPs (30 mg) were
dissolved in 5 ml formamide. DTX (6 mg) (Shanghai Aladdin Bio-Chem
Technology Co. Ltd.) was dissolved in 200 µl ethyl alcohol and
added dropwise to the GA-HA solution. Subsequently, the mixtures
were stirred at room temperature (25˚C) overnight and dialyzed
against distilled water for 24 h. Finally, the DTX/GA-HA-NPs were
sonicated with a probe-type ultrasonicator (working power was 5%;
active every 2 sec for a 3-sec duration) in an ice bath for 0.5 h
and then lyophilized with a lyophilizer.
Preparation of FITC-labeled
GA-HA-NPs
The synthesis of FITC-labeled GA-HA-NPs
(FITC-GA-HA-NPs) was based on the reaction between the
isothiocyanate group of FITC and the amino group of HA.
FITC-GA-HA-NPs were prepared by a dialysis method. The FITC-GA-HA
copolymers were synthesized via two steps. First, GA-HA-NPs (50 mg)
were dissolved in 10 ml mixture buffer solution (0.1 M
Na2CO3, 0.1 M NaHCO3, pH 9.5).
FITC (5 mg) was dissolved in 1 ml ethyl alcohol and added dropwise
to the GA-HA solution. The mixtures were stirred at room
temperature for 24 h. Subsequently, the FITC-GA-HA was dialyzed
against distilled water for 3 days and lyophilized. All procedures
were performed in the dark.
Particle size and zeta potential
The DTX/GA-HA-NPs were then characterized. The
particle size distribution and zeta potential of DTX/GA-HA were
determined using a Zetasizer Nano ZS 90 laser particle analyzer
(Malvern Panalytical). Tests were performed three times to
calculate average values.
Morphological characterization
A JEM1400 transmission electron microscope (TEM;
JEOL, Ltd.) was used to observe the morphology of DTX/GA-HA-NPs.
First, a drop of the DTX/GA-HA-NP suspension was placed onto a
super-thin, carbon-coated copper grid. Subsequently, the grid was
allowed to dry at room temperature and was dyed with
phosphotungstic acid for 2 min. Finally, the grid was examined with
the TEM.
Drug encapsulation efficiency (EE) and
loading capacity (LC)
According to the requirements of the Chinese
Pharmacopoeia for the determination of DTX content, and with
reference to the literature (29-31),
the content of DTX in DTX/GA-HA NPs was determined by
high-performance liquid chromatography (HPLC; LC-2010; Shimadzu
Corporation) with UV detection at 232 nm. In brief, a standard
curve of DTX at 232 nm was drawn using octadecyl silane-bonded
silica gel as the filler and 0.043 mol/l ammonium acetate and
acetonitrile (45:55) as the mobile phase. A known amount of
freeze-dried DTX-NPs was dissolved in distilled water and diluted
with methanol. The amount of DTX was measured using the optical
density of the DTX/GA-HA-NPs at 232 nm. The EE and LC of DTX were
calculated according to the following equations: EE (%) =
(M2/M1) x100; and LC (%) =
(M2/Mt) x100, where M1 is the
initial weight of DTX, M2 is the weight of DTX in NPs
and Mt is the weight of lyophilized DTX/GA-HA-NPs.
In vitro drug release study
The in vitro release of DTX from DTX/GA-HA
was investigated using the dialysis diffusion method (32-34)
in PBS (pH 7.4). In brief, 5 ml of DTX/GA-HA NPs was added to a
dialysis bag (molecular weight, 3,500 Da). The dialysis bag was
kept in a conical flask containing 50 ml PBS at 37±0.5˚C with
horizontal shaking (1.11 x g). At 0.5, 1, 2, 3, 6, 9, 12, 24 and 48
h, the release medium outside the dialysis bag was replaced with
fresh PBS and the removed release medium was examined by HPLC. The
concentration of the released drug was determined from the
absorbance intensity of DTX at 232 nm.
Cell culture
The human liver cancer cell line HepG2 was purchased
from the Chinese Typical Culture Preservation Center (School of
Life Sciences, Wuhan University) and the human breast cancer cell
line MCF-7 was acquired from the Experimental Center at Weifang
Medical University. All cell lines were cultured using DMEM/high
glucose medium (Thermo Fisher Scientific, Inc.) containing 10%
fetal bovine serum (FBS; ExCell Bio) at 37˚C in a cell incubator
with 5% CO2.
The human liver cancer cell line HepG2 was
authenticated. An appropriate amount of HepG2 cells
(1x106) was used to extract DNA with Chelex100 resin
(Bio-Rad Laboratories, Inc.), 21 CELLID System (Sigma-Aldrich;
Merck KGaA) was used to amplify 20 short tandem repeat loci and sex
identification sites, and an ABI3130x1 genetic analyzer (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was utilized for PCR
product detection. Gene Mapper IDX software (cat. no. A39978;
Applied Biosystems; Thermo Fisher Scientific, Inc.) was used to
analyze the test results and compare them with database Cellosaurus
(https://web.expasy.org/cellosaurus/).
In vitro cellular uptake
The liver-targeting ability of GA-HA-NPs was
evaluated with an in vitro cellular uptake assay. The
near-infrared fluorescent dye FITC was used as a probe and observed
by confocal laser scanning microscopy (CLSM; TCS SP8; Leica
Microsystems). First, HepG2 cells and MCF-7 cells harvested in the
exponential growth phase were seeded in 6-well plates at a density
of 2x105 cells/well and incubated overnight at 37˚C.
Subsequently, the cells were incubated with fresh DMEM containing
FITC-GA-HA-NPs for 2 h at 37˚C. The cells were then washed three
times with PBS and fixed with a 4% paraformaldehyde for 10 min at
25˚C. Finally, the cells were counterstained with Hoechst 33342 for
15 min and observed by CLSM.
In vitro cytotoxicity assay and colony
formation assay
The cytotoxicity of the DTX/GA-HA-NPs to HepG2 cells
was evaluated using the Cell Counting Kit-8 (CCK-8) assay. HepG2
cells were seeded in 96-well plates (6x103 cells/well).
After incubation overnight, 100 µl of DMEM containing different
concentrations of GA-HA-NPs, free DTX or DTX/GA-HA NPs
(concentrations of free DTX: 1, 2, 5, 10 and 20 µg/ml) were added,
followed by incubation for 24 or 48 h. Subsequently, 10 µl CCK-8
solution was added to each well and the plates were incubated for
another 4 h at 37˚C in a cell incubator with 5% CO2.
Finally, to quantify the live cells, the 96-well plates were read
in a microplate reader (Perkin Elmer) to measure optical density at
450 nm. Untreated cells were used as controls. Cell viability was
calculated according to the following equation: Cell inhibition
rate (%) = [1-(A/B)] x100, where A is the optical density of cells
incubated with DMEM containing different concentrations of
GA-HA-NPs, free DTX or DTX/GA-HA-NPs, and B is the optical density
of cells incubated with DMEM alone.
For the colony formation assay, HepG2 cells were
seeded in 6-well plates (0.5x103 cells/well) and
maintained until adherent. Subsequently, 2 ml free DTX (15.00
µg/ml) or DTX/GA-HA-NPs (17.65 µg/ml) diluted with DMEM containing
10% FBS were added, followed by incubation for 24 h. Subsequently,
the cells were cultured for 12 days with DMEM containing 10% FBS
until colonies were generated. Finally, the colonies were fixed
with 4% paraformaldehyde for 15 min at 25˚C and stained with 0.1%
crystal violet solution for 30 min. The number of cell colonies for
different treatments were counted using ImageJ v.1.8.0_172
(National Institutes of Health).
Cell apoptosis detection using flow
cytometry and fluorescence microscopy
Treated cells were fixed with 4% paraformaldehyde
for 20 min at 25˚C, permeabilized with 0.2% Triton X-100 for 20 min
and stained with DAPI for 10 min at 25˚C. Next, images of the cells
were acquired using a fluorescence microscope. Cell apoptosis was
determined as previously demonstrated (35).
Microtubule cytoskeleton detection
using immunofluorescence staining
Immunofluorescence staining was used to observe
changes in α-tubulin. In brief, HepG2 cells were seeded on 12-mm
coverslips coated with poly-lysine (1x105 cells/well) in
a 24-well plate. Following overnight incubation, the cells were
treated with free DTX or DTX/GA-HA-NPs for 24 h. Next, the cells
were fixed with 4% paraformaldehyde for 15 min and permeabilized
with 0.5% Triton X-100 (Sigma-Aldrich; Merck KGaA) for 15 min at
room temperature after being washed 3 times with PBS on a shaker.
After blocking with 5% bovine serum albumin (Beijing Solarbio
Science & Technology Co., Ltd.) in PBS at room temperature for
1 h, the cells were incubated with the Tubulin-Tracker Red antibody
(1:250 dilution; cat. no. C1050; Beyotime Institute of Technology)
for 1 h at room temperature. After rinsing for 5 min three times
with PBS, cell nuclei were counterstained with DAPI (0.2 µg/ml) for
10 min at 25˚C, examined using CLSM and images were acquired.
Statistical analysis
Values are expressed as the mean ± standard
deviation and all data were evaluated separately from at least
three independent experiments. Statistical comparisons were
analyzed using GraphPad 5 (GraphPad Software Inc.). Statistical
analysis was performed by application of Student's unpaired t-test
and one-way ANOVA followed by Tukey's post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Particle size and zeta potential of
DTX/GA-HA-NPs
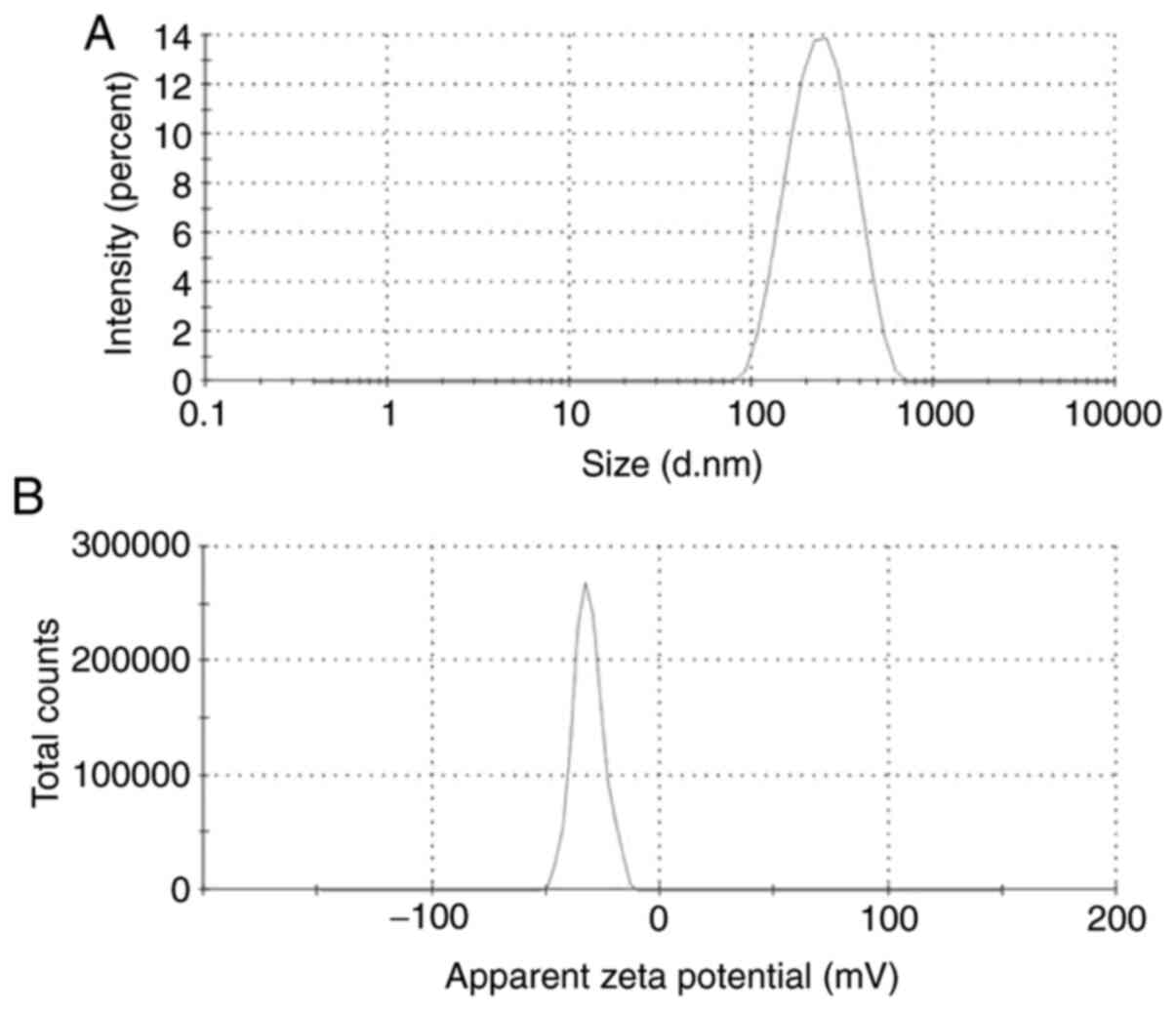
First, the physicochemical properties of the NPs
were determined. The mean diameter, zeta potential and size
distribution of DTX/GA-HA-NPs in the aqueous medium were measured
using a laser particle analyzer and the results are provided in
Table I and Fig. 1A and B. DTX/GA-HA-NPs had a negative zeta
potential (-27.83 mV), which is caused by ionization of the
carboxyl groups of HA and had favorable dispersibility in water
[particle dispersity index (PDI), 0.21].
 | Table IPhysicochemical property of
DTX/GA-HA-NPs. |
Table I
Physicochemical property of
DTX/GA-HA-NPs.
| Sample | DLS, nm | PDI | SEM, nm | Zeta potential,
mV | EE, % | LC, % |
|---|
| DTX/GA-HA-NPs | 208.73±5.00 | 0.21±0.12 | 64.3±10.6 | -27.83±3.14 | 85.38±4.62 | 17.59±0.68 |
Morphological characterization
The morphological characterization of DTX/GA-HA-NPs
was performed by TEM and an electron photomicrograph is provided in
Fig. 2. The results indicated that
DTX/GA-HA-NPs possessed an almost spherical shape and exhibited a
relatively monodisperse distribution. The average NP diameter, as
estimated from the TEM micrographs, was shown in Table I. This is smaller than the
hydrodynamic diameter measured by the laser particle size
analyzer.
Drug EE and LC
The EE and LC of DTX in DTX/GA-HA-NPs were measured
by a simple dialysis method and by HPLC. A representative
chromatogram of DTX is depicted in Fig.
3A. The results indicated that DTX had a retention time of 8.43
min. The standard curve for DTX exhibited linearity over the range
of 10-80 µg/ml (the concentration of standards are 10, 20, 40, 60
and 80 µg/ml) with a regression equation of y=19.794x-1.023
(Fig. 3B). The regression
coefficient (R2) for DTX over the specified range was
calculated to be R2=0.9999. According to the standard
curve, the EE and LC of DTX were determined to be 85.38±4.62 and
17.59±0.68%, respectively, as indicated in Table I.
Drug release profile
The DTX/GA-HA-NPs exhibited an initial fast release
within 12 h, which may be attributed to the drug adhering to the NP
surface. Within 12 to 24 h, DTX was also released slowly and the
cumulative release amount reached an approximate maximum value
(40%) at 24 h (Fig. 3C).
GA-HA-NPs have liver-targeting
ability
A cell uptake study was performed for the
qualitative estimation of the targeting ability of GA-HA-NPs and
CLSM was used to observe the cellular localization of the
GA-HA-NPs. To demonstrate the effect of GA-HA-NP targeting,
receptor-mediated cellular uptake of FITC-GA-HA-NPs was studied in
HepG2 cells and MCF-7 cells [negative for GA receptor (GA-R)
expression] (36). Fluorescence
images of the cells taken after 2 h of incubation with
FITC-GA-HA-NPs are provided in Fig.
4A and B. The green
fluorescence intensity was stronger in HepG2 than in MCF-7
cells.
HepG2 cells are more sensitive to
DTX/GA-HA-NPs than free DTX
The CCK-8 assay is frequently used to detect the
toxic effects of drugs on cells. It was thus used to evaluate the
cytotoxic effects of free DTX and DTX/GA-HA-NPs on HepG2 cells.
IC50 is the dose which led to a 50% reduction in viable
cells compared with the control, reflecting the sensitivity of
cells to drugs. The IC50 values of free DTX and
DTX/GA-HA-NP after 24 h were 15.7 and 4.3 µg/ml (equivalent free
DTX), respectively (Table II), and
the IC50 of DTX was 3.6 times that of DTX/GA-HA-NPs.
After 48 h of incubation, the IC50 values of the free
DTX and DTX/GA-HA-NPs cells were 3.8 and 1.6 µg/ml (equivalent free
DTX), respectively. Cell survival was lower with DTX/GA-HA-NPs
compared with DTX as presented in Fig.
5. Therefore, HepG2 cells are more sensitive to DTX/GA-HA-NPs
than free DTX.
 | Table IIIC50 values of DTX and
DTX/GA-HA-NPs on HepG2 cells following 24 and 48 h of incubation,
respectively. |
Table II
IC50 values of DTX and
DTX/GA-HA-NPs on HepG2 cells following 24 and 48 h of incubation,
respectively.
| | IC50,
µg/ml |
|---|
| Time (h) | DTX | DTX/GA-HA-NPs |
|---|
| 24 | 15.7±0.81 | 4.3±0.29 |
| 48 | 3.8±0.20 | 1.6±0.23 |
DTX/GA-HA-NPs inhibit the colony
formation ability of HepG2-cell
The effects of free DTX and DTX/GA-HA-NPs on the
colony formation ability of HepG2 cells were also evaluated. Cells
were incubated with an equivalent dose of free DTX or
DTX/GA-HA-NPs. As presented in Fig.
6, free DTX and DTX/GA-HA-NPs inhibited the colony formation of
HepG2 cells in a dose- and time-dependent manner. However, the
colony formation of HepG2 cells treated with DTX/GA-HA-NPs was
significantly lower than that of cells treated with free DTX. In
addition, a colony formation assay was performed to examine the
antitumor effects of DTX/GA-HA-NPs against HepG2 cells. The number
of colonies of HepG2 cells treated with DTX/GA-HA-NPs was
significantly lower than that of control cells and cells treated
with free DTX (Fig. 6). These
results suggested that DTX/GA-HA-NPs significantly inhibited the
colony formation ability of HepG2 cells.
DTX/GA-HA-NPs induce apoptosis of
HepG2 cells
Fluorescence micrographs indicated that the
morphology of cells treated with DTX/GA-HA-NPs changed, and in
certain cases, the nucleus was crescent-shaped or even broken
(Fig. 7). Therefore, the percentage
of apoptotic cells was measured using flow cytometry to assess
whether DTX/GA-HA-NPs induced apoptosis in HepG2 cells. The results
indicated that DTX/GA-HA-NPs significantly increased the percentage
of apoptotic HepG2 cells (Fig. 8).
It was thus indicated that DTX/GA-HA-NPs induce apoptosis in HepG2
cells.
DTX/GA-HA-NPs cause α-tubulin
polymerization
According to previous studies, DTX binds to the
α-tubulin subunit of microtubulin to cause tubulin polymerization.
Immunofluorescence staining technology was utilized to examine the
effects of DTX/GA-HA-NPs on HepG2 microtubule cytoskeletons. As
presented in Fig. 9, red
fluorescent staining of tubulin in untreated cells indicated intact
cell morphology and generally cytoplasmic distribution, while
staining of cells treated with DTX revealed partial polymerization
and a slight change in cell morphology. By contrast, cells treated
with DTX/GA-HA-NPs exhibited a large degree of tubulin
polymerization around their nuclei, less tubulin in the cytoplasm
and a markedly distorted morphology.
Discussion
NPs are nano-scale solid colloidal particles made of
natural or synthetic polymer carrier materials. As a drug delivery
carrier, NPs have unique advantages such as low toxicity,
controlled release, good stability and strong targeting. NPs have
unique advantages and potential application value in the field of
NP-targeted drug delivery systems. Ligand-functionalized NPs are
able to deliver drugs to targets. The preparation materials of NPs
include natural polymer materials, synthetic polymer materials and
non-degradable polymer materials. Zhang et al (37) coupled HA with aminated GA and
prepared GA-HA-NPs as a carrier to deliver PTX. Their results
indicated that GA-HA-NPs easily encapsulate PTX, with drug LC and
EE of as high as 31.16 and 92.02%, respectively, and the
cytotoxicity of HepG2 cells was greater than that of B16F10
cells.
In the present study, DTX/GA-HA-NPs were indicated
to have a smaller particle size compared to GA/HA-NPs. This smaller
particle size may be due to the hydrophobicity of the DTX
encapsulated in the GA-HA-NPs. Hydrophobic interactions between the
component materials give the DTX/GA-HA-NPs a more compact core.
Furthermore, the particle size of DTX/GA-HA-NPs measured by TEM was
smaller than that obtained with the particle size analyzer. This
difference may be due to different sample preparation techniques;
the laser particle analyzer measurements were made under aqueous
conditions, while the TEM images were obtained with dried samples
in which the hydrophilic shells of the DTX/GA-HA-NPs may have
shrunk.
During the preparation of GA-HA and DTX/GA-HA, the
excess organic solvent was removed by dialysis. Dialysis is a
purification technique used to prepare biomacromolecules, featuring
desalination, removal of small amounts of organic solvents, removal
of small biomolecule impurities and sample concentration. The
dialysis method is able to separate the excess materials, drugs and
related solvents (such as formamide, ethanol) used during the
synthesis of DTX/GA-HA-NPs from the product and thus purify
DTX/GA-HA-NPs. Therefore, dialysis was used to remove excess drug,
materials and related solvents.
DTX enters cells through passive diffusion. In the
DTX/GA-HA-NPs prepared in the present study, GA has a role in liver
targeting and GA-R-mediated endocytosis may be a key mechanism by
which DTX/GA-HA-NPs target the liver (38). Using GA-modified NPs as a carrier of
liver-targeted drugs provides a novel solution for the treatment of
liver cancer. Studies have indicated that, whether it is the
introduction of GA molecules on the C30-carboxyl group or the
C3-hydroxyl group, GA-modified NPs have the same tendency to target
the liver. The most common modification is the amidation and
esterification of GA and C30-carboxy to obtain more active
compounds, this was also the method used in the present study. In
the modified C3-hydroxyl group of GA, the C30-carboxyl group should
be protected first because of its high activity and then the
C3-hydroxyl group should be amidated and esterified.
To examine the uptake and intracellular localization
of FITC-loaded GA-HA-NPs, two cell lines were selected for
comparison. The results indicated that the fluorescence of
FITC-GA-HA in HepG2 was stronger than that in MCF-7 cells. Among
these cells, GA-R is widely expressed on human liver cancer HepG2
cells (33,34) . Therefore, GA receptor-mediated
endocytosis may be a key mechanism by which GA-HA-NPs target the
liver. In 1990, Aruffo et al (39) reported that CD44 is the major cell
surface receptor of HA and that HA is able to actively target the
surfaces of liver cancer cells to bind to the CD44 receptor and be
taken up by endocytosis. Zhang et al (37) coupled HA with aminated GA and
prepared GA-HA-NPs as carriers to deliver PTX. Confocal microscopy
indicated that the in vitro cellular uptake of FITC-labeled
GA-HA-NPs was higher than that of free FITC and the green
fluorescence intensity of HepG2 cells and B16F10 cells was higher
than that of HELF cells (normal fibroblasts), indicating that the
mechanism of GA-HA targeting may be the interaction between HA and
the CD44 receptor. Therefore, the breast cancer cell line MCF-7
with no GA receptors expressed was selected for an uptake study and
a relatively smaller amount of fluorescence was observed in the
cytoplasm. Uptake by MCF-7 cells may have been due to the binding
affinity of HA to the CD44 receptor. Therefore, HepG2 cells were
used for subsequent studies. In a preliminary experiment for the
present study, GA was added in advance and incubation was performed
for 2 h, followed by the addition of DTX/GA-HA, and it was
indicated that the fluorescence intensity was decreased compared
with the one with no GA incubation, suggesting that during the
pre-incubation, GA combined with the GA-R on the surface of liver
cancer cells, competitively inhibiting the binding of DTX/GA-HA to
GA-R, thereby inhibiting the uptake of DTX/GA-HA (data not
shown).
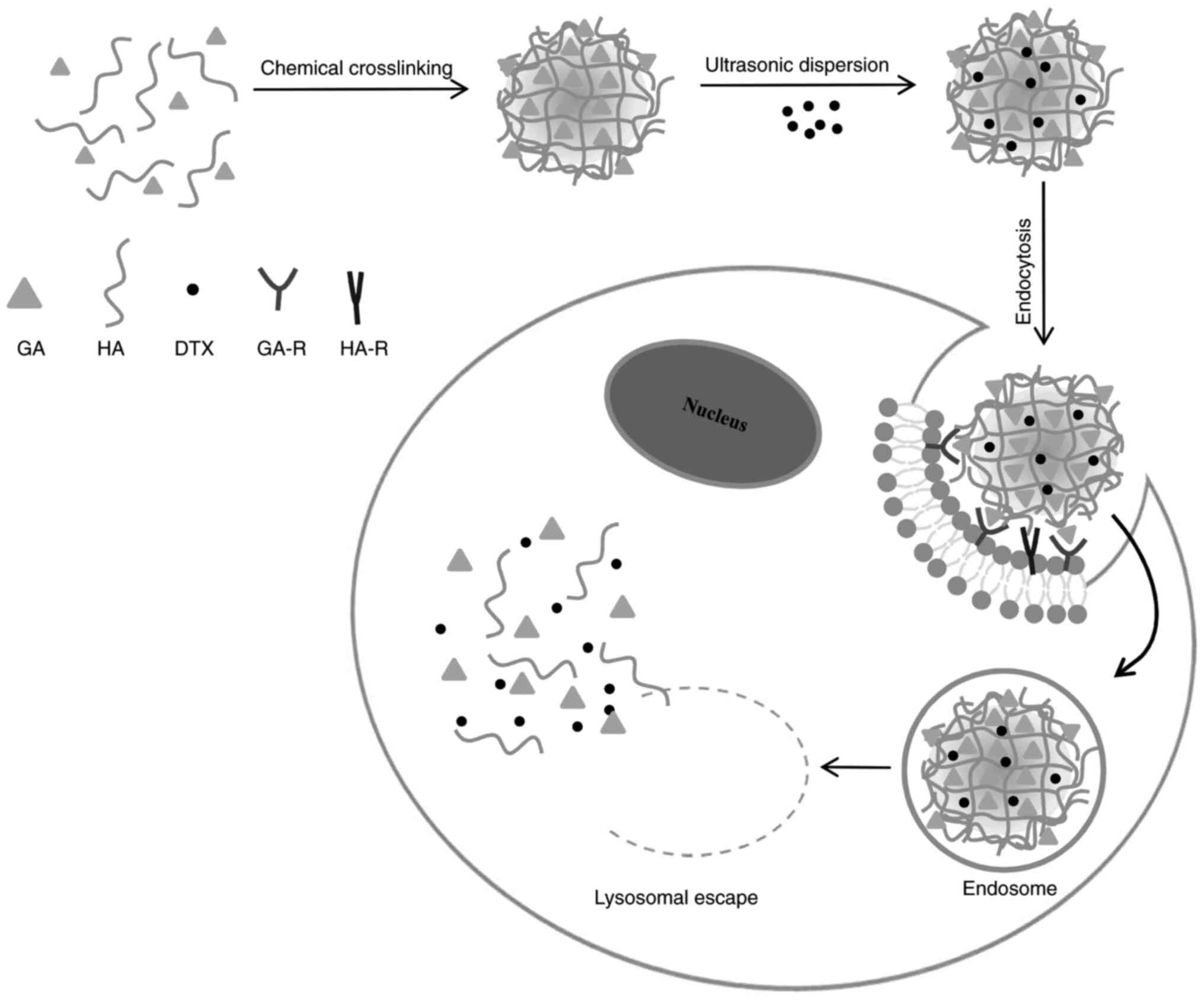
A mechanism for the cellular uptake of DTX/GA-HA and
release of DTX in cancer cells was proposed and illustrated in a
schematic in Fig. 10. GA and HA
self-aggregate to form GA-HA, which is packaged with DTX to form
DTX/GA-HA-NPS. GA directs NPS to the surface of liver/liver cancer
cells, binds to GA-R receptors, enters cells through endocytosis
and exocytosis and releases DTX through lysosome to exert its
efficacy (Fig. 10). However, the
process by which FITC-GA-HA is taken up by cells and the associated
biochemical events warrant further study.
All drugs known to bind human tubulin are associated
with β-tubulin, including DTX. Previous studies have confirmed that
DTX binds the β-tubulin unit, resulting in tubulin polymerization
(40,41). Therefore, α-tubulin was chosen for
verification and it was determined that DTX is also able to enhance
the polymerization of cellular tubulin by binding α-tubulin.
Therefore, the polymerization of DTX on the cellular microtubule
system is able to halt the cell cycle by preventing mitosis. It was
hypothesized that DTX and DTX/GA-HA-NPs act on microtubules,
affecting spindle formation and causing cells to lose their
dividing dynamics, thereby inhibiting cell proliferation. Wang
et al (42) concluded that
this blocking of the mitotic phase is the cause of taxane-induced
cytotoxicity. However, the biochemical events associated with
taxanes binding to microtubules and downstream effects to cause
apoptosis remain to be fully elucidated. Further research by our
group will aim to verify the effects of DTX on cell cycle
regulation to confirm its mechanism of action.
In the present study, the ability of DTX to inhibit
tumor cell proliferation was examined. Inhibitory effects on
proliferation were examined by CCK-8 and cell colony formation
assays. It was first observed that the viability of HepG2 cells
treated with empty GA-HA-NPs was >90%, which was consistent with
previous studies (35) and
indicated the biosafety of the nanocarriers. The calculated
IC50 values indicated that HepG2 cells were more
sensitive to DTX/GA-HA-NPs than free DTX. Furthermore, the CCK-8
assay indicated that the viability of HepG2 cells significantly
decreased after incubation with DTX and DTX/GA-HA-NPs. After 24 h,
the cell viability was <60% compared with the control. After 48
h, the cell viability in the DTX group was <40%, while cell
viability in the DTX/GA-HA-NP group was <10% compared with the
control group. Thus, docetaxel effectively inhibits HepG2 cell
proliferation and this effect was enhanced by the delivery of the
drug via DTX/GA-HA-NPs. Similarly, it was further confirmed by cell
colony formation assay that DTX/GA-HA-NPS can significantly inhibit
the growth of HepG2 cells.
Wang et al (42) observed multiple roles of
microtubules in cell cycle and apoptosis regulation. In the present
study, it was observed that treatment with DTX and DTX/GA-HA-NPs
induced nuclear contraction and deformation in the cells stained
with DAPI. In addition, flow cytometric analysis was performed and
it was determined that the apoptotic rate of HepG2 cells after
incubation with DTX and DTX/GA-HA-NPs was 18 and 24%, respectively.
The result of the present study was consistent with a previous
study (43), which also indicated
DTX/GA-HA-NPs had higher delivery efficacy to liver cancer cells
compare to free drugs.
The present study only verified the liver targeting
and anti-tumor effects of DTX/GA-HA at the cellular level and did
not study the metabolism and kinetic effects of the drug in
vivo. Next, tumor-bearing experiments and in vivo small
animal imaging experiments will be performed by our group to study
the liver targeting and anti-tumor activity of DTX/GA-HA in
vivo.
In summary, DTX/GA-HA was successfully prepared,
which had good physical and chemical properties. Furthermore, it
had a good liver cancer cell targeting effect in vitro, but
the GA-mediated liver targeting transmembrane mechanism requires
further research. Subsequent to the study of its in vitro
anti-cancer activity, it is necessary to clarify the role of
DTX/GA-HA in inhibiting tumor activity and how it affects the cell
cycle, which may provide a good foundation for further research on
its anti-tumor effect in vivo.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by grants from the National
Natural Science Foundation of China (grant nos. 81274093 and
81871892) and the Natural Science Foundation of Shandong Province
(grant no. ZR2019MC042).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZG, YW and HX were responsible for the conception of
this work. FW, BL and HX performed the preliminary synthesis of
GA-HA and DTX/GA-HA-NPs. TS, XL, CW and LZ performed the
characterization of NPs. WD, JB, CZ and LQ contributed to cell
experiments (including cell culture, cell proliferation
experiments, clonogenicity experiments and apoptosis experiments).
HX, BJ, MQ and WW performed experiments on the microtubule
aggregation effect of DTX/GA-HA and HX was a major contributor in
writing the manuscript. JW and WY assisted with the data analysis
and drafted the discussion part of the manuscript. YW and ZG
confirmed the authenticity of all the raw data and approved the
final manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Natarajan Y, Kramer JR, Yu X, Li L, Thrift
AP, El-Serag HB and Kanwal F: risk of cirrhosis and hepatocellular
cancer in patients with non-alcoholic fatty liver disease and
normal liver enzymes. Hepatology. 72:1242–1252. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sagnelli E, Macera M, Russo A, Coppola N
and Sagnelli C: Epidemiological and etiological variations in
hepatocellular carcinoma. Infection. 48:7–17. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dimitroulis D, Damaskos C, Valsami S,
Davakis S, Garmpis N, Spartalis E, Athanasiou A, Moris D,
Sakellariou S, Kykalos S, et al: From diagnosis to treatment of
hepatocellular carcinoma: An epidemic problem for both developed
and developing world. World J Gastroenterol. 23:5282–5294.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sun W, Wang Y, Cai M, Lin L, Chen X, Cao
Z, Zhu K and Shuai X: Codelivery of sorafenib and GPC3 siRNA with
PEI-modified liposomes for hepatoma therapy. Biomater Sci.
5:2468–2479. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lu B, Yu GJ and Cheng M: Expression and
clinical significance of Snail and Claudin-3 in primary
hepatocellular carcinoma. Shandong Yiyao. 58:6–9. 2018.(In
Chinese).
|
|
6
|
Zhang H, Wu Y, Hu Y, Li X, Zhao M and Lv
Z: Targeted nanoparticle drug delivery system for the enhancement
of cancer immunotherapy. J Biomed Nanotechnol. 15:1839–1866.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wu Y, Xu Z, Sun W, Yang Y, Jin H, Qiu L
and Chen J and Chen J: Co-responsive smart cyclodextrin-gated
mesoporous silica nanoparticles with ligand-receptor engagement for
anti-cancer treatment. Mater Sci Eng C. 103(109831)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lin A, Chen J, Liu Y, Deng S, Wu Z, Huang
Y and Ping Q: Preparation and evaluation of N-caproyl chitosan
nanoparticles surface modified with glycyrrhizin for hepatocyte
targeting. Drug Dev Ind Pharm. 35:1348–1355. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sun Y, Dai C, Yin M, Lu J, Hu H and Chen
D: Hepatocellular carcinoma-targeted effect of configurations and
groups of glycyrrhetinic acid by evaluation of its
derivative-modified liposomes. Int J Nanomedicine. 13:1621–1632.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yan T, Cheng J, Liu Z, Cheng F, Wei X,
Huang Y and He J: Acid-sensitive polymeric vector targeting to
hepatocarcinoma cells via glycyrrhetinic acid receptor-mediated
endocytosis. Mater Sci Eng C. 87:32–40. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sakurai Y and Harashima H:
Hyaluronan-modified nanoparticles for tumor-targeting. Expert Opin
Drug Deliv. 16:915–936. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tavianatou AG, Caon I, Franchi M,
Piperigkou Z, Galesso D and Karamanos NK: Hyaluronan: Molecular
size-dependent signaling and biological functions in inflammation
and cancer. FEBS J. 286:2883–2908. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tirella A, Kloc-Muniak K, Good L, Ridden
J, Ashford M, Puri S and Tirelli N: CD44 targeted delivery of siRNA
by using HA-decorated nanotechnologies for KRAS silencing in cancer
treatment. Int J Pharm. 561:114–123. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li W, Yan R, Liu Y, He C, Zhang X, Lu Y,
Khan MW, Xu C, Yang T and Xiang G: Co-delivery of Bmi1 small
interfering RNA with ursolic acid by folate receptor-targeted
cationic liposomes enhances anti-tumor activity of ursolic acid in
vitro and in vivo. Drug Deliv. 26:794–802. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yu Y, Wang J, Kaul SC, Wadhwa R and Miyako
E: Folic acid receptor-mediated targeting enhances the
cytotoxicity, efficacy, and selectivity of withania somnifera leaf
extract: In vitro and in vivo evidence. Front Oncol.
9(602)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cai Y, Xu Y, Chan HF, Fang X, He C and
Chen M: Glycyrrhetinic acid mediated drug delivery carriers for
hepatocellular carcinoma therapy. Mol Pharm. 13:699–709.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
He ZY, Zheng X, Wu XH, Song XR, He G, Wu
WF, Yu S, Mao SJ and Wei YQ: Development of glycyrrhetinic
acid-modified stealth cationic liposomes for gene delivery. Int J
Pharm. 397:147–154. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gao S, Wang J, Tian R, Wang G, Zhang L, Li
Y, Li L, Ma Q and Zhu L: Construction and evaluation of a targeted
hyaluronic acid nanoparticle/photosensitizer complex for cancer
photodynamic therapy. ACS Appl Mater Interfaces. 9:32509–32519.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fang Z, Li X, Xu Z, Du F, Wang W, Shi R
and Gao D: Hyaluronic acid-modified mesoporous silica-coated
superparamagnetic Fe3O4 nanoparticles for
targeted drug delivery. Int J Nanomedicine. 14:5785–5797.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tian G, Sun X, Bai J, Dong J, Zhang B, Gao
Z and Wu J: Doxorubicin loaded dual functional hyaluronic acid
nanoparticles: Preparation, characterization and antitumor efficacy
in vitro and in vivo. Mol Med Rep. 19:133–142. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Duan T, Xu Z, Sun F, Wang Y, Zhang J, Luo
C and Wang M: HPA aptamer functionalized paclitaxel-loaded PLGA
nanoparticles for enhanced anticancer therapy through targeted
effects and microenvironment modulation. Biomed Pharmacother.
117(109121)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Handali S, Moghimipour E, Kouchak M,
Ramezani Z, Amini M, Angali KA, Saremy S, Dorkoosh FA and Rezaei M:
New folate receptor targeted nano liposomes for delivery of
5-fluorouracil to cancer cells: Strong implication for enhanced
potency and safety. Life Sci. 227:39–50. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Patel NR, Piroyan A, Ganta S, Morse AB,
Candiloro KM, Solon AL, Nack AH, Galati CA, Bora C, Maglaty MA, et
al: In vitro and in vivo evaluation of a novel folate-targeted
theranostic nanoemulsion of docetaxel for imaging and improved
anticancer activity against ovarian cancers. Cancer Biol Ther.
19:554–564. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ren J, Chen Y, Song H, Chen L and Wang R:
Inhibition of ZEB1 reverses EMT and chemoresistance in
docetaxel-resistant human lung adenocarcinoma cell line. J Cell
Biochem. 114:1395–1403. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cortes JE and Pazdur R: Docetaxel. J Clin
Oncol. 13:2643–2655. 1995.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Taxanes. In: LiverTox: Clinical and
Research Information on Drug-Induced Liver Injury, 2020.
|
|
27
|
Belderbos BP, Hussaarts KG, van Harten LJ,
Oomen-de Hoop E, de Bruijn P, Hamberg P, van Alphen RJ, Haberkorn
BC, Lolkema MP, de Wit R, et al: Effects of prednisone on docetaxel
pharmacokinetics in men with metastatic prostate cancer: A
randomized drug-drug interaction study. Br J Clin Pharmacol.
85:986–992. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wu F, Zhang LX, Li XC, Jiang B, Zou SY,
Wang C, Mou WQ, Lian B, Wu JL, Yu WJ, et al: Preparation and
proliferation effect on hepatoma cells of adenine loaded
glycyrrhetinic acid modified hyaluronic acid nanoparticles.
Zhongguo Yaolixue Tongbao. 34:706–712. 2018.(In Chinese).
|
|
29
|
Kothari IR, Italiya KS, Sharma S, Mittal A
and Chitkara D: A rapid and precise liquid chromatographic method
for simultaneous determination of alpha lipoic acid and docetaxel
in lipid-based nanoformulations. J Chromatogr Sci. 56:888–894.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lou YM and Huang ZB: Determination of the
related substances in docetaxel for drug materials by HPLC. Pharm
J. 23:41–44. 2011.
|
|
31
|
Cha L, Gu YH and Wang YL: Determination of
docetaxel drug substance by HPLC. J Hubei Univ Sci Technol. 31:3–5.
2017.
|
|
32
|
Stanković V, Mihailović V, Mitrović S and
Jurišić V: Protective and therapeutic possibility of medical herbs
for liver cirrhosis. Rom J Morphol Embryol. 58:723–729.
2017.PubMed/NCBI
|
|
33
|
Li X, Diao W, Xue H, Wu F, Wang W, Jiang
B, Bai J, Lian B, Feng W, Sun T, et al: Improved efficacy of
doxorubicin delivery by a novel dual-ligand-modified liposome in
hepatocellular carcinoma. Cancer Lett. 489:163–173. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li ZP, Tian GX, Jiang H, Pan RY, Lian B,
Wang M, Gao ZQ, Zhang B, Wu JL, et al: Liver-targeting and
pH-sensitive sulfated hyaluronic acid mixed micelles for hepatoma
therapy. Int J Nanomedicine. 14:9437–9452. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jurisic V, Srdic-Rajic T, Konjevic G,
Bogdanovic G and Colic M: TNF-α induced apoptosis is accompanied
with rapid CD30 and slower CD45 shedding from K-562 cells. J Membr
Biol. 239:115–122. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wu F, Xue H, Li X, Diao W, Jiang B, Wang
W, Yu W, Bai J, Wang Y, Lian B, et al: Enhanced targeted delivery
of adenine to hepatocellular carcinoma using glycyrrhetinic
acid-functionalized nanoparticles in vivo and in vitro. Biomed
Pharmacother. 131(110682)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang L, Yao J, Zhou J, Wang T and Zhang
Q: Glycyrrhetinic acid-graft-hyaluronic acid conjugate as a carrier
for synergistic targeted delivery of antitumor drugs. Int J Pharm.
441:654–664. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wu F, Li X, Jiang B, Yan J, Zhang Z, Qin
J, Yu W and Gao Z: Glycyrrhetinic acid functionalized nanoparticles
for drug delivery to liver cancer. J Biomed Nanotechnol.
14:1837–1852. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Aruffo A, Stamenkovic I, Melnick M,
Underhill CB and Seed B: CD44 is the principal cell surface
receptor for hyaluronate. Cell. 61:1303–1313. 1990.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xiao H and Wang L: Effects of X-shaped
reduction-sensitive amphiphilic block copolymer on drug delivery.
Int J Nanomedicine. 10:5309–5325. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Doddapaneni R, Patel K, Chowdhury N and
Singh M: Noscapine chemosensitization enhances docetaxel anticancer
activity and nanocarrier uptake in triple negative breast cancer.
Exp Cell Res. 346:65–73. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wang TH, Wang HS and Soong YK:
Paclitaxel-induced cell death: Where the cell cycle and apoptosis
come together. Cancer. 88:2619–2628. 2000.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen YN, Hsu SL, Liao MY, Liu YT, Lai CH,
Chen JF, Nguyen MT, Su YH, Chen ST and Wu LC: Ameliorative effect
of curcumin-encapsulated hyaluronic acid-PLA nanoparticles on
thioacetamide-induced murine hepatic fibrosis. Int J Environ Res
Public Health. 14(11)2016.PubMed/NCBI View Article : Google Scholar
|