Introduction
Endometrial cancer (EC) is one of the most common
gynecological cancer types, and the incidence has accounted for the
majority of all gynecological malignancies in America and European
countries (1,2). The risk factors of EC include obesity,
hypertension, diabetes, menopause disorder and long-term
stimulation of estrogen (3,4). In recent years, with the rapid
economic development of China, the incidence of uterine cancer was
significantly increased due to changes in people's living habits
and diet structure. Furthermore, it has become a serious threat to
the health of women, a severe social issue and has a huge financial
burden on the country (5,6). The first choice for the treatment of
EC is hysterectomy and bilateral salpingo-ophorectomy, while
multiple studies have demonstrated that laparoscopic surgery
possesses more advantages than laparotomy, including lower degree
of blood loss, fewer wound complications and shorter hospital stays
(7,8). However, there is no difference in the
5-year overall survival rate between robotic surgery and
laparotomy. Furthermore, it was reported that in patients with
stage I intermediate-risk EC, robotic surgery was associated with a
higher recurrence rate than laparotomy (9-11).
The majority of patients with advanced stage EC or for those at
high risk of recurrence require comprehensive therapy, including
drug treatment, adjuvant chemotherapy and radiotherapy (12,13).
However, the management of endometrial cancer remains a clinical
challenge (14-16).
Therefore, it is essential to enrich the strategies of prevention
and treatment for endometrial carcinogenesis. More therapeutic
methods and effective drugs require investigation. The present
study focused on alternate treatment options, including
phytochemicals such as natural herb compound, which has few side
effects and a higher specificity.
Osthole is a natural compound mainly extracted from
Fructus Cnidii, which has been widely used in traditional
Chinese medicine. It possesses a variety of pharmacological
properties, including anti-inflammatory, anti-angiogenic,
stimulates bone formation and ameliorates cartilage degradation
(17-20).
A large number of studies have proven that osthole has an effective
anticancer effect in various tissues, including gallbladder cancer
(21), ovarian cancer (22), head and neck carcinoma (23), pancreatic cancer (24), esophageal squamous cell carcinoma
(25) and breast cancer (26,27).
However, the effects of osthole on EC have not been fully
investigated.
The PI3K/AKT pathway is a major survival pathway in
numerous cancer types (28). It
serves a key role in regulating cell growth, migration, apoptosis
and survival in EC (29).
Activation of the PI3K/AKT pathway has been well studied in the
tumorigenesis and progression of EC (30). It has been proven that a decrease in
PTEN may lead to activation of the PI3K pathway, and loss of PTEN
protein may increase phosphorylation of AKT, which in turn
influences its target genes to increase cell growth and decrease
apoptosis (31). The present study
aimed to investigate the cytotoxic effect of osthole in EC cell
lines (JEC, KLE, and Ishikawa) and its underlying mechanisms
involving the PI3K/AKT pathway.
Materials and methods
Cell culture
Human endometrial cancer KLE and Ishikawa cell lines
were purchased from American Type Culture Collection. The human EC
JEC cell line and the normal human cervical epithelial HcerEpic
cell line were purchased from Shanghai YaJi Biolotechnology Co.,
Ltd. Cells were cultured in Dulbecco's modified Eagle's medium
(Thermo Fisher Scientific, Inc.), supplemented with 10% FBS (Thermo
Fisher Scientific, Inc.) and 1% penicillin/streptomycin (Thermo
Fisher Scientific, Inc.). All cells were cultured at 37˚C and 5%
CO2 atmosphere.
Cell proliferation assay
The effect of osthole on the viability of EC cells
was measured using a CCK-8 kit. CCK-8 kit (kit no. CK04) was
purchased from Dojindo Molecular Technologies, Inc. and Osthole
powder (O9265) was purchased from Sigma-Aldrich (Merck KGaA). In
brief, EC cells (JEC, KLE and Ishikawa) and normal human cervical
epithelial cells (HcerEpic) were seeded onto 96-well plates
overnight. On the second day, cells were treated with various doses
of osthole (0, 25, 50, 100 and 200 µm) for different amounts of
time (24, 48 and 72 h) at 37˚C. At the end of the experiment, CCK-8
(10 µl) was added and the cells were incubated for another 4 h at
room temperature. Finally, the absorbance was measured at 450 nm
(OD 450).
Cell apoptosis analysis
Apoptosis analysis was performed by flow cytometry.
An Annexin V-FITC/PI apoptosis detection kit (cat. no. 556547) was
purchased from BD Biosciences. EC JEC cells were treated with
paclitaxel (10 µm; Sigma-Aldrich; Merck KGaA) or different doses of
osthole (25, 50 and 100 µm; Sigma-Aldrich; Merck KGaA) for 48 h at
37˚C. A total of 1x105 cells were then collected and washed twice
with PBS. Subsequently, cells were resuspended in 100 µl binding
buffer containing 5 µl Annexin V and 5 µl PI. Cells were kept at
room temperature in the dark for 15 min, and a total of 400 µl 1X
binding buffer was subsequently added and flow cytometric analysis
was performed (FACSCalibur™; BD Biosciences). Data were analyzed by
Flow Jo v10 (BD Biosciences).
Western blotting
JEC cells were exposed to different doses (0, 25, 50
and 100 µm) of osthole for 48 h at 37˚C. Then cells were extracted
by RIPA buffer [50 mM Tris-Cl (pH 7.5), 150 mM NaCl, 2 mM EDTA,
0.5% Nonidet P-40, 1 mM NaF and 1 mM PMSF Protease Inhibitor
Cocktail 0.02% (v/v; pH 7.4)]. The concentration of protein was
analyzed using a BCA Protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). The protein samples (50 µg per lane) were loaded
onto 8 or 10% SDS-polyacrylamide gel, prior to being transferred to
PVDF membranes. Membranes were blocked with 5% skimmed milk at 37˚C
for 2 h and then incubated with specific primary antibodies at 4˚C
overnight. The primary antibodies: phosphorylated (p)-AKT (1:1,000;
cat. no. 9271S); AKT (1:1,000; cat. no. 4685S); p-mTOR (1:1,000;
cat. no. 2971S); and mTOR (1:1,000; cat. no. 2983S) were purchased
from Cell Signaling Technology, Inc. The following antibodies:
Bcl-2 (1:1,000; cat. no. ab196495); PTEN (1:1,000; cat. no.
ab170941); PI3K (1:1,000; cat. no. ab191606); p-PI3K (1:1,000; cat.
no. ab182651); cleaved-caspase3 (1:1,000; cat. no. ab2302);
cleaved-caspase-9 (1:1,000; cat. no. ab2324); and Bax (1:1,000;
cat. no. ab32503) were purchased from Abcam. A GAPDH antibody
(1:1,000; cat. no. sc-47724) was purchased from Santa Cruz
Biotechnology, Inc. Following washing twice with TBS with 0.1%
Tween-20, membranes were exposed to the following HRP-conjugated
secondary antibodies for 2 h at 37˚C: Anti-rabbit (1:5,000; cat.
no. ab97051; Abcam) and anti-mouse (1:5,000; cat. no. ab97023;
Abcam). The signal was visualized using chemiluminescent detection
reagent (cat. no. ab133406; Abcam).
ELISA assay
AKT ELISA kit (cat. no. ab126433) and PI3K ELISA kit
(cat. no. ab207484) were purchased from Abcam. In brief, JEC cells
were exposed to different doses (0, 25, 50 and 100 µm) of osthole
at 37˚C for 48 h. Next, cells were collected and solubilized in
cell lysis buffer taken from the aforementioned ELISA kit (Abcam),
prior to lysates being resuspended and incubated at 4˚C for 30 min.
Following centrifugation (10,000 x g for 10 min at 4˚C), 100 µl of
each supernatant was collected in appropriate wells and incubated
overnight at 4˚C. A total of 100 µl antibody taken from the
aforementioned ELISA kit (Abcam) was diluted 55-fold with 1X assay
diluent according to the manufacturer's protocol, and added to each
well for 1 h at 37˚C after washing with wash buffer. Subsequently,
100 µl HRP-conjugated anti-rabbit IgG [diluted 500-fold with 1X
assay diluent taken from the aforementioned ELISA kit (Abcam),
according to manufacturer's protocol] was added to each well for 1
h at 37˚C. The solution was discarded and the washing was repeated.
Next, 100 µl 3,3',5,5'-tetramethylbenzidine one-step substrate
reagent taken from the aforementioned ELISA kit (Abcam) was added,
followed by 50 µl stop solution being added to each well. The
optical density was immediately measured at 450 nm.
Caspase-9 activity assay
The Caspase-Glo® 9 assay kit (kit no.
G8211) was purchased from Promega Corporation. In brief, JEC cells
were plated into 96-well plates and cultured overnight. Next, cells
were exposed to different doses of osthole (0, 25, 50, 100 and 200
µm) for different time periods (24, 48 and 72 h) at 37˚C. The
96-well plates containing cells were removed from the incubator and
100 µl Caspase-Glo 9 Reagent was added to each well. Following
gently mixing contents of wells using a plate shaker at 40 x g for
30 sec at 4˚C, the plate was incubated at 37˚C for another 1 h. The
luminescence was measured using a luminometer.
In vivo tumorigenicity assays
Female nude mice aged 5 weeks (weight, 19-21 g) were
purchased from SPF (Beijing) Biotechnology Co., Ltd. In brief,
suspension of JEC cells (2x106) was subcutaneously
injected into the dorsal of nude mice. A total of 12 mice were used
in the experiment, and two of them were excluded because they did
not bear evident tumors. After 7 days, the mice bearing evident
tumors were randomly distributed into two groups (n=5 each). The
behavior and growth of tumors among the mice were observed daily.
Each mouse was treated with either fresh medium for the control
group or 20 mg/kg of osthole for the treatment groups through
intraperitoneal injection every other day. The indoor temperature
was controlled at 20-25˚C, the humidity was controlled at 30-60%,
the light/dark cycle was 12 h and free access to food and water.
Animal waste was regularly removed and fresh air was regularly
introduced. Tumor volume was calculated as V=length x
width2/2. The maximum tumor volume was 982.8
mm3, and no multiple tumors occurred. All procedures
were performed in accordance with the Institutional Animal Care and
Use of the 980th Hospital of the Joint Logistic Support Force of
the Chinese People's Liberation Army.
Evaluation of biochemical
parameters
To assess changes in hepatic and renal function
following in vivo experiment, blood samples were collected
from the right retroorbital plexus of anesthetized mice two days
prior to the animals being sacrificed. Anesthesia was induced by 50
mg/kg intraperitoneal injection of 1% pentobarbital sodium resolved
in PBS. The levels of serum alanine transaminase, creatinine and
blood urea nitrogen were used to assess changes in liver and renal
function. These biochemical parameters were analyzed using the
Roche Hitachi 911 Chemistry Analyzer (Roche Diagnostics GmbH) as
described previously (32).
Anesthetics and euthanasia
At the end of the in vivo experiment, blood
samples were collected from the right retroorbital plexus of
anesthetized mice. Anesthesia was induced by 50 mg/kg
intraperitoneal injection of 1% pentobarbital sodium resolved in
PBS. Additionally, the animals were euthanized by carbon dioxide
anesthesia after 2 days. In brief, the animals were placed in the
euthanasia box and then 100% CO2 was introduced. The
filling rate was ~10-30% CO2 per min of the chamber
volume. After the animals stopped breathing, which was observed by
minimal chest undulation and the eyeballs turning white,
CO2 flow was maintained for at least 1 min, prior to the
death of the animals being declared.
Statistical analysis
All experiments were conducted at least three times,
and all values are provided as the means ± standard error of the
mean of three independent experiments. Comparisons between two
groups were analyzed using a Student's t-test. A one-way analysis
of variance, followed by Tukey's post hoc test was used to analyze
differences among multiple groups. GraphPad Prism 6.0 software was
used to perform calculations, and P<0.05 was considered to
indicate a statistically significant difference
(*P<0.05, **P<0.01,
***P<0.001).
Results
Osthole suppresses the growth of JEC,
KLE and Ishikawa cells in vitro
Human endometrial cancer cell lines (JEC, KLE and
Ishikawa) and a normal human cervical epithelial cells (HcerEpic)
were exposed to different doses of osthole for 24, 48 or 72 h in
order to determine the effects of osthole on cell growth. As shown
in Fig. 1A, none of these
treatments with osthole revealed significant toxicity on HcerEpic
cells, except the high dose (200 µM osthole) at a long exposure
time (48 and 72 h), which indicated that the appropriate dose of
osthole had little effect on normal human cervical epithelial cell.
Furthermore, it was found that osthole exposure suppressed the
growth of all three cancer cell lines in a dose- and time-dependent
manner, and osthole demonstrated the most toxicity on JEC cells
(Fig. 1B-D). Therefore, following
these experiments, JEC cells were selected for subsequent
research.
Osthole provokes apoptosis in JEC
cells
To further investigate the anticancer potential of
osthole, cell apoptosis of JEC cells was measured by flow
cytometry. Following being treated with paclitaxel or a different
dose of osthole, JEC cells were collected and handled for flow
cytometry detection. As shown in Fig.
2, the proportion of apoptotic JEC cells increased gradually
while the concentration of osthole increased. Specifically, the
proportion of apoptotic cells in the positive control group (10 µM
paclitaxel group) was 48.1±6.4% and that in the negative control
group was 2.0±0.9%, while the number increased to 7.4±0.9% in the
25 µM osthole treatment group, 28.2±4.2% in the 50 µM group and
42.9±2.2% in 100 µM group. These results suggested that osthole
induced apoptotic cell death in JEC cells.
Osthole induces activation of
caspase-9 in JEC cells
The activation of caspase-9 is a critical process in
the occurrence of apoptosis. The present study further investigated
the effects of osthole on caspase-9 activities in JEC cells. As
shown in Fig. 3, the exposure of
osthole activated caspase-9 in a time- and dose-dependent manner.
It was found that four different concentrations of osthole showed
no obvious activation effect after 24 h treatment. However,
caspase-9 activity was increased significantly following 48 h
exposure to osthole, including low-dose osthole (25 µM). These
results implied that osthole treatment may induce the activation of
caspase-9; therefore, the anticancer effect mediated by osthole on
JEC cells is relevant to caspase-involved apoptotic pathways.
Osthole exhibits anticancer effect of
JEC cells through inhibiting the PI3K/AKT signaling pathway
To gain a deep understanding of the underlying
regulatory mechanisms involved in the anticancer effect of osthole,
JEC cells treated with different concentrations of osthole were
collected and Western blot analysis was performed. Annexin
V-FITC/PI staining revealed that osthole provokes apoptosis of JEC;
therefore, the expression of several apoptotic markers was
investigated. As shown in Fig. 4A,
expression of cleaved caspase-3, cleaved caspase-9, cleaved-PARP
and Bax were increased by osthole treatment, particularly in the
100 µM group, while protein expression of Bcl-2 was decreased in a
concentration-dependent manner. The PI3K/AKT signaling pathway
serves a key role in tumor metastasis, and activation of the
PI3K/AKT pathway was associated with protection of cells from
apoptosis. The present study investigated whether osthole inhibits
PI3K/AKT activity and consequently leads to apoptosis of JEC cells.
As shown in Fig. 4, the expression
of PTEN increased following cells being treated with osthole, while
the phosphorylated PI3K and phosphorylated AKT were significantly
decreased. Furthermore, to better quantify the size of the effect,
an ELISA-based system was used to determine the ratio of
phospho-AKT/AKT and phospho-PI3K/PI3K, and a decrease in AKT and
PI3K phosphorylation was observed in osthole-treated JEC cells,
particularly in the 100 µM group (Fig.
4C). These results indicated that osthole may induce apoptosis
of JEC cells via downregulation of the PI3K/AKT signaling
pathway.
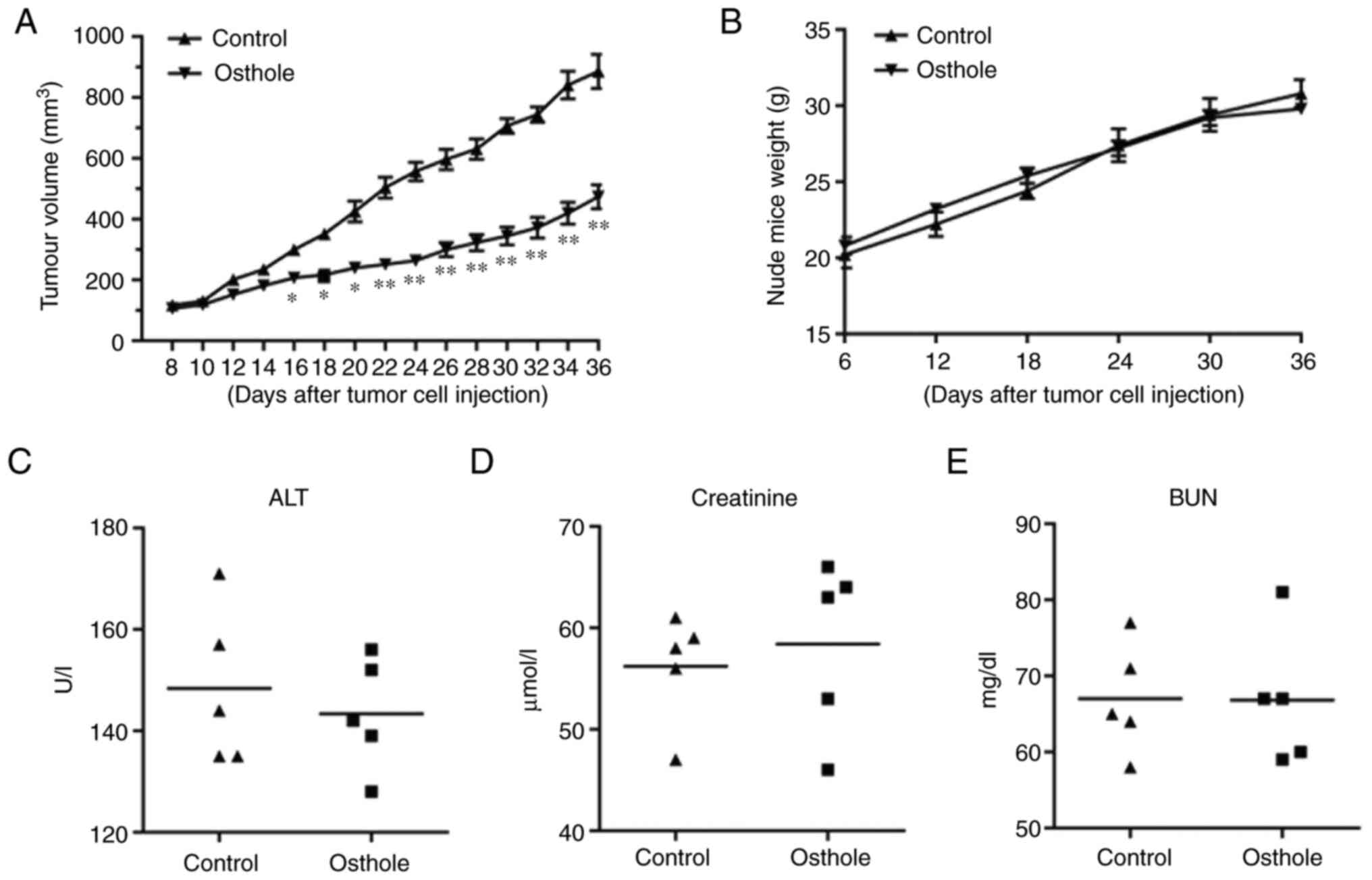
Osthole inhibits the growth of JEC
xenografts in nude mice
To further investigate the tumor-suppressing
potential of osthole, a model for tumorigenicity of JEC cells in
nude mice was established. Each mouse bearing JEC cell xenografts
was treated with either fresh medium for the control group or 20
mg/kg osthole for the treatment groups through intraperitoneal
injection. As shown in Fig. 5, the
tumor volume decreased significantly from day 16 after treatment
with osthole, compared with the control group. Furthermore, osthole
treatment had little effect on the body weight of the mice.
Additionally, there was no significant differences in serum alanine
transaminase, creatinine and blood urea nitrogen between mice
treated with fresh medium or osthole, indicating that neither renal
toxicity nor hepatotoxicity was induced by the indicated
concentration of osthole in vivo.
Discussion
Even though there have been great improvements in
surgical equipment and new anticancer drugs, the outcome of
patients with EC has not notably improved over recent decades
(33,34). There are an estimated 319,500
patients diagnosed with EC annually, which accounts for >76,000
deaths each year (35).
Furthermore, patients with EC, particularly in the late stages,
also require adjuvant therapy to improve progression-free and
overall survival rates (36-39).
However, acquired resistance to current chemotherapeutic drugs has
greatly impaired the successful treatment of EC. Herbal medications
have attracted the interest of clinicians recently due to its
safety, limited side-effect and effectiveness.
Osthole has been reported to exert proliferation
inhibiting effects and to induce the apoptosis of Ishikawa and KLE
cells (40). The present study
found that, compared with Ishikawa and KLE cells, EC JEC cells were
more sensitive to osthole. Furthermore, osthole exhibited
significant cytotoxicity in all three EC cell lines in a
concentration and time-dependent manner. All these types of
treatment with osthole revealed no significant toxicity on HcerEpic
cells, except at a high dose (200 µM osthole) for a long exposure
time (48 and 72 h), indicating that the appropriate dose of osthole
was safe to normal human cervical epithelial cells. The results
obtained from the assays used in the present study (caspase-9
activity, apoptosis analysis and western blotting) also proved that
osthole improved JEC cell apoptosis through a caspase-dependent
pathway. After JEC cells were treated with osthole, the percentage
of apoptotic cells increased significantly, particularly in the 100
µM group. Anti-apoptotic and pro-apoptotic proteins of the Bcl-2
family are key regulators of apoptosis (41-43).
The present study reported that expression of pro-apoptotic
proteins, Bax, cleaved-caspase-3, -caspase-9 and -PARP were
increased while expression of the anti-apoptotic protein, Bcl-2,
was decreased. Additionally, the data indicated that caspase-9
activity was increased significantly after 48 h exposure of
osthole, including low-dose osthole (25 µM). Furthermore, it was
noted that regardless of duration, caspase-9 activation in the 100
µM osthole treatment group was higher than that in the 200 µM
group, indicating that a high concentration of osthole may induce
types of cell death other than apoptosis. The results implied that
osthole treatment may induce the activation of caspase-9;
therefore, the anticancer effect mediated by osthole on JEC cells
is associated with the caspase-involved apoptotic pathway.
Numerous studies have proven that the PI3K/AKT
signal pathway is associated with the progression of EC, and AKT
activation may affect numerous pathways, including cell
proliferation, migration and apoptosis (44-46).
Therefore, this pathway was selected for further study, and it was
found that, with the increased expression of PTEN, the expression
of p-PI3K and p-AKT was decreased following exposure to osthole,
suggesting that this pathway is associated with the anticancer
effect of osthole in JEC cells.
Last but not least, the in vivo effect of
osthole in EC cells was investigated. The results demonstrated that
tumor volume had decreased significantly since day 16 after
treatment with osthole, compared with the control group, while
osthole treatment had little effect on body weight. Furthermore,
there was no significant difference in serum alanine transaminase,
creatinine and blood urea nitrogen between mice treated with fresh
medium or osthole. Further in vivo studies are required, for
example, combined use of osthole with chemotherapeutic drugs, to
elucidate its exact mechanism and clinical therapeutic
potential.
In conclusion, the present study investigated the
anticancer effects of osthole in endometrial cancer cell lines. It
was found that osthole within a certain concentration range may
inhibit cell proliferation in all three EC cells, but exhibited no
significant cytotoxic effect on HcerEpic. Furthermore, flow
cytometry revealed that treatment of osthole significantly
increased JEC cell apoptosis, while the expression of pro-apoptotic
proteins, Bax and cleaved caspase-3, caspase-9 and PARP, was
increased. Additionally, osthole significantly increased the
expression of PTEN, and decreased the activation of phosphorylation
PI3K and AKT, suggesting that the PI3K/AKT signaling pathway was
involved in this process. Furthermore, osthole treatment suppressed
the growth of JEC tumor cells in a nude mouse xenograft model in
vivo, without obvious renal toxicity or hepatotoxicity.
The present study aimed to contribute toward an
improved understanding of the role of osthole in EC and it may be a
safe and effective therapeutic agent for EC. However, whether
osthole may induce types of cell death other than apoptosis,
including autophagy or pyroptosis, remain unknown. The present
study did not investigate target genes other than PTEN and the
PI3K/AKT signaling pathway in EC, and whether knockdown of PTEN may
reverse the antitumor effect of osthole has not been elucidated.
Further experiments are required to verify the anticancer effect of
osthole in EC.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Hebei Province
Key Project of Medical Science Research (grant no. -20160005).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the author on reasonable request.
Authors' contributions
LL and LS designed the study and carried out the
experiments. LL and YW performed the analysis. BY participated in
the design of the study. LL and YW confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
All procedures were performed in accordance with the
Institutional Animal Care and Use of the 980th Hospital of the
Joint Logistic Support Force of the Chinese People's Liberation
Army.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Suarez AA, Felix AS and Cohn DE: Bokhman
Redux: Endometrial cancer ‘types’ in the 21st century. Gynecol
Oncol. 144:243–249. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Temkin SM, Minasian L and Noone AM: The
end of the hysterectomy epidemic and endometrial cancer incidence:
What are the unintended consequences of declining hysterectomy
rates? Front Oncol. 6(89)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bendifallah S, Ouldamer L, Lavoue V,
Canlorbe G, Raimond E, Coutant C, Graesslin O, Touboul C, Collinet
P, Daraï E, et al: Groupe de Recherche FRANCOGYN: Patterns of
recurrence and outcomes in surgically treated women with
endometrial cancer according to ESMO-ESGO-ESTRO Consensus
Conference risk groups: Results from the FRANCOGYN study Group.
Gynecol Oncol. 144:107–112. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Colombo N, Creutzberg C, Amant F, Bosse T,
González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza
MR, et al: ESMO-ESGO-ESTRO Endometrial Consensus Conference Working
Group: ESMO-ESGO-ESTRO consensus conference on endometrial cancer:
Diagnosis, treatment and follow-up. Radiother Oncol. 117:559–581.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Galaal K, Donkers H, Bryant A and Lopes
AD: Laparoscopy versus laparotomy for the management of early stage
endometrial cancer. Cochrane Database Syst Rev.
10(CD006655)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Scaletta G, Dinoi G, Capozzi V, Cianci S,
Pelligra S, Ergasti R, Fagotti A, Scambia G and Fanfani F:
Comparison of minimally invasive surgery with laparotomic approach
in the treatment of high risk endometrial cancer: A systematic
review. Eur J Surg Oncol. 46:782–788. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gueli Alletti S, Cianci S, Perrone E,
Fanfani F, Vascone C, Uccella S, Gallotta V, Vizzielli G, Fagotti
A, Monterossi G, et al: Technological innovation and personalized
surgical treatment for early-stage endometrial cancer patients: A
prospective multicenter Italian experience to evaluate the novel
percutaneous approach. Eur J Obstet Gynecol Reprod Biol.
234:218–222. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gueli Alletti S, Rossitto C, Cianci S and
Scambia G: Telelap ALF-X total hysterectomy for early stage
endometrial cancer: New frontier of robotic gynecological surgery.
Gynecol Oncol. 140:575–576. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Song J, Le T, Hopkins L, Fung-Kee-Fung M,
Lupe K, Gaudet M, E C and Samant R: A comparison of disease
recurrence between robotic versus laparotomy approach in patients
with intermediate-risk endometrial cancer. Int J Gynecol Cancer.
30:160–166. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Crosbie E and Morrison J: The emerging
epidemic of endometrial cancer: Time to take action. Cochrane
Database Syst Rev. 12(ED000095)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Crosbie EJ, Zwahlen M, Kitchener HC, Egger
M and Renehan AG: Body mass index, hormone replacement therapy, and
endometrial cancer risk: A meta-analysis. Cancer Epidemiol
Biomarkers Prev. 19:3119–3130. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
MacKintosh ML and Crosbie EJ: Prevention
strategies in endometrial carcinoma. Curr Oncol Rep.
20(101)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kieszkowski P, Dąbruś D, Grabarek BO and
Boroń D: Differences in the expression pattern of mRNA protein
SEMA3F in endometrial cancer in vitro under cisplatin treatment.
Curr Pharm Biotechnol. 21:1119–1128. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Opławski M, Dziobek K, Adwent I, Dąbruś D,
Grabarek B, Zmarzły N, Plewka A and Boroń D: Expression profile of
endoglin in different grades of endometrial cancer. Curr Pharm
Biotechnol. 19:990–995. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Leung YM, Kuo YH, Chao CC, Tsou YH, Chou
CH, Lin CH and Wong KL: Osthol is a use-dependent blocker of
voltage-gated Na+ channels in mouse neuroblastoma N2A
cells. Planta Med. 76:34–40. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wu SN, Lo YK, Chen CC, Li HF and Chiang
HT: Inhibitory effect of the plant-extract osthole on L-type
calcium current in NG108-15 neuronal cells. Biochem Pharmacol.
63:199–206. 2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
You L, Feng S, An R and Wang X: Osthole: A
promising lead compound for drug discovery from a traditional
Chinese medicine (TCM). Nat Prod Commun. 4:297–302. 2009.PubMed/NCBI
|
|
20
|
Zhang ZR, Leung WN, Cheung HY and Chan CW:
Osthole: A review on its bioactivities, pharmacological properties,
and potential as alternative medicine. Evid Based Complement
Alternat Med. 2015(919616)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Le Zou T, Wang HF, Ren T, Shao ZY, Yuan
RY, Gao Y, Zhang YJ, Wang XA and Liu YB: Osthole inhibits the
progression of human gallbladder cancer cells through JAK/STAT3
signal pathway both in vitro and in vivo. Anticancer Drugs.
30:1022–1030. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jiang G, Liu J, Ren B, Tang Y, Owusu L, Li
M, Zhang J, Liu L and Li W: Anti-tumor effects of osthole on
ovarian cancer cells in vitro. J Ethnopharmacol. 193:368–376.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang J, Zhu XJ, Jin MZ, Cao ZW, Ren YY and
Gu ZW: Osthole induces cell cycle arrest and apoptosis in head and
neck squamous cell carcinoma by suppressing the PI3K/AKT signaling
pathway. Chem Biol Interact. 316(108934)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang B, Zheng X, Liu J, Zhang Z, Qiu C,
Yang L, Zhang L, Zhang Q, Gao H and Wang X: Osthole inhibits
pancreatic cancer progression by directly exerting negative effects
on cancer cells and attenuating tumor-infiltrating M2 macrophages.
J Pharmacol Sci. 137:290–298. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhu X, Li Z, Li T, Long F, Lv Y, Liu L,
Liu X and Zhan Q: Osthole inhibits the PI3K/AKT signaling pathway
via activation of PTEN and induces cell cycle arrest and apoptosis
in esophageal squamous cell carcinoma. Biomed Pharmacother.
102:502–509. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dai X, Yin C, Zhang Y, Guo G, Zhao C, Wang
O, Xiang Y, Zhang X and Liang G: Osthole inhibits triple negative
breast cancer cells by suppressing STAT3. J Exp Clin Cancer Res.
37(322)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Park W, Park S, Song G and Lim W:
Inhibitory effects of osthole on human breast cancer cell
progression via induction of cell cycle arrest, mitochondrial
dysfunction, and ER stress. Nutrients. 11(11)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen J: Multiple signal pathways in
obesity-associated cancer. Obes Rev. 12:1063–1070. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Slomovitz BM and Coleman RL: The
PI3K/AKT/mTOR pathway as a therapeutic target in endometrial
cancer. Clin Cancer Res. 18:5856–5864. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li Y, Zhang Z, Zhang X, Lin Y, Luo T, Xiao
Z and Zhou Q: A dual PI3K/AKT/mTOR signaling inhibitor miR-99a
suppresses endometrial carcinoma. Am J Transl Res. 8:719–731.
2016.PubMed/NCBI
|
|
31
|
Chen J, Zhao KN, Li R, Shao R and Chen C:
Activation of PI3K/Akt/mTOR pathway and dual inhibitors of PI3K and
mTOR in endometrial cancer. Curr Med Chem. 21:3070–3080.
2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li CJ, Chu CY, Huang LH, Wang MH, Sheu LF,
Yeh JI and Hsu HY: Synergistic anticancer activity of triptolide
combined with cisplatin enhances apoptosis in gastric cancer in
vitro and in vivo. Cancer Lett. 319:203–213. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Arnold M, Rutherford MJ, Bardot A, Ferlay
J, Andersson TM, Myklebust TÅ, Tervonen H, Thursfield V, Ransom D,
Shack L, et al: Progress in cancer survival, mortality, and
incidence in seven high-income countries 1995-2014 (ICBP
SURVMARK-2): A population-based study. Lancet Oncol. 20:1493–1505.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fortner RT, Hüsing A, Dossus L, Tjønneland
A, Overvad K, Dahm CC, Arveux P, Fournier A, Kvaskoff M, Schulze
MB, et al: Theoretical potential for endometrial cancer prevention
through primary risk factor modification: Estimates from the EPIC
cohort. Int J Cancer. 147:1325–1333. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Creutzberg CL, van Putten WL, Koper PC,
Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, De Winter KA, Lutgens
LC, van den Bergh AC, van de Steen-Banasik E, et al: Surgery and
postoperative radiotherapy versus surgery alone for patients with
stage-1 endometrial carcinoma: Multicentre randomised trial. PORTEC
Study Group. Post operative radiation therapy in endometrial
carcinoma. Lancet. 355:1404–1411. 2000.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Greven K, Winter K, Underhill K,
Fontenesci J, Cooper J and Burke T: Final analysis of RTOG 9708:
Adjuvant postoperative irradiation combined with
cisplatin/paclitaxel chemotherapy following surgery for patients
with high-risk endometrial cancer. Gynecol Oncol. 103:155–159.
2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li R, Jiang W, Dou S, Zhong L, Sun J,
Zhang C and Zhu G: A phase II trial of chemoradiotherapy using
weekly docetaxel for high-risk postoperative oral squamous cell
carcinoma patients. Int J Radiat Oncol Biol Phys. 107:462–468.
2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Susumu N, Sagae S, Udagawa Y, Niwa K,
Kuramoto H, Satoh S and Kudo R: Japanese Gynecologic Oncology
Group. Randomized phase III trial of pelvic radiotherapy versus
cisplatin-based combined chemotherapy in patients with
intermediate- and high-risk endometrial cancer: A Japanese
Gynecologic Oncology Group study. Gynecol Oncol. 108:226–233.
2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lu K, Lin J and Jiang J: Osthole inhibited
cell proliferation and induced cell apoptosis through decreasing
CPEB2 expression via up-regulating miR-424 in endometrial
carcinoma. J Recept Signal Transduct Res. 40:89–96. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326.
1998.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Delbridge AR, Grabow S, Strasser A and
Vaux DL: Thirty years of BCL-2: Translating cell death discoveries
into novel cancer therapies. Nat Rev Cancer. 16:99–109.
2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang X, Liu X, Zhou D and Zheng G:
Targeting anti-apoptotic BCL-2 family proteins for cancer
treatment. Future Med Chem. 12:563–565. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
LoRusso PM: Inhibition of the
PI3K/AKT/mTOR Pathway in Solid Tumors. J Clin Oncol. 34:3803–3815.
2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Luo J, Manning BD and Cantley LC:
Targeting the PI3K-Akt pathway in human cancer: Rationale and
promise. Cancer Cell. 4:257–262. 2003.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhang Y, Kwok-Shing Ng P, Kucherlapati M,
Chen F, Liu Y, Tsang YH, de Velasco G, Jeong KJ, Akbani R,
Hadjipanayis A, et al: A Pan-cancer proteogenomic atlas of
PI3K/AKT/mTOR pathway alterations. Cancer Cell. 31:820–832.e3.
2017.PubMed/NCBI View Article : Google Scholar
|