Introduction
Gastric cancer (GC) is one of the most common cancer
types, which originates from the gastric mucosa, neuroendocrine,
lymphoid or connective tissue of the gastric wall (1). Helicobacter pylori infection
(1,2), cigarette smoking (3), alcohol (4) and genetic syndromes (5) are common risk factors for GC. Surgical
resection is the optimal therapeutic option for GC. Increasing
evidence has demonstrated that genetic variants, such as those in
p53, adenomatous polyposis coli and human epidermal growth factor
receptor-2 (HER-2), contribute to the development of GC (6,7).
Targeted therapies against HER-2 have been applied for the
treatment of GC patients with HER-2 amplification (8). However, there is still a demand to
identify novel biomarkers and triggers of GC progression.
Podofilox, which is isolated from the North American
plant Podophyllum peltatum L., belongs to the lignan family,
and one of its derivatives is a potential antitumor factor
(9). This natural product has also
been isolated from Podophyllum emodi (9). According to previous studies,
podofilox derivatives exhibit inhibitory effects on the
proliferation and growth of various types of cancer. For example,
XWL-1-48, a novel podofilox derivative, induces apoptosis and
suppresses cell proliferation in breast cancer (10). Another derivative, 4β-NH-(benzo
heterocycles)-4-desoxy-podofilox, exhibits anticancer effects by
improving tubulin binding affinity in MCF-7, A549 and HeLa cells
(11).
Currently, the effects of podofilox and its
potential underlying molecular mechanism of action in GC remain
unclear. In this context, the present study aimed to investigate
whether podofilox may exert an anticancer effect in GC, and the
potential molecular mechanisms therein.
Materials and methods
The Cancer Genome Atlas (TCGA) data
analysis
For the analysis of public data, 375 GC tissue and
non-cancer tissues were analyzed using a dataset from (TCGA)
database (http://cancergenome.nih.gov).
Cell lines and culture
The human GC cell lines AGS and HGC-27 were
purchased from the American Type Culture Collection and maintained
according to the supplier's instructions. Cells were maintained in
DMEM supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin at 37˚C with 5%
CO2.
IC50 measurement
The IC50 of podofilox was determined
using the Cell Counting Kit-8 (CCK-8; Shanghai Yeasen Biotechnology
Co., Ltd.) assay. GC cells were seeded into 96-well plates in
triplicate at a density of 2,000 cells per well and treated with
various concentrations (0, 0.3125, 0.625, 1.25, 2.5, 5, 10, 20, 40
and 80 nM; dissolved in DMSO) of podofilox (cat. no. S18-28-5;
MedChemExpress). The plates were maintained at 37˚C with 5%
CO2. The CCK-8 assay was subsequently performed to
assess cell viability after 48 h. The culture medium was removed,
and 100 µl culture medium was added to each well with 10 µl Cell
CCK-8 reagent (Beyotime Institute of Biotechnology). After
incubation for 3 h at room temperature, cell viability was analyzed
by measuring the OD at 450 nm, using a microplate reader (Thermo
Fisher Scientific, Inc.).
ATG10 interference
Small interfering (si)RNAs against ATG10 (siATG10)-1
(5'-GAGUUCAUGAGUGCUAUAAGA-3') and siATG10-2
(5'-GCAACAGGAACAUCCAAUACU-3') and a control siRNA (siCtrl;
5'-UUCUCCGAACGUGUCACGU-3') were synthesized by Huzhou Hippo
Biotechnology Co., Ltd. A total of 3x105 cells were
seeded in 6-well plates and transfected with siRNAs (50 nM/well)
using Lipofectamine® RNAiMAX reagent (4 µl/well; Thermo
Fisher Scientific, Inc.) for 48 h at 37˚C, according to the
manufacturer's protocol. At 48 h post-transfection, knockdown
efficacy was determined by western blotting and the cells were
subjected to cell function experiments.
Cell viability assay
A total of 2x103/well AGS and HGC-27
cells were seeded into 96-well plates containing 100 µl DMEM (10%
FBS) and treated with 3.4 nM podofilox at 37˚C for 1, 2, 3, 4 and 5
days. CCK-8 reagent (10%) was subsequently added to each well and
cellular proliferation was analyzed at a wavelength of 450 nm after
incubation for 3 h at 37˚C.
Colony formation assay
AGS and HGC-27 cells were seeded at a density of
1,000 cells/well and incubated at 37˚C (5% CO2) with
vehicle or 3.4 nM podofilox. The culture medium, vehicle and
podofilox were changed every 2 days. After 7 days, the colonies
were washed with PBS, fixed with 100% methanol at room temperature
for 15 min, and then stained with 0.2% crystal violet solution at
room temperature for 30 min. Images were captured using a camera
(Nikon Corporation), and cell colonies >50 cells were counted
manually.
Western blotting
AGS and HGC-27 cells treated with or without
podofilox were lysed using a lysis buffer (Beyotime Institute of
Biotechnology). The concentration of total protein was detected
using a BCA Protein Assay Kit. Proteins (30 µg) were separated by
SDS-PAGE (12%), transferred on PVDF membranes (MilliporeSigma) and
blocked with non-fat milk at room temperature for 1 h. The
membranes were incubated with primary antibodies against
hexokinase-2 (HK2; cat. no. 2867; Cell Signaling Technology, Inc.),
pyruvate kinase M1/2 (PKM2; cat. no. 4053; Cell Signaling
Technology, Inc.), ATG10 (cat. no. ab124711; Abcam), p53 (cat. no.
sc-126; Santa Cruz Biotechnology, Inc.), c-Myc (cat. no. sc-40;
Santa Cruz Biotechnology, Inc.) and GAPDH (cat. no. sc-47724; Santa
Cruz Biotechnology, Inc.) at 4˚C overnight. Following primary
antibody incubation, the membranes were washed with TBST (0.1%
Tween) three times at room temperature, and incubated with
secondary antibodies (Santa Cruz Biotechnology, Inc. both 1:10,000;
normal mouse IgG, cat. no. sc-2748; and normal rat IgG, cat. no.
sc-2750) at room temperature for 3 h. Protein bands were visualized
using SuperSignal™ West Pico PLUS Chemiluminescent Substrate
(Thermo Fisher Scientific, Inc.). The western blot bands were
quantified using ImageJ software (v1.8.0).
Cell cycle analysis
AGS and HGC-27 cells (1x105) were seeded
and incubated with vehicle or podofilox (3.4 nM) for 48 h. The
cells were collected and incubated with 70% iced ethanol overnight.
Cell cycle distribution was assessed using the propidium iodide
(Shanghai Yeasen Biotechnology Co., Ltd.) staining kit, according
to the manufacturers' protocols, and analyzed via flow
cytometry.
Microarray analysis
Total RNA was extracted from AGS cells treated with
vehicle or podofilox (3.4 nM) using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The quality and quantity of the RNA were
determined using NanoDrop 2000. PrimeView Human Gene Expression
Array (Affymetrix, cat. no. 902487) was used to profile gene
expression. The following criteria were used to determine
statistical significance: P<0.05 and fold-change >1.3. The
Kyoto Encyclopedia of Genes and Genomes (KEGG) and HALLMARK
databases were used to perform pathway enrichment analyses
(12).
Statistical analysis
Statistical analysis was performed using GraphPad
prism 8.0 software (GraphPad Software, Inc.). All experiments were
performed in triplicate and data are presented as the mean ±
standard error of the mean. Student's t-test was used to compare
differences between two groups, whereas one way ANOVA followed by
Tukey's post hoc test were used to compare differences among
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Podofilox effectively inhibits cell
proliferation
To study the effects of podofilox in GC cells, the
IC50 of podofilox was determined. AGS and HGC-27 cells
were treated with various concentrations of podofilox ranging
between 0 and 80 nM, and the cell viability was determined at 48 h
post-treatment. The results demonstrated that 50% of AGS and HGC-27
cells died following incubation with 3.409 and 3.394 nM podofilox,
respectively (Fig. 1A and B). Subsequently, the present study
investigated whether podofilox exhibited a time-dependent
inhibitory effect on cell proliferation. GC cells were incubated
with vehicle or 3.4 nM podofilox. Cell viability was analyzed
between days 1 and 5. The results of the CCK-8 assay demonstrated
that 3.4 nM podofilox significantly suppressed cell proliferation
compared with that observed in the vehicle-treated cells (Fig. 1C and D). Consistent with these results, cell
colony formation was inhibited following treatment with podofilox
(Fig. 2A and B). Taken together, these results suggested
that podofilox effectively suppressed the proliferation and GC
cells for 5 days.
Podofilox inhibits cell cycle
progression
Next, the cell cycle was analyzed following
treatment with vehicle or podofilox by PI staining and flow
cytometric analysis. As presented in Fig. 3A and B, treatment with podofilox significantly
increased the numbers of AGS and HGC-27 cells in the
non-proliferative G0/G1 phase. These results
suggested that podofilox promoted cell cycle arrest.
Molecular changes following treatment
with podofilox
To determine the molecular mechanisms underlying the
inhibitory effects of podofilox in GC, the cells were treated with
vehicle and podofilox for 48 h, and total RNA was extracted for
microarray analysis. A total of 566 upregulated and 713
downregulated genes were identified following treatment with
podofilox (Fig. 4A and Table S1). The expression levels of ATG10,
an autophagy-related gene, were suppressed by podofilox treatment
compared with those detected in the vehicle control group. Based on
pathway enrichment analysis, ‘KEGG_cell_cycle’ and
‘Hallmark_MYC_Targets_v1’ pathways were also suppressed by
podofilox, while ‘Hallmark_p53_Pathway’ was activated by podofilox
(Fig. 4B).
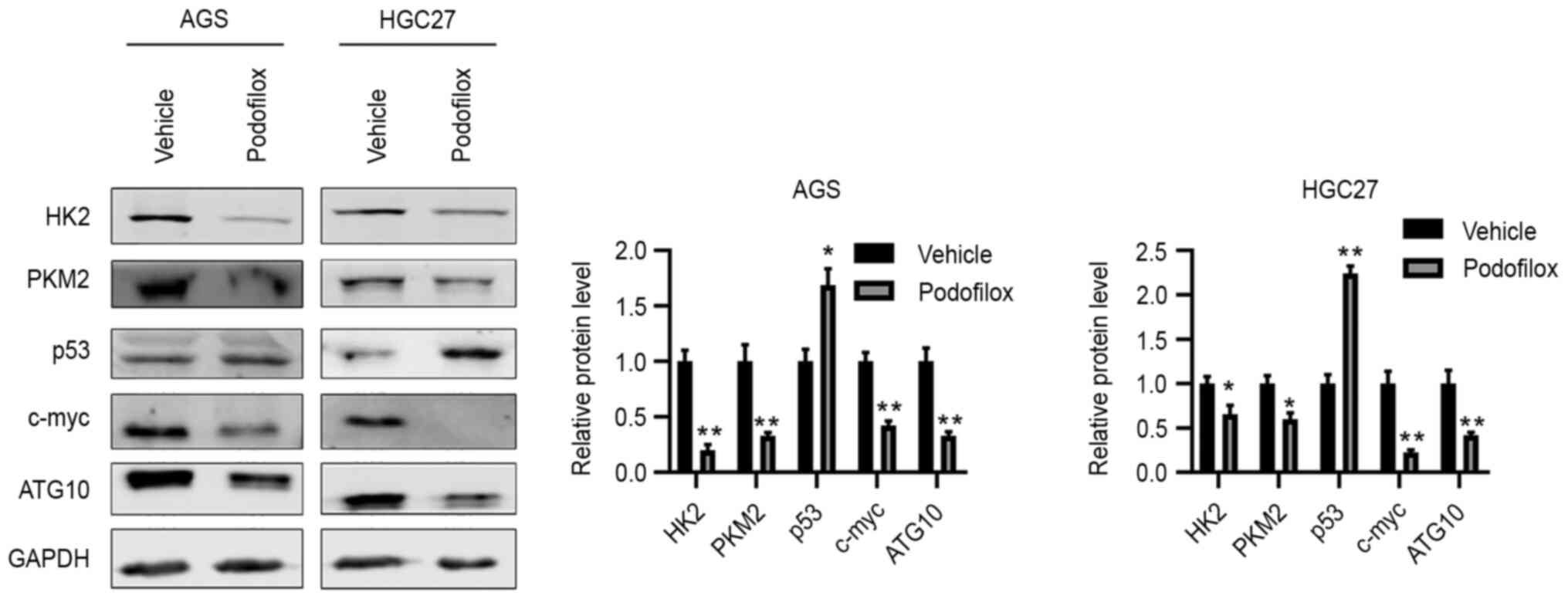
To validate the microarray results, western blot
analysis was performed, and the results demonstrated that compared
with the protein levels in the vehicle control group, podofilox
downregulated the protein expression levels of HK2, PKM2, c-Myc and
ATG10, and upregulated those of p53 (Fig. 5). Taken together, these results
suggested that podofilox may suppress the cell cycle and the
c-Myc/ATG10 axis, and activate p53.
ATG10 knockdown inhibits cell
proliferation
The role of ATG10 in cell proliferation was
subsequently investigated. The Cancer Genome Atlas (TCGA) database
revealed that ATG10 was upregulated in GC samples compared with
adjacent non-cancerous tissue (Fig.
6A). To determine the function of ATG10 in GC, ATG10 was
silenced in GC cells, and proliferation was assessed by CCK-8
assay. Western blot analysis was performed to assess the
transfection efficiency (Fig. 6B).
The results demonstrated that ATG10 knockdown decreased the
proliferation of HGC-27 cells compared with those in cells
transfected with siCtrl (Fig. 6C).
In AGS cells, ATG10 interference by siATG10-1 exhibited notable
inhibition; however, siATG10-2 had a marginal inhibitory effect on
the proliferation of AGS cells compared with that in the control
group (Fig. 6D). The results of the
colony formation assay demonstrated that both siRNAs targeting
ATG10 significantly suppressed colony formation in GC cells
(Fig. 6E and F). Thus, ATG10 may act as an oncogene in
GC.
Discussion
Advanced GC is an incurable malignancy as the
current therapeutic strategies, including surgery, targeted
therapies and immunotherapy, are ineffective against this disease
(13). Despite recent advances, the
prognosis of patients with GC remains poor (768,793 new deaths in
2020), and effective treatment is limited (14). The results of the present study
demonstrated that podofilox significantly suppressed the
proliferative ability of GC cells. In addition, cell cycle arrest
was induced by podofilox at the G0/G1 phase.
Taken together, these results suggested that podofilox may be used
as a therapeutic drug for the treatment of GC.
Podofilox, also termed podophyllotoxin, exhibits an
antiviral function against herpes viruses, warts and influenza, as
well as antitumor effects (15,16).
According to previous studies, its derivatives are widely used in
different types of cancer. For example, dendrimer-conjugated
podophyllotoxin suppresses the development of chemical-induced
hepatocellular carcinoma (HCC) in mice (17). In another study, 14 biotinylated
podophyllotoxin derivatives were designed and synthesized, which
exhibited potent cytotoxic effects against a number of cancer cell
lines, such as H1299 and H1975(18). Furthermore, the
4β-acetamidobenzofuranone-podophyllotoxin hybrids exhibit
anticancer effects in various types of cancer cells, such as
breast, lung and prostate cancer (19). The results of the present study
demonstrated that podofilox exhibited anticancer effects against GC
cells. The IC50 of podofilox in AGS and HGC-27 cells was
3.409 and 3.394 nM, respectively. Treatment with 3.4 nM podofilox
notably suppressed cell proliferation and colony formation compared
with those observed in the vehicle control groups. Collectively,
these results suggested that podofilox may be a promising drug
against GC. Podofilox has been reported to disrupt the microtubules
and inhibit cell cycle progression at the G1 phase
(20). In addition,
deoxypodophyllotoxin suppresses the cell cycle progression and
promotes apoptosis in human glioma (21). Consistent with these findings, the
results of the present study demonstrated that podofilox promoted
cell cycle arrest at the G0/G1 phase in GC
cells. Thus, podofilox may induce G0/G1 cell
cycle arrest in various types of cancer cells. However, these
results were insufficient to conclude whether cell cycle arrest may
be a general function of the drug or if it may be cell
line-specific. This should be investigated in other cancer cell
lines in the future.
The molecular basis by which podofilox inhibits
cancer cell proliferation and tumor growth has been investigated in
previous studies. For example, podofilox inhibits HCCLM3 and HepG2
cell proliferation and migration by inactivating the PI3K/AKT/mTOR
signaling pathway (22). In HCC
cells, podofilox inhibits the PI3K/AKT/mTOR pathway and activates
p53(23). However, the molecular
mechanisms underlying the effects of podofilox in GC remain
unknown. Thus, microarray analysis was performed in the present
study to determine the dysregulated genes following treatment with
podofilox. A total of 1,279 genes were dysregulated in
podofilox-treated AGS cells, including 566 upregulated and 713
downregulated genes. KEGG and HALLMARK pathway enrichment analyses
demonstrated that the cell cycle and the c-Myc pathway were
inhibited by podofilox. By contrast, the p53 signaling pathway was
activated by podofilox. Western blot analysis confirmed that the
protein expression levels of c-Myc and ATG10 were downregulated in
GC cells following treatment with podofilox compared with those in
vehicle-treated cells. The levels of glycolysis hallmark genes HK2
and PKM2, which are frequently upregulated in cancers (24), were downregulated by podofilox
compared with those in the control groups. A previously study
demonstrated that ATG10 was a downstream effector of c-Myc
(25). Notably, ATG10 was
upregulated in GC compared with normal tissues in samples from TCGA
database.
The podophyllotoxin-indirubin hybrid termed Da-1
suppresses the proliferation of leukemia cells and induces
autophagy (26). The
autophagy-inducing effect of podophyllotoxin has also been
demonstrated in HCC cells (27).
Since c-Myc signaling and its downstream effector ATG10 were both
inhibited by podofilox in the present study, the involvement of
ATG10 in GC cell proliferation was subsequently assessed. ATG10 was
silenced in GC cells by siRNA, and the results of the CCK-8 and
colony formation assays demonstrated that ATG10 knockdown
significantly suppressed the proliferation of HGC-27 and AGS cells.
Taken together, these results suggested that ATG10 may act as an
oncogene in GC, and podofilox may suppress the proliferation of GC
cells by downregulating ATG10.
In conclusion, the results of the present study
demonstrated the antitumor effects of podofilox in GC. The cell
cycle was arrested by podofilox at the G0/G1
phase, whereas apoptosis was unaffected. In addition, podofilox
induced autophagy by regulating ATG10.
Supplementary Material
List of dysregulated genes in
microarray analysis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Key R&D and
transformation program of Qinghai Province-Special project of
science and technology assistance (grant no.2021-QY-213), the
Applied Basic Research of Qinghai (grant no. 2018-ZJ-744), the
Light of the West (grant no. 2019-33), the National Natural Science
Foundation of China (grant no. 81460429), the Chunhui Plan of
Ministry of Education of China (grant no. Z2017037), the Open
Project of State Key Laboratory of Plateau Ecology and Agriculture,
Qinghai University (grant no. 2019-ZZ-07), the Team Project of
Qinghai University Medical College (grant no. 2020-KYT-2).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request. The datasets generated and/or analyzed during the current
study are available in the Gene Expression Omnibus repository,
under the accession number GSE172408.
Authors' contributions
JA and ZS conceived the present study, performed the
experiments and prepared the manuscript. YL and SD performed the
cell cycle analysis. XM and LA performed the western blot analysis.
Yun Y, DJ and Yup Y performed the microarray analysis. QC performed
the colony formation assay. JA and ZS wrote and revised the
manuscript draft. All authors read and approved the final
manuscript. JA and ZS confirmed the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Joshi S and Badgwell B: Current treatment
and recent progress in gastric cancer. CA Cancer J Clin.
71:264–279. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ajani J, Lee J, Sano T, Janjigian Y, Fan D
and Song S: Gastric adenocarcinoma. Nat Rev Dis Primers.
3(17036)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang LY, Zhao S, Lv GJ, Ma XJ and Zhang
JB: Mechanisms of resveratrol in the prevention and treatment of
gastrointestinal cancer. World J Clin Cases. 8:2425–2437.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Deng W, Jin L, Zhuo H, Vasiliou V and
Zhang Y: Alcohol consumption and risk of stomach cancer: A
meta-analysis. Chem Biol Interact. 336(109365)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lerner B and Llor X: Genetic gastric
cancer risk syndromes. Curr Treat Options Gastroenterol.
18:604–615. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Huang K, Ramnarayanan K, Zhu F, Srivastava
S, Xu C, Tan ALK, Lee M, Tay S, Das K, Xing M, et al: Genomic and
epigenomic profiling of high-risk intestinal metaplasia reveals
molecular determinants of progression to gastric cancer. Cancer
Cell. 33:137–150.e5. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Machlowska J, Baj J, Sitarz M, Maciejewski
R and Sitarz R: Gastric Cancer: Epidemiology, risk factors,
classification, genomic characteristics and treatment strategies.
Int J Mol Sci. 21(4012)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shitara K, Bang Y, Iwasa S, Sugimoto N,
Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, et al:
Trastuzumab deruxtecan in previously treated HER2-positive gastric
cancer. N Engl J Med. 382:2419–2430. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu YQ, Tian J, Qian K, Zhao XB,
Morris-Natschke SL, Yang L, Nan X, Tian X and Lee KH: Recent
progress on C-4-modified podophyllotoxin analogs as potent
antitumor agents. Med Res Rev. 35:1–62. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang Y, Sun H, Xiao Z, Zhang G, Zhang D,
Bao X, Li F, Wu S, Gao Y and Wei N: DNA damage and apoptosis
induced by a potent orally podophyllotoxin derivative in breast
cancer. Cell Commun Signal. 16(52)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhao W, He L, Xiang TL and Tang YJ:
Discover 4β-NH-(6-aminoindole)-4-desoxy-podophyllotoxin with
nanomolar-potency antitumor activity by improving the tubulin
binding affinity on the basis of a potential binding site nearby
colchicine domain. Eur J Med Chem. 170:73–86. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Smyth E, Nilsson M, Grabsch H, van Grieken
N and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhao W, Cong Y, Li H, Li S, Shen Y, Qi Q,
Zhang Y, Li YZ and Tang YJ: Challenges and potential for improving
the druggability of podophyllotoxin-derived drugs in cancer
chemotherapy. Nat Prod Rep. 38:470–488. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cohen T, Schwarz T, Vigant F, Gardner T,
Hernandez R, Lee B and Tortorella D: The microtubule inhibitor
podofilox inhibits an early entry step of human cytomegalovirus.
Viruses. 8(295)2016.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Tracz-Gaszewska Z and Dobrzyn P:
Stearoyl-CoA desaturase 1 as a therapeutic target for the treatment
of cancer. Cancers (Basel). 11(948)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zi CT, Gao YS, Yang L, Feng SY, Huang Y,
Sun L, Jin Y, Xu FQ, Dong FW, Li Y, et al: Design, synthesis, and
biological evaluation of novel biotinylated podophyllotoxin
derivatives as potential antitumor agents. Front Chem.
7(434)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Paidakula S, Nerella S, Vadde R, Kamal A
and Kankala S: Design and synthesis of
4β-Acetamidobenzofuranone-podophyllotoxin hybrids and their
anti-cancer evaluation. Bioorg Med Chem Lett. 29:2153–2156.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hao SY, Feng SL, Wang XR, Wang Z, Chen SW
and Hui L: Novel conjugates of podophyllotoxin and coumarin:
Synthesis, cytotoxicities, cell cycle arrest, binding CT DNA and
inhibition of Topo IIβ. Bioorg Med Chem Lett. 29:2129–2135.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang W, Gao W, Zhang L, Zhang D, Zhao Z
and Bao Y: Deoxypodophyllotoxin inhibits cell viability and
invasion by blocking the PI3K/Akt signaling pathway in human
glioblastoma cells. Oncol Rep. 41:2453–2463. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li Y, Huang T, Fu Y, Wang T, Zhao T, Guo
S, Sun Y, Yang Y and Li C: Antitumor activity of a novel dual
functional podophyllotoxin derivative involved PI3K/AKT/mTOR
pathway. PLoS One. 14(e0215886)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li Y, Wang T, Sun Y, Huang T and Li C, Fu
Y, Li Y and Li C: p53-mediated PI3K/AKT/mTOR pathway played a role
in ptox-induced EMT inhibition in liver cancer cell lines. Oxid Med
Cell Longev. 2019(2531493)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Guo W, Qiu Z, Wang Z, Wang Q, Tan N, Chen
T, Chen Z, Huang S, Gu J, Li J, et al: MiR-199a-5p is negatively
associated with malignancies and regulates glycolysis and lactate
production by targeting hexokinase 2 in liver cancer. Hepatology.
62:1132–1144. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sun W, Li J, Zhou L, Han J, Liu R, Zhang
H, Ning T, Gao Z, Liu B, Chen X and Ba Y: The
c-Myc/miR-27b-3p/ATG10 regulatory axis regulates chemoresistance in
colorectal cancer. Theranostics. 10:1981–1996. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang J, Long L, Chen Y, Xu Y and Zhang L:
Design, synthesis and antineoplastic activity of novel hybrids of
podophyllotoxin and indirubin against human leukaemia cancer cells
as multifunctional anti-MDR agents. Bioorg Med Chem Lett.
28:1817–1824. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ren J, Liu Y, Li L, Zhao Y, Li Z, Wu C,
Chen L and Hu K: OAMDP, a novel podophyllotoxin derivative, induces
apoptosis, cell cycle arrest and autophagy in hepatoma HepG2 cells.
Cell Biol Int. 42:194–204. 2018.PubMed/NCBI View Article : Google Scholar
|