Introduction
Endometriosis is an estrogen-dependent condition
that is characterized by the ectopic growth of endometrial tissue,
which can result in symptoms such as pelvic pain and dysmenorrhea
and problems such as infertility (1,2).
Although endometriosis is known to be a benign disease, it exhibits
characteristics common of malignant tumors, such as infinite
growth, angiogenesis, infiltration and destruction of surrounding
tissues, and local or distant metastasis (3,4).
Furthermore, the migratory and invasive properties of ectopic
endometrial stromal cells (ESCs) were found to be similar to those
of malignant cancer cells (5).
However, to the best of our knowledge, the molecular mechanisms
underlying the proliferation of ectopic ESCs remain unclear, thus
further studies on endometriosis are required to accelerate the
development of effective treatment strategies.
MicroRNAs (miRNAs/miRs) are small, non-coding RNA
molecules of ~20-22 nucleotides in length, which are involved in
cell proliferation and regulating the expression of target genes
(6,7). Previous studies have suggested that
miRNAs were associated with several types of human disease,
including cancer, and cardiovascular, gynecological and
inflammatory diseases (8-11).
Prislei et al (12)
demonstrated that miR-200c affected the proliferation of ovarian
cancer cell lines by regulating tubulin β 3 class III expression.
Furthermore, He et al (13)
confirmed the role of miR-155 in pathological cardiac remodeling,
as it was discovered that miR-155-induced targeting of tumor
protein p53-inducible nuclear protein 1 improved pathological
cardiac remodeling (13). Previous
studies have indicated that several types of miRNA participate in
the progression of endometriosis, as the expression profiles of
miRNAs were demonstrated to be different (abnormal decrease and
increase in contrast) between non-ectopic and ectopic endometrial
tissues (14,15). Of these, miR-139-5p has been
demonstrated to play key roles in several types of disease. For
example, Song et al (16)
reported that miR-139-5p was a potential tumor suppressor and
prognostic factor for colorectal cancer. Furthermore, miR-139-5p
was shown to play a regulatory role in numerous types of disease,
such as neurological diseases, atherosclerosis and endometriosis
(17-19).
Therefore, it is suggested that miR-139-5p plays important roles in
the regulation of cellular functions. In addition, a previous study
reported that miR-139-5p expression was significantly upregulated
in ectopic stroma and ectopic stroma cells (19); however, to the best of our
knowledge, its underlying molecular mechanism remains unclear. As
ESCs have been found to play crucial roles in endometriosis, we
hypothesized that miR-139-5p may be involved in endometriosis by
regulating ESC function.
The present study aimed to investigate whether
miR-139-5p served a role in endometriosis by regulating the
malignant behaviors (apoptosis, viability, migration and invasion)
of ectopic ESCs. Furthermore, the study sought to determine its
molecular mechanism of action to provide novel therapeutic
strategies for endometriosis.
Materials and methods
Patient samples
The present study was approved by the Ethics
Committee of The Second Affiliated Hospital of Shenzhen University
(Shenzhen, China) and written informed consent was provided by all
participants prior to the study start. A total of 15 paired ectopic
and non-ectopic endometrial tissue samples were collected from
premenopausal women (mean age, 30 years; age range: 26-36 years)
who underwent gynecological procedures for benign indications at
The Second Affiliated Hospital of Shenzhen University from April
2018 to April 2019. Normal endometrial tissue samples were
collected from 15 premenopausal women (age range, 26-36 years) with
hysteromyoma at The Second Affiliated Hospital of Shenzhen
University (Shenzhen Baoan District People's Hospital; Shenzhen,
China) from April 2018 to April 2019 and used as the control
samples. The inclusion criteria for patients with endometriosis
were as follows: i) Patients in the mid-to-late proliferative phase
of the disease; and ii) patients who had not received hormonal
treatment 3 months prior to surgery. Patients who had malignancy or
other diseases, such as submucous myoma, endometrial hyperplasia,
adenomyosis or chronic pelvic inflammation were excluded from the
present study.
Extraction and culture of normal,
non-ectopic and ectopic ESCs
Non-ectopic ESCs were isolated from non-ectopic
endometrial tissue, ectopic ESCs were isolated from ectopic
endometrial tissue and the normal ESCs were isolated from normal
tissue samples. Tissues samples were washed three times with PBS
and dissected, prior to digestion with 0.1% type IV collagenase
solution and 0.25% trypsin at 37˚C for 2 h. Subsequently, normal,
non-ectopic and ectopic ESCs were extracted from tissue samples as
previously described (20), and
cultured in Ham's F12/DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific,
Inc.) at 37˚C for 24 h in 5% CO2. The purity of the ESCs
was determined following the third passage, and ESCs with >99%
purity were selected for subsequent experimentation.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from normal, non-ectopic and
ectopic ESCs and endometrial tissues using
RNAqueousTM-96 Total RNA Isolation kit (Invitrogen;
Thermo Fisher Scientific, Inc.). Total RNA was reverse transcribed
into first strand cDNA using a RevertAid First Strand cDNA
Synthesis kit (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. qPCR was subsequently
performed on a Prism 7000 Real-Time PCR Detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using a SYBR qPCR
Master Mix (Vazyme Biotech Co., Ltd.), according to the
manufacturer's protocol. The primer sequences used for the qPCR
were designed by Sangon Biotech Co., Ltd. and were as follows:
GAPDH forward, 5'-CTTTGGTATCGTGGAAGGACTC-3' and reverse,
5'-GTAGAGGCAGGGATGATGTTCT-3'; U6 forward,
5'-GCTTCGGCAGCACATATACTAAAAT-3' and reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'; miR-139-5p forward,
5'-AGCGGCATGATCAACATTTGCTAAAG-3' and reverse,
5'-TCTGTATGGTTTTCCTTCCGGCTT-3'; BBC3 forward,
5'-ACGACCTCAACGCGCAGTA-3' and reverse, 5'-CTAGTTGGGCTCCATTTCTGG-3'.
The following thermocycling conditions were used for the qPCR:
Initial denaturation at 95˚C for 5 min; followed by 40 cycles of
95˚C for 10 sec, 60˚C for 30 sec and 72˚C for 30 sec. Relative mRNA
expression levels of miR-139-5p and Bcl-2 binding component 3
(BBC3) were quantified using the 2-ΔΔCq method (21) and normalized to the internal
reference genes, U6 or GAPDH, respectively. All experiments were
performed in triplicate.
Cell transfection
Ectopic ESCs were seeded into 6-well plates at a
density of 5x104 cells/ml and cultured at 37˚C
overnight. A total of 50 nM inhibitor control
(5'-GGACCAAATCTCGAGATTTGG-3'; Shanghai GenePharma, Co., Ltd.), 50
nM miR-139-5p inhibitor (5'-ACTGGAGACACGTGCACTGTAGA-3'; Shanghai
GenePharma, Co., Ltd.), 1 µM control-small interfering (si)RNA
(cat. no. sc-36869; Santa Cruz Biotechnology, Inc.), 1 µM
BBC3-siRNA (cat. no. sc-37153; Santa Cruz Biotechnology, Inc.), 50
nM miR-139-5p inhibitor + 1 µM control-siRNA or 50 nM miR-139-5p
inhibitor + 1 µM BBC3-siRNA were transfected into ectopic ESCs
using Lipofectamine® 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Following 48 h of transfection at 37˚C, cells were collected
(centrifugation at 4˚C at 12,000 x g for 15 min) and the
transfection efficiency was determined using RT-qPCR. Subsequent
experiments were performed 48 h after transfection.
miRNA target analysis and dual
luciferase reporter assay
The binding relationship between miR-139-5p and BBC3
was determined using TargetScan software (version 7.1; www.targetscan.org/vert_71). The 3'-untranslated
region (UTR) of BBC3 that contained the target sequence of
miR-139-5p was attained via RT-qPCR and integrated into the pmirGLO
vector (Promega Corporation) to construct the wild-type (WT)-BBC3
reporter vector. Similarly, a mutant (MUT)-BBC3 reporter vector was
also constructed using a QuikChange Site-Directed Mutagenesis kit
(Stratagene; Agilent Technologies, Inc.) according to the
manufacturer's instructions. Following incubation at 37˚C for 24 h,
293T cells (ATCC) were co-transfected with WT-BBC3 or MUT-BBC3
luciferase reporter vectors and miR-139-5p mimic
(5'-UCUACAGUGCACGUGUCUCCAG-3'; Shanghai GenePharma Co., Ltd.) or
mimic control (5'-UCUCCGAACGUGUCACGU-3'; Shanghai GenePharma Co.,
Ltd.) using Lipofectamine 2000 at 37˚C for 48 h. Following the
transfection, firefly and Renilla luciferase activities were
detected using a Dual Luciferase Reporter assay system (Promega
Corporation), according to the manufacturer's protocol. Firefly
luciferase activity was normalized to Renilla luciferase
activity. All experiments were performed in triplicate.
Western blotting
Total protein from endometrial tissues and ESCs was
extracted using a Radio Immunoprecipitation Assay (RIPA) Lysis
Solution (Beyotime Institute of Biotechnology; cat. no. P0013B) and
quantified using a BCA assay kit (Pierce; Thermo Fisher Scientific,
Inc.). Total protein (40 µg/lane) was separated via 10% SDS-PAGE
and then transferred onto a polyvinylidene difluoride membrane.
Membranes were blocked with 5% non-fat milk at room temperature for
1 h and subsequently incubated with primary antibodies against BBC3
(1:1,000; cat. no. ab9643; Abcam) and GAPDH (1:1,000; cat. no.
ab9485; Abcam) overnight at 4˚C. Following the primary antibody
incubation, the membranes were washed three times with PBS-0.1%
Tween-20 and incubated with an HRP-conjugated secondary antibody
(1:2,000; cat. no. 7074; Cell Signaling Technology, Inc.) at room
temperature for 1 h. Protein bands were visualized using enhanced
chemiluminescence (Pierce; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. All experiments were
performed in triplicate.
MTT assay
Following transfection for 48 h at 37˚C, ectopic
ESCs were seeded into 96-well plates and incubated at 37˚C
overnight. Subsequently, 10 µl MTT solution (Beyotime Institute of
Biotechnology) was added to each well and incubated at 37˚C for a
further 4 h. Following the MTT incubation, the purple formazan
crystals were dissolved in 100 µl DMSO and cell viability was
subsequently analyzed at a wavelength of 490 nm using a microplate
reader (Bio-Rad Laboratories, Inc.). Relative cell viability of
each experimental group was normalized to the control group using
optical density values. All experiments were performed in
triplicate.
Flow cytometric analysis of
apoptosis
Following transfection for 48 h at 37˚C, ectopic
ESCs were seeded into 6-well plates at 37˚C overnight, collected
via trypsinization by centrifugation at 1,000 x g at 4˚C for 5 min,
washed with PBS and then re-suspended in 1X binding buffer
(Beyotime Institute of Biotechnology) from the Annexin-V/propidium
iodide (PI) Apoptosis Detection kit according to the manufacturer's
protocol. A total of 100 µl cell suspension was transferred into a
5 ml tube, and cells were respectively stained with 5 µl Annexin
V-FITC and 5 µl PI (Beyotime Institute of Biotechnology) at room
temperature in the dark for 15 min, according to the manufacturer's
protocol. Apoptotic cells (the percentage of early + late apoptotic
cells) were analyzed using a FACSCalibur flow cytometer (BD
Biosciences) and the data were analyzed using FlowJo software
(version 7.6.1; FlowJo LLC). All experiments were performed in
triplicate.
Transwell assays
For the cell invasion and migration assays,
following transfection for 48 h at 37˚C, ectopic ESCs
(2x104 cells/well) were plated into the upper chambers
of Transwell plates in serum-free DMEM (Gibco; Thermo Fisher
Scientific, Inc.). Transwell membranes were precoated with Matrigel
(BD Biosciences) at 37˚C for 30 min for the invasion assay only.
DMEM supplemented with 10% FBS was plated into the lower chambers.
Following incubation at 37˚C for 24 h, the migratory or invasive
cells in the lower chambers were fixed with 4% paraformaldehyde at
room temperature for 30 min and subsequently stained with 0.1%
crystal violet at room temperature for 15 min. Stained cells were
counted in five randomly selected fields of view using a light
microscope (magnification, x100). All experiments were performed in
triplicate.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 18.0; SPSS, Inc.). Data are presented as the mean
± SD. Statistical differences between two groups were assessed
using an unpaired Student's t-test (for luciferase activity
analysis), while a one-way ANOVA followed by a Tukey's post hoc
test was used for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference. All experiments
were performed in triplicate.
Results
miR-139-5p expression levels are
upregulated in and ectopic endometriosis tissues and ESCs
miR-139-5p expression levels in patients with or
without endometriosis were detected via RT-qPCR. The results
demonstrated that miR-139-5p expression was significantly
upregulated in ectopic endometrial samples compared with the normal
endometrium samples (Fig. 1A).
Subsequently, miR-139-5p expression levels were assessed in ESCs,
and the results revealed that the expression levels of miR-139-5p
were significantly upregulated in ectopic ESCs compared with normal
ESCs (Fig. 1B).
BBC3 is a direct target of
miR-139-5p
To determine the underlying molecular mechanisms of
miR-139-5p in endometriosis, its candidate targets were predicted
using TargetScan software, which identified BBC3 as a potential
target of miR-139-5p (Fig. 2A). A
dual luciferase reporter assay was subsequently performed in 293T
cells to identify the binding sites of miR-139-5p in the BBC3
3'-UTR. The results demonstrated that the co-transfection with the
miR-139-5p mimic significantly decreased the relative luciferase
activity of the WT-BBC3 3'-UTR compared with co-transfection with
the mimic control; however, no significant differences were
observed in the relative luciferase activities of the MUT-BBC3
3'-UTRs in the cells co-transfected with the mimic control or
miR-139-5p mimic (Fig. 2B). Taken
together, these results suggested that BBC3 is a direct target of
miR-139-5p.
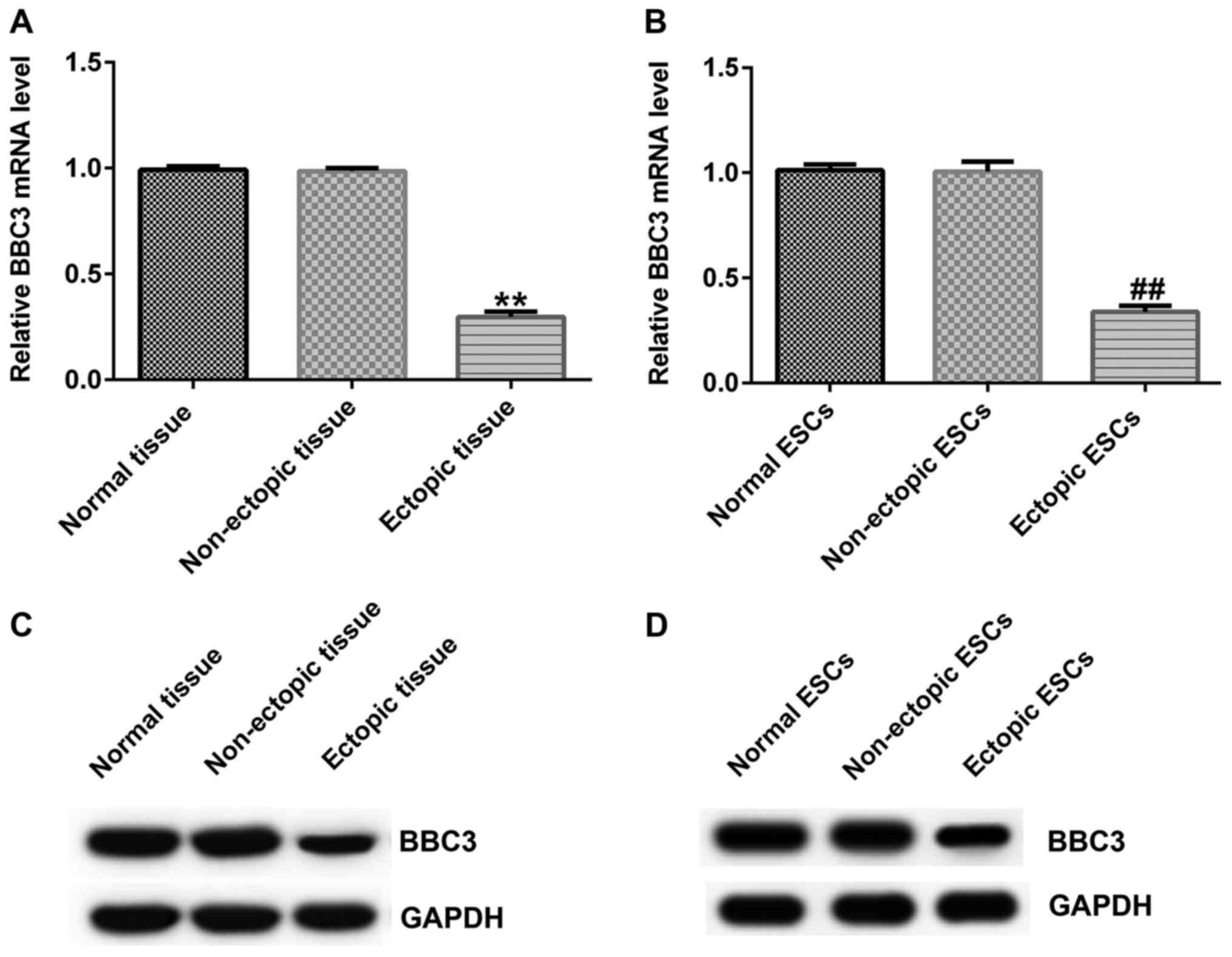
BBC3 expression levels are
downregulated in ectopic endometriosis tissues and ESCs
BBC3 mRNA expression levels in tissue samples and
ESCs were determined via RT-qPCR analysis. The results demonstrated
that BBC3 mRNA expression levels were significantly downregulated
in ectopic endometrial tissues and ESCs compared with the normal
endometrium tissues and ESCs, respectively (Fig. 3A and B). Furthermore, BBC3 protein expression
levels were determined using western blotting. Similarly, BBC3
protein expression levels were markedly downregulated in ectopic
tissues and ESCs compared with the normal endometrium tissues and
ESCs, respectively (Fig. 3C and
D).
BBC3 is negatively regulated by
miR-139-5p
To determine the roles of miR-139-5p and BBC3 in
endometriosis, ectopic ESCs were transfected with inhibitor
control, miR-139-5p inhibitor, control-siRNA or BBC3-siRNA. mRNA
and protein expression levels of miR-139-5p and BBC3 were detected
via RT-qPCR and western blotting, respectively. The results
demonstrated that transfection with the miR-139-5p inhibitor
significantly downregulated miR-139-5p expression in ectopic ESCs
compared with the inhibitor control group (Fig. 4A). Similarly, compared with the
control-siRNA group, BBC3-siRNA significantly downregulated BBC3
mRNA expression levels in ectopic ESCs (Fig. 4B). Conversely, transfection with the
miR-139-5p inhibitor significantly upregulated BBC3 expression
levels in ectopic ESCs compared with the inhibitor control group,
which was reversed following co-transfection with BBC3-siRNA
(Fig. 4C and D).
BBC3 reverses the effects of
miR-139-5p on viability, apoptosis, migration and invasion in
ectopic ESCs
Flow cytometric analysis, and MTT and Transwell
assays were performed to determine the potential underlying
molecular mechanism of miR-139-5p in the regulation of cell
apoptosis, viability, and migration and invasion in endometriosis.
The results of the MTT assay demonstrated that transfection with
the miR-139-5p inhibitor significantly decreased the viability of
ectopic ESCs compared with the inhibitor control group, which was
reversed following the combined knockdown of BBC3 (Fig. 5A). Similarly, the results of the
Transwell assays indicated that the inhibitory effects of the
miR-139-5p inhibitor on the migration and invasion of ectopic ESCs
were suppressed following the knockdown of BBC3 (Figs. 5B and C, and S1
and S2). Furthermore, transfection
with the miR-139-5p inhibitor significantly increased the levels of
apoptosis in ectopic ESCs, which were partially reversed following
co-transfection with BBC3-siRNA (Fig.
5D and E).
Discussion
A common histological feature of endometriosis is
the presence of dense fibrous tissue around the endometrial glands
and stroma (22). Furthermore, the
migration and invasion of ectopic ESCs were found to be similar to
that observed of malignant cancer cells (5). Previous studies have demonstrated that
the aberrant expression of miRNAs was implicated in the progression
of endometriosis (23,24). Both the migratory and invasive
abilities of endometriotic cells were discovered to play key roles
in the development of endometriosis (25). In fact, miR-139-5p was found to
serve an important role in the progression and development of
several types of tumor. For example, miR-139-5p expression levels
were reported to be downregulated in endometrial, colorectal and
small cell lung cancer (17,26,27).
Conversely, Rekker et al (19) reported that miR-139-5p expression
was significantly upregulated in ectopic endometrial tissues and
ESCs. The present study extracted ectopic, non-ectopic and normal
ESCs from related endometrial samples in premenopausal women.
Consistent with the latter finding, the results of the present
study demonstrated that miR-139-5p expression levels were
significantly upregulated in ectopic endometrial samples and ESCs,
suggesting that miR-139-5p may be involved in the progression of
endometriosis.
It is well-known that miRNAs exert their biological
function by binding to the 3'-UTR of target genes, and they have
been found to be involved in the development of endometriosis
(6,7,23,24).
To further investigate the underlying molecular mechanism of
miR-139-5p in the development of endometriosis, BBC3 was verified
as a direct target of miR-139-5p and was negatively regulated by
miR-139-5p in endometriosis. BBC3, also known as p53 upregulated
modulator of apoptosis, is a member of the Bcl-2 family, which is
involved in regulating cell apoptosis (28). BBC3 is a positive regulator of cell
death and induces apoptosis when cells are appropriately stimulated
(29,30). The results of the present study
demonstrated that the expression levels of BBC3 were downregulated
in ectopic endometrial tissues and ESCs. The migratory and invasive
properties of ectopic ESCs were found to be similar to those of
malignant cancer cells (5). Thus,
to determine whether miR-139-5p affected the apoptosis, viability,
migration and invasion of ectopic ESCs by directly targeting BBC3,
a series of experiments were performed in ectopic ESCs following
the knockdown of miR-139-5p or BBC3 expression. The results
demonstrated that transfection with the miR-139-5p inhibitor
suppressed the viability, migration and invasion of ectopic ESCs,
and induced cell apoptosis; however, all these effects were
reversed following the knockdown of BBC3. The data indicated that
the miR-139-5p inhibitor may negatively regulate viability,
migration and invasion, while inducing apoptosis in ectopic ESCs by
targeting BBC3 to participate in the occurrence and development of
endometriosis.
However, there were several limitations to the
present study. First, the sample size used in the study was small
and larger sample sizes should be used to further investigate the
expression levels of miR-139-5p in patients with endometriosis and
ectopic ESCs. In addition, normal tissue samples collected from
patients with hysteromyoma were used as the control samples in this
study. Unfortunately, fibroids can alter the local environment in
the uterine cavity and the patients are typically older (31), therefore, selecting the endometrial
tissue of patients with uterine fibroids as the control group may
not be ideal. Moreover, the current study focused on the effect of
miR-139-5p on ectopic ESCs. To further verify the present results,
the expression levels of miR-139-5p in endometrial epithelial cells
and endometrial stromal cells in normal, non-ectopic and ectopic
samples should be further analyzed, which is an aim of future
studies.
In conclusion, the results of the present study
indicated that miR-139-5p regulated the viability, migration,
invasion and apoptosis of ectopic ESCs by directly targeting BBC3.
These findings suggested that miR-139-5p may represent a potential
target for the treatment of endometriosis and provide novel
opportunities for clinical therapies for endometriosis.
Supplementary Material
Representative images of the Transwell
migration assay for each group. Magnification, x200. miR, microRNA;
BBC3, Bcl-2 binding component 3; siRNA, small interfering RNA.
Representative images of the Transwell
invasion assay for each group. Magnification, x200. miR, microRNA;
BBC3, Bcl-2 binding component 3; siRNA, small interfering RNA.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LF contributed to the study design, data collection,
statistical analysis, data interpretation and manuscript
preparation. XC, SZ and YC contributed to the data collection and
statistical analysis. YY contributed to the data collection,
statistical analysis and manuscript preparation. LF and YY
confirmed the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Second Affiliated Hospital of Shenzhen University
(Shenzhen, China) and written informed consent was provided by all
participants prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Muñoz-Hernando L, Muñoz-Gonzalez JL,
Marqueta-Marques L, Alvarez-Conejo C, Tejerizo-García Á,
Lopez-Gonzalez G, Villegas-Muñoz E, Martin-Jimenez A and
Jiménez-López JS: Endometriosis: Alternative methods of medical
treatment. Int J Women's Health. 7:595–603. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Marí-Alexandre J, Barceló-Molina M,
Olcina-Guillem M, García-Oms J, Braza-Boïls A and Gilabert-Estellés
J: MicroRNAs: New players in endometriosis. World J Obstet Gynecol.
5:28–38. 2016.
|
|
3
|
Yerlikaya G, Balendran S, Pröstling K,
Reischer T, Birner P, Wenzl R, Kuessel L, Streubel B and Husslein
H: Comprehensive study of angiogenic factors in women with
endometriosis compared to women without endometriosis. Eur J Obstet
Gynecol Reprod Biol. 204:88–98. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ferrero S, Barra F and Leone Roberti
Maggiore U: Current and emerging therapeutics for the management of
endometriosis. Drugs. 78:995–1012. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Diao R, Wei W, Zhao J, Tian F, Cai X and
Duan YG: CCL19/CCR7 contributes to the pathogenesis of
endometriosis via PI3K/Akt pathway by regulating the proliferation
and invasion of ESCs. Am J Reprod Immunol.
78(e12744)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hébert SS, Wang WX, Zhu Q and Nelson PT: A
study of small RNAs from cerebral neocortex of pathology-verified
Alzheimer's disease, dementia with lewy bodies, hippocampal
sclerosis, frontotemporal lobar dementia, and non-demented human
controls. J Alzheimers Dis. 35:335–348. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li M, Marin-Muller C, Bharadwaj U, Chow
KH, Yao Q and Chen C: MicroRNAs: Control and loss of control in
human physiology and disease. World J Surg. 33:667–684.
2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Farazi TA, Spitzer JI, Morozov P and
Tuschl T: miRNAs in human cancer. J Pathol. 223:102–115.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sayed AS, Xia K, Salma U, Yang T and Peng
J: Diagnosis, prognosis and therapeutic role of circulating miRNAs
in cardiovascular diseases. Heart Lung Circ. 23:503–510.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Santamaria X and Taylor H: MicroRNA and
gynecological reproductive diseases. Fertil Steril. 101:1545–1551.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xia G, Bao L, Gao W, Liu S, Ji K and Li J:
Differentially expressed miRNA in inflammatory mucosa of chronic
rhinosinusitis. J Nanosci Nanotechno. 15:2132–2139. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Prislei S, Martinelli E, Mariani M,
Raspaglio G, Sieber S, Ferrandina G, Shahabi S, Scambia G and
Ferlini C: miR-200c and HuR in ovarian cancer. BMC Cancer.
13(72)2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
He W, Huang H, Xie Q, Wang Z, Fan Y, Kong
B, Huang D and Xiao Y: miR-155 knockout in fibroblasts improves
cardiac remodeling by targeting tumor protein p53-inducible nuclear
protein 1. J Cardiovasc Pharmacol Ther. 21:423–435. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lee JW, Park YA, Choi JJ, Lee YY, Kim CJ,
Choi C, Kim TJ, Lee NW, Kim BG and Bae DS: The expression of the
miRNA-200 family in endometrial endometrioid carcinoma. Gynecol
Oncol. 120:56–62. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hsu CY, Hsieh TH, Tsai CF, Tsai HP, Chen
HS, Chang Y, Chuang HY, Lee JN, Hsu YL and Tsai EM: miRNA-199a-5p
regulates VEGFA in endometrial mesenchymal stem cells and
contributes to the pathogenesis of endometriosis. J Pathol.
232:330–343. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Song M, Yin Y, Zhang J, Zhang B, Bian Z,
Quan C, Zhou L, Hu Y, Wang Q, Ni S, et al: miR-139-5p inhibits
migration and invasion of colorectal cancer by downregulating AMFR
and NOTCH1. Protein Cell. 5:851–861. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu H, Yin Y, Hu Y, Feng Y, Bian Z, Yao S,
Li M, You Q and Huang Z: miR-139-5p sensitizes colorectal cancer
cells to 5-fluorouracil by targeting NOTCH-1. Pathol Res Pract.
212:643–649. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
van den Akker EK, Dor FJ, IJzermans JN and
de Bruin RW: MicroRNAs in kidney transplantation: Living up to
their expectations. J Transplant. 2015(354826)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rekker K, Tasa T, Saare M, Samuel K,
Kadastik Ü, Karro H, Götte M, Salumets A and Peters M:
Differentially-expressed miRNAs in ectopic stromal cells contribute
to endometriosis development: The plausible role of miR-139-5p and
miR-375. Int J Mol Sci. 19(3789)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu H, Zhang Z, Xiong W, Zhang L, Xiong Y,
Li N, He H, Du Y and Liu Y: Hypoxia-inducible factor-1α promotes
endometrial stromal cells migration and invasion by upregulating
autophagy in endometriosis. Reproduction. 153:809–820.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Apostol R, Sirota I, Mrkaic A and Nezhat
FR: Benign ovarian tumors and endometriosis (106). Obstet Gynecol.
125:S39–S40. 2015.
|
|
23
|
Olson MR, Vadlapatla NM, Khoo SK,
Gadisetti C, Resau JH and Fazleabas AT: Early changes in micro RNA
(miRNA) expression in the eutopic endometrium (EUE) in a baboon
model of induced endometriosis. Fertil Steril. 94
(Suppl)(S200)2010.
|
|
24
|
Bjorkman S and Taylor HS: MicroRNAs in
endometriosis: Biological function and emerging biomarker
candidates. Biol Reprod. 100:1135–1146. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Qiu JJ, Lin YY, Tang XY, Ding Y, Yi XF and
Hua KQ: Extracellular vesicle-mediated transfer of the
lncRNA-TC0101441 promotes endometriosis migration/invasion. Exp
Cell Res. 388(111815)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu J, Li C, Jiang Y, Wan Y, Zhou S and
Cheng W: Tumor-suppressor role of miR-139-5p in endometrial cancer.
Cancer Cell Int. 18(51)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu H, Zhang G, Guo L and Tan S:
Expression of miR-139-5p in small cell lung cancer tissue and its
clinical significance. J Jilin Univ. 42:942–948. 2016.
|
|
28
|
Harder JM and Libby RT: BBC3 (PUMA)
regulates developmental apoptosis but not axonal injury induced
death in the retina. Mol Neurodegener. 6(50)2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nakano K and Vousden KH: PUMA, a novel
proapoptotic gene, is induced by p53. Mol Cell. 7:683–694.
2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yu J and Zhang L: PUMA, a potent killer
with or without p53. Oncogene. 27 (Suppl 1):S71–S83.
2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
De La Cruz MS and Buchanan EM: Uterine
fibroids: Diagnosis and treatment. Am Fam Physician. 95:100–107.
2017.PubMed/NCBI
|