1. Introduction to fungal
rhinosinusitis
PubMed was searched using the key words ‘sinus and
aspergilloma’ and limited the search to free full text available
articles. All 20 articles identified were included in this review
and other specific aspects were clarified also using free full text
sources on PubMed or established reference ENT books. Moreover,
concerning the time frame, all references were not older than 1973.
Applying other inclusion criteria would have limited the scope of
this review.
There are numerous types of sinusitis caused by
fungal strains, some of which already colonize the nasal cavity.
Mild forms present fungus balls growing inside a preexisting sinus
cavity. The invasive type ranges from chronic manifestations to
acute aggravated episodes. The latter scenario is encountered in
cases with reduced immune responses, such as patients with
diabetes, individuals receiving any form of transplant, AIDS cases
and chemotherapy patients. Without the control of
immunosuppression, the infection is aggravated and extends to the
orbit and inside the skull base, regardless of the prompt surgical
and medical treatment. Conversely, the cases with chronic invasive
sinusitis in individuals with a competent immune response present a
slow evolution with minimum vascular invasion (1,2).
Infectious organisms involved in fungal aggressive
sinusitis are most frequently members of the Aspergillus
family, Mucor, Alternaria, Auricularia,
Bipolaris, Candida, Drechslera, Sporothrix
Schenckii and Pseudallescheria Boydii. One of the first
classification systems is based on the histology aspect. Therefore,
the cases with fungal rhinosinusitis lacking a granulomatous
response are associated with a reserved prognosis due to a high
rate of persistence and recurrence. However, these data are not
available during the clinical exam and should not influence the
first line of treatment. The pathology result is key to a final
diagnosis and granuloma-related prognosis in fungal rhinosinusitis
(3,4).
Noninvasive forms of fungal rhinosinusitis were
recently defined as allergic fungal rhinosinusitis (AFRS). Although
there are still debates regarding its pathogenesis, this appears to
be the most common type.
For defining AFRS, the following criteria must be
used: type I hypersensitivity, nasal polyposis, specific computed
tomography (CT) scan aspect of a dense sinus cavity content
(Fig. 1), positive fungal culture,
or a positive pathology slide stained accordingly and lack of
tissue invasion. Supplementary data are elevated total IgE serum
values, the presence of specific IgE antigens and peripheral blood
eosinophilia (5,6).
AFRS is mostly unilateral and may produce bone
erosions with orbit and skull base invasion in advanced stages.
When surgically opening the fungal cavity, the content is greenish
and with high consistency. From a pathological point of view, this
secretion contains eosinophils, Charcot-Leyden crystals, and fungal
hyphae. The fungal hyphae are thin, uniform and regularly septate,
with dichotomous branching at 45 degrees. The host response is
minimal, with no inflammatory aspects of the tissue and no fungal
invasion, although cases of invasive and extensive aspergillosis
have been reported (Fig. 2)
(7). The detail differentiating
AFRS from chronic eosinophilia rhinosinusitis is the presence of a
fungal allergy. AFRS is not related to the presence of fungi in the
cavity but more to the aberrant response to environmental naturally
occurring fungus. Using laboratory procedures with high
sensitivity, fungi colonizing the nasal cavity may be detected in
almost any individual. Given the wide range of fungi involved in
developing AFRS, it is necessary to identify the precise type to
prepare patient desensitization. Another aspect is that patients
rarely present associated dermal mycosis (8).
2. General data on rhinosinusal
aspergilloma
Aspergillus is a fungus present worldwide,
and a member of the ascomycota class. Along with
Penicillium, they form the Aspergillaceae family.
This fungus usually evolves in moist areas and agricultural
environments or on the surface of decomposing organic residues. Its
growth is through the process of budding or branching. It can be
easily carried by wind and inhaled. The branching microorganisms
are easily identified with silver stains. This is the most common
pathogenic fungus at the level of the nasal sinuses. Occasionally,
it can enter the sinus cavity during dental procedures. The
pathogenesis is enhanced by anaerobic conditions in poorly
ventilated sinus cavities (9,10).
After the intake of spores of Aspergillus
fumigates, Aspergillus flavus, or Aspergillus niger
an infection is generated, usually localized in the lungs of
immunocompromised patients. However, destructive lesions
occasionally appear in the paranasal sinuses, anterior palate, or
nasal cavities. There have been cases of aspergilloma recorded even
in immunocompetent hosts (Fig. 3).
Chronic manifestations occur in healthy subjects, while acute
manifestations burden immunocompromised hosts. Invasive forms in
healthy subjects are extremely rare. Since the first description in
1953 as an opportunistic infection, numerous cases have been
frequently documented during the time of an autopsy (11,12).
Aspergilloma defines infections in the upper airways
or lungs, cutaneous manifestations, or extrapulmonary sites, along
with allergic reactions produced by the Aspergillus species.
The most frequent site of infection are the lungs, with a maximum
of 20% of cases also presenting with sinus infections according to
some studies. Fungal sinusitis accounts for ~6% of the cases with
sinusitis. The most common pathogen is Aspergillus fumigatus
followed by Aspergillus flavus and Aspergillus niger.
The most common site of infection is the maxillary sinus (13,14).
Rhinosinusal aspergilloma is also classified into
invasive and noninvasive, depending on the mucosa involvement and
bony erosion. Invasive aspergilloma may evolve into a malignant
state, with the destruction of the adjacent orbit or skull base
(15,16).
Differential diagnosis includes Wegener
granulomatosis and Pseudomonas lesions. Pathology reveals
the progression of the infection in deep tissues. Moreover, there
is a vascular invasion with tissue necrosis (Fig. 4). The prognosis of such cases with a
poor immune response is uncertain (17,18).
3. Principles of diagnosis in rhinosinusal
aspergilloma
Common complaints are nasal obstruction, rhinorrhea,
and smell dysfunction. The clinical exam should underline the fact
that the symptoms appear only on one side of the nasal cavity.
Facing such a scenario, the possibility of sinusitis with dental
origin or fungal sinusitis associated with nasal polyposis should
be considered. Occasionally, these two scenarios may intertwine
(19). Unilateral, persistent
headache, trigeminal neuralgia in the V2 territory, posterior
rhinorrhea and unilateral cacosmia are clinically strong arguments
for a possible odontogenic rhinosinusitis associated with
aspergilloma.
Chronic evolution of a sinus infection due to a
dental problem in an immunocompetent patient may lead to
aspergilloma through a range of mechanisms: repeated use of
antibiotics, closed sinus cavity due to ostium obstruction,
exhaustion of the immune resources of the host due to chronic
bacterial infection (20).
Clinical examination of the ENT with nasal endoscopy
may reveal the presence of a purulent secretion in the middle
meatus that drains on the tail of the lower cornet, marked edema or
polyps in the middle meatus. After anemia and aspiration, it is
possible to highlight aspergilloma even in the middle meatus, in
the nasal fossa or in the sphenoethmoidal recess (Fig. 5). It is important to gently clean
the nostrils before the examination, in order to avoid bleeding and
highlight the lesions. Careful examination of the teeth on the
homonymous hemimaxillary that can identify the presence of a
mobile, painful tooth or an old dental work under which the source
of origin for odontogenic sinusitis and aspergilloma can be hidden
should not be overlooked.
Unilateral nasal polyposis indicates either AFRS or
an inverted papilloma. Even nasal carcinomas should not be ruled
out from the differential diagnosis. There are situations in which
the initial evolution of the tumor generates the required changes
in the local environment leading to the development of a fungal
infection (21).
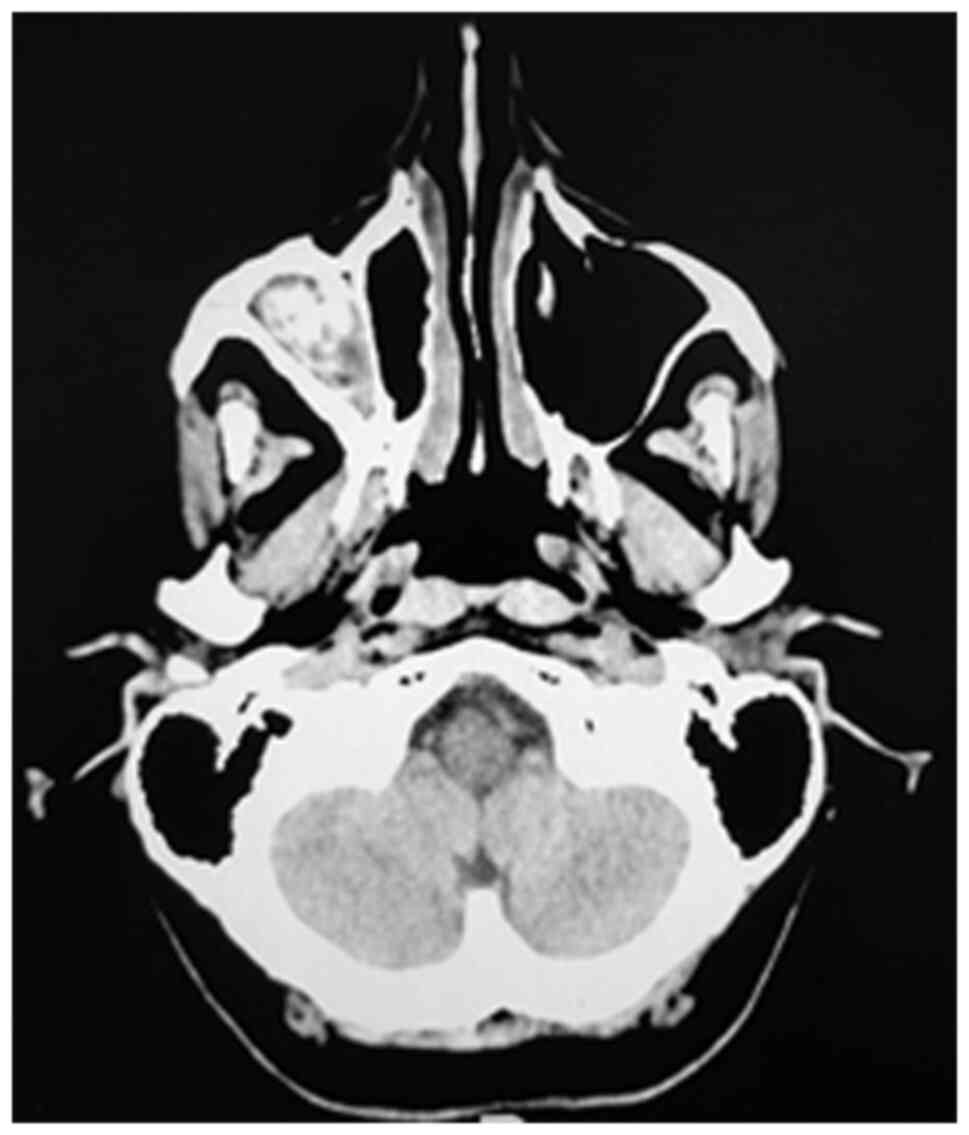
Imaging exploration is mandatory to support the
diagnosis of fungal rhinosinusitis and reveals unilateral
maxillo-ethmoidal rhinosinusal damage with hyperostosis of the
walls of the maxillary sinus, endosinusal calcifications and
obstruction of the osteomeatal complex (Fig. 6). However, the characteristic
imaging lesions for an aspergilloma consists of the lysis of the
bony walls of the maxillary sinus, especially of the medial wall,
of the orbital floor, but also of the bony septum. A
high-resolution CT scan underlines the bone involvement with
erosions. Magnetic resonance imaging (MRI) should also be performed
in cases where the erosion is tackling the skull base to rule out
meningeal involvement. In addition, MRI is useful in delineating
the level of fluid retention inside the sinus cavity secondary to
tumor obstruction (22).
Performing rhinosinusal imaging should always be
completed when there is unilaterally suspected clinical and imaging
unilateral rhinosinusitis with orthopantomogram or cone beam
computed tomography (CBCT) to highlight a dental outbreak in the
sinus teeth to be treated concomitantly with surgical treatment of
aspergilloma or more rarely conservatively, shortly after
aspergilloma treatment to prevent a recurrence of sinus fungal
injury (Fig. 7). Surgery represents
the option only after receiving the clinical, endoscopic and
imaging results (23).
The biological picture is not very suggestive in the
immunocompetent host which presents an increase in erythrocyte
sedimentation rate (ESR) and occasionally an eosinophilia
associated with aspergillomas can be revealed in the context of
AFRS. Numerous cases also associate with uncontrolled diabetes
(24).
In the exacerbation of a chronic fungal
rhinosinusitis or when the immunity of the patient becomes
precarious, the clinical onset can be witnessed through a most
common oculo-orbital complication with orbital cellulite associated
especially with a violent nosebleed, with an intense headache,
possible fever and a general impaired condition (Fig. 8). In such cases, the biological
picture reveals an acute inflammatory syndrome, and the therapeutic
attitude requires emergency surgical drainage and massive
antibiotic therapy. On such occasions, aspergilloma is an
intraoperative discovery that may require the perioperative
association with a systemic antifungal. Voriconazole is one of the
compounds used on a wide scale for emergency cases due to its
diminished systemic toxicity (25).
4. Principles of treatment in rhinosinusal
aspergilloma
In general, the treatment is based on surgical
debridement and systemic steroids. Allergic sensitization reduction
and antifungal treatment are controversial with benefits in
specific cases (26).
The surgical approach should be either external or
combined with endoscopy taking into consideration the site of
infection, patient status, as well as the experience of the
surgeon. For small aspergillomas of the middle meatus or
spheno-ethmoidal recess, endoscopic extraction is possible with
correct vision and radical ablation of the lesion associated,
ensuring the drainage and ventilation of the involved sinus
(Fig. 9). In aspergillomas of the
maxillary sinus, the external approach conveys some advantages
compared with Functional Endoscopic Sinus Surgery (FESS), by
enabling complete removal of the fungus and drainage of the remnant
sinus cavity (Fig. 10). Moreover,
wide external exposure allows complete ablation of the sinus mucosa
containing all aspergillus colonies and preventing recurrence
(Fig. 11) (27). The bacteriological examination with
antibiogram of the secretion collected intraoperatively from the
maxillary sinus for aerobic and anaerobic flora for targeted
antibiotic therapy is mandatory, as well as mycological
examination. Nevertheless, histological examination is what
provides certainty, by describing Aspergillus colonies in
intraoperative samples. A fungus ball is the characteristic
non-invasive aspect of aspergilloma, histologically characterized
by colonies with hyphae and so called ‘fruiting’ (conidial) heads
(Fig. 12). Frequently,
aspergillomas in the maxillary sinus develop due to dental
pathology and it is frequently necessary to extract the affected
teeth and close oral fistulas. This is another situation that
requires the external approach or a combined endoscopic approach
(28).
Medical treatment includes antibiotics covering the
spectrum of anaerobic microorganisms. The combination of 3rd
generation cephalosporins with metronidazole is useful. This
combination penetrates the blood-brain barrier and prevents
endocranial and orbit extension of the infectious process. Such
combinations should be administered even in cases without
complications upon admission (29).
Antifungal compounds should be used in cases with
compromised immune response. Given the availability in the
hospital, voriconazole, caspofungin, or amphotericin B should be
opted for (30). Voriconazole is
most widely used due to its favorable tolerance by the patients and
high efficiency improving survival with diminished systemic
toxicity (31).
In cases with efficient immune response,
administering oral antifungal compounds with fewer side effects
should be considered. Compounds like itraconazole or posaconazole
should be administered up to 10 days after surgery. However, these
compounds are inefficient without surgical removal of the fungus
ball (32).
For the endoscopic approach of the rhinosinusal
aspergilloma, special equipment with wide angle nasal endoscopes is
necessary, for a clear view of the sinus cavities (33). Moreover, the endoscopic approach
enables reaching pathology positioned deeper in the ethmoid cells
(34). This is a minimally invasive
approach (35). In cases of
hematologic malignancies and higher risk of bleeding, the
endoscopic approach appears safer (36). The recovery after the endoscopic
approach appears to be more rapid and thorough in chronic cases
(37). Fortunately, this type of
pathology appears to have a lower incidence in Eastern Europe and
the cases should be referred to regional tertiary specialized
units, with experience in the management of such cases (38).
5. Conclusions
Rhinosinusal aspergilloma has a slow, insidious
evolution over months and even years. Our experience revealed the
presence of both a dental problem and previous self-administered
antibiotic regimens in almost every case. The initial symptoms are
common with sinusitis of dental origin, but aspergilloma should be
considered when a patient with a competent immune system does not
respond to standard antibiotic treatment.
A comprehensive review of a special type of fungal
rhinosinusitis caused by Aspergillus strains is presented.
There are other fungal species that can cause rhinosinusitis as
well. There is a lot of confusion regarding the classification of
fungal rhinosinusitis. The most accepted classification includes
acute invasive fungal rhinosinusitis, granulomatous invasive fungal
rhinosinusitis, chronic invasive fungal rhinosinusitis, fungus
ball, AFRS and saprophytic fungal infestation (39).
Aspergillus can cause invasive or
non-invasive disease. The differentiation between invasive and
non-invasive disease is important because the two entities have
different treatment options and prognosis.
Laboratory results are in the normal range, except
occasionally elevated ESR, and this should raise the question why
the organism is not correctly fighting the fungus infection.
The final diagnosis of rhinosinusal aspergilloma is
conducted on a pathology sample with silver staining. The
bacteriology exam of the sinus secretion rarely reveals a fungal
infection, but as revealed in our clinical experience, it may
indicate a coinfection with other multidrug-resistant bacteria.
Early diagnosis and emergency surgical debridement,
along with administering systemic antifungal compounds, represent
in some cases the key to successful treatment of invasive
aspergilloma. Surgical treatment must establish a wide exposure of
the sinus cavity and correct drainage, regardless of the external
or combined endoscopic approach. Voriconazole is one of the
compounds used on a wide scale for emergency cases, due to its
diminished systemic toxicity.
Acknowledgements
Professional editing, linguistic and technical
assistance was performed by Irina Radu, Individual Service
Provider, certified translator in Medicine and Pharmacy
(certificate credentials: E0048/2014).
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DV, MD and TP contributed substantially to the
conception and design of the study, the acquisition, analysis, and
interpretation of the data, and were involved in the drafting of
the manuscript. OMP and AC contributed substantially to the
acquisition, analysis and interpretation of the data and were
involved in the drafting of the manuscript. RC contributed
substantially to the acquisition of the data and was involved in
the critical revisions of the manuscript for important intellectual
content. All authors agreed to be accountable for all aspects of
the work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved. All the authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
The study followed the international regulations in
accordance with the Declaration of Helsinki.
Patient consent for publication
Patient informed consent for publication of the
data/images associated with the manuscript was obtained. The
authors followed the international regulations in accordance with
the Declaration of Helsinki and all identifying information was
removed.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Neville BW, Damm DD, Allen CM and Bouquot
JE: Fungal and protozoal diseases. In: Oral and Maxillofacial
Pathology. 2nd edition. Saunders, Philadelphia PA, pp189-211,
2005.
|
|
2
|
Lin SJ, Schranz J and Teutsch SM:
Aspergillosis case-fatality rate: Systematic review of the
literature. Clin Infect Dis. 32:358–366. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Nikolaizik WH, Weichel M, Blaser K and
Crameri R: Intracutaneous tests with recombinant allergens in
cystic fibrosis patients with allergic bronchopulmonary
aspergillosis and aspergillus allergy. Am J Respir Crit Care Med.
165:916–921. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hartl D, Latzin P, Zissel G, Krane M,
Krauss-Etschmann S and Griese M: Chemokines indicate allergic
bronchopulmonary aspergillosis in patients with cystic fibrosis. Am
J Respir Crit Care Med. 173:1370–1376. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kraemer R, Deloséa N, Ballinari P, Gallati
S and Crameri R: Effect of allergic bronchopulmonary aspergillosis
on lung function in children with cystic fibrosis. Am J Respir Crit
Care Med. 174:1211–1220. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Frisvad JC, Rank C, Nielsen KF and Larsen
TO: Metabolomics of aspergillus fumigatus. Med Mycol. 47 (Suppl
1):S53–S71. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Guarner J and Brandt ME: Histopathologic
diagnosis of fungal infections in the 21st century. Clin Microbiol
Rev. 24:247–280. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Arndt S, Aschendorff A, Echternach M,
Daemmrich TD and Maier W: Rhino-orbital-cerebral mucormycosis and
aspergillosis: Differential diagnosis and treatment. Eur Arch
Otorhinolaryngol. 266:71–76. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Walsh TJ, Anaissie EJ, Denning DW,
Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH,
Steinbach WJ, Stevens DA, et al: Treatment of aspergillosis:
Clinical practice guidelines of the infectious diseases society of
america. Clin Infect Dis. 46:327–360. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Tamgadge AP, Mengi R, Tamgadge S and
Bhalerao SS: Chronic invasive aspergillosis of paranasal sinuses: A
case report with review of literature. J Oral Maxillofac Pathol.
16:460–464. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sharma OP and Chwogule R: Many faces of
pulmonary aspergillosis. Eur Respir J. 12:705–715. 1998.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Warder FR, Chikes PG and Hudson WR:
Aspergillosis of the paranasal sinusis. Arch Otolaryngol.
101:683–685. 1975.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sapp JP, Eversole LR and Wysocki GP: Oral
infections. In: Contemporary Oral, Maxillofacial Pathology. 2nd
edition. Mosby, St. Louis, MO, pp207-251, 2004.
|
|
14
|
Dayananda BC, Vandana R, Rekha K and Kumar
GS: Aspergillosis of the maxillary antrum: A case report. J Oral
Maxillofac Pathol. 1:26–29. 2002.
|
|
15
|
Chambers MS, Lyzak WA, Martin JW, Lyzak JS
and Toth BB: Oral complications associated with aspergillosis in
patients with a hematologic malignancy. Presentation and treatment.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 79:559–563.
1995.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Veress B, Malik OA, el-Tayeb AA, el-Daoud
S, Mahgoub ES and el-Hassan AM: Further observations on the primary
paranasal aspergillus granuloma in the Sudan: A morphological study
of 46 cases. Am J Trop Med Hyg. 22:765–772. 1973.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Samaranayake LP, Keung Leung W and Jin L:
Oral mucosal fungal infections. Periodontol 2000. 49:39–59.
2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jeican II, Barbu Tudoran L, Florea A,
Flonta M, Trombitas V, Apostol A, Dumitru M, Aluaș M, Junie LM and
Albu S: Chronic Rhinosinusitis: MALDI-TOF mass spectrometry
microbiological diagnosis and electron microscopy analysis;
Experience of the 2nd otorhinolaryngology clinic of Cluj-Napoca,
Romania. J Clin Med. 9(3973)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Denning DW: Invasive aspergillosis. Clin
Infect Dis. 26:781–803. 1998.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Regezi JA, Sciubba JJ and Jordan RC:
Ulcerative conditions. In: Oral pathology-Clinical Pathologic
Correlations. 4th edition. Saunders, St. Louis, MO, pp23-74,
2003.
|
|
21
|
Kwon J, Park KH, Park SI and Jin SY:
Aspergillosis of the paranasal sinuses-diagnostic significance of
the computed tomography. Yonsei Med J. 30:294–297. 1989.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ciobanu IC, Motoc A, Jianu AM, Cergan R,
Banu MA and Rusu MC: The maxillary recess of the sphenoid sinus.
Rom J Morphol Embryol. 50:487–489. 2009.PubMed/NCBI
|
|
23
|
DelGaudio JM, Swain RE, Kingdom TT, Muller
S and Hudgins PA: Computed Tomographic findings in patients with
invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg.
129:236–240. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rusu E, Jinga M, Rusu F, Ciurtin C, Enache
G, Dragomir A, Cristescu V, Stoica V, Costache A, Cheta D and
Radulian G: Statin therapy in patients with diabetes and hepatitis
C. Farmacia. 61:1204–1215. 2013.
|
|
25
|
Pasqualotto AC, Xavier MO, Andreolla HF
and Linden R: Voriconazole therapeutic drug monitoring: Focus on
safety. Expert Opin Drug Saf. 9:125–137. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Berghi NO, Dumitru M, Vrinceanu D,
Ciuluvica RC, Simioniuc-Petrescu A, Caragheorgheopol R, Tucureanu
C, Cornateanu RS and Giurcaneanu C: Relationship between chemokines
and T lymphocytes in the context of respiratory allergies (Review).
Exp Ther Med. 20:2352–2360. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Stammberger H, Jakse R and Beaufort F:
Aspergillosis of the paranasal sinuses; X-ray diagnosis,
histopathology, and clinical aspects. Ann Otol Rhinol Laryngol.
93:251–256. 1984.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rowe-Jones JM and Moore-Gillon V:
Destructive noninvasive paranasal sinus aspergillosis: Component of
a spectrum of disease. J Otolaryngol. 23:92–96. 1994.PubMed/NCBI
|
|
29
|
Myoken Y, Sugata T, Fujita Y, Fujihara M,
Iwato K, Murayama SY and Mikami Y: Early diagnosis and successful
management of atypical invasive aspergillus sinusitis in a
hematopoietic cell transplant patient: A case report. J Oral
Maxillofac Surg. 64:860–863. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sungkanuparph S, Sathapatayavongs B,
Kunachak S, Luxameechanporn T and Cheewaruangroj W: Treatment of
invasive fungal sinusitis with liposomal amphotericin B: A report
of four cases. J Med Assoc Thai. 84:593–601. 2001.PubMed/NCBI
|
|
31
|
Peral-Cagigal B, Redondo-González LM and
Verrier-Hernández A: Invasive maxillary sinus aspergillosis: A case
report successfully treated with voriconazole and surgical
debridement. J Clin Exp Dent. 6:e448–e451. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Herbrecht R, Denning DW, Patterson TF,
Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P,
Lortholary O, et al: Voriconazole versus amphotericin B for primary
therapy of invasive aspergillosis. N Engl J Med. 347:408–415.
2002.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee JH, Oh DH and Lee DH: A single
small-sized fungus ball in the maxillary sinus: An endoscopic view.
Ear Nose Throat J. 99:165–166. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lee JH: Endoscopic view of fungus ball in
the most posterior ethmoid cell via the supreme meatus. Ear Nose
Throat J. 98:319–320. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mitroi M, Albulescu D, Capitanescu A,
Docea AO, Musat G, Mitroi G, Zlatian O, Tsatsakis A, Tzanakakis G,
Spandidos DA and Calina D: Differences in the distribution of CD20,
CD3, CD34 and CD45RO in nasal mucosa and polyps from patients with
chronic rhinosinusitis. Mol Med Rep. 19:2792–2800. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gletsou E, Ioannou M, Liakopoulos V,
Tsiambas E, Ragos V and Stefanidis I: Aspergillosis in
immunocompromised patients with haematological malignancies. J
Buon. 23:7–10. 2018.PubMed/NCBI
|
|
37
|
Jeican II, Trombitas V, Crivii C, Dumitru
M, Aluas M, Dogaru G, Gheban D, Junie LM and Albu S: Rehabilitation
of patients with chronic rhinosinusitis after functional endoscopic
sinus surgery. Balneo and PRM Research Journal. 12:65–72. 2021.
|
|
38
|
Leszczyńska J, Stryjewska-Makuch G,
Lisowska G, Kolebacz B and Michalak-Kolarz M: Fungal sinusitis
among patients with chronic rhinosinusitis who underwent endoscopic
sinus surgery. Otolaryngol Pol. 72:35–41. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Deutsch PG, Whittaker J and Prasad S:
Invasive and non-invasive fungal Rhinosinusitis-A review and update
of the evidence. Medicina (Kaunas). 55(319)2019.PubMed/NCBI View Article : Google Scholar
|