Introduction
Joint cartilage damage derived from trauma, disease
and lifetime physical wear affects 10-12% of the world's population
(1). This percentage is
continuously growing due to an aging and increasingly overweight
society (2). Cartilage consists of
an extracellular matrix secreted by chondrocytes (3). This tissue provides multiple
mechanical properties, such as elasticity, water retention and
friction reduction in joints. The treatment for chondral diseases
usually focuses on symptomatic relief by local or systemic drug
treatments and repair procedures such as cartilage autograft,
autologous chondrocyte implantation and subchondral bone
microfracture (4). These treatments
improve the short-term life quality of patients but lack long-term
effects due to their progression to fibrocartilage tissue without
the elastic properties of the native cartilage (5,6).
Novel alternative therapies for long-term
restoration of cartilage damage are in constant development. Among
these, the use of human mesenchymal stem cells (MSCs), alone or in
combination with biological scaffolds, is the most promising
procedure. MSCs have been proposed as an innovative method in
regenerative medicine for the development of cell-based therapies
and are currently being evaluated in a large number of clinical
trials for cartilage damage repair (7). In vitro, MSCs may be
differentiated into a chondrocyte-like phenotype by the use of
chemical stimuli such as insulin-like growth factors (IGF), bone
morphogenic proteins (BMPs) and TGF (8).
Multiple growth factors have been identified as
promoters of differentiation from MSCs to chondrocytes, such as
fibroblast growth factor 2 (FGF2), TGFβ1, TGFβ3, BMP2, BMP7 and
IGF1 (9-11).
Other differentiation techniques include co-culture with native
chondrocytes and microenvironment modulation, such as the use of
bioactive matrices that simulate the biological microenvironment of
native chondrocytes, in monolayer conditions or more suitable
three-dimensional (3D) scaffolding culture conditions (12). However, these approaches frequently
lose effectiveness over time due to hypertrophy of differentiated
chondrocytes (13). This
hypertrophic process is modulated by a decrease in the expression
of the transcription factor SOX9, which serves as the major
promoter of chondrogenic differentiation and their metabolic
processes, stimulating factors such as collagen type II and
proteoglycans, and acting as a suppressor of runt-related
transcription factor (RUNX2), which is responsible for the
initiation of the ossification mechanism (14). RUNX2 is a key transcription factor
in the osteoblastic differentiation process, bone formation and
skeletal morphogenesis (2,14). Long-term culture of MSC-derived
chondrocytes leads to inhibition of SOX9 and to hypertrophic
progression, increasing the expression levels of RUNX2 and matrix
replacement by secretion of proteins such as collagen type X, VEGF,
MMP13 and alkaline phosphatase (ALPL) (15).
To achieve MSC differentiation into phenotypically
stable chondrocytes, and cartilage restoration, it is hypothesized
that a precise combination of chondrogenic and osteogenic factors
and temporal changes in the local microenvironment is required,
along with a 3D scaffold ready for its use in the repair of human
cartilage damage (16,17).
To investigate this, a 3D scaffold that emulates the
biological conditions and microenvironment typical for native
chondrocytes was developed to allow human adipose-derived (Ad)-MSC
differentiation and maintenance of phenotypic stability under
culture conditions free of animal components (xeno-free). The
present results were promising for providing an advanced medical
therapy product in areas of chondral damage. In addition, analysis
of the differentiation process was performed to study the
biological mechanisms involved, which may be helpful in future
experiments of MSC differentiation using growth factors (18).
Materials and methods
Patients and samples
The present study was approved by the Institutional
Ethics and Research Committees of the Faculty of Medicine and
University Hospital ‘Dr José E. González’ (HU) of the Autonomous
University of Nuevo Leon (Monterrey, México; registry no.
PI17-00360). Lower abdominal adipose tissue was collected from the
surgical lipoaspirate waste of three healthy females (BMI <30),
that were >18 years of age and demonstrated negative serology
(HIV, hepatitis B and C virus). Tissue was obtained at the HU
between May 2017 and June 2018. The adipose tissue was collected in
sterile 50-ml conical tubes (cat. no. 430290; Corning, Inc.) and
maintained at 4˚C for a maximum of 6 h until processing. All
patients provided written informed consent for the use of their
tissue.
Ad-MSC isolation under xeno-free
conditions
To isolate Ad-MSCs, a modified version of the
isolation protocol described in Moncada-Saucedo et al
(19) was followed (20). To achieve this, lipoaspirate was
centrifuged for 10 min at 1,000 x g in a 4˚C refrigerated
centrifuge (5804R; Eppendorf), the aqueous phase was discarded and
the adipose tissue was washed with PBS (pH 7.4). Tissue was placed
in 200-ml sterile bottles and incubated with collagenase I 0.1%
(cat. no. 17018029; Gibco; Thermo Fisher Scientific, Inc.) for 30
min at 37˚C with low agitation for tissue digestion and then left
to rest without agitation for 5 min to allow cells to form a
sediment at the bottom of the bottles. The fat phase was removed
and the aqueous phase and cells were centrifuged at 1,000 x g for 5
min at 4˚C to obtain the cell pellet. After washing with sterile
PBS, the supernatant was discarded and cells were resuspended in
DMEM (cat. no. 11995065; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% human serum (HS; serotype AB+,
obtained from healthy donors after informed consent was signed) and
antibiotic-antifungal (penicillin 100 U/ml, streptomycin 100 µg/ml
and amphotericin B 0.25 µg/ml; cat. no. 15240062; Gibco; Thermo
Fisher Scientific, Inc.). Cells were maintained in
25-cm2 culture flasks (cat. no. 430639; Corning, Inc.)
at 37˚C with 5% CO2 for 24 h. The supernatant with
unattached cells was obtained from the culture flasks and incubated
in a new 25-cm2 flask at 37˚C and 5% CO2
(subculture) for 5 days. The subculture was washed with sterile PBS
to remove traces of erythrocytes and other suspended cells and was
finally maintained at 37˚C and 5% CO2 with a change of
medium every 2 days until cells reached 90% confluence; they were
then trypsinized (cat. no. 15050065; Gibco; Thermo Fisher
Scientific, Inc.) and expanded in 150-cm2 culture flasks
(cat. no. 430823; Corning, Inc.).
Proliferation analysis
Proliferation analysis of Ad-MSCs under HS or FBS
conditions was performed with alamarBlue (cat. no. DAL1100;
Invitrogen; Thermo Fisher Scientific, Inc.). Proliferation was
tested in biological triplicate every 24 h for 6 days. Cells were
seeded in 96-well plates (cat. no. 3300; Corning, Inc.) at 10,000
cells/well with DMEM (cat. no. 11995065; Gibco; Thermo Fisher
Scientific, Inc.) and 10% HS or 10% FBS (cat. no. 16140071; Gibco;
Thermo Fisher Scientific, Inc.) and incubated at 37˚C with 5%
CO2. For each time point, medium was replaced with 10%
alamarBlue solution in DMEM, after which cells were incubated for 2
h at 37˚C and the supernatant was transferred to 96-well black
plates (cat. no. 3915; Corning, Inc.). The fluorescence signal of
the supernatant was measured at 525 nm using a Glo-Max multi
detection system (Promega Corporation).
Expression of multipotential MSC
markers
To evaluate the undifferentiated stage
(multipotential) of the isolated MSCs, Nanog homeobox (NANOG) and
octamer-binding transcription factor (OCT3/4), which are
transcription factors involved in self-renewal and maintenance of
the undifferentiated stage of MSCs (21,22),
were evaluated by immunofluorescence (IF). Stage-specific embryonic
antigen 4 (SSEA4), a cell surface antigen of embryonic stem cells,
was included as a negative control (23,24).
The isolated cells in passage 3 were seeded in duplicate with a
seeding density of 10,000 cells/well in 8-well culture chambers
(cat. no. 154534; Nunc; Thermo Fisher Scientific, Inc.). Once they
reached 90% confluence, the culture medium was removed and the
cells were washed with PBS followed by 5 min of incubation at room
temperature with cytoskeleton buffer (CB) composed of the
following: 2-ethanesulfonic acid (10 mM), NaCl (150 mM), EGTA (5
mM), MgCl (5 mM) and glucose 5 (mM). CB was then removed and
fixation was performed with 3% formaldehyde (cat. no. HT501128;
Sigma-Aldrich; Merck KGaA) diluted in CB for 10 min at room
temperature. Cells were permeabilized with 0.05% Triton X-100 (cat.
no. T8787; Sigma-Aldrich; Merck KGaA) for 5 min, then washed with
PBS and blocked for non-specific sites by treatment with 0.5%
bovine serum albumin (cat. no. A3294; Sigma-Aldrich; Merck KGaA)
for 30 min at room temperature.
Cells were incubated overnight at 4˚C with a 1:100
dilution of specific primary antibodies against NANOG (cat. no.
sc-293121), OCT3/4 (cat. no. sc-5279) and SSEA4 (cat. no. sc-21704;
all from Santa Cruz Biotechnology, Inc.) in a wet chamber. Samples
were then washed with PBS-Tween 20 (0.01%) and incubated with
anti-mouse IgG secondary antibody conjugated with Alexa
Fluor® 488 (cat. no. ab150113; 1:1,000) and rhodamine
phalloidin (cat. no. ab235138; 1:60; all from Abcam) for 2 h at
room temperature to stain actin filaments. After the final wash,
assembly of the culture slides was performed with VectaShield (cat.
no. H-1200-10; Vector Laboratories, Inc.; Maravai LifeSciences)
mounting medium containing DAPI as a counterstain for nuclei. The
slides were observed under a fluorescence microscope
(magnification, x20; Olympus AX70; Olympus Corporation) and whole
sections were analyzed. Selected markers were expected to exhibit
green fluorescence for NANOG and OCT3/4, with a counterstain of
blue fluorescence for the nuclei and red for actin filaments of the
cytoskeleton.
Biphasic scaffold manufacturing
Bovine cartilage matrix was obtained from the
scrapings of femoral condyles from 19 fresh bovine knees obtained
through a certified slaughterhouse and decellularized with
hypotonic buffers (19). Full
decellularization was assured by H&E staining. Bovine bone
chips were obtained by perforation of the same bovine knees after
cross-sectional slicing of the condyle area, followed by
decellularization with hydrogen peroxide according to the
decellularization method described by Pérez-Silos et al
(25) to avoid host rejection of
the material (26).
The 3D biphasic scaffolds were assembled as
previously described (19) to
generate a 2-mm cartilage upper phase and a 4-mm bone phase at the
bottom. The 1:1 mixture of decellularized bovine cartilage matrix
and NaCl with a particle size of 77-177 µm, selected by sifting
through sieves (Mont Inox), was placed inside
polytetrafluoroethylene (PTFE) molds with an internal diameter of 6
mm, previously sealed at the bottom with sealing film (cat. no.
P7793-1EA; Parafilm). The mixture was slightly compacted at the
bottom of the mold and 8% of silk fibroin (cat. no. 5154-20ML;
Advanced BioMatrix), previously lyophilized and dissolved in
hexafluoroisopropanol (cat. no. 105228-1006; Sigma-Aldrich; Merck
KGaA), was added and the mixture was left to rest for 2 min to
achieve a homogeneous distribution between the NaCl and cartilage
matrix. Subsequently, a bone chip was submerged in 90% methanol;
the excess of methanol was removed and the bone chip was slightly
compressed over the mixture in the PTFE mold to maintain the union
between all of the components. PTFE molds with the mix were covered
and left to rest for 24 h, and then the sealing film was removed
and molds were submerged in 90% methanol for 1 h. Biphasic
scaffolds were removed from the molds and washed for 3 days in
ultrapure water to remove the NaCl and obtain pores of the desired
size (77-177 µm). The scaffolds were freeze-dried, sterilized with
ethylene oxide and stored at room temperature until use.
Ad-MSC chondrogenic differentiation
protocol
Ad-MSCs were initially divided into five groups (A
to E; Fig. 1) and differentiated in
DMEM with added dexamethasone 100 nM (cat. no. D2915-100MG;
Sigma-Aldrich; Merck KGaA), L-ascorbic acid 50 µg/ml (cat. no.
013-19641; FUJIFILM Wako Pure Chemical Corporation) and
insulin-transferrin-selenium 10 µg/ml (cat. no. 25-800-CR; Gibco;
Thermo Fisher Scientific, Inc.).
 | Figure 1Growth factor treatment schedules
designed for chondrogenic differentiation of adipose-derived
mesenchymal stem cells. Schematic diagram of the five
differentiation scheme groups. Triplicate samples were set up for
each time point of marker expression analysis (0, 5, 9, 12, 19, 26,
33 and 40 days) and duplicates for each histology analysis
timepoint (12, 19, 33 and 40 days). At day 9, cells were
transferred from the monolayer to the biphasic scaffold. IGF,
insulin-like growth factor; FGF, fibroblast growth factor. |
In the first step, all groups were exposed to human
recombinant FGF2 (cat. no. SRP4037-50UG; Sigma-Aldrich; Merck KGaA)
at 10 ng/ml for 5 days until cells reached 90% confluence in
150-cm2 culture flasks (cat. no. 430823; Corning, Inc.).
After this period, human recombinant growth factors IGF1 (100
ng/ml; cat. no. SRP3069-100UG; Sigma-Aldrich; Merck KGaA) and TGFβ1
(10 ng/ml; cat. no. T7039-2UG; Sigma-Aldrich; Merck KGaA) alone or
in combination were added to each one of the five groups, following
the scheme presented in Fig. 1.
Exposure to growth factors was performed in a monolayer until day
9, following which the cell monolayer was trypsinized and cells
were seeded in the chondral phase of the biphasic scaffolds by
injection of 300,000 cells/scaffold. The cells in the scaffold were
maintained with the exposure scheme in 48-well cell culture plates
(cat. no. 142475; Nunc; Thermo Fisher Scientific, Inc.) until day
40. The scaffolds were processed, and reverse
transcription-quantitative PCR (RT-qPCR) for chondrogenic and
osteogenic markers (by triplicate) and histological techniques at
different time points (by duplicate) were performed.
RNA isolation and expression
analysis
Total RNA was isolated with TRIzol®
reagent (cat. no. 15596018; Invitrogen; Thermo Fisher Scientific,
Inc.). The two-phase scaffold was separated into its cartilage and
bone phases, cartilage phase was homogenized by cutting it into
small pieces with sterile scissors followed by mechanical
disaggregation with TissueRuptor II (cat. no. 9002756; Qiagen GmbH)
using a steel probe. The homogenized sample was then centrifuged at
14,000 x g for 10 min at 4˚C and the supernatant was collected and
processed according to the manufacturer's protocol. The RNA was
resuspended in 50 µl ultrapure water and the integrity was analyzed
via electrophoresis in 1% agarose gel. The purity and concentration
of RNA were determined using a NanoDrop™ 1000 (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.).
RNA was retrotranscribed from 1 µg of total RNA with
the High-Capacity cDNA Reverse Transcription Kit (cat. no. 4368813;
Applied Biosystems; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol with the use of random hexamers at a final
volume of 20 µl. The temperature program was as follows: 25˚C for
10 min, 37˚C for 120 min and 85˚C for 5 min. cDNA stored at -20˚C
until use.
Expression analysis of the differentiation groups
was performed at 0, 5, 9, 12, 26, 33 and 40 days using three
scaffolds for each time point (biological triplicate). The
expression levels of the genes SOX9, aggrecan (ACAN), cartilage
oligomeric matrix protein (COMP), collagen type II α 1 chain
(COL2A1), COL10A1 (chondrogenic markers), RUNX2, ALPL, MMP13,
COL1A2, COL10A1 and osteopontin (SPP1) (osteogenic markers) were
determined. Thy-1 cell surface antigen (THY1; CD90) gene expression
(MSC marker) was also assessed. qPCR was performed with
mRNA-specific primers previously designed with Oligo 7 software
v7.6 (Table I) using 100 ng of cDNA
and PowerUp SYBR Green Master Mix (cat. A25776; Applied Biosystems;
Thermo Fisher Scientific, Inc.). qPCR was conducted in a
StepOnePlus thermal cycler (cat. 4376600; Applied Biosystems;
Thermo Fisher Scientific, Inc.) with the following cycling
conditions: 50˚C for 2 min, and 40 cycles of denaturation at 95˚C
for 15 sec and annealing/extension at 60˚C for 1 min. Results were
analyzed with the 2-∆ΔCq method (27) using GAPDH as an endogenous
control.
 | Table IPCR primers designed for mesenchymal,
chondrogenic and osteogenic markers. |
Table I
PCR primers designed for mesenchymal,
chondrogenic and osteogenic markers.
| A, Chondrogenic
markers |
|---|
| Gene | Forward primer
(5'-3') | Reverse primer
(5'-3') |
|---|
| SOX9 |
AACGGCTCCAGCAAGAACAAG |
GCTCCGCCTCCTCCACGAAG |
| ACAN |
CAACAATGCCCAAGACTACCAG |
TTCCACTCGCCCTTCTCGTG |
| COMP |
CAGACAATGAACAGCGACCC |
GCCTGCCAATACGTTTGCTC |
| COL2A1 |
TCATCCAGGGCTCCAATGACGTG |
AACAGTCTTGCCCCACTTACCG |
| B, Osteogenic
markers |
| Gene | Forward primer
(5'-3') | Reverse primer
(5'-3') |
| RUNX2 |
GAACTCGTCCGCACCGACAG |
ATCGTTACCCGCCATGACAGT |
| ALPL |
CGGCCTGGACCTCGTTGACA |
ACGTTGTTCCTGTTCAGCTCGTA |
| MMP13 |
CGCCAGACAAATGTGACCCTT |
AAAACAGCTCCGCATCAACC |
| COL10A1 |
CGCCAGACAAATGTGACCCTT |
AAAACAGCTCCGCATCAACC |
| COL1A2 |
TAGAAAGAACCCAGCTCGCACA |
GGTTTCGCCAGTAGAGAAATCACA |
| SPP1 |
AAGAAGTTTCGCAGACCTGACATCC |
TGCACCATTCAACTCCTCGCTTT |
| C, Mesenchymal
marker |
| Gene | Forward primer
(5'-3') | Reverse primer
(5'-3') |
| THY1 |
CACACATACCGCTCCCGAAC |
CTGATGCCCTCACACTTGACCA |
| D, Endogenous
gene |
| Gene | Forward primer
(5'-3') | Reverse primer
(5'-3') |
| GAPDH |
ACAACAGCCTCAAGATCATCAGC |
TCACGCCACAGTTTCCCGGAG |
Histological analysis
Histological analysis in duplicate was performed on
the cellularized scaffolds at different time points (12, 19, 33 and
40 days). The scaffolds were withdrawn from the growth medium,
washed with PBS and fixed in 10% formalin solution for 48 h at room
temperature. Scaffolds were decalcified with 14% EDTA for 7 days,
followed by gradual dehydration with acetone-xylol, to be embedded
in paraffin. Longitudinal sections (5 µm) of the paraffin scaffolds
were stained with H&E (28) to
evaluate the cell distribution after 3 days of scaffold
cellularization, as well as new matrix formation within the
scaffold at the specified time points (12, 19, 36 and 40 days).
Collagen formation and extracellular matrix fibrosis were evaluated
with Masson's trichrome staining (29) at the specified time points (12, 19,
36 and 40 days). Slides were observed under a microscope
(magnification, x10 and x20; Leica DMRA; Leica Microsystems GmbH)
and histological qualitative results were determined by blinded
analysis of at least 3 optical fields by a pathology expert.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical analysis was performed with GraphPad Prism 5
software (GraphPad Software, Inc.) using one-way ANOVA with Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Isolation and standardization of the
xeno-free cell culture
Isolation of Ad-MSCs was performed from
lipoaspirate. Cells obtained after the subculture process exhibited
typical MSC characteristics, such as adherence to plastic substrate
and fibroblastoid morphology, reaching a high confluence after 10
days of isolation (Fig. 2A).
Identification of MSC surface markers CD73, CD90 and CD44, and
positive three-lineage differentiation, assessed previously by our
research group by Moncada-Saucedo (20).
Ad-MSC proliferation was analyzed in the presence of
FBS and HS. The relative cell proliferation in HS medium was almost
two times higher than that in FBS medium on day 6 of proliferation
(Fig. 2B), thereby improving the
culture of the cells under xeno-free conditions.
In addition, IF detection of principal transcription
factors present in adult isolated stem cells (NANOG and OCT3/4),
along with SSEA4 as a negative control, was performed to evaluate
the multipotent characteristics of the Ad-MSCs (Fig. 3). IF analysis of isolated Ad-MSCs
demonstrated the presence of NANOG and OCT3/4 in the cells; NANOG
was observed in both the nuclei and cytoplasm, while OCT3/4 had a
specific nuclear compartmentalization. SSEA4 was confirmed to be
negative in the adult isolated stem cells.
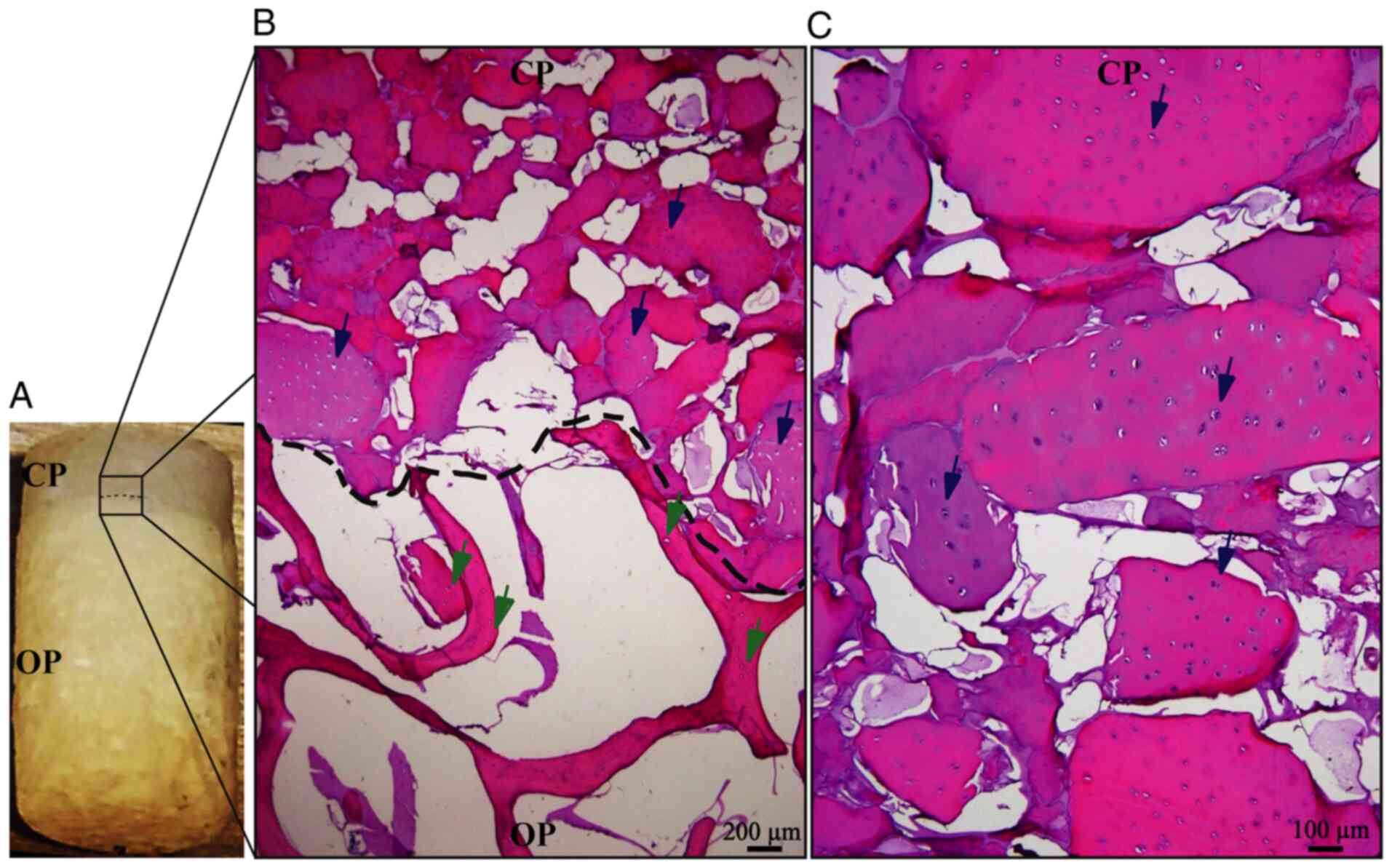
Osteochondral biphasic scaffold
manufacture and cell migration evaluation
The described method allows for the generation of
easily reproducible biphasic scaffolds (Fig. 4A), able to maintain the cartilage
structure until the differentiated cells secrete the new cartilage
matrix. Migration analysis of pre-differentiated cells indicated
that these cells have a strong preference for the chondral phase
(Fig. 4B), maintaining tropism for
the bioactive material of the chondral phase (decellularized bovine
cartilage matrix), as indicated in previous experiments performed
by our research group (19).
H&E staining suggested that Ad-MSC cells migrated into the
scaffold and 1 or 2 cells were located in the empty spaces
corresponding to the native chondral lagoons, forming isogenic
groups (Fig. 4C). These results
demonstrated that these cells were able to adopt a cellular
conformation similar to that of native cartilage tissue.
Sequential exposure differentiation
and expression analysis
Evaluation of differentiation and hypertrophy of
Ad-MSCs was performed by measuring the relative expression of
chondrogenic and osteogenic mRNA markers via RT-qPCR. The
expression levels of the mesenchymal marker THY1 increased during
preconditioning with FGF2, with a subsequent decrease when FGF2 was
removed at early culture times in the 3D scaffold to finally
increase to its maximum at 26 days of differentiation and decrease
in the late stages of differentiation (Fig. 5A).
Analysis of chondrogenic marker expression (Fig. 5B) demonstrated maximal expression of
SOX9 between 12 and 19 days of exposure, decreasing over time in
all scheme groups. Furthermore, the expression of the chondrogenic
markers ACAN and COL2A1 increased for up to 19 days of
differentiation, reaching its maximum level at 26 days, to decrease
at 33 days. At this point, ACAN and COL2A1 (two of the main
components of the cartilage matrix) had an increased expression in
group B compared with other differentiation schemes. COMP
(chondrogenic marker) underwent a ‘reset’ or decreased expression
during the monolayer culture transfer to the biphasic scaffold,
before increasing in the 3D scaffolds; high expression was
sustained in groups B and E. The expression patterns of the
chondrogenic markers indicated that all groups underwent a process
of chondrogenic differentiation; however, group B [FGF2 (10 ng/ml)
followed by IGF1 (100 ng/ml)/TGFβ1 (10 ng/ml) followed by TGFβ1 (10
ng/ml)] had the highest capacity towards chondrocyte
differentiation.
On the other hand, analysis of osteogenic markers
(Fig. 6) provided different
expression signatures depending on the differentiation protocol.
RUNX2 expression was increased since the preconditioning with FGF2
and maintained across the differentiation stages. Expression
analysis indicated a linear and steady expression of RUNX2 until
day 40. The osteogenic marker ALPL maintained a relatively low
expression for 33 days with a high increase at 40 days, with
notably increased expression in the D group [IGF1 (100 ng/ml)]. The
expression of the metalloprotease MMP13 increased to a maximum at
26 days, while COL10A1 expression started at day 19, increasing to
a maximum between 26 and 33 days, particularly in group E [TGFβ1
(10 ng/ml)] and decreasing at day 40. COL1A2 and SPP1 displayed a
similar expression pattern, maintaining reduced expression
throughout the differentiation process with an increase at day 40,
where group A exhibited the biggest increase of these markers.
Histological analysis
Histological analysis revealed new acidophilic
amorphous material formed over time, which produced a homogeneous
tissue similar to native cartilage (Fig. 7). In addition, the biodegradability
of silk fibroin was observed over time, with only traces on day 40.
The remodeling of the cartilage matrix was evaluated with Masson's
trichrome staining, where collagen was stained blue and fibrous
tissue turned into a reddish color. In most groups, collagen
decreased over time, while the amount of fibrous matrix increased
at later stages (Fig. 8).
Group B maintained the most stable collagen matrix
until day 33; however, an apparent fibrotic process began between
days 33 and 40. In groups A, C, D and E, the fibrotic process
started before day 33, with a small amount of collagen and high
calcification observed at day 40. These observations suggested
that, despite the presence of certain hypertrophic markers, group B
maintained the lowest levels of osteogenic expression, while it had
higher expression of chondrogenic markers compared with the other
groups, retaining its properties until day 33 at the histological
level. It was indicated that group B was subjected to the most
optimal protocol of growth factor application for chondrogenic
differentiation of Ad-MSCs with conditions suitable for human
use.
Discussion
Native cartilage properties hinder its regenerative
abilities once tissue reaches maturity and, despite being generated
by a single cellular lineage, the medical approaches to regenerate
a damaged area face a big challenge that has not been surpassed
(30,31). MSCs are undifferentiated cells with
the ability to proliferate, migrate and differentiate into
different cell lineages. In vivo, these cells support the
maintenance, renewal and regeneration of human tissues (32). For this reason, isolation and in
vitro differentiation of MSCs is a promising therapy for the
regeneration of most human tissues (18,33,34).
However, optimal culture and differentiation conditions for this
purpose are still in development (35,36).
MSCs may be isolated from multiple human tissues
(bone marrow, dental pulp and umbilical cord) (37). However, adipose tissue, usually
obtained from surgical waste, is easily accessible and contains a
relatively high number of MSCs, with adequate properties for cell
culture, proliferation, differentiation and minimum ethical
considerations (38), which may be
used to generate a chondrocyte-like phenotype through its
stimulation for chondrogenesis.
Biological chondrogenesis is regulated by signaling
molecules (growth factors), and by cell-cell and cell-matrix
interactions. In vitro models of MSCs are capable of
inducing chondrogenic differentiation by the use of growth factors
and/or biomaterials (39,40). In the present study, it was proposed
that the simultaneous use of a combination of growth factors and
matrix stimuli (bioactive scaffold) on Ad-MSCs may achieve stable
chondrogenic differentiation, with the aim of developing a
long-term therapy for human cartilage injuries, in the form of a
medical implant loaded with pre-differentiated Ad-MSCs, to generate
a medical implant capable of assisting in cartilage regeneration.
The scaffolds should have the following properties: i)
Biocompatibility with the adjacent tissue; ii) biodegradability;
iii) allow cellular migration and adhesion; and iv) ability to
resist the mechanical load until the tissue is fully regenerated
(5,41). To achieve these properties, a
biphasic scaffold with decellularized cartilage as a bioactive
ingredient was developed by our group, which has been demonstrated
to promote MSC migration, adhesion and cell-matrix signaling that
may improve chondrogenic differentiation (19,42).
Silk fibroin was used as the adhesive material to hold together the
fragments of cartilage. It had the properties of being able to
generate pores for cell migration and hold together bone and
cartilage phases, as well as flexibility to allow mechanical load
(43).
In the present study, the biphasic scaffolds
developed had an upper 2-mm thick phase that simulates native
cartilage conditions for further implantation, with native
cartilage characteristics, such as flexibility, water retention and
mechanical resistance to compression (25). The lower 4-mm thick phase of
decellularized bone matrix chips served as support for the
cartilage phase implantation and integration of cartilage with
adjacent tissue. Pre-differentiated Ad-MSCs were introduced into
the biphasic scaffold via direct injection of a cell suspension
into the chondral phase of the biphasic scaffold, where the pore
size, growth conditions and cartilage substrate allowed the
migration of cells within the whole scaffold.
For efficient differentiation, a precise balance of
chondrogenic and osteogenic factors with temporal changes in the
local microenvironment is required (41). RUNX2 is necessary at the first step
of differentiation (together with SOX9) to achieve chondrogenic
differentiation, also acting as an osteogenic marker after
differentiation and at the beginning of the hypertrophic process
(44). In addition, native
embryonic cells express RUNX2 during the early stages of
chondrocyte differentiation, and the generation of mice deficient
in RUNX2 leads to a lack of chondrocyte maturation (45). Thus, a proper combination of
microenvironment modulation, the appropriate stimuli and an
adequate period of exposure are necessary for the maintenance of a
stable phenotype.
Previous research has demonstrated that the addition
of FGF2 in the expansion phase of MSC culture increases their
proliferation, and predisposes cells to undergo chondrocyte
differentiation and matrix secretion in a SOX9-independent
mechanism, by its coupling to the FGF receptor (46-48).
In addition, IGF1, a growth factor commonly used for chondrogenic
differentiation, is capable of MSC differentiation and increase of
extracellular matrix secretion at the first stages of
differentiation, but is also one of the principal promoters of the
hypertrophic process and matrix replacement in the late stages of
chondrogenic differentiation; that is why IGF1 needs to be removed
from the differentiation scheme after the first stages. On the
other hand, TGFβ1 inhibits hypertrophic differentiation (49), and promotes SOX9 activation and
chondrogenic differentiation, with a synergistic effect with
IGF1(50) in the first stages of
biological MSC differentiation to chondrocytes (9,51).
In the present study, all of the tested schemes
achieved differentiation of Ad-MSCs to a chondrocyte-like
phenotype; however, there were differences between group schemes,
depending on the sequential order and concentration of the growth
factors. The expression analysis and histological results indicated
that group B [FGF2 (10 ng/ml), followed by a combination of IGF1
(100 ng/ml)/TGFβ1 (10 ng/ml) and a final exposure step of TGFβ1
alone (10 ng/ml)] led to a more efficient chondrogenesis and
decreased hypertrophic progression.
The use of IGF1 at a continuous dose led to the
lowest degree of differentiation and matrix generation, while the
synergistic effect of the use of IGF1 plus TGFβ1 in the
differentiation of MSCs to the chondrocyte phenotype (50), which was validated by the expression
analysis of group D (with IGF1 alone) and group E (with TGFβ1), the
combination of which (group B) achieved preferable differentiation
results.
Maintaining differentiated cells with low levels of
TGFβ1 (1 ng/ml) (group A) failed to prevent endochondral
ossification, leading to the highest levels of certain osteogenic
markers. This may be due to the following: i) An insufficient
concentration of TGFβ1 to maintain the chondrogenic phenotype and
to avoid endochondral ossification; ii) natural degradation of the
growth factor over time; iii) a lower rate of diffusion inside the
biphasic scaffold; or iv) a low ratio between the TGFβ1
concentration and cell number after proliferation inside the
scaffold. Finally, a higher concentration of TGFβ1 (10 ng/ml) in
the candidate scheme (group B) maintained chondrogenic
differentiation while cell hypertrophy was decreased, even under
extensive growing times (until >33 days).
The unexpected behavior of the CD90 mesenchymal
marker along the exposure period is consistent with the study by
Hagmann et al (46), who
reported that the addition of FGF2 to the culture medium alters the
expression patterns of the CD90 marker in MSCs.
Comparison among the test conditions for
differentiation used in the present study indicated that group B
had the most effective differentiation potential towards more
phenotypically stable chondrocytes. However, an important
limitation of the present study was the lack of a
non-differentiated group for RT-qPCR and histology, to determine
the absolute values in the differentiation groups in comparison to
MSCs without differentiation.
For the adequate clinical use of regenerative
therapy, it is important that isolation and culture methods are
performed under conditions that may be extrapolated for medical use
(xeno-free conditions), which allows the use of Ad-MSCs for a wide
range of clinical applications, minimizing the possibility of an
adverse reaction or host rejection, in addition to inhibiting
changes in molecular signaling pathways. The present study focused
on chondrocyte differentiation under clinically relevant
conditions, with the use of decellularized materials and
animal-free medium conditions with the use of HS (to avoid cellular
signaling changes and immune response), which also demonstrated
improved proliferation compared with FBS conditions.
In conclusion, the present results demonstrated that
the use of clinically relevant conditions, together with an
improved differentiation protocol of growth factors in sequence and
concentrations to emulate biological chondrogenesis (group B), in a
3D scaffold that simulates native cartilage, allows the possible
use of these differentiated cells in an implant for cartilage
damage. Future perspectives of this work include pre-clinical
testing of the present method, the use of decellularized human
cartilage and bone materials, and cell differentiation in a porcine
model of cartilage damage to examine the safety, biodegradability
and regenerative capacity of the technique of differentiation in
the 3D scaffold.
Acknowledgements
The authors thank Dr Sergio Lozano-Rodriguez
(Faculty of Medicine and University Hospital ‘Dr José E. González’,
Autonomous University of Nuevo Leon) for his help in reviewing the
manuscript.
Funding
Funding: This work was supported by CONACyT (grant no.
SALUD-2014-01-233365).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JLA and LFM conceptualized the study, acquired
funding and administered the project. AGR, VPS, NKMS performed the
experiments. AGR, VPS, NKMS, VJRD and RRP performed the formal
analysis. VJRD and AGR performed histological analysis. YCG
examined the patients, collected the samples and was involved in
the isolation of Ad-MSCs. CNSD, AMRE, RST, HL, VPM, ACM, RRP and
IAMM contributed to the study conception and design. VPS and NKMS
analyzed and approved the authenticity of the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethics and Research Committees of the Faculty of Medicine and
University Hospital ‘Dr José E. González’ of the Autonomous
University of Nuevo Leon (Monterrey, México; registry no.
PI17-00360). All patients provided written informed consent for the
use of their tissues.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Medvedeva EV, Grebenik EA, Gornostaeva SN,
Telpuhov VI, Lychagin AV, Timashev PS and Chagin AS: Repair of
damaged articular cartilage: Current approaches and future
directions. Int J Mol Sci. 19(2366)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Musumeci G, Mobasheri A, Trovato FM,
Szychlinska MA, Graziano AC, Lo Furno D, Avola R, Mangano S,
Giuffrida R and Cardile V: Biosynthesis of collagen I, II, RUNX2
and lubricin at different time points of chondrogenic
differentiation in a 3D in vitro model of human mesenchymal stem
cells derived from adipose tissue. Acta Histochem. 116:1407–1417.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sanchez Naranjo JC: Fisiología del
condrocito articular. Rev Colomb Reumatol. 15:21–33. 2008.
|
|
4
|
Devitt BM, Bell SW, Webster KE, Feller JA
and Whitehead TS: Surgical treatments of cartilage defects of the
knee: Systematic review of randomised controlled trials. Knee.
24:508–517. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li YY, Cheng HW, Cheung KM, Chan D and
Chan BP: Mesenchymal stem cell-collagen microspheres for articular
cartilage repair: Cell density and differentiation status. Acta
Biomater. 10:1919–1929. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Delanois RE, Etcheson JI, Sodhi N, Henn RF
III, Gwam CU, George NE and Mont MA: Biologic therapies for the
treatment of knee osteoarthritis. J Arthroplasty. 34:801–813.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Walker JM: Mesenchymal Stem Cells. 2nd
edition. Gnecchi M (ed). Springer, New York, NY, 2016.
|
|
8
|
Augustyniak E, Trzeciak T, Richter M,
Kaczmarczyk J and Suchorska W: The role of growth factors in stem
cell-directed chondrogenesis: A real hope for damaged cartilage
regeneration. Int Orthop. 39:995–1003. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cicione C, Muiños-López E, Hermida-Gómez
T, Fuentes-Boquete I, Díaz-Prado S and Blanco FJ: Alternative
protocols to induce chondrogenic differentiation: Transforming
growth factor-β superfamily. Cell Tissue Bank. 16:195–207.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhou N, Li Q, Lin X, Hu N, Liao JY, Lin
LB, Zhao C, Hu ZM, Liang X, Xu W, et al: BMP2 induces chondrogenic
differentiation, osteogenic differentiation and endochondral
ossification in stem cells. Cell Tissue Res. 366:101–111.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sheykhhasan M, Qomi RT and Ghiasi M:
Fibrin scaffolds designing in order to human adipose-derived
mesenchymal stem cells differentiation to chondrocytes in the
presence of TGF-β3. Int J Stem Cells. 8:219–227. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zuo Q, Cui W, Liu F, Wang Q, Chen Z and
Fan W: Co-cultivated mesenchymal stem cells support chondrocytic
differentiation of articular chondrocytes. Int Orthop. 37:747–752.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhong L, Huang X, Karperien M and Post J:
The regulatory role of signaling crosstalk in hypertrophy of MSCs
and human articular chondrocytes. Int J Mol Sci. 16:19225–19247.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hata K, Takahata Y, Murakami T and
Nishimura R: Transcriptional network controlling endochondral
ossification. J Bone Metab. 24:75–82. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mueller MB and Tuan RS: Functional
characterization of hypertrophy in chondrogenesis of human
mesenchymal stem cells. Arthritis Rheum. 58:1377–1388.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang X, Li Y, Han R, He C, Wang G, Wang J,
Zheng J, Pei M and Wei L: Demineralized bone matrix combined bone
marrow mesenchymal stem cells, bone morphogenetic protein-2 and
transforming growth factor-β3 gene promoted pig cartilage defect
repair. PLoS One. 9(e116061)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Solorio LD, Dhami CD, Dang PN, Vieregge EL
and Alsberg E: Spatiotemporal regulation of chondrogenic
differentiation with controlled delivery of transforming growth
factor-β1 from gelatin microspheres in mesenchymal stem cell
aggregates. Stem Cells Transl Med. 1:632–639. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Krinner A and Roeder I: Quantification and
modeling of stem cell-niche interaction. Adv Exp Med Biol.
844:11–36. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Moncada-Saucedo NK, Marino-Martínez IA,
Lara-Arias J, Romero-Díaz VJ, Camacho A, Valdés-Franco JA,
Pérez-Silos V, García-Ruiz A, Lin H, Tuan RS, et al: A bioactive
cartilage graft of IGF1-transduced adipose mesenchymal stem cells
embedded in an alginate/bovine cartilage matrix tridimensional
scaffold. Stem Cells Int. 2019(9792369)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Saucedo M and Karina N: Diseño y
evaluación in vitro de un implante bifásico bioactivo para la
reparación de defectos osteocondrales. Universidad Autónoma de
Nuevo León, 2019.
|
|
21
|
Haghighi F, Dahlmann J, Nakhaei-Rad S,
Lang A, Kutschka I, Zenker M, Kensah G, Piekorz RP and Ahmadian MR:
bFGF-mediated pluripotency maintenance in human induced pluripotent
stem cells is associated with NRAS-MAPK signaling. Cell Commun
Signal. 16(96)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang W, Sui Y, Ni J and Yang T: Insights
into the Nanog gene: A propeller for stemness in primitive stem
cells. Int J Biol Sci. 12:1372–1381. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Damdimopoulou P, Rodin S, Stenfelt S,
Antonsson L, Tryggvason K and Hovatta O: Human embryonic stem
cells. Best Pract Res Clin Obstet Gynaecol. 31:2–12.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Henderson JK, Draper JS, Baillie HS,
Fishel S, Thomson JA, Moore H and Andrews PW: Preimplantation human
embryos and embryonic stem cells show comparable expression of
stage-specific embryonic antigens. Stem Cells. 20:329–337.
2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pérez-Silos V, Moncada-Saucedo NK,
Peña-Martínez V, Lara-Arias J, Marino-Martínez IA, Camacho A,
Romero-Díaz VJ, Lara Banda M, García-Ruiz A, Soto-Dominguez A, et
al: A cellularized biphasic implant based on a bioactive silk
fibroin promotes integration and tissue organization during
osteochondral defect repair in a porcine model. Int J Mol Sci.
20(5145)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Blaudez F, Ivanovski S, Hamlet S and
Vaquette C: An overview of decellularisation techniques of native
tissues and tissue engineered products for bone, ligament and
tendon regeneration. Methods. 171:28–40. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Feldman AT and Wolfe D: Tissue processing
and hematoxylin and eosin staining. Methods Mol Biol. 1180:31–43.
2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rieppo L, Janssen L, Rahunen K, Lehenkari
P, Finnilä MAJ and Saarakkala S: Histochemical quantification of
collagen content in articular cartilage. PLoS One.
14(e0224839)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Carballo CB, Nakagawa Y, Sekiya I and
Rodeo SA: Basic science of articular cartilage. Clin Sports Med.
36:413–425. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang L, Hu J and Athanasiou KA: The role
of tissue engineering in articular cartilage repair and
regeneration. Crit Rev Biomed Eng. 37:1–57. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Dulak J, Szade K, Szade A, Nowak W and
Józkowicz A: Adult stem cells: Hopes and hypes of regenerative
medicine. Acta Biochim Pol. 62:329–337. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xian CJ and Foster BK: Repair of injured
articular and growth plate cartilage using mesenchymal stem cells
and chondrogenic gene therapy. Curr Stem Cell Res Ther. 1:213–229.
2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Somoza RA, Welter JF, Correa D and Caplan
AI: Chondrogenic differentiation of mesenchymal stem cells:
Challenges and unfulfilled expectations. Tissue Eng Part B Rev.
20:596–608. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Danišovič Ľ, Varga I and Polák Š: Growth
factors and chondrogenic differentiation of mesenchymal stem cells.
Tissue Cell. 44:69–73. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Almalki SG and Agrawal DK: Key
transcription factors in the differentiation of mesenchymal stem
cells. Differentiation. 92:41–51. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fujii S, Miura Y, Iwasa M, Yoshioka S,
Fujishiro A, Sugino N, Kaneko H, Nakagawa Y, Hirai H, Takaori-Kondo
A, et al: Isolation of mesenchymal stromal/stem cells from
cryopreserved umbilical cord blood cells. J Clin Exp Hematop.
57:1–8. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Argentati C, Morena F, Bazzucchi M,
Armentano I, Emiliani C and Martino S: Adipose stem cell
translational applications: From bench-to-bedside. Int J Mol Sci.
19(3475)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kwon H, Paschos NK, Hu JC and Athanasiou
K: Articular cartilage tissue engineering: The role of signaling
molecules. Cell Mol Life Sci. 73:1173–1194. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jiang X, Huang X, Jiang T, Zheng L, Zhao J
and Zhang X: The role of Sox9 in collagen hydrogel-mediated
chondrogenic differentiation of adult mesenchymal stem cells
(MSCs). Biomater Sci. 6:1556–1568. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Demoor M, Ollitrault D, Gomez-Leduc T,
Bouyoucef M, Hervieu M, Fabre H, Lafont J, Denoix JM, Audigié F,
Mallein-Gerin F, et al: Cartilage tissue engineering: Molecular
control of chondrocyte differentiation for proper cartilage matrix
reconstruction. Biochim Biophys Acta. 1840:2414–2440.
2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xia C, Mei S, Gu C, Zheng L, Fang C, Shi
Y, Wu K, Lu T, Jin Y, Lin X and Chen P: Decellularized cartilage as
a prospective scaffold for cartilage repair. Mater Sci Eng C Mater
Biol Appl. 101:588–595. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kundu B, Rajkhowa R, Kundu SC and Wang X:
Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv
Rev. 65:457–470. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Komori T: Runx2, an inducer of osteoblast
and chondrocyte differentiation. Histochem Cell Biol. 149:313–323.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Mikasa M, Rokutanda S, Komori H, Ito K,
Tsang YS, Date Y, Yoshida CA and Komori T: Regulation of Tcf7 by
Runx2 in chondrocyte maturation and proliferation. J Bone Miner
Metab. 29:291–299. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hagmann S, Moradi B, Frank S, Dreher T,
Kämmerer PW, Richter W and Gotterbarm T: FGF-2 addition during
expansion of human bone marrow-derived stromal cells alters MSC
surface marker distribution and chondrogenic differentiation
potential. Cell Prolif. 46:396–407. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kabiri A, Esfandiari E, Hashemibeni B,
Kazemi M, Mardani M and Esmaeili A: Effects of FGF-2 on human
adipose tissue derived adult stem cells morphology and
chondrogenesis enhancement in transwell culture. Biochem Biophys
Res Commun. 424:234–238. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Correa D, Somoza RA, Lin P, Greenberg S,
Rom E, Duesler L, Welter JF, Yayon A and Caplan AI: Sequential
exposure to fibroblast growth factors (FGF) 2, 9 and 18 enhances
hMSC chondrogenic differentiation. Osteoarthritis Cartilage.
23:443–453. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Grafe I, Alexander S, Peterson JR, Snider
TN, Levi B, Lee B and Mishina Y: TGF-β family signaling in
mesenchymal differentiation. Cold Spring Harb Perspect Biol.
10(a022202)2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhou Q, Li B, Zhao J, Pan W, Xu J and Chen
S: IGF-I induces adipose derived mesenchymal cell chondrogenic
differentiation in vitro and enhances chondrogenesis in vivo. In
Vitro Cell Dev Biol Anim. 52:356–364. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kim YI, Ryu JS, Yeo JE, Choi YJ, Kim YS,
Ko K and Koh YG: Overexpression of TGF-β1 enhances chondrogenic
differentiation and proliferation of human synovium-derived stem
cells. Biochem Biophys Res Commun. 450:1593–1599. 2014.PubMed/NCBI View Article : Google Scholar
|