1. Introduction
Orthopedic injuries and associated pathologies are
an important public health issue worldwide as well as a major
global burden of disability and suffering. It is estimated that the
musculoskeletal conditions affect approximately 1.71 billion
individuals globally (1). The
Global Burden of Disease, Injuries, and Risk Factors Study (GBD)
2019 reported for the 1990-2019 time period musculoskeletal
disorders as one of the 10 significant causes of enhancing burden
from teenage years to older age with a 30.7% increase in DALY rates
(Disability-Adjusted Life-Years) (2). These conditions or orthopedic trauma
often require surgery and the use of permanent, temporary or
biodegradable medical devices that include various natural or
synthetic biomaterials able to substitute or repair different
tissues (ligaments, tendons, cartilage, bone). The design of the
medical device, functional properties of biomaterials and the
bioresponse are some of the key players that determine the clinical
success of the orthopedic intervention. Apart from their
mechanical, microstructural and chemical characteristics,
biomaterials are screened for their innocuity, biocompatibility,
safety and efficacy for the clinical efficacy (3).
Biocompatibility, one of the most important features
of biomaterials, is defined as ‘the ability of a biomaterial to
perform its desired function with respect to a medical therapy,
without eliciting any undesirable local or systemic effects on the
recipient or beneficiary of that therapy, but meanwhile generating
the most optimized clinically relevant performance of that therapy’
or ‘the ability of a material to perform with an appropriate host
response in a specific situation’ (3,4). Thus,
biocompatibility testing is a primary requirement in the
development and the approval of orthopedic materials for clinical
use by regulatory agencies. Biomaterials need to meet basic
biocompatibility criteria as set by the International Standards
Organization (ISO 10993). They must be nontoxic, nonthrombogenic,
noncarcinogenic, nonantigenic and nonmutagenic in order to exhibit
an appropriate biological response (5).
In this regard, biocompatibility testing is a
complex process that include in vitro and in vivo
specific tests depending on the end-use application of the
biomaterials. The goal of this review is to provide a roadmap for
the practical approach to the biocompatibility testing for
orthopedic materials. The most important assays in this area are
discussed based on the current findings. In addition, the review
summarizes the main categories of biomaterials in orthopedics and
their biocompatibility issues.
2. Biomaterials in orthopedics
According to the American National Institute of
Health, a biomaterial is ‘any substance or combination of
substances, other than drugs, synthetic or natural in origin, which
can be used for any period of time, which augments or replaces
partially or totally any tissue, organ or function of body in order
to maintain or improve the quality of life of the individual’
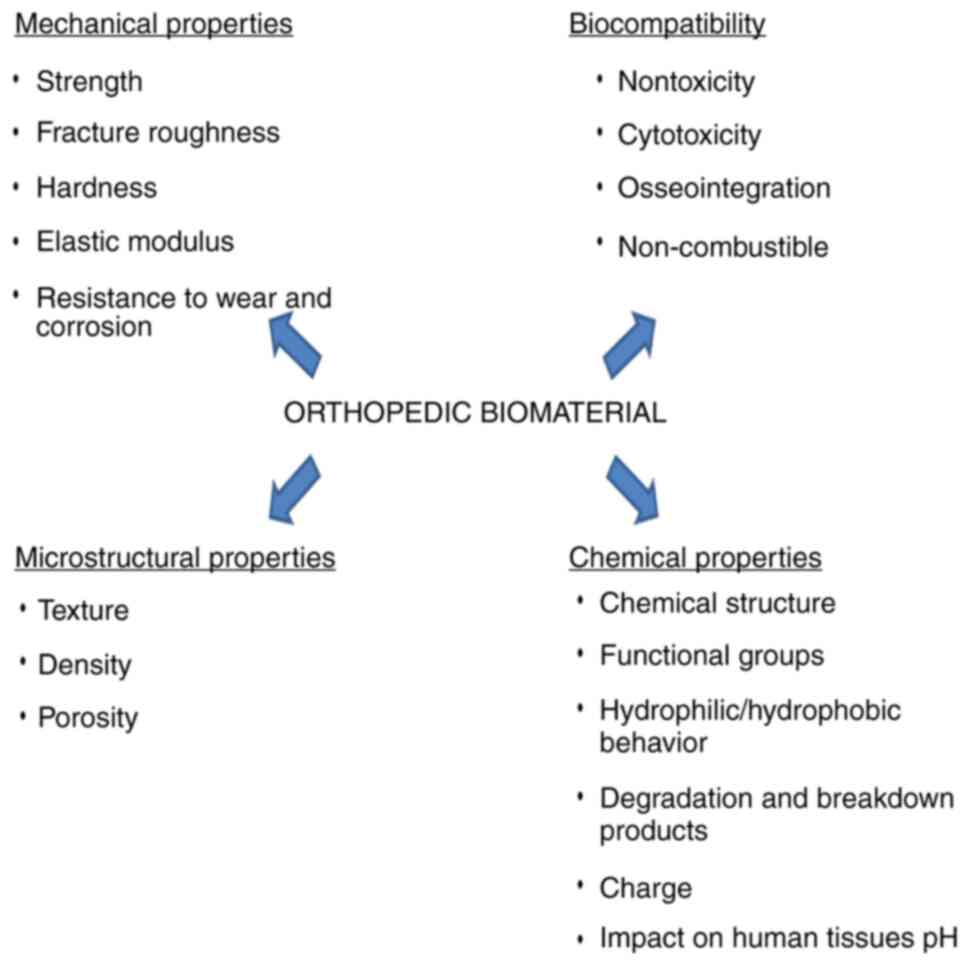
(6). The main required properties
of orthopedic biomaterials are good mechanical abilities including
high resistance to corrosion and wear, biocompatibility, chemical
stability and appropriate microstructural characteristics (Fig. 1) (7,8). A
high longevity of materials expressed by unaltered properties after
a long time of contact with the biological surroundings are
tremendously essential.
Depending on their nature, orthopedic materials can
be classified as metals, ceramics, polymers and composites.
Metallic materials
Due to their superior mechanical properties, metals
have been mostly used in prostheses stems, fracture plates, or as
load-bearing components in total joint replacement. The most
commonly used metals include stainless steels (316L), titanium and
titanium-base alloys (Ti-6Al-4V, TiAl4VELI, Ti6Al17Nb) and cobalt
alloys (Co-Cr-Mo) (Table I)
(7-9).
Among these, titanium and its alloys show an excellent corrosion
resistance and a high biocompatibility as well as good long-term
behavior (10). In addition, Co-Cr
based alloys exhibit good mechanical properties including excellent
wear resistance, but they have a low biocompatibility (11). The main biocompatibility issues
related to metallic materials and their alloys are bioactivity,
corrosion byproducts, hypersensitivity reactions (mostly in the
case of nickel alloys), and lipid uptake (5).
 | Table IVarious important biomaterials used
in orthopedic applications. |
Table I
Various important biomaterials used
in orthopedic applications.
| Materials |
Characteristics | Disadvantages | Applications | (Refs.) |
|---|
| Metallic
materials | | | | |
|
Stainless
steel (316L) | Excellent corrosion
resistance | High modulus of
elasticity | Fracture
plates | (9,11) |
| | Relative good
biocompatibility | Rigidity | Screws | |
| | Relative low
cost | Allergic reactions
(nickel alloys) | Cerclage
cables | |
| | Easy
processing | | | |
|
Titanium and
its alloys (Ti, Ti6A14V) | Good corrosion
resistance | Bacterial
adherence | Prostheses
stems | (9,11,12) |
| | Moderate elastic
modulus | High hardness | | |
| | Good
mechano-chemical properties | Low wear
resistance | | |
| |
Osseointegration | | | |
| | Immunological
resistance | | | |
| | Excellent
biocompatiblity | | | |
|
Co-Cr-Mo
alloys | Excellent corrosion
resistance | Lower
biocompatibility | Prostheses
stems | (9,11) |
| | Good wear
resistance | Immunogenic | Load-bearing
components in | |
| | Superior fatigue
strength | Expensive to
manufacture | total joint
replacement | |
| | Low ductility | Limited
intra-operative manipulation | | |
| | High surface
smoothness | | | |
| Ceramics | | | | |
|
Alumina | Good corrosion
resistance | | Femoral head
implants | (8,11) |
| | Low friction/wear
coefficients | | | |
| | Surface
smoothness | | | |
|
Zirconia | Good fracture
toughness | | Hip
replacement | (8,11) |
| | High strength | | Bone
reconstruction | |
| | High
biocompatibility | | Endosseous
implants | |
|
Hydroxyapatite
(HA) | High compressive
strength | Low tensile | Coating of
orthopedic implants | (11,15, |
| | High
biocompatibility | Low fatigue
strength | Bone grafts | 18,19) |
| | Good
immunotolerance | | | |
| |
Osseoconduction | | | |
| |
Osseointegration | | | |
|
Tricalcium
phosphate (ß-TCP) | Bioresorbable | Poor mechanical
properties | Femoral knee | (13,17, |
| | Biocompatible | Brittleness | Hip prostheses | 20) |
| | Similar composition
to bone | | Tibial
components | |
| | Good
osteoconductive properties | | Bone plates and
screws | |
| Polymers | | | | |
|
Polyethylene
(PE), ultrahigh | Excellent
toughness | Low wear
resistance | Liner of acetabular
cups | (11,19) |
|
molecular
weight PE (UHMWPE) | Low density | Release of
particles and the occurrence of | in hip
arthroplasties | |
| | Availability | inflammatory
reactions/osteolysis | Bone joint
replacement (UHMWPE) | |
|
Poly(methylmethacrylate),
PMMA | Good
mechanical | Low tension
resistance | Orthopedic
surgeries | (11,12, |
| | properties | Thermal
degradation | Vertebroplasty | 21,22) |
| | Non-porous
structure | Systemic reactions
(inflammatory, embolism) | Space filler for
the proper | |
| | Excellent optical
properties | Poor
osseointegration | anchorage of the
implant to | |
| | Ease of
manipulation | | bone surface | |
| | Less expensive | | | |
|
Polylactic
acid (polylactide), |
Biodegradability | Weak thermal
stability | Bioabsorbable | (11,23) |
|
PA | Long term
biocompatibility | Low toughness | Fixation
devices | |
| | Thermoplastic
properties | Low ductility | Porous scaffolds
for the | |
| | | | grow of
neo-tissue | |
The coating of metals with bioactive ceramics and
the chemical modification of metal surface by binding of polymers
and biomolecules allow the biofunctionalization of metallic
materials and the control of the biodegradability rate and
biocompatibility (8,12). In this regard, magnesium alloys
(Mg-Ca, Mg-Zn) and magnesium matrix composites (Mg-calcium
phosphate particle, Mg-hydroxyapatite, Mg-tricalcium phosphate) are
one of the metallic materials which have recently attracted growing
interest due to their improved mechanical and biological properties
(9,13). Magnesium is ideal for biodegradable
orthopedic implants owing to its high biocompatibility,
osteogenesis ability and biodegradable behavior (14).
Ceramics
Ceramics represent a family of
inorganic/non-metallic products with a broad range of composition
that can be dense and resorbable like tricalcium phosphate or
dense, non-porous and chemically binding to the bone such as
hydroxyapatite (HA) (15,16). They are widely used in orthopedic
applications as bone replacement in hip and knee reconstruction.
The main advantages of these materials are high corrosion
resistance, hardness, wear resistance, low friction, significant
biocompatibility and osseointegration with the host tissue. On the
basis of tissue-material interface reaction, bioceramics are
generally classified into three categories: i) bioactive
(hydroxyapatite, bioactive glasses); ii) bioresorbable (calcium
phosphate) and iii) bioinert (alumina, zirconia) (Table I) (8,9,17-20).
It is of interest to modulate the chemistry of ceramics that they
become osteoinductive but also to enhance the bone regeneration
rate. The biocompatibility issues that could be considered in the
ceramics category are related to ADME profile (adsorption,
deposition, excretion, and metabolism), bioactivity, lipid uptake
and thromboresistance (5).
Polymers
Some of the most known polymers of first generation
are acrylic resins, polyethylene (PE) and ultrahigh molecular
weight PE (UHMWPE) and polymethacrylate (PMMA) (Table I) (11,12,19,21-23).
They are characterized by structural stability, low cost but a
relative biocompatibility. Their main use refers to joint
replacement, anchorage of prostheses and hip arthroplasties. The
second generation of polymers are biodegradable and resorbable
materials that are of interest in orthopedic practice for the
purpose of bone substitution, and repair of bone fractures,
cartilage, or membranes. Some examples of biodegradable polymers
include polyglicolide (PGA), polylactide (PLA), polydioxanone
(PDS), poly(e-caprolactone) (PCL) or chitosan (5,11). The
biocompatibility of polymeric materials is influenced by various
factors as structural properties (chemical structure, molecular
weight, functional groups, hydrophobicity), surface morphology,
wetting abilities, or electrical charge. Thus, the increase in
molecular weight of polyethylene glycol is associated with a
decrease in protein adsorption (9).
The biocompatibility issues associated with these biomaterials
differ slightly between the two polymer types; thus, in the case of
synthetic polymers, these issues refer to the calcification,
extractables, hypersensitivity reactions, lipid uptake, protein
adsorption and sterilization residuals. For the biodegradable
polymers, the biodegradation products, ADME, effect of infection
(acid pH on biodegradation particulates), effect of hematoma
(alkaline pH) are the specific biocompatibility issues (5).
Composite biomaterials
Composites comprise at least two components, namely
matrix material and a filler (reinforcement) and they are widely
used in structural and automotive applications. The addition of the
filler enhances structural properties, biocompatibility and
bioactivity of the matrix. These biomaterials have been classified
into metal matrix composites (Ti/HA, Mg/HA, Ti6Al4V/HA), ceramic
matrix composites (HA/stainless steel) and polymer matrix
composites (high density polyethylene/HA, carbon fiber/polyether
ether ketone). Fiber-reinforced polymers and PMMA-composites are
largely used in orthopedic devices (7,9,16,17).
The major biocompatibility issues described for composites include
hypersensitivity, lipid uptake, matching tissue biomechanics, and
surface exposure of compounded particles (5).
3. Biocompatibility testing
The designed materials for orthopedic uses should be
capable to function in vivo without exhibiting any
undesirable local or systemic effects as immune, allergic,
inflammatory and carcinogenic responses (5). Biocompatibility includes not only
bio-inertia, but also biofunctionality and biostability. It is a
key concept that strongly depends on the material properties
(texture, crystallinity, wettability, surface chemistry, breakdown
products, charges, stiffness), interaction with the biological
environment of targeted tissues (adsorption of proteins,
inflammatory processes, contact with blood), period of the device
application, and type of application (24-26).
Taking all this into account, the biological
evaluation of biomaterials includes a broad spectrum of in
vitro and in vivo tests related to the
cytocompatibility, genotoxicity, sensitization, irritation, acute
and chronic toxicity, hemocompatibility, reproductive and
developmental toxicity, carcinogenicity, implantation and
degradation as specified in different international standards
(Table II) (3).
 | Table IIInternational standards for
biocompatibility testing of biomaterials (4,5). |
Table II
International standards for
biocompatibility testing of biomaterials (4,5).
| |
Standardsa |
|---|
| Biological
response | ISO | ASTM |
|---|
| Cytotoxicity | 10993-5 | F813-07; F895-84;
F1027-06 |
| Sensitization | 10993-10 | F720-81; F2147-01;
F2148-07 |
| Irritation | 10993-10 | F719-81;
F749-98 |
| Acute systemic
toxicity | 10993-11 | F750-87 |
| Subacute
toxicity | 10993-11 | - |
| Genotoxicity | 10993-3 | E1262-88 |
|
Immunoresponsiveness | 10993-20 | F1906-98 |
|
Hemocompatibility | 10993-4 | F756-08 |
| Chronic
toxicity | 10993-11 | - |
|
Carcinogenicity | 10993-3 | F1439-03 |
| Degradation | 10993-9;
10993-13 | F1983-14 |
| | 10993-14;
10993-15 | |
| Implantation | 10993-6 | F1408-97; F763-04;
F1904-98 F981-04; F1983-99 |
Before biocompatibility testing, the biomaterials
from a final form of the medical device, are extracted using a
semiphysiological medium (saline solution, cottonseed or sesame
seed oil) or cell culture medium in small tubes. Then, they are
usually incubated for 24-72 h at 37˚C. Furthermore, the resulting
extract solutions are decanted into sterile glass tubes and used in
the biological tests (3). The
extraction procedure is very gentle. Only some compounds from the
surface of materials can be extracted. Its main limitations include
low extraction yield (<10%), short-time contact between material
and cells/tissue fluids compared to the in vivo conditions,
and unsuitable extraction medium for organic hydrophobic compounds.
The use of the extraction vehicle with low concentrations of
dimethylsulphoxide (<0.5%) allows solubilization of both
hydrophilic and hydrophobic compounds (4).
Cytocompatibility tests
Cytocompatibility tests evaluate the biological
reactivity of living cells to the biomaterial extract solutions
including cell viability, growth, and metabolic activity (4). Toxic agents derived from biomaterials
such as metal ions, reactive agents, residual monomers, may exert
toxic effects on cell functions and viability. The cellular damage
involves structural disintegration and alteration of cell
morphology, reduction in cell adhesion and proliferation, decrease
in metabolic activity and cell lysis (27).
Cytotoxicity elution test
Cytotoxicity elution test (MEM elution) is an in
vitro qualitative assay. It involves the incubation of L-929
mouse fibroblast cells with an extract of the test material for 48
h. After incubation, the cells are microscopically examined in
terms of morphological changes (deformed and lysed cells). The cell
responses to material are scored on a scale of 0 to 4. The
biomaterial is considered biocompatible if the cell response to the
test material is not greater than grade 2 (mild reactivity)
(3).
MTT assay
MTT assay is the most commonly used test to evaluate
cell viability and proliferation. It relies on the enzymatic
reduction of yellow
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) to purple formazan in metabolically active cells. The color
intensity of formed formazan is directly proportional to the number
of viable cells. The measuring absorbance of formazan at 570 nm
allows quantitative evaluation of living cells. The material is
considered cytocompatible if the percentage of viable cells is
equal to or higher than 70% (3,28).
Although MTT is considered the ‘gold standard’ in cytotoxicity
studies, the test has several limitations. Thus, the cell culture
conditions may affect the metabolism and rate of MTT reduction, and
the interaction between test material and MTT can modify the final
results. The carbon nanotubes as well as calcium phosphate
scaffolds are able to reduce MTT. In addition, carbon nanotubes can
bind the formed formazan altering the test outcome (4,28).
Agar overlay assay
Agar overlay assay is a qualitative test that
evaluates cytotoxicity by indirect contact. Subconfluent cell
cultures (mouse fibroblasts L929, NIH 3T3) are overlaid by an agar
layer. The test material is placed on the agar layer and after
24-72 h exposure time, it is removed. Then, the cells under and
around the test material are exposed to Neutral Red
(3-amino-7-dimethylamino-2-methylphenazine hydrochloride) staining.
The detachment, vacuolization and lysis of cells can be
semi-quantitatively scored. Only the living cells accumulate the
Neutral Red dye and they appear red colored to light-microscope
analysis. The agar overlay assay is suitable for high density
biomaterials. The limits of the test are related to the short time
exposure to biomaterial (only acute cytotoxicity detection) and the
binding or adsorption of Neutral Red on test or released compounds
(3,4,29).
Genotoxicity evaluation
Genotoxicity evaluation is an essential part of the
safety assessment of biomaterials as damage of genetic material may
result in the induction of carcinogenesis or may alter the
reproductive function if germ cell DNA is impaired. The sensitive
genotoxicity endpoints comprise DNA damage, gene mutations and
chromosomal damage (30,31). The genotoxic risk assessment of
biomaterials is performed in mammalian and non-mammalian systems
(3).
Mouse lymphoma assay
Mouse lymphoma assay (MLN) is an in vitro
mammalian cell gene mutation test that is able to detect both gene
mutations (point mutations) and clastogenic injuries (deletions,
translocations, mitotic recombination/gene conversion, and
aneuploidy). Basically, the test quantifies alterations of
thymidine kinase (TK) gene expression located on chromosome
11. The L5178YTK+/--3.7.2 C mouse lymphoma cell line is
used for the assay. The cells deficient in the TK gene as a
result of the mutation TK+/- to TK-/- induced
by a genotoxicant are resistant to cytostatic activity of
pyrimidine analogue triflurothymidine (TFT). In the presence of TFT
in the medium, the mutant cells are able to survive and proliferate
whereas the normal cells are not. Cells are incubated with test
material for 3 to 4 h in the presence and absence on an metabolic
activation system. In addition, the cells are exposed to test agent
for 24 h without exogenous metabolic activation. A
co-factor-supplemented post-mitochondrial fraction obtained from
hepatic tissue of rodents is used for metabolic activation. After
treatments, the cells are subcultured for two days to allow
expression of the mutant phenotype. Then, the cells are seeded into
96-well plates with and without TFF to detect mutant cells and to
evaluate cloning efficiency. Previously, the dose range of the test
material is established depending on the cytotoxicity assessment.
The occurrence of mutant colonies (small and large colonies) and
the increase in mutant frequency (more than Global Evaluation
Factor, 126x10-6) is associated with the induction of
chromosomal aberrations and the mutagenicity of the test material.
The outcome of the test is significantly influenced by the cell
line viability per se, solubilization of the test material,
changes in the pH, osmolality, and high levels of cytotoxicity
(3,32,33).
In vitro chromosomal aberration
assay
In vitro chromosomal aberration assay
evaluates structural aberrations (chromosome/chromatid breaks;
chromosome/chromatid exchanges) but it also detects polyploidy and
endoreduplication. The test is performed in primary human
peripheral blood lymphocytes or in established cell lines such as
Chinese hamster ovary (CHO) cells or Chinese hamster fibroblasts.
In this method, the proliferating cells are exposed to the extract
test dilutions with and without metabolic activation for 3 to 6 h.
The same metabolic activation system as in the MLA test is used.
After exposure to the test material, the cell cultures are treated
for 1-3 h with a metaphase-arresting compound (colcemid) at
predetermined time intervals in relation with cell cycle length.
The cells are harvested for the preparation of chromosomes by
hypotonic treatment, fixation and staining with Giemsa. Chromosomal
aberrations (number and types) in the mitotic cells are scored. The
percentage of cells with this chromosomal damage is evaluated for
test material dilutions as compared to the value of the negative
controls. Test conditions (pH changes, osmolality) may influence
the final results leading to a positive outcome. In addition,
established cell lines should be characterized by stability in the
modal chromosome number. The solvent used for the extraction of
biomaterial should not interfere with the cell response (3,34,35).
Reverse mutation assay
Reverse mutation assay (Ames test) is a
nonmammalian test system that use mutagenicity in bacteria as an
end point. The assay is performed with strains of Salmonella
typhimurium and Escherichia coli that have point
mutations in the genes of the histidine and tryptophan operon,
respectively. The exposure of mutant bacterial cells to a mutagenic
agent causes a reversal of initial mutation (back mutation)
restoring the ability of the bacteria to grow on the media lacking
in histidine. Bacterial cultures are exposed to the biomaterial in
the presence and the absence of metabolic activation system, using
either plate incorporation or preincubation before plating. After 2
to 3 days of incubation at 37˚C, the revertant colonies are counted
compared to the control plates. An increased number of revertant
colonies indicates the mutagenic potential of the tested material.
The preincubation method is recommended for some materials such as
divalent metals (3,36,37).
Irritation (intracutaneous reactivity)
testing
Irritation (intracutaneous reactivity) testing is an
in vivo assay that evaluates the potential of biomaterials
to cause irritation on the exposed area of the body. Saline and
vegetable biomaterial extracts are administered by intracutaneous
injection into multiple sites on the back of albino rabbits. The
skin reactions (erythema, edema, scabbing, bleeding) are evaluated
24, 48 and 72 h following injection using a standardized scoring
scheme. The biomaterials meets the criteria of the test if the
difference between its average irritation score and the value of
control is 1 or <1. The use of rancid vegetable oil as a vehicle
for biomaterials could determine an extreme reactivity hiding the
true effect of the biomaterial (3,38).
Skin sensitization assay
Skin sensitization assay evaluates the allergenic
potential of biomaterials. Guinea pig maximization test is the most
commonly used assay. It is based on the induction of an immune
response of the skin and it comprises 3 phases. Stage I of the test
(Induction) implies an intradermal injection of the biomaterial
extract in guinea pigs on the test area. Then, a topical patch is
applied after 7 days (Phase II, Induction II) and 14 days (Phase
III, Challenge). Skin reactions (erythema, swelling) are evaluated
at 24 and 48 h following patch removal using a scoring system.
Sensitization potential is associated with a score value of 1 or
greater in the test group (3,39).
Acute systemic toxicity testing
Acute systemic toxicity testing estimates the hazard
potential of a biomaterial following short-time exposure in
animals. The extracts of the test material and negative controls
are injected intravenously (saline extract) or intraperitoneally
(cottonseed oil extract) into Swiss Albino mice. Then, the animals
are observed 4, 24, 48, and 72 h after treatment. The body weight,
survival, and animal behavior are recorded. The biomaterial meets
the test requirements if its biological reaction is lower than the
negative control (3).
Hemocompatibility
The biomaterial components of prosthetic devices in
contact with the bloodstream regardless of contact duration must be
hemocompatible; they do not cause clinically significant
blood-related adverse events such as thrombosis, hemolysis,
platelet and complement activation. Mostly the artificial surfaces
can induce clotting, and high levels of coagulation is associated
with acute thrombosis or thromboembolism that determine the failure
of biomaterials/device (40,41).
The hemocompatibility depends on the material characteristics but
also on the fluid mechanics of the device and the blood
coagulability. The key points in hemocompatibility testing are
coagulation, hemolysis, hematology, platelets, and complement
system. The assessment involves static, agitated or shear flow
in vitro models for the incubation of fresh human blood with
biomaterials. The hemocompatibility markers are determined before
and after the incubation of the test material (42).
Coagulation
Coagulation is evaluated in vitro by
measuring the rate of clot formation or the partial thromboplastin
time (PTT) of plasma exposed to the biomaterials during an
incubation time. A shortening of PTT induced by contact with
biomaterials compared to the negative control shows the activation
of the internal coagulation pathway. The amount of thrombin, the
main enzyme of the coagulation pathway can also be determined by
measuring thrombin-antithrombin complex (TAA) and prothrombin
fragment 1+2 using ELISA techniques. The TAA complex reflects a
functional state of the coagulation, and prothrombin fragments are
released during thrombin formation. An overall picture of the
clotting process can be obtained by thromboelastography, an in
vitro whole-blood viscoelastic test (40,42).
Hemolysis analysis. Hemolysis analysis deals with
the evaluation of the degree of erythrocyte lysis and the release
of hemoglobin induced by the tested biomaterial. The determination
of plasma hemoglobin is performed by spectrophotometric methods.
Immunonephelometry and ELISA techniques may be other options. For
device components having direct contact with the blood, hemolysis
testing is recommended by both direct and indirect methods. Only an
indirect method is indicated in the case of devices having indirect
contact with circulating blood. In direct testing, the blood is
incubated with biomaterial and in indirect method, the blood is
exposed to the biomaterial extract. Depending on the hemolysis
testing results, the material are classified as hemolytic (over 5%
hemolysis), slightly hemolytic (between 5 and 2%) and nonhemolytic
(below 2%) (40,42).
Hematology testing
Hematology testing includes the assessment of the
complete blood count and the activation of leukocytes as a result
of biomaterial-induced inflammatory response. Hematology analyzer
and ELISA method are used in this direction. Since the activation
of leukocytes leads to an enhanced oxygen metabolism, the excessive
reactive oxygen species generation can be evaluated using
fluorogenic or chemiluminogenic agents. In addition, the release of
polymorphonuclear leukocyte elastase, an another event induced by
leukocyte activation, can be quantified by ELISA or fluorescence
(40,42).
Platelet activation testing
Several points can be used for the evaluation of
undesired platelet activation induced by the blood-biomaterial
contact. They involve the quantification of degranulation proteins
(platelet factor 4, b-thromboglobulin, thromboxane B2) released
after platelet activation by ELISA, detection of P-selectin (CD62P)
or activated GPIIb/IIIa using flow cytometry (40,42).
Complement system activation
The complement system is an important component of
the innate immune response and it is primarily involved in the
first-line host defense against pathogenic factors through three
major pathways (classical, alternative and lectin pathway).
Biomaterials act primarily upon the alternative pathway of
complement, and the complement activation is associated with the
generation of complement proteins (C3, C5b, C6, C7, C8, C9) that
can be determined using ELISA. In addition, the complement
activation can be analyzed by the evaluation of the 50% complement
hemolytic activity (CH50). In general, the hydrophobic surfaces of
biomaterials cause an increased complement activation compared to
hydrophilic surfaces. The binding of complement components to the
biomaterial surfaces can reduce their plasma content and can alter
the final result (40,42).
Implantation tests
Implantation tests evaluate the local pathological
effects on living tissues that constitute the target of the
biomaterial/medical device used for implantation. The biological
response of the surrounding tissues (number and distribution of
inflammatory cells, vascularity of fibrous capsule, granuloma,
fatty infiltration, material debris, endothelialization, presence
of necrosis) is commonly characterized by histological analysis.
Both the tissue from the immediate vicinity of the implant and more
distant tissues are studied.
In implantation tests, the safety of materials is
assessed not only dose-dependently but also time-dependently. The
tests are performed using mice, rats, guinea pigs or rabbits for
short-term evaluation studies (12 weeks) (43-45).
The rabbits are preferred for the musculoskeletal implantation
studies since they have faster skeletal change, a high bone turn
over and are less expensive and easy to handle (12). The animals with relatively long life
expectancy such as dogs, sheep, goats, are used in longer-term
studies. The chemistry, size and the degradation pattern of the
materials significantly influence the biological response (43-45).
4. Conclusions
It is undeniable that the biocompatibility concept
is a major concern in the design of biomaterials in orthopedics. A
wide array of specific tests must be performed in order to ensure
safety and efficacy of the biomaterials as this paper has reviewed.
The development of new materials, the new paradigm of
biocompatibility (‘do no harm’ approach is transformed into one of
doing ‘good’) (5) and the
understanding of the role of biomaterial surface physicochemistry
accentuate the need for a calibration of regulatory criteria
depending on new technologies and concepts. The design of
biomaterials with high biocompatibility and functional properties
(antimicrobial, osteoinductive) is highly desirable.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
BH wrote the initial draft of the manuscript,
contributed to the conception and design of the article and
performed the literature data collection. BP and RMN contributed to
the design of the article. BP, RMN, SG, GP and AF consulted
relevant references and performed the literature data collection.
OA and PDS revised the manuscript in light of the literature
findings. All authors have read and approved the final version of
the manuscript for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cieza A, Causey K, Kamenov K, Hanson SW,
Chatterji S and Vos T: Global estimates of the need for
rehabilitation based on the global burden of disease study 2019: A
systematic analysis for the global burden of disease study 2019.
Lancet. 396:2006–2017. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
GBD 2019 Diseases and Injuries
Collaborators. Global burden of 369 diseases and injuries in 204
countries and territories, 1990-2019: A systematic analysis for the
global burden of disease study 2019. Lancet. 396:1204–1222.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Assad M and Jackson N: Biocompatibility
evaluation of orthopedic biomaterials and medical devices: A review
of safety and efficacy models. In: Encyclopedia of Biomedical
Engineering. Vol 2. 1st edition. Narayan RJ (ed). Elsevier,
Amsterdam, pp281-309, 2019.
|
|
4
|
Bruinink A and Luginbuehl R: Evaluation of
biocompatibility using in vitro methods: Interpretation and
limitations. Adv Biochem Eng Biotechnol. 126:117–152.
2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Helmus MN, Gibbons DF and Cebon D:
Biocompatibility: Meeting a key functional requirement of
next-generation medical devices. Toxicol Pathol. 36:70–80.
2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bergmann CP and Stumpf A: Biomaterials.
In: Dental ceramics. Topics in mining, metallurgy and materials
engineering. Bergmann CP (ed) Springer, Heidelberg, pp9-15,
2013.
|
|
7
|
Rodríguez-González ÁF: Biomaterials in
Orthopaedic Surgery. ASM International, pp1-10, 2009.
|
|
8
|
Shekhwat D, Singh A, Banerjee MK, Singh T
and Patnaik A: Bioceramics composites for orthopaedic applications:
A comprehensive review of mechanical, biological, and
microstructural properties. Ceram Int. 47:3013–3030. 2021.
|
|
9
|
Kiradzhiyaska DD and Mantcheva RD:
Overview of biocompatible materials and their use in medicine.
Folia Med (Plovdiv). 61:34–40. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gobbi SJ, Gobbi JV and Rocha Y:
Requirements for selection/development of a biomaterial. Biomed J
Sci Tech Res. 14:10674–10679. 2019.
|
|
11
|
Navarro M, Michiardi A, Castaño O and
Planell JA: Biomaterials in orthopaedics. J R Soc Interface.
5:1137–1158. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vandana U, Nancy D, Sabareeswaran A, Remya
NS, Rajendran N and Mohanan PV: Biocompatibility of strontium
incorporated ceramic coated titanium oxide implant indented for
orthopaedic applications. Mater Sci Eng B. 264(114954)2021.
|
|
13
|
Bommala VK, Krishna MG and Rao CT:
Magnesium matrix composites for biomedical applications: A review.
J Magnes Alloy. 7:72–79. 2019.
|
|
14
|
Kumar K, Das A and Prasad SB: Recent
developments in biodegradable magnesium matrix composites for
orthopaedic applications: A review based on biodegradability,
mechanical and biocompatibility perspective. Mater Today Proc.
44:2038–2042. 2021.
|
|
15
|
Nuss KMR and von Rechenberg B:
Biocompatibility issues with modern implants in bone-a review for
clinical orthopedics. Open Orthop J. 2:66–78. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Victor SP and Muthu J: Polymer ceramic
composite materials for orthopedic applications-relevance and need
for mechanical match and bone regeneration. J Mechatron. 2:1–10.
2014.
|
|
17
|
Aherwar A, Singh AK and Patnaik A: Current
and future biocompatibility aspects of biomaterials for hip
prosthesis. AIMS Bioeng. 3:23–43. 2016.
|
|
18
|
Kattimani VS, Kondaka S and Lingameneni
KP: Hydroxyapatite-past, present, and future in bone regeration.
Bone Tissue Regen Insights: Sep 11, 2016 (Epub ahead of print).
|
|
19
|
Rufino Senra M and Marques FV: Synthetic
polymeric materials for bone replacement. J Compos Sci.
4(191)2020.
|
|
20
|
Liu B and Lun DX: Current application of
β-tricalcium phosphate composites in orthopaedics. Orthop Surg.
4:139–144. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Samavedi S, Poindexter LK, Van Dyke M and
Goldstein AS: Synthetic biomaterials for regenerative medicine
applications. In: Regenerative Medicine Applications in Organ
Transplantation. Orlando G, Lerut J, Soker S and Stratta RJ (eds).
Academic Press, Boston, pp81-99, 2014.
|
|
22
|
Webb JC and Spencer RF: The role of
polymethylmethacrylate bone cement in modern orthopaedic surgery. J
Bone Joint Surg Br. 89:851–857. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cipurković A, Horozić E, Đonlagić N, Marić
S, Saletović M and Ademović Z: Biodegradable polymers: Production,
properties and application in medicine scientific review paper.
Technol Acta. 11:25–35. 2018.
|
|
24
|
Quinn J, McFadden R, Chan CW and Carson L:
Titanium for orthopedic applications: An overview of surface
modification to improve biocompatibility and prevent bacterial
biofilm formation. iScience. 28(10174)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rahmati M, Silva EA, Reseland JE, Heyward
CA and Haugen HJ: Biological responses to physicochemical
properties of biomaterial surface. Chem Soc Rev. 49:5178–5224.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cvrček L and Horáková M: Plasma modified
polymeric materials for implant applications. In: Non-Thermal
Plasma Technology for Polymeric Materials. Applications in
Composites, Nanostructured Materials and Biomedical Fields. Thomas
S, Mozetič M, Cvelbar U, Špatenka P and Praveen KM (eds). Elsevier,
Amsterdam, pp376-407, 2019.
|
|
27
|
Groth T, Falck P and Miethke RR:
Cytotoxicity of biomaterials-basic mechanisms and in vitro test
methods: A review. ATLA. 23:790–799. 1995.
|
|
28
|
Iqbal HMN and Keshavarz T: The challenge
of biocompatibility evaluation of biocomposites. In: Woodhead
Publishing Series in Biomaterials, Biomedical composites (second
edition). Ambrosio L (ed). Woodhead Publishing, Cambridge,
pp303-334, 2017.
|
|
29
|
Li W, Zhou J and Xu Y: Study of the in
vitro cytotoxicity testing of medical devices. Biomed Rep.
3:617–620. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kohl Y, Rundén-Pran E, Mariussen E, Hesler
M, El Yamani N, Longhin EM and Dusinska M: Genotoxicity of
nanomaterials: Advances in vitro models and high throughput methods
for human hazard assessment-a review. Nanomaterials (Basel).
10(1911)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Raghavendra GM, Varaprasad K and
Jayaramudu T: Biomaterials: Design, development and biomedical
applications. In: Nanotechnology Applications for Tissue
Engineering. Thomas S, Grohens Y and Ninan N (eds). Elsevier,
Amsterdam, pp21-44, 2015.
|
|
32
|
Moore MM, Honma M, Clements J, Bolcsfoldi
G, Burlinson B, Cifone M, Clarke J, Delongchamp R, Durward R,
Fellows M, et al: Mouse lymphoma thymidine kinase gene mutation
assay: Follow-up meeting of the international workshop on
genotoxicity testing-aberdeen, scotland, 2003-assay acceptance
criteria, positive controls, and data evaluation. Environ Mol
Mutagen. 47:1–5. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
OECD Guideline for the Testing of
Chemicals. In vitro mammalian cell gene mutation assays using the
thymidine kinase gene, 2014. Available from: https://www.oecd.org/env/ehs/testing/In%20Vitro%20Mammalian%20Cell%20Gene%20Mutation%20Thymidine%20Kinase%20.pdf.
|
|
34
|
OECD/OCED Guideline for the Testing of
Chemicals. In vitro mammalian chromosomal aberration test, 2016.
Available from: https://www.oecd-ilibrary.org/docserver/9789264264649-en.pdf?expires=1615635244&id=id&accname=guest&checksum=AD494BA2A538E6C1302075C64D4C7BA5.
|
|
35
|
Registre M and Proudlock R: The in vitro
aberration test. In: Genetic toxicology testing. Proudlock R (ed).
Academic Press, Amsterdam, pp207-267, 2016.
|
|
36
|
Jain AK, Singh D, Dubey K, Maurya R,
Mittal S and Pandey AK: Models and methods for in vitro toxicity.
In: In vitro Toxicology. Dhawan A and Kwon S (eds). Academic Press,
Amsterdam, pp45-65, 2018.
|
|
37
|
Guy RC: Ames test. In: Encyclopedia of
Toxicology. 2nd edition. Wexler P (ed). Elsevier, Amsterdam,
pp88-91, 2005.
|
|
38
|
De Jong WH, Caraway JW and Geertsma RE: In
vivo and in vitro testing for the biological evaluation of
biomaterials and medical devices. In: Woodhead Publishing Series in
Biomaterials, Biocompatibility and Performance of Medical Devices.
Boutrand JP (ed). Woodhead Publishing, Cambridge, pp120-158,
2012.
|
|
39
|
Kimber I, Basketter DA, Berthold K, Butler
M, Garrigue JL, Lea L, Newsome C, Roggeband R, Steiling W, Stropp
G, et al: Skin sensitization testing in potency and risk
assessment. Toxicol Sci. 59:198–208. 2001.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nalezinková M: In vitro hemocompatibility
testing of medical devices. Thromb Res. 195:145–190.
2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Brănișteanu DE, Nichifor M, Dorobăț CM,
Brănișteanu DC, Petrariu FD, Molodoi AD, Radu DC and Boda D: Use of
textile biomaterials for the topic treatment of chronic venous
disease. Rom Biotechnol Lett. 20:10618–10625. 2015.
|
|
42
|
Weber M, Steinle H, Golombek S, Hann L,
Schlensak C, Wendel HP and Avci-Adali M: Blood-contacting
biomaterials: In vitro evaluation of the hemocompatibility. Front
Bioeng Biotechnol. 6(99)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Anderson JM and Schoen FJ: In vivo
assessment of tissue compatibility. In Biomaterials Science. 3rd
edition. Ratner BD, Hoffman AS, Schien FJ and Lemons JE (eds).
Academic Press, Amsterdam, pp609-617, 2013.
|
|
44
|
Anderson JM and Jiang S: Animal models in
biomaterial development. In: Encyclopedia of biomedical
engineering. Narayan R (ed). Elsevier, Amsterdam, pp237-241,
2019.
|
|
45
|
Hakimi O, Vollrath F and Carr AJ:
Evaluation of silk as a scaffold for musculoskeletal
regeneration-the path from the laboratory to clinical trials. In:
Comprehensive biotechnology. 2nd edition. Moo-Young M (ed).
Academic Press, Amsterdam, pp341-351, 2011.
|