Introduction
At present, lung cancer is the leading cause of
cancer-associated mortality, resulting in 30-40 deaths per 100,000
(1,2). Histologically, lung cancer can be
divided into small and non-small cell lung cancer (2). As one of the most common subtypes of
non-small cell lung cancer, the incidence of lung adenocarcinoma
accounts for >40% of the total incidence of lung cancer
(3). Moreover, in the past few
decades the incidence of lung adenocarcinoma has demonstrated an
upward trend, which has posed a great threat to human life and
health (4). Therefore, the early
diagnosis and treatment of lung adenocarcinoma is important for the
prevention and treatment of lung cancer. However, early diagnosis
of lung adenocarcinoma is difficult, as the early symptoms of lung
adenocarcinoma are insignificant, and no specific markers exist
(5). When cancer occurs, patients
are usually in a locally advanced stage, but the patients are
usually diagnosed in the terminal stage of cancer. Therefore,
patients miss the optimal opportunity for surgical treatment
(6). Although traditional
anti-tumor treatments, such as radiotherapy and chemotherapy, can
exhibit certain curative effects, they cannot markedly change the
mortality rate of lung adenocarcinoma due to their toxic effects on
normal cells and multi-drug resistance (7). Previously, tumor-targeted therapeutic
drugs, such as gefitinib and cetuximab, have achieved certain
effects, but their application range is narrow, and various side
effects have also occurred and resistance has emerged (8). Therefore, the search for novel and
effective approaches for early diagnosis and treatment still
constitute an important element for the prevention and treatment of
lung adenocarcinoma.
Axl is a member of the receptor tyrosine kinase
family. It binds to its ligand growth arrest-specific protein 6
(Gas6) and activates its own tyrosine kinase activity (9). It activates downstream signal
transduction pathways and serves an important role in cell
adhesion, proliferation, migration and inhibition of apoptosis
(9). Gas6 and Axl have been
indicated to be highly expressed in various tumor tissues, and
activation of the Gas6/Axl signaling pathway has been revealed to
serve an important role in tumor cell proliferation and inhibition
of apoptosis, tumor angiogenesis, tumor invasion and metastasis
(10,11). Sawabu et al (12) demonstrated that the expression of
Gas6 and Axl mRNA and protein was higher in gastric cancer tissues
compared with adjacent healthy tissues. In a study on hepatoma
cells lines, Lee et al (13)
indicated that Gas6 induced Axl phosphorylation to activate the
MAPK/ERK signaling pathway and upregulate the downstream factor
Slug. Slug belongs to the Snail transcriptional repressor
superfamily, and its upregulation can enhance cell motility,
thereby enhancing hepatoma cell invasive ability (13). However, in lung adenocarcinoma, the
role of the Gas6/Axl signaling pathway has rarely been studied.
Therefore, the current study used human lung adenocarcinoma tissues
and the human lung adenocarcinoma cell line A549 to study the
effects of the Gas6/Axl signaling pathway on lung adenocarcinoma.
The present study demonstrated that TP-0903, which is an inhibitor
of the Gas6/Axl signaling pathway, may be a targeted drug for the
Gas6/Axl signaling pathway for the treatment of lung adenocarcinoma
as a better alternative to other drugs, such as gefitinib and
cetuximab.
Materials and methods
Patient tissue samples
All lung adenocarcinoma tissues were obtained from
patients undergoing lung adenocarcinoma resection. The present
study was approved by the Ethics Committee of the Affiliated
Hospital of Hebei University (Baoding, China) and written informed
consent was provided by all patients. All patients were diagnosed
with lung adenocarcinoma by imaging and based on pathological
findings. A total of 22 lung adenocarcinoma tissues (age, 55-65
years; 17 males and 5 females) and adjacent healthy tissues
(distance, 1 cm) were obtained from January 2017 to September 2019.
Patients with first-onset disease were included; patients who had
undergone radiotherapy or chemotherapy were excluded. During the
operation, the lung adenocarcinoma and adjacent healthy tissues
were immediately removed and stored in liquid nitrogen for
subsequent experiments.
Cell culture and drug treatment
The lung adenocarcinoma cell line A549 was purchased
from American Type Culture Collection. Cells were cultured in
RPMI-1640 medium (Thermo Fisher Scientific, Inc.) containing
1x105 µmol/l penicillin and streptomycin and 10% FBS
(Thermo Fisher Scientific, Inc.). Cells were cultured in an
incubator at 37˚C with 5% CO2. Then, 0.1 µM Axl
inhibitor (cat. no. TP-0903; Selleck Chemicals) (14), 1 µg/ml recombinant human Gas6
(ACROBiosystems, Inc.) (15), Gas6
+ siRNA-Axl or an equal volume of PBS was used to treat cells.
Control cells were treated with sterile phosphate buffer or
negative control siRNA.
Small interfering (si)RNA
transfection
A549 cells were transfected with siRNA-Axl or
siRNA-negative control (Thermo Fisher Scientific, Inc.). A total of
95% of the cells were viable 12 h after transfection. The cells
were incubated for another day before subsequent experiments. Axl
expression level was decreased after transfection with siRNA-Axl.
The transfection efficiency was examined via reverse
transcription-quantitative PCR (RT-qPCR). The siRNA-Axl sequence
was as follows: siRNA-Axl-sense, 5'-UAUCACAGGUGCCAGAGGA-3';
siRNA-Axl-antisense, 5'-UCCUCUGGCACCUGUGAUA-3'. Negative control
siRNA sequence was 5'-GCAAGCTGACCCTGAAGTT-3'.
The cells were transfected after cell growth density
had reached 30%. A total of 50 pmol siRNA were mixed with
serum-free RPMI-1640 medium in 25 µl final volume, and 1 µl
Entranster-R4000 (Engreen Biosystem, Ltd.) was mixed with 24 µl
serum-free RPMI-1640 medium and incubated at room temperature for
15 min. The RNA and Entranster-R4000 solutions were subsequently
mixed and further incubated at room temperature for 15 min. The 50
µl transfection solution was mixed with 0.45 ml complete RPMI-1640
medium and added into the cells. A total of 6 h after transfection
at 37˚C, the cell medium was replaced with fresh complete RPMI-1640
medium and the cells were cultured.
Western blot analysis
A549 cells were seeded in six-well plates at
1x105 cells/well. After the cells had reached the
appropriate confluency at 2 days, total protein was extracted using
RIPA lysis buffer (Beyotime Institute of Biotechnology), and
protein concentration was measured using the BCA method (Thermo
Fisher Scientific, Inc.). SDS-PAGE was subsequently performed with
10% gels and 30 µg protein loaded per lane, followed by transfer of
the proteins to a PVDF membrane. After blocking with 5% BSA
(Beyotime Institute of Biotechnology) for 2 h at room temperature,
the PVDF membrane was incubated with the following primary
antibodies: Gas6 (rabbit; 1:3,000; Abcam; cat. no. ab264098), Axl,
(rabbit; 1:3,000; Abcam; cat. no. ab259831), p21 (rabbit; 1:3,000;
Abcam; cat. no. ab109520), p53 (rabbit; 1:3,000; Abcam; cat. no.
ab32389), caspase-3 (rabbit; 1:1,000; Cell Signaling Technology,
Inc.; cat. no. 9662S), caspase-9 (rabbit; 1:1,000; Abcam; cat. no.
ab32539) and β-actin (1:10,000; ProteinTech Group, Inc.; cat. no.
66009-1-Ig) overnight at 4˚C. Following three washes with TBS-0.1%
Tween 20 (TBST), the membrane was incubated in the secondary
antibody solution (horseradish peroxidase-conjugated goat
anti-rabbit IgG H&L; 1:2,000; Abcam; cat. no. ab205718) on a
shaker for 1 h at room temperature. After washing the membrane
three times using TBST, the protein bands were visualized and
detected using an electrochemiluminescence system (Sigma-Aldrich;
Merck KGaA). β-actin was used as the internal control.
RT-qPCR
A549 cells were seeded into 6-well plates at
1x105 cells/well, and the cells were treated as
aforementioned. TRIzol® reagent (Thermo Fisher
Scientific, Inc.) was used to extract total RNA from the cells. A
spectrophotometer (UV-670; Shanghai Mapada Instruments Co., Ltd.)
was used to measure the concentration of the extracted RNA, and the
RNA concentration was diluted to 200-500 ng/µl with RNase free
water. Following measurement of the RNA concentration, RNA was
reverse transcribed to cDNA using the PrimeScript™ RT Master Mix
(Thermo Fisher Scientific, Inc.). Temperature protocol was 15 min
at 37˚C, 5 sec at 85˚C and 30 min at 4˚C. Subsequently, qPCR was
performed to quantify Gas6, Axl, p21, p53, caspase-3 and caspase-9
expression level using TaqMan Fast Advanced Master Mix (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions, and GAPDH was used for normalization. The primers
used for RT-qPCR are presented in Table
I. Relative mRNA expression levels were calculated using the
2-ΔΔCq method (16).
 | Table IPrimers used for reverse
transcription-quantitative PCR. |
Table I
Primers used for reverse
transcription-quantitative PCR.
| Gene name | Primer type | Sequence (5'-3') | Length (base
no.) |
|---|
| Axl | Forward |
TCAAGGTGGCTGTGAAGACGA | 21 |
| | Reverse |
CGTTCAGAACCCTGGAAACAGAC | 23 |
| Gas6 | Forward |
GCCTTCTACAGCCTGGACTAC | 21 |
| | Reverse |
TCTTGAGTTTCTTCGTGGAGTG | 22 |
| p21 | Forward |
AGTATGCCGTCGTCTGTTCG | 20 |
| | Reverse |
GACTGCAAGACAGCGACAAG | 21 |
| p53 | Forward |
GGTTCCTGCCCCAGGATGTTG | 21 |
| | Reverse |
GGAACATCTCGAAGCGCTCA | 20 |
| Caspase-3 | Forward |
CAGAATCATAAGCCCCTGGA | 20 |
| | Reverse |
TCTGCGAGTCAGGCATTTG | 19 |
| Caspase-9 | Forward |
TTCTTGAGCAACACCCTC | 18 |
| | Reverse |
CGCATACACTGTCTACCT | 18 |
| GAPDH | Forward |
ACAACTTTGGTATCGTGGAAGG | 22 |
| | Reverse |
GCCATCACGCCACAGTTTC | 19 |
Immunocytofluorescence (IF)
staining
Cell slides were placed into a 24-well plate and
A549 cells were seeded on the slides at 5x104
cells/well. After cell confluence reached 50%, IF staining was
performed. After being washed with PBS, the cells were fixed with
4% paraformaldehyde for 20 min at room temperature and incubated in
1% Triton for 15 min at room temperature, followed by blocking
non-specific antigens with 10% goat serum (Beyotime Institute of
Biotechnology) for 1 h at room temperature. The cells were then
incubated overnight at 4˚C with caspase 8 (rabbit; 1:500; Abcam;
cat. no. ab25901). The following day, after washing the cells with
PBS, a fluorescent secondary antibody [goat anti-rabbit IgG H&L
(Alexa Fluor® 488); 1:500; Abcam; cat. no. ab150077] was
added to the cells for 2 h at room temperature. Finally, DAPI was
added and incubated for 10 min at room temperature. The cells were
visualized using a fluorescence microscope (magnification x200;
Carl Zeiss AG).
Cell Counting Kit-8 (CCK-8) assay
A total of 5,000 A549 cells/well were added to a
96-well plate and placed in a 37˚C cell culture incubator. After
the cells became adherent, the cells were treated as
aforementioned. Following treatment for 24 h, 10 µl CCK-8 reagent
(Dojindo Molecular Technologies, Inc.) was added to each well. The
96-well plate was then placed in the cell culture incubator for
another 2 h. Finally, a microplate reader was used to measure the
absorbance of each well at 450 nm.
Flow cytometry
A total of 1x105 A549 cells/well were
seeded in a six-well plate. After cell confluence reached 50%, flow
cytometry was performed. The medium was discarded following
treatment as aforementioned for 24 h. Following washing of the
cells with PBS, trypsin without EDTA was used to digest the cells
for 2 min at 37˚C. Following collection by centrifugation (1,000 x
g, 5 min, 20˚C), the cells were washed with cold PBS and were
suspended in 400 µl 1X Annexin V binding solution at
~1x106 cells/ml. Subsequently, 5 µl Annexin V-FITC
(Beyotime Institute of Biotechnology) was added to each tube of
cells, which was gently mixed and incubated in the dark for 15 min
at room temperature, followed by addition of 10 µl PI in each tube
and incubation for 5 min at room temperature. Finally, flow
cytometry (Thermo Fisher Scientific, Inc.; cat. no. A28997) was
used to detect apoptosis with Attune NxT software version 3.1.2
(Thermo Fisher Scientific, Inc.).
Wound-healing assay
A549 cells were seeded into a six-well plate at
1x105 cells/well. When cell confluence reached 100%, a
10-µl pipette tip was used to create wounds vertically in the
wells. Following removal of the floating cells using PBS, the cells
were cultured in serum-free RPMI-1640 medium containing
aforementioned treatments. Photos (magnification x100) of the cells
at the same position of the wound were captured under a light
microscope at 0, 6, 12 and 24 h after wound creation to compare the
healing speed of each group.
Transwell invasion assay
A total of 50 µl Matrigel were added into the
Transwell chambers (8 µm) and incubated at 37˚C for 30 min to allow
Matrigel precoating. The treated cells were digested using trypsin
for 2 min at room temperature, and the cell suspensions with
1x105 cells diluted in RPMI-1640 medium were added to
the Transwell chambers. The serum-free medium was placed in the
upper chamber and medium with 30% FBS was placed in the lower
chamber. After incubation for 24 h at 37˚C with 5% CO2,
the cells in the upper layer of the chamber filter were removed
with a cotton swab. Subsequently, 0.1% crystal violet was used to
stain the invaded cells for 15 min at room temperature. The cells
attached to the bottom layer of the chamber filter were observed by
a light microscope (magnification x400), the number of cells was
counted in four fields of view per sample, and the effects of
treatment as aforementioned on the invasive ability of A549 cells
were examined.
Colony formation assay
A549 cells (5x103 cells/well) in 24-well
culture plates for each group were cultured for 7 days until the
colonies (diameter, 0.3-1.0 mm) were visible. 0.1% crystal violet
staining was performed for 30 min at room temperature, and the
number of colonies was counted using ImageJ 1.52 software (National
Institutes of Health).
Statistical analysis
STATA v12.0 software (StataCorp LP) was used for
statistical analysis. Measurement data are presented as the mean ±
SD. A paired Student's t-test was used to compare data between two
groups. For comparisons among >2 groups, one-way ANOVA followed
by Bonferroni's post hoc test was used. All experiments were
repeated three times. P<0.05 was considered to indicate a
statistically significant difference.
Results
High expression of Gas6 and Axl in
lung adenocarcinoma tissues
Lung adenocarcinoma tissues removed during surgery
were compared with adjacent healthy tissues, and the expression
level of Gas6 and Axl was detected by western blotting (Fig. 1A) and RT-qPCR (Fig. 1B and C). The results revealed that the
expression level of Gas6 and Axl in lung adenocarcinoma tissues was
notably higher compared with that of the adjacent healthy
tissues.
Gas6/Axl signaling pathway promotes
proliferation in A549 cells
The effect of Gas6/Axl signaling pathway on lung
adenocarcinoma cells was demonstrated using two different ways of
inhibiting the Gas6/Axl signaling pathway. TP-0903 and siRNA-Axl
were used as inhibitors of the Gas6/Axl signaling pathway. RT-qPCR
revealed that transfection with siRNA-Axl efficiently reduced the
expression level of the Axl mRNA compared with the siRNA-control
(Fig. 1A). The results of western
blotting (Fig. 2B) and RT-qPCR
(Fig. 2C and D) demonstrated that TP-0903 increased,
while recombinant human Gas6 decreased the expression level of p21
and p53 compared with control cells. Furthermore, the expression
level of p21 and p53 in the Gas6 + siRNA-Axl group higher compared
with that of the Gas6 group. In addition, CCK-8 assay (Fig. 2E) indicated that the inhibitor
TP-0903 decreased and recombinant human Gas6 increased cell
viability when compared with the control group. Furthermore, cell
viability was decreased in the Gas6 + siRNA-Axl group compared with
the Gas6 group. The results of the colony formation assay were
similar to those of the CCK8 assay (Fig. 2F and G). The results revealed that the
proliferation of A549 cells also decreased or increased by
inhibiting or activating the Gas6/Ax1 signaling pathway,
respectively.
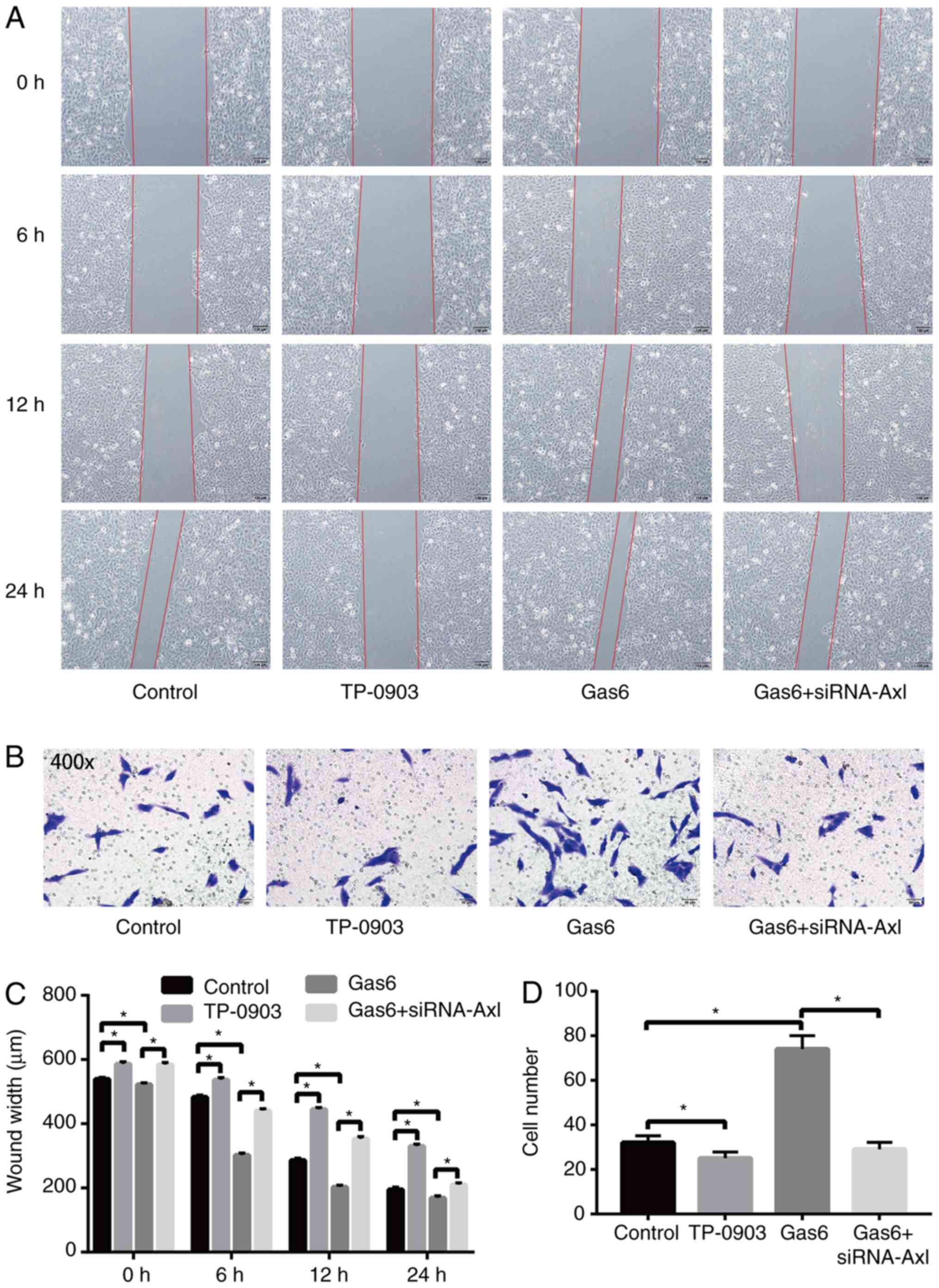
Gas6/Axl signaling pathway promotes
the migration and invasion of A549 cells
The effect of the Gas6/Axl signaling pathway on the
migratory and invasive ability of A549 cells was examined using
wound-healing and Transwell invasion assays. The results of the
wound-healing assay (Fig. 3A and
C) demonstrated that the cell
scratch healing rate was lower in the TP-0903 group and higher in
the Gas6 group when compared with the control group, and the cell
scratch rate of the Gas6 + siRNA-Axl group was lower when compared
with the Gas6 group. The Transwell invasion assay (Fig. 3B and D) revealed that when compared with the
control group, the cell invasive ability of the TP-0903 group was
decreased and that of the Gas6 group was increased. Furthermore,
the cell invasive ability of the Gas6 + siRNA-Axl group was reduced
compared with that of the Gas6 group. Therefore, the results
indicated that the migratory and invasive ability of A549 cells
decreased or increased by inhibiting or activating the Gas6/Axl
signaling pathway, respectively.
Gas6/Axl signaling pathway inhibits
apoptosis in A549 cells
The apoptosis of A549 cells was examined by flow
cytometry (Fig. 4A and B). The results revealed that the apoptotic
level of the TP-0903 group was higher and that of the Gas6 group
was lower compared with that of the control group, and the level of
apoptosis in the Gas6 + siRNA-Axl group was higher compared with
that of the Gas6 group. Western blot analysis (Fig. 4C) and RT-qPCR (Fig. 4D and E) demonstrated that the expression level
of caspase-3 and caspase-9 was increased in the TP-0903 group and
decreased in the Gas6 group compared with the control, but the
expression level of caspase-3 and caspase-9 in the Gas6 + siRNA-Axl
group was higher than that of the Gas6 group. The results of cell
immunofluorescence (Fig. 4F)
indicated that TP-0939 increased and Gas6 reduced the expression of
caspase-8, while siRNA-Axl could attenuate the effect of Gas6.
These results indicated that when the Gas6/Ax1 signaling pathway
was inhibited or activated, the level of apoptosis of A549 cells
increased or decreased, respectively.
Discussion
Lung adenocarcinoma is the most common subtype of
lung cancer, accounting for 65% of non-small cell lung cancer cases
and >40% of all lung cancers (17). Therefore, understanding the
carcinogenic signaling mechanisms of lung adenocarcinoma is
necessary to discover novel therapeutic targets for the treatment
of this disease. In the present study, the Gas6/Axl signaling
pathway was demonstrated to increase the proliferation, invasion
and migration of A549 cells, and inhibit the apoptosis of A549
cells. In addition, high expression level of Gas6 and Axl was also
observed in the lung tissues of patients with lung adenocarcinoma.
These data confirmed the pro-tumorigenic role of the Gas6/Axl
signaling pathway. These results are similar to those by Kariolis
et al (18), who
demonstrated that inhibiting the Gas6/Axl signaling pathway
potentiated the anti-tumor effect of chemotherapy. Wang et
al (19) also revealed that the
Gas6/Axl axis can promote breast cancer resistance and metastasis
via the AKT/GSK-3β/β-catenin signaling pathway. These results
indicated that the Gas6/Axl signaling pathway promoted malignant
tumorigenesis.
As a common neoplastic disease, lung adenocarcinoma
has a great impact on human health (20). When patients visit their doctor are
often in the late stages of the disease; therefore, it is
particularly important to discover targets for the treatment of
lung adenocarcinoma (21). The
present study revealed that the Gas6/Axl signaling pathway
exhibited an important effect on lung adenocarcinoma. When the
Gas6/Axl signaling pathway was inhibited, the proliferation and
invasive ability of lung adenocarcinoma cells were significantly
decreased and the apoptotic level was increased. These results are
similar to those of previous studies. For instance, in an in
vitro study in osteosarcoma cell lines, Han et al
(22) demonstrated that the
expression of phosphorylated (p)-AKT in the osteosarcoma MG63 and
U2OS cell lines was associated in a concentration-dependent manner
with exogenous Gas6 and p-AKT expression, and that p-Axl was
positively associated with p-AKT. It was also demonstrated that
exogenous Gas6 attenuated the apoptosis rate of these osteosarcoma
cell lines by activating Axl, and it was hypothesized that the
Gas6/Axl signaling pathway may promote osteosarcoma by activating
the downstream PI3K/AKT signaling pathway (22). In the A549 lung adenocarcinoma cell
line of the present study, the inhibitory effect of the Gas6/Axl
pathway on tumor cell apoptosis was also demonstrated. However, it
is unclear whether the Gas6/Axl signaling pathway exerts an
anti-apoptotic effect by activating the PI3K/AKT signaling pathway
in lung adenocarcinoma. Therefore, the mechanism of the
anti-apoptotic effect of the Gas6/Axl signaling pathway on tumor
cells will be further explored in future studies. Rankin et
al (23) revealed that
downregulation of Axl expression in ovarian cancer cells inhibited
MMP-2 gene transcription by decreasing the activity of the MMP-2
promoter, whereas decreased MMP-2 expression resulted in decreased
invasion, metastasis and tumorigenic ability of the ovarian cancer
cells. The study also demonstrated that the expression level of
p-AKT was decreased when Axl expression was downregulated in
ovarian cancer cells, while the expression level of p-ERK1/2 was
not affected (23). Therefore, it
was speculated that the Gas6/Axl axis promoted the activation of
the PI3K/AKT pathway, which in turn enhanced the expression and
activity of MMP-2. As a result, MMP-2 promoted the invasion and
metastasis of tumor cells. Sainaghi et al (24) demonstrated that when exogenous Gas6
was added into the culture medium of the DU145 prostate cancer cell
line during serum starvation, the uptake of
[3H]-thymidine by tumor cells increased, and the
expression level of p-AKT and p-p38 MAPK also increased. This
suggested that Gas6 may enhance the mitotic ability of tumor cells
by activating the PI3K/AKT and p38 MAPK signaling pathways.
Therefore, existing studies have indicated that the Gas6/Axl
signaling pathway can promote tumor cell migration and invasion,
while increasing tumor cell proliferation in various types of
tumors.
A previous study has also demonstrated that Gas6 and
Axl were highly expressed in lung adenocarcinoma tumor tissues,
indicating that the Gas6/Axl signaling pathway was activated in
lung adenocarcinoma tumor tissues (25). This also provided a theoretical
basis for the present study to investigate the use the Gas6/Axl
signaling pathway as a target for the treatment of lung
adenocarcinoma. At present, the treatment of advanced lung
adenocarcinoma mainly includes chemoradiotherapy, but it can only
serve a role in delaying the course of the disease (26). Therefore, the Gas6/Axl signaling
pathway may be the focus of future clinical research as a possible
therapeutic target for lung adenocarcinoma.
However, there are also certain limitations to the
present study. EGFR, echinoderm microtubule-associated protein-like
4, anaplastic lymphoma kinase and VEGF have been indicated to be
the most important driver genes of lung adenocarcinoma. However,
the specific targets of the Gas6/Axl signaling pathway in lung
adenocarcinoma and whether they are associated with the
aforementioned driver genes are unknown. Therefore, the specific
targets of the Gas6/Axl signaling pathway in lung adenocarcinoma
will be explored in subsequent studies to provide a strong
theoretical basis for the clinical treatment of lung
adenocarcinoma.
In summary, the Gas6/Axl signaling pathway was
revealed to be highly expressed in lung adenocarcinoma tissues and
cells, and inhibition of the Gas6/Axl signaling pathway attenuated
the proliferation and invasion of lung adenocarcinoma cells. The
present study provided a potential molecular target for future
tumor-targeted therapy and indicated that TP-0903 may be an
effective drug to treat lung adenocarcinoma. In addition, the
current study preliminarily demonstrated the role of the Gas6/Axl
signaling pathway in the development of lung cancer. However, its
deeper function and potential molecular mechanisms remain unclear
and require further investigation. In conclusion, the Gas6/Axl
signaling pathway was indicated to promote the proliferation,
migration and invasion and reduce the apoptosis of lung
adenocarcinoma cells, thereby serving an important role in the
progression of lung adenocarcinoma.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DW, LB, JR, NX, LZ and XL were responsible for the
study design, resources, data curation and analysis. DW and LB were
responsible for the manuscript preparation. NX, LZ and XL reviewed
and edited the manuscript. DW and XL confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Hebei University (Baoding,
China) and written informed consent was provided by all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Howlader N, Forjaz G, Mooradian MJ, Meza
R, Kong CY, Cronin KA, Mariotto AB, Lowy DR and Feuer EJ: The
effect of advances in lung-cancer treatment on population
mortality. N Engl J Med. 383:640–649. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Schwartz AG and Cote ML: Epidemiology of
lung cancer. Adv Exp Med Biol. 893:21–41. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yang F, Li H and Wang J: The precision
surgical treatment strategies of lung adenocarcinoma under the
guidance of the WHO new classification. Zhonghua Wai Ke Za Zhi.
55:49–53. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
4
|
Morales-Oyarvide V and Mino-Kenudson M:
Tumor islands and spread through air spaces: Distinct patterns of
invasion in lung adenocarcinoma. Pathol Int. 66:1–7.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kometani T, Sugio K, Osoegawa A, Seto T
and Ichinose Y: Clinicopathological features of younger (aged ≤50
years) lung adenocarcinoma patients harboring the EML4-ALK fusion
gene. Thoracic Cancer. 9:563–570. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Senan S, Paul MA and Lagerwaard FJ:
Treatment of early-stage lung cancer detected by screening: Surgery
or stereotactic ablative radiotherapy? Lancet Oncol. 14:e270–e274.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jiang N and Xu X: Exploring the survival
prognosis of lung adenocarcinoma based on the cancer genome atlas
database using artificial neural network. Medicine (Baltimore).
98(e15642)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chang CH, Lee CH and Wang JY: Gefitinib or
erlotinib for previously treated lung adenocarcinoma: Which is
superior? J Clin Oncol. 35:1374–1375. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gustafsson A, Fritz HKM and Dahlbäck B:
Gas6-Axl signaling in presence of Sunitinib is enhanced,
diversified and sustained in renal tumor cells, resulting in
tumor-progressive advantages. Exp Cell Res. 355:47–56.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Antony J, Tan TZ, Kelly Z, Low J, Choolani
M, Recchi C, Gabra H, Thiery JP and Huang RYJ: The GAS6-AXL
signaling network is a mesenchymal (Mes) molecular subtype-specific
therapeutic target for ovarian cancer. Sci Signal.
9(ra97)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jin Y, Nie D, Li J, Du X, Lu Y, Li Y, Liu
C, Zhou J and Pan J: Gas6/AXL signaling regulates self-renewal of
chronic myelogenous leukemia stem cells by stabilizing
beta-catenin. Clin Cancer Res. 23:2842–2855. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sawabu T, Seno H, Kawashima T, Fukuda A,
Uenoyama Y, Kawada M, Kanda N, Sekikawa A, Fukui H, Yanagita M, et
al: Growth arrest-specific gene 6 and Axl signaling enhances
gastric cancer cell survival via Akt pathway. Mol Carcinog.
46:155–164. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Lee HJ, Jeng YM, Chen YL, Chung L and Yuan
RH: Gas6/Axl pathway promotes tumor invasion through the
transcriptional activation of slug in hepatocellular carcinoma.
Carcinogenesis. 35:769–775. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sinha S, Boysen J, Nelson M, Secreto C,
Warner SL, Bearss DJ, Lesnick C, Shanafelt TD, Kay NE and Ghosh AK:
Targeted Axl inhibition primes chronic lymphocytic leukemia B cells
to apoptosis and shows synergistic/additive effects in combination
with BTK inhibitors. Clin Cancer Res. 21:2115–2126. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Morizono K, Xie Y, Olafsen T, Lee B,
Dasgupta A, Wu AM and Chen IS: The soluble serum protein Gas6
bridges virion envelope phosphatidylserine to the TAM receptor
tyrosine kinase Axl to mediate viral entry. Cell Host Microbe.
9:286–298. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Testa U, Castelli G and Pelosi E: . Lung
cancers: Molecular characterization, clonal heterogeneity and
evolution, and cancer stem cells. Cancers (Basel).
10(248)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kariolis MS, Miao YR, Diep A, Nash SE,
Olcina MM, Jiang D, Jones DS II, Kapur S, Mathews II, Koong AC, et
al: Inhibition of the GAS6/AXL pathway augments the efficacy of
chemotherapies. J Clin Invest. 127:183–198. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Wang C, Jin H, Wang N, Fan S, Wang Y,
Zhang Y, Wei L, Tao X, Gu D, Zhao F, et al: Gas6/Axl axis
contributes to chemoresistance and metastasis in breast cancer
through Akt/GSK-3β/β-catenin signaling. Theranostics. 6:1205–1219.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhao CC, Chen J, Niu RF, Liu Y and Zhang
CG: Increased resistin suggests poor prognosis and promotes
development of lung adenocarcinoma. Oncol Rep. 40:3392–33404.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Faget J, Contat C, Zangger N, Peters S and
Meylan E: RANKL Signaling sustains primary tumor growth in
genetically engineered mouse models of lung adenocarcinoma. J
Thorac Oncol. 13:387–398. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Han J, Tian R, Yong B, Luo C, Tan P, Shen
J and Peng T: Gas6/Axl mediates tumor cell apoptosis, migration and
invasion and predicts the clinical outcome of osteosarcoma
patients. Biochem Biophys Res Commun. 435:493–500. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rankin EB, Fuh KC, Taylor TE, Krieg AJ,
Musser M, Yuan J, Wei K, Kuo CJ, Longacre TA and Giaccia AJ: AXL is
an essential factor and therapeutic target for metastatic ovarian
cancer. Cancer Res. 70:7570–7579. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sainaghi PP, Castello L, Bergamasco L,
Galletti M, Bellosta P and Avanzi GC: Gas6 induces proliferation in
prostate carcinoma cell lines expressing the Axl receptor. J Cell
Physiol. 204:36–44. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Seike M, Kim CH, Zou F, Noro R, Chiba M,
Ishikawa A, Κunugi S, Kubota K and Gemma A: AXL and GAS6
co-expression in lung adenocarcinoma as a prognostic classifier.
Oncol Rep. 37:3261–3269. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Scafoglio CR, Villegas B, Abdelhady G,
Bailey ST, Liu J, Shirali AS, Wallace WD, Magyar CE, Grogan TR,
Elashoff D, et al: Sodium-glucose transporter 2 is a diagnostic and
therapeutic target for early-stage lung adenocarcinoma. Sci Transl
Med. 10(eaat5933)2018.PubMed/NCBI View Article : Google Scholar
|