EVO is considered to be a novel class of
multi-target compounds that can be used to treat different types of
cancer (16). In addition to
topoisomerases, transient receptor potential cation channel
subfamily V member 1(TRPV1) and the aryl hydrocarbon receptor (AhR)
are considered direct protein targets of EVO (16). TRPV1 has been linked to processes
mediating inflammation, cancer and cardiovascular and other disease
(22,23). AhR serves a functional role in
physiology and toxicology, especially in cell proliferation and
differentiation, the adaptive and toxic responses and
immunomodulation (24,25). However, the potential application
of EVO in the clinic is hindered by its poor bioavailability due to
limited absorption (26).
Furthermore, similar to several anticancer drugs, such as
doxorubicin, erlotinib, sunitinib and sorafenib, that can induce
cardiotoxicity (27-29),
EVO has been reported to induce cardiovascular side effects,
including oxidative stress, both in vitro and in vivo
(30). The cardiotoxicity of EVO
has been investigated on primary cultured neonatal rat
cardiomyocytes in vitro, and on zebrafish in vivo;
EVO induced cardiac malfunction, which was evidenced by a decrease
in the heart rate, as well as in circulation and pericardial
malformations (30). In addition
to cardiotoxicity, the issue of hepatotoxicity has also been raised
with regards to the application and safety of EVO. It has been
reported that the alkaloid-rich extract of Evodiae fructus
is likely to cause hepatic injury in mice (31). As a major alkaloid compound
contained within Evodiae fructus, hepatotoxicity mediated by
EVO is induced by enhancing the activity of aspartate
aminotransferase, alanine transaminase, lactate dehydrogenase and
alkaline phosphatase (32).
Drug metabolism serves crucial roles in the
bioavailability and subsequent pharmacological activities of poorly
soluble, naturally occurring therapeutic agents, which significant
impacts their toxicity profile and side effects (33). Previous studies have reported that
the toxicity of EVO may be associated with its mechanism of
metabolism in vivo and pharmacokinetics (34,35).
EVO is readily susceptible to metabolism, P450 enzymes-mediated
dehydrogenation reactions may cause toxicity via formation of
electrophilic intermediates, then produce glutathione
(GSH)-conjugated metabolites to exert more potent cytotoxic effects
than EVO itself (34). In
addition, 10-hydroxyevodiamine and 11-hydroxyevodiamine are two
primary toxic metabolites of EVO (36). Therefore, there is a demand to
identify suitable EVO delivery systems for enhancing its
bioavailability and bioactivity, in addition to alleviating the
toxicity of EVO by regulating its metabolism and pharmacokinetics.
The present review aims to summarize the potential mechanism of EVO
in cancer therapeutics and discuss its pharmacokinetic
characteristics. Finally, strategies designed to improve its oral
bioavailability and alleviating its side effects are also
mentioned.
Apoptosis is a programmed mechanism of cell death
that can eliminate foreign or malignant cells to protect organisms
against cancer, in addition to being important for normal
development (40). EVO is a potent
inducer of apoptosis in human non-small-cell lung cancer A549 cells
(41), where EVO-induced apoptosis
has been observed to occur downstream of mitotic arrest and
subsequent mitotic slippage (42).
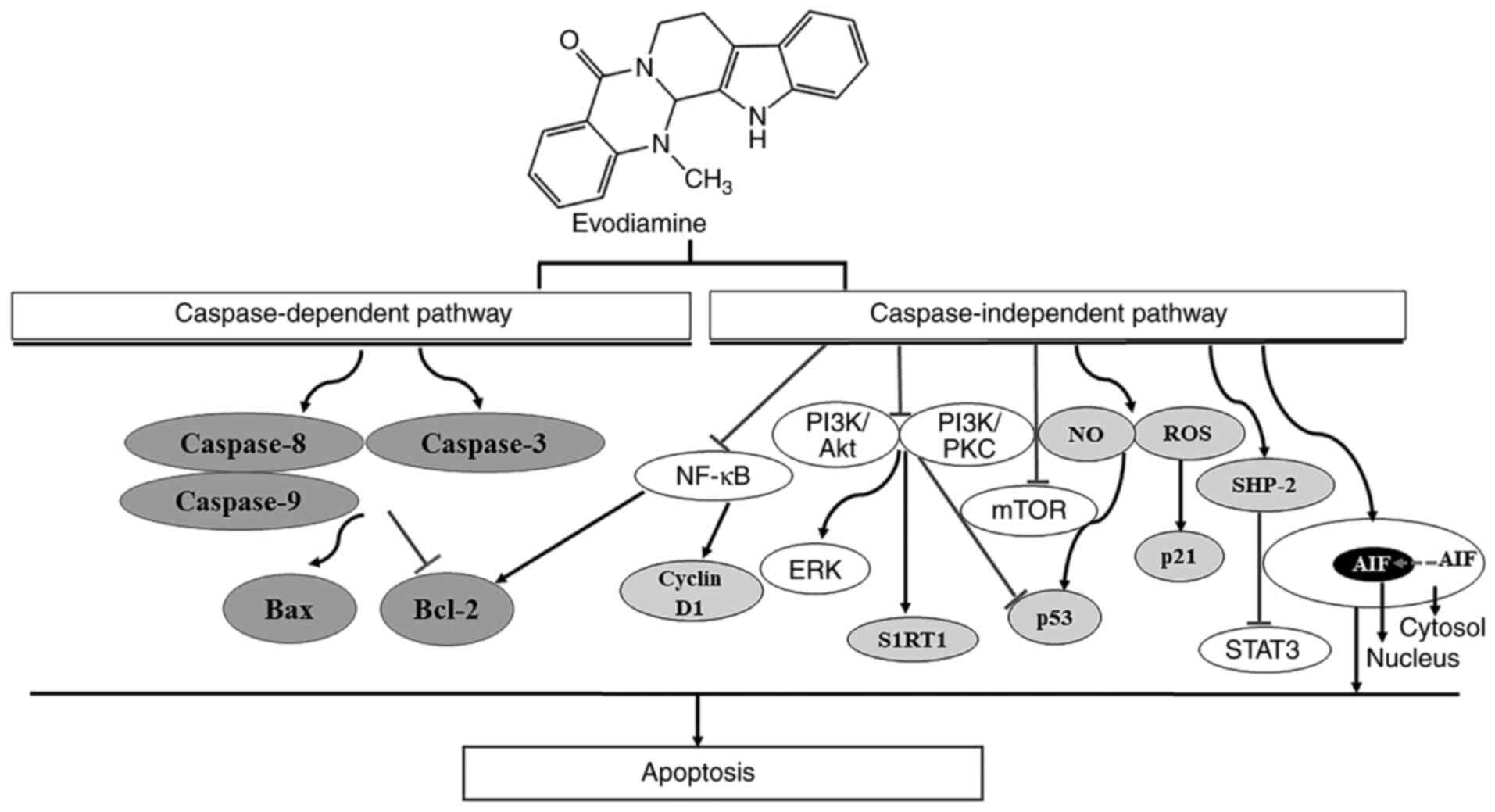
The potential underlying mechanism of EVO on the induction of tumor
cell apoptosis is presented in Fig.
3. EVO has been reported to induce apoptosis through both
caspase-dependent and caspase-independent pathways (43). Apoptotic induction by EVO in human
cervical cancer cells can be completely blocked by Pan-caspase
inhibitors, suggesting that EVO induces cell apoptosis via the
mitochondrial caspase-dependent pathway (44). In another study, EVO-induced
apoptosis in human melanoma cells was not completely reversed by
caspase inhibitor z-VAD-fmk (45).
EVO has also been found to induce apoptosis in gastric cancer cells
by inhibiting the mTOR signaling pathway, both in the presence and
absence of z-VAD-fmk (46),
suggesting that the caspase-independent cell death pathway is also
involved in the proapoptotic mechanism of EVO. EVO can decrease the
activity of the apoptosis suppressor Bcl-2 whilst increasing that
of the apoptosis inducer Bax in tumor cells (47,48),
thereby activating initiator caspases (caspase-8 and 9) and the
effector caspase (caspase-3) (49,50).
Activation of the caspase-independent apoptotic pathway has been
observed to be mediated by translocation of the apoptosis-inducing
factor into the nuclei of human leukemia cells pretreated with EVO
(43). Taken together, these
findings suggest the role of the caspase-independent pathway in EVO
against certain types of cancer cell line. Recently, EVO has been
reported to induce human cholangiocarcinoma cell apoptosis by
blocking the STAT3 signaling pathway, which is mediated by
upregulating shatterproof 2 expression (51). In addition, generation of reactive
oxygen species, nitric oxide (52,53)
and inactivation of the PI3K/AKT (19,47,54-57)
or PI3K/Protein Kinase C (PKC) (58) signaling pathways have been reported
to serve promoting roles in EVO-induced apoptosis.
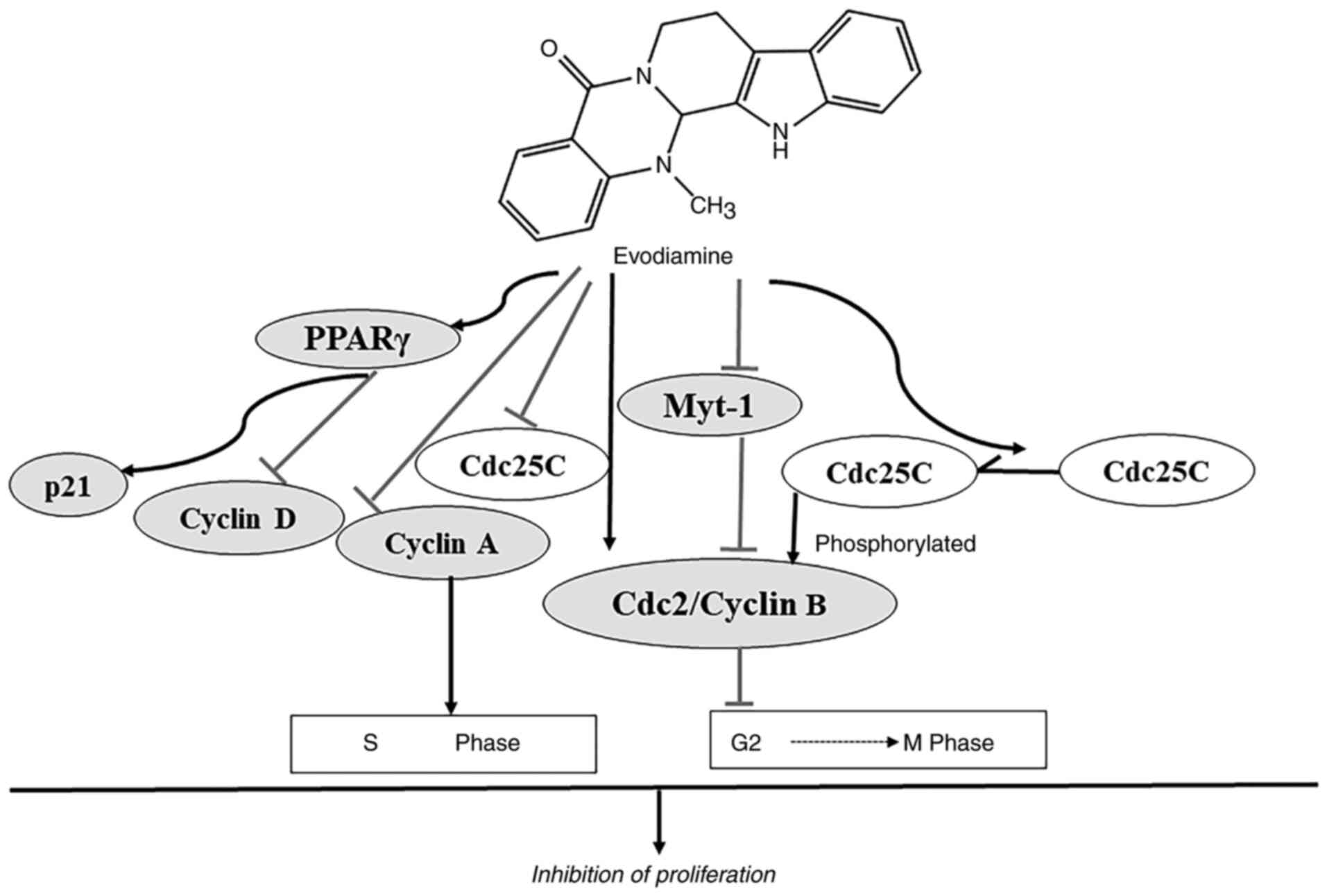
EVO exerts potent antiproliferative effects on the
cervical cancer cell line HeLa at the same concentration as those
mediated by 2.4-dihydroxy-5-fluoropyrimidine (44). The reported underlying mechanism of
action of EVO on the inhibition of proliferation and the cell cycle
progression is summarized in Fig.
4. EVO suppresses proliferation by cell cycle arrest at the
G2/M phase (50,59-62).
EVO can block the cell cycle at the G2/M phase of human
gastric adenocarcinoma SGC-7901 and breast cancer NCI/ADR-RES cells
in a dose-dependent manner, with prominent arrest at both the
sub-G1 and G2/M phases observed in
NCI/ADR-RES cells with longer incubation times (62,63).
The phosphorylation of Cdc2 occurs on three regulatory sites:
Threonine 14 (Thr14), tyrosine 15 (Try15, the inactive form) and
threonine 161 (Thr161, the active form) (64). Phosphorylation of Cdc2 on Thr161
results from the concurrent inhibition of Wee-1 and Myt-1 and
activation of Cdc25C phosphatase, leading to activation of
Cdc2/cyclin B complex (60,65).
The G2/M arrest is accompanied by an increase in the
protein expression of cyclin B1 and phosphorylated form of Cdc2
(Thr161) (60). EVO has been shown
to arrest cell cycle at the G2/M phase in prostate
cancer cells by significantly increasing the protein expression
levels of activated Cdc2 (Thr161) and cyclin B1 whilst diminishing
the activity of Myt-1 and unphosphorylated Cdc25C (59-61).
The inhibitory effect of EVO on the proliferation of colon cancer
LoVo cells occurred by S-phase arrest by decreasing the protein
expression levels of cyclin A and Cdc25C (49). In addition, the peroxisome
proliferator-activated receptor γ (PPARγ) signaling pathway may be
involved in the EVO-induced inhibition of the proliferation of
leukemia cells. EVO exerts an inhibitory effect on proliferation of
leukemia cells by enhancing expression of PPARγ via stimulating p21
and inhibiting cylin D1(66).
Metastasis is a major cause of cancer-associated
mortality, where EVO may be a promising candidate for antitumor
therapy by inhibiting metastasis (38). The roles of EVO in suppressing the
invasion and metastasis of cancer cells are presented in Fig. 5. Hepatocyte growth factor (HGF) can
promote the invasion and migration of different types of tumor
cells (67). EVO has been reported
to reverse HGF-stimulated invasion of colon 26-L5 carcinoma,
B16-F10 melanoma and Lewis lung carcinoma cells in a dose-dependent
manner, whereby 100% inhibition of HGF activity was achieved using
30 µM EVO (68). Inhibition of
MMP-2 expression and attenuation of ERK1/2 activation have also
been documented to contribute to the anti-metastasis and
anti-invasion effects of EVO on tumor cells (69). Previous studies found that EVO can
inhibit the migration and invasion of colorectal cancer cells both
in vitro and in vivo by decreasing MMP-9 expression
and suppressing NF-κB/p65 nuclear translocation and acetylation
(69,70).
Autophagy serves a synchronized role with apoptosis
in the cytotoxic activities mediated by EVO (63). Blockade of angiogenesis also
contributes to the anticancer activity of EVO (71,72).
EVO exerts antitumor effects by inhibiting the activation of NF-κB
and therefore inhibits the transcription of NF-κB-regulated gene
products, including those that can mediate proliferation (cyclin D1
and c-Myc), inhibit apoptosis (survivin and TNF receptor associated
factor 1), immunomodulation (chemokines and IL) and metastasis
(intracellular adhesion molecule-1 and MMP-9) (73). Previously, EVO has been reported to
promote hepatocellular carcinoma cell death by downregulating
hypoxia-inducible factor-1α (HIF-1α) expression (74). In addition, bone morphogenetic
protein 9 has been found to mediate the anticancer effects of EVO
by upregulation of HIF-1α/p53 in colon cancer cells (75). Suppressing the Wnt and Notch
signaling pathways, which serve crucial roles in cancer stem cell
signaling, may also be involved in the anticancer activity of EVO
(76-79).
EVO is highly soluble in acetone, but barely soluble
in ether or diluted alcohol and is insoluble in water, benzene or
chloroform (80). Poor solubility
and absorption means that EVO has low bioavailability (81). Shyr et al (26) reported that the bioavailability of
EVO was only 0.1%, with a maximum plasma concentration
(Cmax) of 49.0 ± 19.0 ng/ml following oral
administration in rats (500 mg/kg) (26). A pharmacokinetic study of EVO
previously performed in rabbits demonstrated that 5 min after
intravenous administration of 4 mg/kg EVO, the plasma concentration
of EVO was 877.0 ± 96.6 ng/ml (82). Pharmacokinetic parameters simulated
in beagle dog models demonstrated that the area under the curve
(AUC)0-24 h and the Cmax of EVO were 45.85 ±
29.17 ng h/ml and 30.94 ± 12.16 ng/ml, respectively, after
administration with capsules encapsulated with 10 mg/kg EVO
(83). The pharmacokinetics of EVO
were also assessed following the oral administration of
[3H] EVO in rats (84).
The radioactivity levels in the plasma reached their maximum level
within 1 h of oral administration, which declined in a biphasic
manner, with half-times of 1.6 and 78.4 h (84). EVO has also been reported to reach
a maximum plasma concentration in rat 3.38 h (Tmax)
after oral administration (35).
EVO is predominantly distributed in the liver, kidney, heart and
lungs, where 19 and 63% of orally administered EVO is eliminated in
the urine and bile after 24 h, respectively (84). The pharmacokinetic process
following the intravenous administration of EVO was characterized
by a two-compartment model, which appears to be a biphasic
phenomenon with a rapid distribution phase followed by a slower
elimination phase (85). The
absorption of EVO by rats with headaches induced by Nitroglycerin
was significantly higher compared with that in the healthy group
(86). Compared with the crude
drug, the Wu-Zhu-Yu extract appeared to increase the
bioavailability of EVO, with increased purities (16-80%),
suggesting that some ingredients in the Wu-Zhu-Yu extract may
promote the efficacy of EVO in vivo (81). At present, the low bioavailability
of EVO has been confirmed in different animal models. These
differences in the pharmacokinetic parameters may be due to the
diversity of measurements and estimation methods. However, further
studies are required to understand the distribution in the body and
excretion of EVO.
Aromatic, aliphatic hydroxylation, N-demethylation,
oxygenation, dehydrogenation, glucuronidation and GSH conjugation
are involved in the metabolic pathways of EVO, where a methyl group
in position 14 of EVO may contribute to its metabolic properties
(87,88). In vitro incubation of EVO
with human and rat liver microsomes, in the presence of NADPH,
resulted in the formation of four mono-hydroxylated metabolites and
one N-demethylated metabolite (87). Cytochrome (CYP)3A4, CYP2C9 and
CYP1A2 have been identified to be the main CYP isoforms involved in
the metabolism of EVO in human liver microsomes (87). Several GSH conjugates of EVO were
also found after the addition of sulfhydryl nucleophiles to EVO
(34). Recently, a total of 12
phase I metabolites of EVO were found in human liver microsomes,
whilst 12 phase I metabolites and seven phase II conjugated
metabolites were identified and quantified in human hepatocytes
(88). In vivo experiments
demonstrated that the metabolites of EVO, 10-hydroxyevodiamine and
18-hydroxyevodiamine, were rapidly converted from EVO within 0.167
h after oral administration in rats, which further conjugated with
glucuronide to form 10-hydroxyevodiamine-glucuronide and
18-hydroxy-evodiamine-glucuronide (35). A cocktail method was used to
investigate the metabolism of EVO involving the P450 family of
metabolizing enzymes (89). The
results demonstrated that EVO inhibited the activities of the
metabolizing enzymes, CYP1A2, CYP2C9 and CYP2D6, which increases
the half-life (t1/2),
Cmax and AUC(0–∞), whilst decreasing the
clearance of the corresponding enzymatic substrates (89).
The metabolism of low-solubility natural medicines
partly depend on drug-drug interactions and the delivery systems
(33). Appropriate drug delivery
systems can contribute to increased absorption, improved
bioavailability, prolonged residence time and minimized side
effects of low-solubility natural medicines (33).
The solid dispersion technique has been used to
enhance the dissolution rate and solubility of EVO. A previous
study demonstrated that solid dispersion of EVO in hard capsules
has a greater absorption rate compared with that of enriched
samples of EVO in physical hard capsules (90). Following oral administration of EVO
(57.5 mg), the Cmax of solid dispersion (27.85 ± 13.78
mg/l) was notably higher compared with that of physical mixtures
(10.48 ± 7.28 mg/l). Notably, solid dispersion also contributed to
an advanced Tmax and a shorter
T1/2 of EVO in beagle dogs
(90). Phospholipids also possess
the potential for improving oral bioavailability and biological
efficacy of drugs with low aqueous solubility, by forming
noncovalently bonded drug-phospholipid complexes (91,92).
Tan et al (91) previously
designed a novel EVO-phospholipid complex (EPLC), with a higher
bioavailability than free EVO. Compared with free EVO, the relative
bioavailability of EPLC was significantly increased to 218.82%
(91).
Cytotoxic drug-carrying nano-carriers are a viable
strategy for enhancing cancer cell cytotoxicity and minimizing side
effects. Nano-emulsion, which includes lipid nano-emulsion,
inclusion complexes, nanoparticles and noisomes, is a vital
component of nano-carriers (33).
A novel type of water-in-oil nano-emulsion containing
EVO-phospholipid nanocomplexes (NEEPN) was considered to be a good
carrier for the oral delivery of EVO due to its favorable in
vivo kinetic characteristics in rat (93). It markedly enhanced the oral
bioavailability of EVO to 630.35% by increasing gastrointestinal
absorption and the effective permeability of NEEPN in the colon was
increased 8.64-fold compared with free EVO (93). Woody oil-based emulsive nanosystem
was also used to increase the sensitivity of lung cancer cells to
EVO (93,94). In addition, the bioavailability of
EVO was increases following formation of inclusion complexes
(95,96). EVO hydroxypropyl-β-cyclodextrin
complexes have been reported to improve the oral bioavailability of
EVO by 2.56-fold, increase the Cmax and extend the
Tmax by 1.57- and 1.01-folds, respectively (95).
Recently, mesoporous silica nanoparticles (MSNs)
have become an attractive type of carrier for hydrophobic and
hydrophilic agents due to their site-specific functionalization
prominent biocompatibility and large loading surface areas
(97,98). EVO is loaded with berberine using a
novel temperature- and pH-responsive dual drug delivery
platform-coated MSN to improve efficacy and biocompatibility in the
low pH and high temperature microenvironment of the tumor (99). This MSN-loaded drug pair (EVO and
berberine) not only possessed optimal synergistic therapeutic
effects in vitro (cytotoxicity, cell migration, invasion and
angiogenesis) and in vivo (growth of tumor grafts in mice),
but also exhibited low systemic toxicity (99). In another study, a delivery system
based on poly (lactic-co-glycolic acid) nanoparticles was
established to deliver EVO to overcome its drawbacks of limited
solubility and low bioavailability (100). It persistently controlled the
release of EVO for >180 h, suggesting that it can became a
potential agent for improving anticancer efficacy of EVO in breast
cancer therapy (100). In
addition, EGFR-targeting EVO-encapsulated poly (amino acid)
nanoparticles have been developed as a new class of
nanotherapeutics (101). These
nanoparticles exert significantly improved cytotoxicity on colon
cancer cells, resulting in the downregulation of EGFR and extension
of the tumor-bearing survival duration (101). However, the application of
niosomes for the delivery of EVO is yet to be investigated.
Novel carriers, micelles and microspheres have been
applied to load natural medicine with low solubility and high
permeability. Polymeric micelles, including paclitaxel micelles
(102) and tanshinone IIA
micelles (103,104), exhibit high capacity for drug
loading and have the ability to improve the bioavailability of
drugs (4.33-fold higher than free paclitaxel and 5.60-fold higher
than free tanshinone IIA). Chitosan microspheres are biocompatible
and readily biodegradable, which can enhance the absolute
bioavailability of paclitaxel by 1.52-fold (105) whilst increasing the oral
bioavailability of capsaicin by 1.53-fold (106). However, these drug delivery
systems are yet to be applied for the bioavailability of EVO.
TCM is considered a promising source of potential
anticancer agents and novel adjuvant therapies to improve the
efficacy of chemotherapy with little to no side effects. A previous
study has demonstrated that EVO can enhance doxorubicin sensitivity
in doxorubicin-resistant breast cancer cells by synchronously
affecting apoptosis and survival signaling transduction pathways,
thereby enhancing the apoptotic action of doxorubicin (107). In addition, combined treatment of
erlotinib with EVO, an oral epidermal growth factor receptor
tyrosine kinase inhibitor, successfully inhibited cell
proliferation and survival in erlotinib-resistant wild-type EGFR
non-small cell lung cancer cells (108). These favorable anti-cancer
properties of EVO would contribute to the killing of resistant
cancer cells in the clinic with minimal toxicity. Previous studies
on the antitumor mechanisms of EVO have suggested an exciting
future for the pursuit of anticancer therapies. Although EVO is
currently used in the clinic as a TCM to treat headaches, abdominal
pain, vomiting and colds in Southeast Asia (109,110), it has not been clinically
approved as an anticancer agent. Considering the therapeutic
potential of EVO for antitumor treatment, an approach to improve
the oral bioavailability and enhance its pharmacological effects
would prove beneficial, thus minimizing the possible adverse
effects due to overdosing.
Since advanced delivery systems have been proposed,
the oral bioavailability of EVO can be notably improved by
increasing absorption whilst avoiding first-pass metabolism. Since
a substantial signaling network involved in apoptosis or
proliferation inside the cell can be stimulated by EVO, exploring
the possible targets of EVO on cell the membrane and any
ligand-receptor interactions would be a potential means of
developing novel transportable compounds to address the defect in
absorption. The inhibitory effects of EVO on various CYP enzymes
may decrease the biotransformation of medicines primarily dependent
on these pathways. The inhibition of metabolic enzymes and
resultant promotion of drug potency by EVO will facilitate the
investigation of potential drug-drug or herb-drug interactions and
evaluation of their clinical safety.
Previous studies have focused on the anticancer
activity and underlying mechanisms of EVO. The present review
discussed the advances in research investigating the anticancer
effects of EVO over the past two decades, highlighting the
pharmacokinetics and practicality of using novel loading carriers
to promote the absorption of EVO and enhance the bioavailability of
drugs with low solubility. It is hoped that all of this will
contribute to the clinical application of EVO and T.
ruticarpum.
Not applicable.
Funding: The present study was supported by the Basic Scientific
Research Project of Guangxi Academy of Agricultural Sciences (grant
no. 2021YT144).
Not applicable.
CL designed and drafted the manuscript, JA collected
the information and prepared the figures. ER collected the
information and prepared Table I.
JL and CF supervised and revised the manuscript. XLi and XLu drew
the chemical structure and prepared Fig. 2. All authors have read and approved
the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Zhang L, Feng YL, Wang YS and Yang SL:
Modern research status on Evodia rutaecarpa. J Jiangxi Univ
Tradit Chin Med. 22(5)2010.
|
|
2
|
Nam EY, Kim SA, Kim H, Kim SH, Han JH, Lee
JH and Kim DI: Akt activation by Evodiae fructus extract
protects ovary against 4-vinylcyclohexene diepoxide-induced
ovotoxicity. J Ethnopharmacol. 194:733–739. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sui H, Liu X, Jin BH, Pan SF, Zhou LH, Yu
NA, Wu J, Cai JF, Fan ZZ, Zhu HR and Li Q: Zuo Jin Wan, a
traditional Chinese herbal formula, reverses P-gp-mediated MDR in
vitro and in vivo. Evid Based Complement Alternat Med.
2013(957078)2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pan J, Xu Y, Song H, Zhou X, Yao Z and Ji
G: Extracts of Zuo Jin Wan, a traditional Chinese medicine,
phenocopies 5-HTR1D antagonist in attenuating Wnt/β-catenin
signaling in colorectal cancer cells. BMC Complement Altern Med.
17(506)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tang QF, Ji Q, Qiu YY, Cao AL, Chi YF,
Liang B, Peng W and Yin PH: Synergistic effect of Zuo Jin Wan on
DDP-induced apoptosis in human gastric cancer SGC-7901/DDP cells.
Evid Based Complement Alternat Med. 2014(724764)2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Guan X, Zheng X, Vong CT, Zhao J, Xiao J,
Wang Y and Zhong Z: Combined effects of berberine and evodiamine on
colorectal cancer cells and cardiomyocytes in vitro. Eur J
Pharmacol. 875(173031)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chou ST, Hsiang CY, Lo HY, Huang HF, Lai
MT, Hsieh CL, Chiang SY and Ho TY: Exploration of anti-cancer
effects and mechanisms of Zuo-Jin-Wan and its alkaloid components
in vitro and in orthotopic HepG2 xenograft immunocompetent mice.
BMC Complement Altern Med. 17(121)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bae JR, Park WH, Suh DH, No JH, Kim YB and
Kim K: Role of limonin in anticancer effects of evodia rutaecarpa
on ovarian cancer cells. BMC Complement Med Ther.
20(94)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Park E, Lee MY, Seo CS, Jang JH, Kim YU
and Shin HK: Ethanol extract of evodia rutaecarpa attenuates cell
growth through caspase-dependent apoptosis in benign prostatic
hyperplasia-1 cells. Nutrients. 10(523)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gong X, Zhou X, Cai Z, Zhang J and Zhou W:
Studies on chemical constituents of Evodia rutaecarpa.
Zhongguo Zhong Yao Za Zhi. 34:177–179. 2009.PubMed/NCBI(In Chinese).
|
|
11
|
Kim D, Lee YH, Park SH, Lee MJ, Kim MJ,
Jang HS, Lee JM, Lee HY, Han BS, Son WC, et al: Subchronic oral
toxicity of evodia fruit powder in rats. J Ethnopharmacol.
151:1072–1078. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hu X, Li D, Chu C, Li X, Wang X, Jia Y,
Hua H and Xu F: Antiproliferative effects of alkaloid evodiamine
and its derivatives. Int J Mol Sci. 19(3403)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wu JY, Chang MC, Chen CS, Lin HC, Tsai HP,
Yang CC, Yang CH and Lin CM: Topoisomerase I inhibitor evodiamine
acts as an antibacterial agent against drug-resistant Klebsiella
pneumoniae. Planta Med. 79:27–29. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Peng J and Li YJ: The vanilloid receptor
TRPV1: Role in cardiovascular and gastrointestinal protection. Eur
J Pharmacol. 627:1–7. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhao Z, Gong S, Wang S and Ma C: Effect
and mechanism of evodiamine against ethanol-induced gastric ulcer
in mice by suppressing Rho/NF-κB pathway. Int Immunopharmacol.
28:588–595. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yu H, Jin H, Gong W, Wang Z and Liang H:
Pharmacological actions of multi-target-directed evodiamine.
Molecules. 18:1826–1843. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yuan SM, Gao K, Wang DM, Quan XZ, Liu JN,
Ma CM, Qin C and Zhang LF: Evodiamine improves congnitive abilities
in SAMP8 and APP(swe)/PS1(ΔE9) transgenic mouse models of
Alzheimer's disease. Acta Pharmacol Sin. 32:295–302.
2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pan X, Hartley JM, Hartley JA, White KN,
Wang Z and Bligh SW: Evodiamine, a dual catalytic inhibitor of type
I and II topoisomerases, exhibits enhanced inhibition against
camptothecin resistant cells. Phytomedicine. 19:618–624.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huang J, Chen ZH, Ren CM, Wang DX, Yuan
SX, Wu QX, Chen QZ, Zeng YH, Shao Y, Li Y, et al: Antiproliferation
effect of evodiamine in human colon cancer cells is associated with
IGF-1/HIF-1α downregulation. Oncol Rep. 34:3203–3211.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhong ZF, Tan W, Wang SP, Qiang WA and
Wang YT: Anti-proliferative activity and cell cycle arrest induced
by evodiamine on paclitaxel-sensitive and -resistant human ovarian
cancer cells. Sci Rep. 5(16415)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang J, Cai X, Lu W, Hu C, Xu X, Yu Q and
Cao P: Evodiamine inhibits STAT3 signaling by inducing phosphatase
shatterproof 1 in hepatocellular carcinoma cells. Cancer Lett.
328:243–251. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wei J, Ching LC, Zhao JF, Shyue SK, Lee
HF, Kou YR and Lee TS: Essential role of transient receptor
potential vanilloid type 1 in evodiamine-mediated protection
against atherosclerosis. Acta Physiol (Oxf). 207:299–307.
2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wanner SP, Garami A, Pakai E, Oliveira DL,
Gavva NR, Coimbra CC and Romanovsky AA: Aging reverses the role of
the transient receptor potential vanilloid-1 channel in systemic
inflammation from anti-inflammatory to proinflammatory. Cell Cycle.
11:343–349. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mezrich JD, Nguyen LP, Kennedy G, Nukaya
M, Fechner JH, Zhang X, Xing Y and Bradfield CA: SU5416, a VEGF
receptor inhibitor and ligand of the AHR, represents a new
alternative for immunomodulation. PLoS One.
7(e44547)2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
O'Donnell EF, Kopparapu PR, Koch DC, Jang
HS, Phillips JL, Tanguay RL, Kerkvliet NI and Kolluri SK: The aryl
hydrocarbon receptor mediates leflunomide-induced growth inhibition
of melanoma cells. PLoS One. 7(e40926)2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shyr MH, Lin LC, Lin TY and Acta TH:
Determination and pharmacokinetics of evodiamine in the plasma and
feces of conscious rats. Anal Chim Acta. 558:16–21. 2006.
|
|
27
|
Lefrak EA, Pitha J, Rosenheim S and
Gottlieb JA: A clinicopathologic analysis of adriamycin
cardiotoxicity. Cancer. 32:302–314. 1973.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Olson RD, Mushlin PS, Brenner DE,
Fleischer S, Cusack BJ, Chang BK and Boucek RJ Jr: Doxorubicin
cardiotoxicity may be caused by its metabolite, doxorubicinol. Proc
Natl Acad Sci USA. 85:3585–3589. 1988.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Stewart DJ, Grewaal D, Green RM, Mikhael
N, Goel R, Montpetit VA and Redmond MD: Concentrations of
doxorubicin and its metabolites in human autopsy heart and other
tissues. Anticancer Res. 13:1945–1952. 1993.PubMed/NCBI
|
|
30
|
Yang W, Ma L, Li S, Cui K, Lei L and Ye Z:
Evaluation of the cardiotoxicity of evodiamine in vitro and in
vivo. Molecules. 22(943)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Huang W, Zhao Y and Sun R: Comparative
study on acute toxicity of different components of Evodia
rutaecarpa in mice. Zhongguo Yao Wu Jing Jie. 7:129–134.
2010.
|
|
32
|
Li F, Dong YZ, Zhang D, Zhang XM, Lin ZJ
and Zhang B: Molecular mechanisms involved in drug-induced liver
injury caused by urate-lowering Chinese herbs: A network
pharmacology study and biology experiments. PLoS One.
14(e0216948)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yan S, Liu Y, Feng J, Zhao H, Yu Z, Zhao
J, Li Y and Zhang J: Difference and alteration in pharmacokinetic
and metabolic characteristics of low-solubility natural medicines.
Drug Metab Rev. 50:140–160. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wen B, Roongta V, Liu L and Moore DJ:
Metabolic activation of the indoloquinazoline alkaloids evodiamine
and rutaecarpine by human liver microsomes: Dehydrogenation and
inactivation of cytochrome P450 3A4. Drug Metab Dispos.
42:1044–1054. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang C, Yue F, Ai G and Yang J:
Simultaneous determination of evodiamine and its four metabolites
in rat plasma by LC-MS/MS and its application to a pharmacokinetic
study. Biomed Chromatogr. 32(e4219)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li L, Liu R, Ye M, Hu X, Qiao W, Bi K and
Guo D: Microbial metabolism of evodiamine by penicillium
janthinellum and its application for metabolite identification in
rat urine. Enzyme Microb Technol. 39:561–567. 2006.
|
|
37
|
Jiang J and Hu C: Evodiamine: A novel
anti-cancer alkaloid from evodia rutaecarpa. Molecules.
14:1852–1859. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ogasawara M, Matsunaga T, Takahashi S,
Saiki I and Suzuki H: Anti-invasive and metastatic activities of
evodiamine. Biol Pharm Bull. 25:1491–1493. 2002.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chien CC, Wu MS, Shen SC, Ko CH, Chen CH,
Yang LL and Chen YC: Activation of JNK contributes to
evodiamine-induced apoptosis and G2/M arrest in human colorectal
carcinoma cells: A structure-activity study of evodiamine. PLoS
One. 9(e99729)2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Rao L and White E: Bcl-2 and the ICE
family of apoptotic regulators: Making a connection. Curr Opin
Genet Dev. 7:52–58. 1997.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zou Y, Qin X, Xiong H, Zhu F, Chen T and
Wu H: Apoptosis of human non-small-cell lung cancer A549 cells
triggered by evodiamine through MTDH-dependent signaling pathway.
Tumour Biol. 36:5187–5193. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhu LH, Bi W, Liu XD, Li JF, Wu YY, Du BY
and Tan YH: Induction of apoptosis by evodiamine involves both
activation of mitotic arrest and mitotic slippage. Oncol Rep.
26:1447–1455. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lee TJ, Kim EJ, Kim S, Jung EM, Park JW,
Jeong SH, Park SE, Yoo YH and Kwon TK: Caspase-dependent and
caspase-independent apoptosis induced by evodiamine in human
leukemic U937 cells. Mol Cancer Ther. 5:2398–2407. 2006.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Fei XF, Wang BX, Li TJ, Tashiro S, Minami
M, Xing DJ and Ikejima T: Evodiamine, a constituent of Evodiae
fructus, induces anti-proliferating effects in tumor cells.
Cancer Sci. 94:92–98. 2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhang Y, Wu LJ, Tashiro S, Onodera S and
Ikejima T: Intracellular regulation of evodiamine-induced A375-S2
cell death. Biol Pharm Bull. 26:1543–1547. 2003.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Liu X, Yang L, Bi Y, Wang LH and Huang H:
Effect of evodiamine in inducing apoptosis of gastric cancer
SGC-7901 cells through mTOR signal pathway. Zhongguo Zhong Yao Za
Zhi. 40:3262–3266. 2015.PubMed/NCBI(In Chinese).
|
|
47
|
Yang F, Shi L, Liang T, Ji L, Zhang G,
Shen Y, Zhu F and Xu L: Anti-tumor effect of evodiamine by inducing
Akt-mediated apoptosis in hepatocellular carcinoma. Biochem Biophys
Res Commun. 485:54–61. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Guo XX, Li XP, Zhou P, Li DY, Lyu XT, Chen
Y, Lyu YW, Tian K, Yuan DZ, Ran JH, et al: Evodiamine induces
apoptosis in SMMC-7721 and HepG2 cells by suppressing NOD1 signal
pathway. Int J Mol Sci. 19(3419)2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhang C, Fan X, Xu X, Yang X, Wang X and
Liang HP: Evodiamine induces caspase-dependent apoptosis and S
phase arrest in human colon lovo cells. Anticancer Drugs.
21:766–776. 2010.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Yang L, Liu X, Wu D, Zhang M, Ran G, Bi Y
and Huang H: Growth inhibition and induction of apoptosis in
SGC-7901 human gastric cancer cells by evodiamine. Mol Med Rep.
9:1147–1152. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhu B, Zhao L, Liu Y, Jin Y, Feng J, Zhao
F, Sun J, Geng R and Wei Y: Induction of phosphatase shatterproof 2
by evodiamine suppresses the proliferation and invasion of human
cholangiocarcinoma. Int J Biochem Cell Biol. 108:98–110.
2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Yang J, Wu LJ, Tashino S, Onodera S and
Ikejima T: Critical roles of reactive oxygen species in
mitochondrial permeability transition in mediating
evodiamine-induced human melanoma A375-S2 cell apoptosis. Free
Radic Res. 41:1099–1108. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Yang J, Wu LJ, Tashino S, Onodera S and
Ikejima T: Reactive oxygen species and nitric oxide regulate
mitochondria-dependent apoptosis and autophagy in
evodiamine-treated human cervix carcinoma HeLa cells. Free Radic
Res. 42:492–504. 2008.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wei WT, Chen H, Wang ZH, Ni ZL, Liu HB,
Tong HF, Guo HC, Liu DL and Lin SZ: Enhanced antitumor efficacy of
gemcitabine by evodiamine on pancreatic cancer via regulating
PI3K/Akt pathway. Int J Biol Sci. 8:1–14. 2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hong Z, Wang Z, Zhou B, Wang J, Tong H,
Liao Y, Zheng P, Jamshed MB, Zhang Q and Chen H: Effects of
evodiamine on PI3K/Akt and MAPK/ERK signaling pathways in
pancreatic cancer cells. Int J Oncol. 56:783–793. 2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wang R, Deng D, Shao N, Xu Y, Xue L, Peng
Y, Liu Y and Zhi F: Evodiamine activates cellular apoptosis through
suppressing PI3K/AKT and activating MAPK in glioma. Onco Targets
Ther. 11:1183–1192. 2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wang C, Li S and Wang MW:
Evodiamine-induced human melanoma A375-S2 cell death was mediated
by PI3K/Akt/caspase and Fas-L/NF-kappaB signaling pathways and
augmented by ubiquitin-proteasome inhibition. Toxicol In Vitro.
24:898–904. 2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wang C, Wang MW, Tashiro S, Onodera S and
Ikejima T: Roles of SIRT1 and phosphoinositide 3-OH kinase/protein
kinase C pathways in evodiamine-induced human melanoma A375-S2 cell
death. J Pharmacol Sci. 97:494–500. 2005.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Huang DM, Guh JH, Huang YT, Chueh SC,
Chiang PC and Teng CM: Induction of mitotic arrest and apoptosis in
human prostate cancer pc-3 cells by evodiamine. J Urol.
173:256–261. 2005.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Kan SF, Yu CH, Pu HF, Hsu JM, Chen MJ and
Wang PS: Anti-proliferative effects of evodiamine on human prostate
cancer cell lines DU145 and PC3. J Cell Biochem. 101:44–56.
2007.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Kan SF, Huang WJ, Lin LC and Wang PS:
Inhibitory effects of evodiamine on the growth of human prostate
cancer cell line LNCaP. Int J Cancer. 110:641–651. 2004.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Liao CH, Pan SL, Guh JH, Chang YL, Pai HC,
Lin CH and Teng CM: Antitumor mechanism of evodiamine, a
constituent from Chinese herb Evodiae fructus, in human
multiple-drug resistant breast cancer NCI/ADR-RES cells in vitro
and in vivo. Carcinogenesis. 26:968–975. 2005.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Rasul A, Yu B, Zhong L, Khan M, Yang H and
Ma T: Cytotoxic effect of evodiamine in SGC-7901 human gastric
adenocarcinoma cells via simultaneous induction of apoptosis and
autophagy. Oncol Rep. 27:1481–1487. 2012.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Graves PR, Yu L, Schwarz JK, Gales J,
Sausville EA, O'Connor PM and Piwnica-Worms H: The Chk1 protein
kinase and the Cdc25C regulatory pathways are targets of the
anticancer agent UCN-01. J Biol Chem. 275:5600–5605.
2000.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Devault A, Martinez AM, Fesquet D, Labbé
JC, Morin N, Tassan JP, Nigg EA, Cavadore JC and Dorée M: MAT1
(‘menage à trois’) a new RING finger protein subunit stabilizing
cyclin H-cdk7 complexes in starfish and Xenopus CAK. EMBO J.
14:5027–5036. 1995.PubMed/NCBI
|
|
66
|
Sun C, Zhang G, Luan S, Luan C, Shao H,
Dong F and Liu X: Evodiamine inhibits the proliferation of leukemia
cell line K562 by regulating peroxisome proliferators-activated
receptor gamma (PPARγ) pathway. J Recept Signal Transduct Res.
36:422–428. 2016.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Moosavi F, Giovannetti E, Saso L and
Firuzi O: HGF/MET pathway aberrations as diagnostic, prognostic,
and predictive biomarkers in human cancers. Crit Rev Clin Lab Sci.
56:533–566. 2019.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Ogasawara M and Suzuki H: Inhibition by
evodiamine of hepatocyte growth factor-induced invasion and
migration of tumor cells. Biol Pharm Bull. 27:578–582.
2004.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Peng X, Zhang Q, Zeng Y, Li J, Wang L and
Ai P: Evodiamine inhibits the migration and invasion of
nasopharyngeal carcinoma cells in vitro via repressing MMP-2
expression. Cancer Chemother Pharmacol. 76:1173–1184.
2015.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Zhou P, Li XP, Jiang R, Chen Y, Lv XT, Guo
XX, Tian K, Yuan DZ, Lv YW, Ran JH, et al: Evodiamine inhibits
migration and invasion by Sirt1-mediated post-translational
modulations in colorectal cancer. Anticancer Drugs. 30:611–617.
2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Shi L, Yang F, Luo F, Liu Y, Zhang F, Zou
M and Liu Q: Evodiamine exerts anti-tumor effects against
hepatocellular carcinoma through inhibiting β-catenin-mediated
angiogenesis. Tumour Biol. 37:12791–12803. 2016.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Shyu KG, Lin S, Lee CC, Chen E, Lin LC,
Wang BW and Tsai SC: Evodiamine inhibits in vitro angiogenesis:
Implication for antitumorgenicity. Life Sci. 78:2234–2243.
2006.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Takada Y, Kobayashi Y and Aggarwal BB:
Evodiamine abolishes constitutive and inducible NF-kappaB
activation by inhibiting IkappaBalpha kinase activation, thereby
suppressing NF-kappaB-regulated antiapoptotic and metastatic gene
expression, up-regulating apoptosis, and inhibiting invasion. J
Biol Chem. 280:17203–17212. 2005.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Li YL, Zhang NY, Hu X, Chen JL, Rao MJ, Wu
LW, Li QY, Zhang B, Yan W and Zhang C: Evodiamine induces apoptosis
and promotes hepatocellular carcinoma cell death induced by
vorinostat via downregulating HIF-1α under hypoxia. Biochem Biophys
Res Commun. 498:481–486. 2018.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Li FS, Huang J, Cui MZ, Zeng JR, Li PP, Li
L, Deng Y, Hu Y, He BC and Shu DZ: BMP9 mediates the anticancer
activity of evodiamine through HIF-1α/p53 in human colon cancer
cells. Oncol Rep. 43:415–426. 2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Kim H, Yu Y, Choi S, Lee H, Yu J, Lee JH
and Kim WY: Evodiamine eliminates colon cancer stem cells via
suppressing notch and Wnt signaling. Molecules.
24(4520)2019.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Wen Z, Feng S, Wei L, Wang Z, Hong D and
Wang Q: Evodiamine, a novel inhibitor of the Wnt pathway, inhibits
the self-renewal of gastric cancer stem cells. Int J Mol Med.
36:1657–1663. 2015.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Yang X, Zhang Y, Huang Y, Wang Y, Qi X, Su
T and Lu L: Evodiamine suppresses Notch3 signaling in lung
tumorigenesis via direct binding to γ-secretases. Phytomedicine.
68(153176)2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Su T, Yang X, Deng JH, Huang QJ, Huang SC,
Zhang YM, Zheng HM, Wang Y, Lu LL and Liu ZQ: Evodiamine, a novel
NOTCH3 methylation stimulator, significantly suppresses lung
carcinogenesis in vitro and in vivo. Front Pharmacol.
9(434)2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Gai L, Rao GX, Song CQ and Hu ZB: Studies
on the chemical constituents of evodia rutaecarpa (Juss.) Benth.
var. officinalis (Dode) Huang. Yao Xue Xue Bao. 36:743–745.
2001.PubMed/NCBI(In Chinese).
|
|
81
|
Xu S, Peng J, Li Y, He L, Chen F, Zhang J
and Ding J: Pharmacokinetic comparisons of rutaecarpine and
evodiamine after oral administration of Wu-Chu-Yu extracts with
different purities to rats. J Ethnopharmacol. 139:395–400.
2012.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Lin C, Pan X, Li W, Ma J, Pan J, Cai J,
Wang X and Lin G: Simultaneous determination of evodiamine and
rutecarpine in rabbit plasma by LC-ESI-MS and its application to
pharmacokinetics. Pharmazie. 66:920–923. 2011.PubMed/NCBI
|
|
83
|
Xia YY, Xu HY, Cai YY, Si DY and Liu CX:
Simultaneous determination of evodiamine and evodine in beagle dog
plasma using liquid chromatography tandem mass spectrometry. J
Asian Nat Prod Res. 15:235–243. 2013.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Komatsu K, Wakame K and Kano Y:
Pharmacological properties of galenical preparation. XVI.
Pharmacokinetics of evodiamine and the metabolite in rats. Biol
Pharm Bull. 16:935–938. 1993.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Jeng KF, Lin YH, Lin LC, Chou CJ, Tsai TH
and Chen CF: High-performance liquid chromatographic determination
of evodiamine in rat plasma: Application to pharmacokinetic
studies. J Chromatogr B Biomed Appl. 668:343–345. 1995.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Xu H, Li Q, Yin Y, Lv C, Sun W, He B, Liu
R, Chen X and Bi K: Simultaneous determination of three alkaloids,
four ginsenosides and limonin in the plasma of normal and headache
rats after oral administration of Wu-Zhu-Yu decoction by a novel
ultra fast liquid chromatography-tandem mass spectrometry method:
Application to a comparative pharmacokinetics and ethological
study. J Mass Spectrom. 48:519–532. 2013.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Sun HZ, Fang ZZ, Cao YF, Sun XY and Hong
M: Investigation of the in vitro metabolism of evodiamine:
Characterization of metabolites and involved cytochrome p450
isoforms. Phytother Res. 27:705–712. 2013.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Zhang Z, Fang T, Zhou H, Yuan J and Liu Q:
Characterization of the in vitro metabolic profile of evodiamine in
human liver microsomes and hepatocytes by UHPLC-Q exactive mass
spectrometer. Front Pharmacol. 9(130)2018.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Zhang YT, Zhang DF, Ge NY, Zhu GH, Hao C,
Zhang Y and Chen RJ: Effect of evodiamine on CYP enzymes in rats by
a cocktail method. Pharmacology. 97:218–223. 2016.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Xu H, Zhang T, Yang H, Xiao X, Bian Y, Si
D and Liu C: Preparation of evodiamine solid dispersions and its
pharmacokinetics. Indian J Pharm Sci. 73:276–281. 2011.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Tan Q, Liu S, Chen X, Wu M, Wang H, Yin H,
He D, Xiong H and Zhang J: Design and evaluation of a novel
evodiamine-phospholipid complex for improved oral bioavailability.
AAPS PharmSciTech. 13:534–547. 2012.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Grattagliano I, Diogo CV, Mastrodonato M,
de Bari O, Persichella M, Wang DQ, Liquori A, Ferri D, Carratù MR,
Oliveira PJ and Portincasa P: A silybin-phospholipids complex
counteracts rat fatty liver degeneration and mitochondrial
oxidative changes. World J Gastroenterol. 19:3007–3017.
2013.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Hu J, Chen D, Jiang R, Tan Q, Zhu B and
Zhang J: Improved absorption and in vivo kinetic characteristics of
nanoemulsions containing evodiamine-phospholipid nanocomplex. Int J
Nanomedicine. 9:4411–4420. 2014.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Zhao J, Liu S, Hu X, Zhang Y, Yan S, Zhao
H, Zeng M, Li Y, Yang L and Zhang J: Improved delivery of natural
alkaloids into lung cancer through woody oil-based emulsive
nanosystems. Drug Deliv. 25:1426–1437. 2018.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Zhang X, Liu HM, Lei TT, Feng J and Zhang
JQ: A preliminary study of pharmacokinetics of evodiamine
hydroxypropyl-β-cyclodextrin inclusion complex. Nan Fang Yi Ke Da
Xue Xue Bao. 36:548–551. 2016.PubMed/NCBI(In Chinese).
|
|
96
|
Qiu C, Gao LN, Yan K, Cui YL and Zhang Y:
A promising antitumor activity of evodiamine incorporated in
hydroxypropyl-β-cyclodextrin: Pro-apoptotic activity in human
hepatoma HepG2 cells. Chem Cent J. 10(46)2016.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Xu L, Li W, Sadeghi-Soureh S, Amirsaadat
S, Pourpirali R and Alijani S: Dual drug release mechanisms through
mesoporous silica nanoparticle/electrospun nanofiber for enhanced
anticancer efficiency of curcumin. J Biomed Mater Res A, Aug 10,
2021 (Online ahead of print).
|
|
98
|
Rastegari E, Hsiao YJ, Lai WY, Lai YH,
Yang TC, Chen SJ, Huang PI, Chiou SH, Mou CY and Chien Y: An update
on mesoporous silica nanoparticle applications in nanomedicine.
Pharmaceutics. 13(1067)2021.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Feng Y, Li NX, Yin HL, Chen TY, Yang Q and
Wu M: Thermo- and pH-responsive, lipid-coated, mesoporous silica
nanoparticle-based dual drug delivery system to improve the
antitumor effect of hydrophobic drugs. Mol Pharm. 16:422–436.
2019.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Zou L, Chen F, Bao J, Wang S, Wang L, Chen
M, He C and Wang Y: Preparation, characterization, and anticancer
efficacy of evodiamine-loaded PLGA nanoparticles. Drug Deliv.
23:908–916. 2016.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Li C, Cai G, Song D, Gao R, Teng P, Zhou
L, Ji Q, Sui H, Cai J, Li Q and Wang Y: Development of
EGFR-targeted evodiamine nanoparticles for the treatment of
colorectal cancer. Biomater Sci. 7:3627–3639. 2019.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Ge Y, Zhao Y and Li L: Preparation of
sodium cholate-based micelles through non-covalent ıbonding
interaction and application as oral delivery systems for
paclitaxel. Drug Deliv. 23:2555–2565. 2016.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Zhang J, Li Y, Fang X, Zhou D, Wang Y and
Chen M: TPGS-g-PLGA/Pluronic F68 mixed micelles for tanshinone IIA
delivery in cancer therapy. Int J Pharm. 476:185–198.
2014.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Chen F, Zhang J, He Y, Fang X, Wang Y and
Chen M: Glycyrrhetinic acid-decorated and reduction-sensitive
micelles to enhance the bioavailability and anti-hepatocellular
carcinoma efficacy of tanshinone IIA. Biomater Sci. 4:167–182.
2016.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Jiang J, Liu Y, Wu C, Qiu Y, Xu X, Lv H,
Bai A and Liu X: Development of drug-loaded chitosan hollow
nanoparticles for delivery of paclitaxel to human lung cancer A549
cells. Drug Dev Ind Pharm. 43:1304–1313. 2017.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Wu S, Pan H, Tan S, Ding C, Huang G, Liu
G, Guo J and Su Z: In vitro inhibition of lipid accumulation
induced by oleic acid and in vivo pharmacokinetics of chitosan
microspheres (CTMS) and chitosan-capsaicin microspheres (CCMS).
Food Nutr Res. 61(1331658)2017.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Wang S, Wang L, Shi Z, Zhong Z, Chen M and
Wang Y: Evodiamine synergizes with doxorubicin in the treatment of
chemoresistant human breast cancer without inhibiting
P-glycoprotein. PLoS One. 9(e97512)2014.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Li YL, Pan YN, Wu WJ, Mao SY, Sun J, Zhao
YM, Dong JY, Zhang DY, Pan JP, Zhang C and Lin NM: Evodiamine
induces apoptosis and enhances apoptotic effects of erlotinib in
wild-type EGFR NSCLC cells via S6K1-mediated Mcl-1 inhibition. Med
Oncol. 33(16)2016.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Pan X, Wang M, Wu Y, Lu X, Shang Y, Xu Y,
Zhai Y, Li J, Li Z and Gong M: Identification of active ingredients
in Wuzhuyu decoction improving migraine in mice by spectral
efficiency association. Mol Med Rep. 12:1524–1534. 2015.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Xu H, Niu H, He B, Cui C, Li Q and Bi K:
Comprehensive qualitative ingredient profiling of chinese herbal
formula Wu-Zhu-Yu decoction via a mass defect and fragment
filtering approach using high resolution mass spectrometry.
Molecules. 21(664)2016.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Wang D, Ge S, Chen Z and Song Y:
Evodiamine exerts anticancer effects via induction of apoptosis and
autophagy and suppresses the migration and invasion of human colon
cancer cells. J BUON. 24:1824–1829. 2019.PubMed/NCBI
|
|
112
|
Zhu LQ, Zhang L, Zhang J, Chang GL, Liu G,
Yu DD, Yu XM, Zhao MS and Ye B: Evodiamine inhibits high-fat
diet-induced colitis-associated cancer in mice through regulating
the gut microbiota. J Integr Med. 19:56–65. 2021.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Ogasawara M, Matsubara T and Suzuki H:
Inhibitory effects of evodiamine on in vitro invasion and
experimental lung metastasis of murine colon cancer cells. Biol
Pharm Bull. 24:917–920. 2001.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Shen H, Zhao S, Xu Z, Zhu L, Han Y and Ye
J: Evodiamine inhibits proliferation and induces apoptosis in
gastric cancer cells. Oncol Lett. 10:367–371. 2015.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Hu C, Gao X, Han Y, Guo Q, Zhang K, Liu M,
Wang Y and Wang J: Evodiamine sensitizes BGC-823 gastric cancer
cells to radiotherapy in vitro and in vivo. Mol Med
Rep. 14:413–419. 2016.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Huang H, Zhang Y, Liu X, Li Z, Xu W, He S,
Huang Y and Zhang H: Acid sphingomyelinase contributes to
evodiamine-induced apoptosis in human gastric cancer SGC-7901
cells. DNA Cell Biol. 30:407–412. 2011.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Sachita K, Kim Y, Yu HJ, Cho SD and Lee
JS: In Vitro assessment of the anticancer potential of evodiamine
in human oral cancer cell lines. Phytother Res. 29:1145–1151.
2015.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Wu Y and Wang J, Zhao J, Zhang Y, Sun Y,
Chen J and Wang J: Gene regulation analysis of the effects of
evodiamine on tongue squamous cell carcinoma. J Cell Biochem.
120:15933–15940. 2019.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Zhao S, Xu K, Jiang R, Li DY, Guo XX, Zhou
P, Tang JF, Li LS, Zeng D, Hu L, et al: Evodiamine inhibits
proliferation and promotes apoptosis of hepatocellular carcinoma
cells via the hippo-yes-associated protein signaling pathway. Life
Sci. 251(117424)2020.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Hu CY, Wu HT, Su YC, Lin CH, Chang CJ and
Wu CL: Evodiamine exerts an anti-hepatocellular carcinoma activity
through a WWOX-dependent pathway. Molecules.
22(1175)2017.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Zhu H, Ge K, Lu J and Jia C: Growth
inhibitor of human hepatic carcinoma HepG2 cells by evodiamine is
associated with downregulation of PRAME. Naunyn Schmiedebergs Arch
Pharmacol. 392:1551–1560. 2019.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Wang XN, Han X, Xu LN, Yin LH, Xu YW, Qi Y
and Peng JY: Enhancement of apoptosis of human hepatocellular
carcinoma SMMC-7721 cells through synergy of berberine and
evodiamine. Phytomedicine. 15:1062–1068. 2008.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Fang C, Zhang J, Qi D, Fan X, Luo J, Liu L
and Tan Q: Evodiamine induces G2/M arrest and apoptosis via
mitochondrial and endoplasmic reticulum pathways in H446 and H1688
human small-cell lung cancer cells. PLoS One.
9(e115204)2014.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Wang T, Qi D, Hu X, Li N, Zhang X, Liu H,
Zhong C and Zhang J: A novel evodiamine amino derivative as a
PI3K/AKT signaling pathway modulator that induces apoptosis in
small cell lung cancer cells. Eur J Pharmacol.
906(174215)2021.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Lin L, Ren L, Wen L, Wang Y and Qi J:
Effect of evodiamine on the proliferation and apoptosis of A549
human lung cancer cells. Mol Med Rep. 14:2832–2838. 2016.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Jiang ZB, Huang JM, Xie YJ, Zhang YZ,
Chang C, Lai HL, Wang W, Yao XJ, Fan XX, Wu QB, et al: Evodiamine
suppresses non-small cell lung cancer by elevating CD8+
T cells and downregulating the MUC1-C/PD-L1 axis. J Exp Clin Cancer
Res. 39(249)2020.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Hong JY, Park SH, Min HY, Park HJ and Lee
SK: Anti-proliferative effects of evodiamine in human lung cancer
cells. J Cancer Prev. 19:7–13. 2014.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Tu YJ, Fan X, Yang X, Zhang C and Liang
HP: Evodiamine activates autophagy as a cytoprotective response in
murine Lewis lung carcinoma cells. Oncol Rep. 29:481–490.
2013.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Mohan V, Agarwal R and Singh RP: A novel
alkaloid, evodiamine causes nuclear localization of cytochrome-c
and induces apoptosis independent of p53 in human lung cancer
cells. Biochem Biophys Res Commun. 477:1065–1071. 2016.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Huang YC, Guh JH and Teng CM: Induction of
mitotic arrest and apoptosis by evodiamine in human leukemic
T-lymphocytes. Life Sci. 75:35–49. 2004.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Zhang Y, Zhang QH, Wu LJ, Tashiro SI,
Onodera S and Ikejima T: Atypical apoptosis in L929 cells induced
by evodiamine isolated from evodia rutaecarpa. J Asian Nat Prod
Res. 6:19–27. 2004.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Khan M, Bi Y, Qazi JI, Fan L and Gao H:
Evodiamine sensitizes U87 glioblastoma cells to TRAIL via the death
receptor pathway. Mol Med Rep. 11:257–262. 2015.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Wu WS, Chien CC, Liu KH, Chen YC and Chiu
WT: Evodiamine prevents glioma growth, induces glioblastoma cell
apoptosis and cell cycle arrest through JNK activation. Am J Chin
Med. 45:879–899. 2017.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Liu AJ, Wang SH, Chen KC, Kuei HP, Shih
YL, Hou SY, Chiu WT, Hsiao SH and Shih CM: Evodiamine, a plant
alkaloid, induces calcium/JNK-mediated autophagy and
calcium/mitochondria-mediated apoptosis in human glioblastoma
cells. Chem Biol Interact. 205:20–28. 2013.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Meng ZJ, Wu N, Liu Y, Shu KJ, Zou X, Zhang
RX, Pi CJ, He BC, Ke ZY, Chen L, et al: Evodiamine inhibits the
proliferation of human osteosarcoma cells by blocking PI3K/Akt
signaling. Oncol Rep. 34:1388–1396. 2015.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Zhou Y and Hu J: Evodiamine induces
apoptosis, G2/M cell cycle arrest, and inhibition of cell migration
and invasion in human osteosarcoma cells via Raf/MEK/ERK signalling
pathway. Med Sci Monit. 24:5874–5880. 2018.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Yang S, Chen J, Tan T, Wang N, Huang Y,
Wang Y, Yuan X, Zhang P, Luo J and Luo X: Evodiamine exerts
anticancer effects against 143B and MG63 cells through the
Wnt/β-catenin signaling pathway. Cancer Manag Res. 12:2875–2888.
2020.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Bai X, Meng H, Ma L and Guo A: Inhibitory
effects of evodiamine on human osteosarcoma cell proliferation and
apoptosis. Oncol Lett. 9:801–805. 2015.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Hwang ST, Um JY, Chinnathambi A, Alharbi
SA, Narula AS, Namjoshi OA, Blough BE and Ahn KS: Evodiamine
mitigates cellular growth and promotes apoptosis by targeting the
c-Met pathway in prostate cancer cells. Molecules.
25(1320)2020.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Zhang T, Qu S, Shi Q, He D and Jin X:
Evodiamine induces apoptosis and enhances TRAIL-induced apoptosis
in human bladder cancer cells through mTOR/S6K1-mediated
downregulation of Mcl-1. Int J Mol Sci. 15:3154–3171.
2014.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Shi CS, Li JM, Chin CC, Kuo YH, Lee YR and
Huang YC: Evodiamine induces cell growth arrest, apoptosis and
suppresses tumorigenesis in human urothelial cell carcinoma cells.
Anticancer Res. 37:1149–1159. 2017.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Du J, Sun Y, Lu YY, Lau E, Zhao M, Zhou QM
and Su SB: Berberine and evodiamine Act synergistically against
human breast cancer MCF-7 cells by inducing cell cycle arrest and
apoptosis. Anticancer Res. 37:6141–6151. 2017.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Wang KL, Hsia SM, Yeh JY, Cheng SC, Wang
PS and Wang SW: Anti-proliferative effects of evodiamine on human
breast cancer cells. PLoS One. 8(e67297)2013.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Du J, Wang XF, Zhou QM, Zhang TL, Lu YY,
Zhang H and Su SB: Evodiamine induces apoptosis and inhibits
metastasis in MDA-MB-231 human breast cancer cells in vitro
and in vivo. Oncol Rep. 30:685–694. 2013.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Wei L, Jin X, Cao Z and Li W: Evodiamine
induces extrinsic and intrinsic apoptosis of ovarian cancer cells
via the mitogen-activated protein
kinase/phosphatidylinositol-3-kinase/protein kinase B signaling
pathways. J Tradit Chin Med. 36:353–359. 2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
146
|
Chen TC, Chien CC, Wu MS and Chen YC:
Evodiamine from evodia rutaecarpa induces apoptosis via activation
of JNK and PERK in human ovarian cancer cells. Phytomedicine.
23:68–78. 2016.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Liu N, Li Y, Chen G and Ge K: Evodiamine
induces reactive oxygen species-dependent apoptosis and necroptosis
in human melanoma A-375 cells. Oncol Lett. 20(121)2020.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Lin H, Lin L, Choi Y and Michniak-Kohn B:
Development and in-vitro evaluation of co-loaded berberine chloride
and evodiamine ethosomes for treatment of melanoma. Int J Pharm.
581(119278)2020.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Yang J, Wu LJ, Tashiro S, Onodera S and
Ikejima T: Nitric oxide activated by p38 and NF-kappaB facilitates
apoptosis and cell cycle arrest under oxidative stress in
evodiamine-treated human melanoma A375-S2 cells. Free Radic Res.
42:1–11. 2008.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Wang C, Wang MW, Tashiro S, Onodera S and
Ikejima T: Evodiamine induced human melanoma A375-S2 cell death
partially through interleukin 1 mediated pathway. Biol Pharm Bull.
28:984–989. 2005.PubMed/NCBI View Article : Google Scholar
|