Introduction
Tumoral involvement of the inferior cava vein has
been described as being the consequence of the presence of primary
lesions at this level such as inferior cava vein leiomyosarcomas or
due to the presence of locally advanced urological malignancies
such as renal cell carcinoma, adrenal carcinoma or retroperitoneal
metastatic adenopathies with urological or gynecological origin
invading the inferior cava vein (1,2); in
such cases advances in the field of surgical techniques allow
performing extended vascular and visceral resections in order to
achieve negative resection margins and therefore to offer a chance
for cure for these patients (3-9).
In the present article, we present the case of a 43-year-old male
successfully submitted to surgery for a primitive leiomyosarcoma of
the cava vein.
Case report
After obtaining approval of the Ethics Committee of
‘Fundeni’ Clinical Institute (no. 311/2020), data concerning the
patient were reviewed and presented in the present article.
The 43-year-old male with no significant medical
history was investigated for diffuse abdominal and dorso-lumbar
pain in association with lower limb edema and was diagnosed at the
preoperative computed tomography with a large retroperitoneal tumor
involving both the cava vein and the right kidney.
After preoperative preparation, the patient was
submitted to surgery, the tumor being resected en bloc with
infra-hepatic, perirenal cava vein resection and right nephrectomy.
The continuity of the cava vein was re-established by using a
polytetrafluoroethylene (PTFE) prosthesis which was anastomosed
with the proximal infra-hepatic cava vein and with the infra-renal
cava vein distally. Due to the presence of an adequate collateral
network at the level of the left kidney through both adrenal and
gonadal veins, the left renal vein was no longer re-implanted at
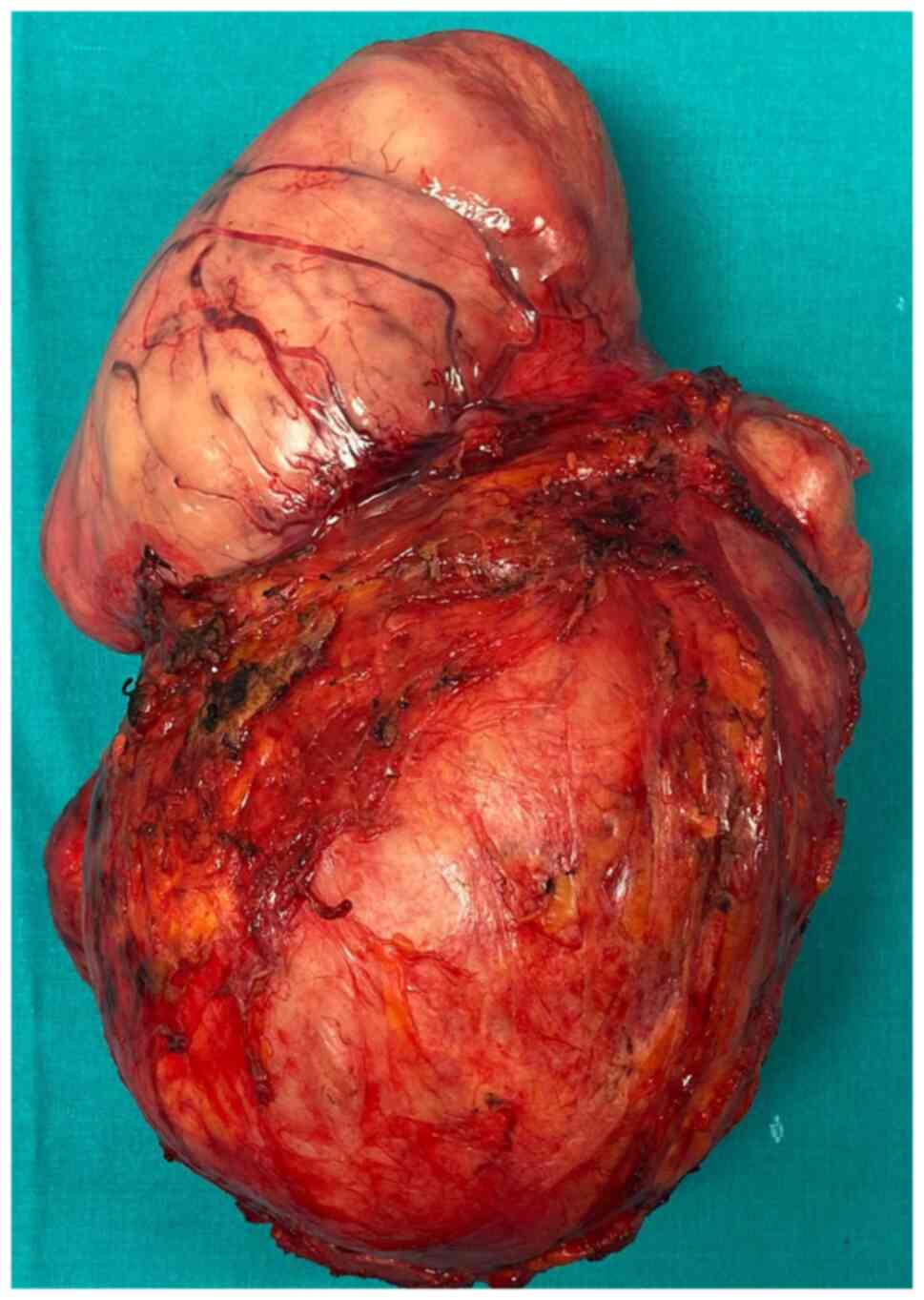
the level of the synthetic graft (Fig.
1, Fig. 2, Fig. 3, Fig.
4 and Fig. 5).
The postoperative Doppler ultrasound revealed a
proper flow at the level of the venous graft while the renal
function proved to be an acceptable one; a slight increase in the
postoperative values of serum creatinine (at 1.6 mg/dl) in the
first postoperative week were encountered. The overall
postoperative outcome was favorable, the patient being discharged
on postoperative day 13. The anticoagulant injectable treatment
consisting of fractioned heparin injections was ended at the time
of discharge and replaced with coumadin oral treatment which was
administered during the next three months; meanwhile an
International Normalized Ratio (INR) value was determined every two
weeks, with target values ranging between 2 and 3. At the 3-month
follow-up, the patient exhibited a good general condition; the
Doppler ultrasound revealed a functional venous graft while the
biological tests showed a serum level of creatinine of 1.4 mg/dl.
The final histopathological report demonstrated the presence of a
caval leiomyosarcoma invading the right kidney; meanwhile negative
resection margins were confirmed.
Discussion
Cava vein resection can be imposed by the presence
at this level of primary caval leiomyosarcomas, by vascular
invasion caused by retroperitoneal sarcomas or other malignant
primaries (the most commonly situation being represented by renal
tumors) or by metastatic lesions (10). Although initially cava vein invasion
was considered as the sign of locally advanced disease and was
therefore considered as a contraindication for surgery, improvement
of vascular surgical techniques in addition to visceral standard
resections have led to the incorporation of such procedures in the
therapeutic armamentarium of these cases (3-13).
Meanwhile, it has been observed that this aggressive surgical
approach remains the only therapeutic option which might increase
the overall survival of these patients, especially when it comes to
tumors with a primary origin at the level of venous structures such
as cava vein leiomyosarcomas (3,12,13).
However, the fact should not be omitted that caval resection in the
absence of a pre-existent caval obstruction can be hardly tolerated
especially in the absence of a well-developed collateral network
(14).
Retroperitoneal sarcomas may cause inferior vena
cava invasion due to extrinsic involvement, and therefore their
resection will impose partial or circumferential venous excision;
while in cases in which lateral invasion is present, partial
resection followed by primary suture or patch repair could be taken
into consideration. Cases presenting circumferential invasion will
necessitate circumferential resection; meanwhile, when it comes to
primary sarcomas of the cava vein, circumferential resection is
usually the option of choice (15).
In this respect, it should not be overlooked that
caval resection may increase the cardiac preload, may increase the
risk of venous congestion and the peripheral venous pressure
resulting in lower extremity edema and deep venous thrombosis
(16).
Depending on the length of the resected segment and
on the presence and patency of collateral circulation, various
methods of reconstruction might be taken into consideration. The
presence of collateral circulation, if patent, may allow performing
caval ligation without further reconstruction; moreover,
interruption of the renal veins at the time of resection might be
well tolerated if an adequate collateral venous return is provided
through the adrenal veins. However, attention should be focused at
the time of resection in order not to destroy the network of
collaterals which is expected to provide an adequate venous return
(3,10,12,13,15,17).
Moreover, this aspect is particularly important in the case of the
left kidney, in which the left gonadal and adrenal veins appear to
play a crucial role in providing an adequate venous return, as for
the right kidney, the absence of these collaterals might pose
significant issues in terms of venous return (15).
According to the length of resection of the cava
vein, various types of reconstruction have been proposed, ranging
from primary repair, patch placement or segmental resection
followed or not by venous reconstruction; as mentioned before,
cases in which an adequate collateral circulation is present may be
candidates for solely resection and no further reconstruction
(10).
When it comes to the types of materials which can be
used for venous replacement, both synthetic and natural grafts have
been proposed. Therefore, using a circular polytetrafluoroethylene
(PTFE) prosthesis may be the option of choice due to the fact that
in a significant number of cases, it is more facile to be obtained.
If this is the option of choice, attention should be paid to the
diameter of the graft; the general recommendations underlining the
fact that a lower diameter prosthesis is more efficient due to the
fact that it seems to provide a faster velocity at its level
(15,18). In cases in which an autologous graft
is available, the superficial femoral vein has been widely used;
meanwhile, cryopreserved grafts can be also used (15). As for the details of surgical
technique, it is considered that the reconstruction should proceed
from distal to proximal; meanwhile the bifurcation of the caval
vein should be preserved as much as possible in order to make more
facile the distal reconstruction (15). In the meantime, if the perirenal
segment is resected, it is recommended to perform first the distal
and proximal anastomoses of the graft followed by renal vein
reimplantation if possible; if the both renal veins are to be
implanted, the right one should be first reinserted due to the lack
of collaterals of the right kidney when compared to the left kidney
(15,19-21).
As for the anastomosis at the level of the proximal end, the most
common site of this anastomosis is the infra-hepatic area; in
certain cases in which the tumor also involves this segment, a
retrohepatic resection and anastomosis are required. However, in
such cases, attention should be focused on the risk of destroying
the venous branches of the caudate lobe, which can cause
significant bleeding (15).
Furthermore, it should not be overlooked that the
only chance for cure in such cases is represented by the
achievement of negative resection margins (22,23),
and therefore the length of the resected segment should be long
enough in order to achieve this desiderate (24). Moreover, it has been demonstrated
that administration of adjuvant treatment can be difficult to be
administered due to the high sensitivity of the healthy tissues
around the field of resection (25). The utility of negative resection
margins is also sustained by the observation that 77% of the
sarcoma-related deaths are caused by the development of local
recurrence in the absence of distal metastases (26).
One of the largest studies which analyzed the
effectiveness and safety of cava vein resection as part of extended
oncological procedures was conducted by Ruiz et al (10); the study included 52 patients
submitted to cava vein resection as part of various oncological
procedures; among these cases there were 5 patients diagnosed with
primary cava vein leiomyosarcomas, 11 renal cell carcinomas, 7
testicular carcinomas, 5 cholangiocarcinomas, 10 retroperitoneal
sarcomas and a variety of other histopathological types and
subtypes of lesions. As for the option of choice for
reconstruction, in 17 cases, primary repair was the option of
choice; in the other 18 cases, a patch angioplasty was required
while in the remaining 17 cases, graft interposition was the option
of choice. Among the latter category, PTFE grafts were used in 13
cases, Dacron grafts were preferred in 2 cases while in the
remaining 3 cases homologous grafts were used; the authors decided
to reinsert the left renal vein at the level of the prosthesis in 3
cases. Postoperatively the overall complication rate was 75%, 10
cases necessitating reoperation; however, among cases in which
graft interposition was performed only 2 cases developed
postoperative graft thrombosis and secondary lower limb lymphedema.
Meanwhile the authors underlined the fact that graft thrombosis was
significantly higher among cases in which non-ringed grafts were
used as well as among cases in which the diameter of the graft was
wider than 18 mm. As for the long-term outcomes, the authors
reported a 2-year survival rate of 64.7% and a 2-year patency rate
of 77.5% demonstrating in this way the effectiveness and safety of
the method (10).
In conclusion, retroperitoneal sarcomas originating
from the cava vein might require extensive resection followed by
demanding reconstruction of the venous contiguity in order to
re-establish a functional venous outflow at this level. In cases in
which circumferential resections are needed, the reconstruction can
be performed by allograft or autologous grafts. In such cases, a
debatable subject is related to the necessity of performing a
reimplantation of the renal veins. The presence of an adequate
collateral network seems to provide an acceptable venous return
especially for the left kidney; the most important collateral
venous drainage pathways being represented by the left adrenal and
gonadal vein.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Further information regarding the case presentation
is available upon request.
Authors' contributions
NB contributed to the conception of the study,
collected, analyzed and interpreted the data from the literature
and critically revised the manuscript. IoB contributed to the
conception of the study, performed the literature research, drafted
the manuscript and was responsible for confirming the authenticity
of all the raw data. VB contributed to the conception of the study,
performed the literature research, drafted the manuscript and was
responsible for confirming the authenticity of all the raw data.
IrB contributed to the interpretation of the data from the
literature, collected, analyzed and interpreted the data
corresponding to the patient and critically revised the manuscript.
IC collected, analyzed and interpreted the data corresponding to
the patient and critically revised the manuscript. All authors read
and approved the final manuscript for publication.
Ethics approval and consent to
participate
The Ethics Committee of ‘Fundeni’ Clinical Institute
(Bucharest, Romania) (no. 311/2020) approved the study.
Patient consent for publication
Patient consent for publication was obtained and
signed by the patient on 23/08/2020.
Competing interests
There are no competing interests to declare
regarding this study.
References
|
1
|
Psutka SP, Boorjian SA, Thompson RH,
Schmit GD, Schmitz JJ, Bower TC, Stewart SB, Lohse CM, Cheville JC
and Leibovich BC: Clinical and radiographic predictors of the need
for inferior vena cava resection during nephrectomy for patients
with renal cell carcinoma and caval tumour thrombus. BJU Int.
116:388–396. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Djaladat H, Ghoreifi A, Basin MF, Hugen C,
Aslzare M, Miranda G, Hwang DH, Schuckman AK, Aron M, Thangathurai
D, et al: Perioperative outcome of suprarenal resection of vena
cava without reconstruction in urologic malignancies: A case series
and review of the literature. Urology. 142:146–154. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Stauffer JA, Fakhre GP, Dougherty MK,
Nakhleh RE, Maples WJ and Nguyen JH: Pancreatic and multiorgan
resection with inferior vena cava reconstruction for
retroperitoneal leiomyosarcoma. World J Surg Oncol.
7(3)2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bacalbasa N, Brezean I, Anghel C, Barbu I,
Pautov M, Balescu I and Brasoveanu V: Successful resection and
vascular ligation of a large hepatic artery aneurysm-a case report
and literature review. In Vivo. 31:979–982. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bacalbasa N, Brezean I, Anghel C, Barbu I,
Pautov M, Balescu I and Brasoveanu V: Management of a fulminant
upper gastrointestinal bleeding exteriorized through hemobilia due
to arteriobiliary fistula between the common bile duct and a right
hepatic artery aneurysm-a case report. In Vivo. 31:983–989.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Brasoveanu V, Anghel C, Barbu I, Pautov M,
Ionescu MI, Motthor M, Balescu I, Dima S and Bacalbasa N:
Pancreatoduodenectomy en bloc with portal and superior mesenteric
artery resection-a case report and literature review. Anticancer
Res. 35:1613–1618. 2015.PubMed/NCBI
|
|
7
|
Bacalbasa N, Balescu I, Tanase A, Brezean
I, Vilcu M and Brasoveanu V: Successful resection of a
non-functional paraganglioma with celiac trunk invasion followed by
common hepatic artery reimplantation-a case report and literature
review. In Vivo. 32:911–914. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bacalbasa N, Balescu I, Tanase A, Pautov
M, Brezean I, Vilcu M and Brasoveanu V: Spleno-Pancreatectomy en
bloc with parcelar gastrectomy for splenic artery aneurysm-a case
report and literature review. In Vivo. 32:915–919. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Brasoveanu V, Dumitrascu T, Bacalbasa N
and Zamfir R: Splenic artery used for replaced common hepatic
artery reconstruction during pancreatoduodenectomy-a case report.
Chirurgia (Bucur). 104:499–504. 2009.PubMed/NCBI
|
|
10
|
Ruiz CS, Kalbaugh CA, Browder SE,
McGinigle KL, Kibbe MR, Farber MA, Crowner JR, Marston WA and
Pascarella L: Operative strategies for inferior vena cava repair in
oncologic surgery. J Vasc Surg Venous Lymphat Disord. 8:396–404.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sarkar R, Eilber FR, Gelabert HA and
Quinones-Baldrich WJ: Prosthetic replacement of the inferior vena
cava for malignancy. J Vasc Surg. 28:75–81. 1998.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hardwigsen J, Baque P, Crespy B,
Moutardier V, Delpero JR and Le Treut YP: Resection of the inferior
vena cava for neoplasms with or without prosthetic replacement: A
14-patient series. Ann Surg. 233:242–249. 2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bower TC, Nagorney DM, Cherry KJ Jr,
Toomey BJ, Hallett JW, Panneton JM and Gloviczki P: Replacement of
the inferior vena cava for malignancy: An update. J Vasc Surg.
31:270–281. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Duty B and Daneshmand S: Venous resection
in urological surgery. J Urol. 180:2338–2342. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Quinones-Baldrich WJ and Farley S:
Techniques for inferior vena cava resection and reconstruction for
retroperitoneal tumor excision. J Vasc Surg Venous Lymphat Disord.
1:84–89. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Vladov NN, Mihaylov VI, Belev NV,
Mutafchiiski VM, Takorov IR, Sergeev SK and Odisseeva EH: Resection
and reconstruction of the inferior vena cava for neoplasms. World J
Gastrointest Surg. 4:96–101. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fiore M, Colombo C, Locati P, Berselli M,
Radaelli S, Morosi C, Casali PG and Gronchi A: Surgical technique,
morbidity, and outcome of primary retroperitoneal sarcoma involving
inferior vena cava. Ann Surg Oncol. 19:511–518. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wachtel H, Jackson BM, Bartlett EK,
Karakousis GC, Roses RE, Bavaria JE and Fraker DL: Resection of
primary leiomyosarcoma of the inferior vena cava (IVC) with
reconstruction: A case series and review of the literature. J Surg
Oncol. 111:328–333. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu D, Ren HL, Liu B, Shao J, Chen YX,
Song XJ, Liu ZL, Chen Y, Li YJ, Liu CW and Zheng YH: Renal function
preservation in surgical resection of primary inferior vena cava
leiomyosarcoma involving the renal veins. Eur J Vasc Endovasc Surg.
55:229–239. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Daylami R, Amiri A, Goldsmith B, Troppmann
C, Schneider PD and Khatri VP: Inferior vena cava leiomyosarcoma:
Is reconstruction necessary after resection? J Am Coll Surg.
210:185–190. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yoshidome H, Takeuchi D, Ito H, Kimura F,
Shimizu H, Ambiru S, Togawa A, Ohtsuka M, Kato A and Miyazaki M:
Should the inferior vena cava be reconstructed after resection for
malignant tumors? Am J Surg. 189:419–424. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lewis JJ and Benedetti F: Adjuvant therapy
for soft tissue sarcomas. Surg Oncol Clin N Am. 6:847–862.
1997.PubMed/NCBI
|
|
23
|
Lewis JJ, Leung D, Woodruff JM and Brennan
MF: Retroperitoneal soft-tissue sarcoma: Analysis of 500 patients
treated and followed at a single institution. Ann Surg.
228:355–365. 1998.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Schwarzbach MH, Hormann Y, Hinz U,
Leowardi C, Böckler D, Mechtersheimer G, Friess H, Büchler MW and
Allenberg JR: Clinical results of surgery for retroperitoneal
sarcoma with major blood vessel involvement. J Vasc Surg. 44:46–55.
2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Brennan MF: Retroperitoneal sarcoma: Time
for a national trial? Ann Surg Oncol. 9:324–325. 2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Stojadinovic A, Yeh A and Brennan MF:
Completely resected recurrent soft tissue sarcoma: Primary anatomic
site governs outcomes. J Am Coll Surg. 194:436–447. 2002.PubMed/NCBI View Article : Google Scholar
|