Introduction
Osteoarthritis (OA) is characterized by the slow,
progressive but irreversible erosion of the articular cartilage
(1,2). It is also one of the most common
musculoskeletal disorders and is a leading cause of disability,
which results in the reduction in the quality of life in older
adults. Among the various causes of OA, cartilage matrix
degradation caused by inflammation is considered to be one of the
main causes, which finally leads to the degradation of the
extracellular matrix (ECM) (3,4). In
particular, the proinflammatory cytokine interleukin (IL)-1β is
considered to be a key inducer of OA acceleration by stimulating
the release of cartilage degrading enzymes, including matrix
metalloproteinases (MMPs), A disintegrin-like and metalloproteinase
with thrombospondin motifs (ADAMTSs) and other inflammatory
mediators, such as nitric oxide (NO) and prostaglandin E2 (PGE2)
(5,6). In addition, elevated levels of IL-1β
have been previously detected in the synovial fluid and cartilage
tissue of patients with OA (7).
The MMP family of matrix-degrading enzymes serves an important role
in OA cartilage degradation, of which MMP-13 is a key component in
the catabolic processes of OA (8).
It hydrolyzes protein structures in the ECM in the articular
cartilage, including collagen type II and aggrecan (9). In addition, aggrecanases such as
ADAMT-4 and -5 have been shown to induce degradation of the ECM by
causing cleavage of proteoglycans and aggrecans within the matrix
(10). These repeated cycles of
inflammation and catabolism impair chondrocyte homeostasis and
promote the irreversible degradation of the ECM in the cartilage,
which occurs during OA (11). At
present, conservative treatment options for OA mainly involve
controlling pain and inflammation using anti-inflammatory agents,
including analgesics and nonsteroidal anti-inflammatory drugs, to
attenuate articular cartilage injury at the early stages of OA
(12,13). However, the majority of these
treatment options are only short term that do not alleviate or
prevent the progression of OA. In addition, they have been
previously reported to cause adverse side effects, including the
gastrointestinal hemorrhage (14)
and renal impairment (15). By
contrast, the other therapeutic strategy available, surgery, can
increase risk (such as postoperative infection) and financial
burden of the patient and family. Since there is currently no
effective treatment strategies available to reverse OA progression,
there is a demand to develop an alternative and efficient strategy
to alleviate, delay or even reverse the process of OA.
Premna fulva Craib (Verbenaceae), also called
‘Zhangu’, is an ethnomedicine of the Zhuang people (a Chinese
minority) that is used for the preparation of Jian-Gu injection
(16). Clinically, Jian-Gu
injection has been widely applied for the treatment of cervical
spondylosis, lumbar sprain and OA (17-19).
However, despite its use in the hospital, the mechanism of action
has not been characterized in detail. Vicenin 3, which is also
called (apigenin 6-C-β-glucopyranosyl-8-C-β-xylopyranoside
(Fig. 1A), is a flavone
di-C-glycoside purified from Premna fulva Craib that has
been reported to be one of the major components in the Jian-Gu
injection (20). Recently,
flavonoid C-glycosides were reported to exert significant
antioxidant, anti-tumor, anti-inflammatory and anti-diabetic
activities (21,22). In general, C-glycosyl flavonoids
exhibit more potent activity compared with that mediated by their
corresponding O-glycosyl flavonoid and aglycone counterparts
(23). Previous studies have shown
that treatment with flavonoid C-glycosides are associated with a
number of health benefits in the prevention and management of
diseases such as cancer, diabetes, and cardiovascular disease
(23), especially when used as a
treatment for antigen-induced arthritis (24,25).
However, the underlying mechanism of the effects of vicenin 3 on
articular cartilage degeneration during OA remain poorly
understood.
MAPK is a super family of intracellular
serine-threonine protein kinases that serves as a major node of
numerous signal transduction pathways (5). The MAPK pathway mainly includes three
signal cascades: JNK, p38 MAPK and ERK (26,27).
Previous studies have shown that the p38 signaling pathway serves a
key role in the progression of several human diseases including
cardiovascular disease, diabetes and cancer, especially in the
development of OA (28,29). In addition, activation of the p38
MAPK signaling pathway may increase the expression of
proinflammatory cytokines (IL-6, TNF-α), chemokines (CCL3, CCL5),
MMPs (MMP-3, MMP-13) and signaling enzymes (iNOS, COX-2) in human
OA chondrocytes (30). Blocking
the p38 MAPK pathway with a p38 inhibitor has been revealed to
inhibit chondrocyte apoptosis and reduce the production of
inflammatory cytokines to prevent the recruitment of inflammatory
cells, which may alleviate bone and cartilage degradation (29). Therefore, in the present study, the
effects of vicenin 3 on chondrocytes in an in vitro model of
OA was investigated. In addition, the underlying mechanism of these
effects, which particular focus on the MAPK signaling cascade, was
also assessed.
Materials and methods
Reagents
Vicenin 3 (purity ≥98%) was isolated from Premna
fulva Craib by recycling counter-current chromatography in
Guangxi Key Laboratory of Functional Phytochemicals Research and
Utilization as previously described (20). FBS and DMEM were purchased from
Gibco (Thermo Fisher Scientific, Inc.). Recombinant human IL-1β was
purchased from R&D Systems, Inc. The Cell Counting Kit-8
(CCK-8) was purchased from Beyotime Institute of Biotechnology. The
Griess reagent for NO estimation was obtained from Nanjing
Jiancheng Bioengineering Institute. The ELISA kits for aggrecan
(cat. no. E-EL-H0294c), collagen type II (cat. no. E-EL-H0777c),
PGE2 (cat. no. E-EL-0034c), MMP-1 (cat. no. E-EL-H6073c) and MMP-13
(cat. no. E-EL-H0134c) were obtained from Elabscience Biotechnology
Co., Ltd. whereas the MMP-3 (cat. no. CSB-E04677h) ELISA kit was
purchased from Cusabio Biotech Co., Ltd. TRIzol reagent was
purchased from Ambion; Thermo Fisher Scientific, Inc. HiScript
QuantiTect Reverse Transcription kit was obtained from Vazyme
Biotech Co., Ltd. Antibodies against JNK (#9252), phosphorylated
(p-)-JNK (#9251), ERK (#9102), p-ERK (#9101), p38 (#8690), p-p38
(#9211) and GAPDH (#5174) were purchased from Cell Signaling
Technology Inc. The p38 inhibitor SB203580 was obtained from
MedChemExpress to block the activation of p38. Cultured
chondrocytes were pretreated with vicenin 3 (20 µM) or SB203580 (10
µM) at 37˚C for 1 h and then treated with 10 ng/ml IL-1β at 37˚C
for 24 h, western blot was used to detect the expression of p38,
p-p38; PGE2, MMP-1, MMP-3, MMP-13, aggrecan and collagen type II
were measured in the culture medium using an ELISA kit.
Cell culture
The human chondrocyte cell line SW1353 was acquired
from the Shanghai Institute of Biochemistry and Cell Biology,
Chinese Academy of Sciences. They were cultured in DMEM containing
10% FBS and 2 mM glutamine, 100 U/ml penicillin and 100 µg/ml
streptomycin at 37˚C in humidified atmosphere with 5%
CO2.
Cell viability assay
The effects of vicenin 3 (6.25-100 µM) on
chondrocytes were determined using a CCK-8 kit according to the
manufacturer's protocols. The SW1353 cells were seeded into 96-well
plates (5,000 cells/well) at 37˚C for 12 h and then treated with
various concentrations of either vicenin 3 or IL-1β (10 ng/ml)
alone at 37˚C for 24 h. In an additional protocol, the cells were
also pretreated with vicenin 3 (5 and 20 µM) at 37˚C for 1 h before
IL-1β treatment (at 37˚C for 24 h). Subsequently, 10 µl CCK-8 was
added to each well and incubated at 37˚C for 4 h. Absorbance in
each well was then measured at 450 nm using a microplate reader
(Leica Microsystems GmbH). All experiments were performed in
triplicate.
NO measurement and ELISA
The SW1353 cells (5,000 cells/well) were pretreated
with different concentrations (5 and 20 M) of vicenin 3 at 37˚C for
1 h, followed by treatment with IL-1β (10 ng/ml) at 37 ˚C for 24 h,
before the levels of NO accumulation in the culture medium were
determined using the Griess reaction according to the
manufacturer's protocols.
The protein levels of PGE2, MMP-1, MMP-3, MMP-13,
aggrecan and collagen type II released from the chondrocyte
cultured medium under the same conditions were evaluated using the
ELISA kits according to the manufacturer's protocols. All assays
were performed in triplicate.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from chondrocytes using
TRIzol reagent (Ambion; Thermo Fisher Scientific, Inc., 15596-026)
according to the manufacturer's protocol. The concentration was
spectrophotometrically measured at 260 nm using NanoDrop 2000
(Thermo Fisher Scientific, Inc.). The A260/A280 ratio was
calculated to test the quality and purity of the RNA samples.
First-strand cDNA was synthesized from 3 µg total RNA with the
HiScript QuantiTect RT kit (Vazyme Biotech Co., Ltd, R101-01/02) at
25˚C for 5 min, 50˚C for 15 min, 85˚C for 5 min and at 4˚C for 10
min. qPCR was performed in the Bio-Rad CFX96 Touch Real-Time PCR
Detection System (Bio Rad Laboratories, Inc., 1855196) under the
following thermocycling conditions: 10 min at 95˚C, followed by 40
cycles of 15 sec at 95˚C and 1 min at 60˚C. The level of target
mRNA was normalized to the level of GAPDH and then compared with
the control. mRNA expression was quantified using the
2-∆∆Cq method (31).
This assay was performed in triplicate. The primer sequences of the
targeted genes are listed in Table
I.
 | Table IPrimer sequences used for reverse
transcription-quantitative PCR in the present study. |
Table I
Primer sequences used for reverse
transcription-quantitative PCR in the present study.
| Gene | Primer | Sequence
(5'-3') | PCR products |
|---|
| GAPDH | Forward |
CAGCCTCAAGATCATCAGCA | 106 bp |
| | Reverse |
TGTGGTCATGAGTCCTTCCA | |
| MMP-1 | Forward |
CCAGGTATTGGAGGGGATG | 273 bp |
| | Reverse |
GTCACACGCTTTTGGGGTT | |
| MMP-13 | Forward |
CCCAACCCTAAACATCCAA | 147 bp |
| | Reverse |
AAACAGCTCCGCATCAACC | |
| MMP-3 | Forward |
TTCCTTGGATTGGAGGTGAC | 248 bp |
| | Reverse |
AGCCTGGAGAATGTGAGTGG | |
| ADAMTS-4 | Forward |
CAATCCTGTCAGCTTGGTGG | 162 bp |
| | Reverse |
GCTGTGTCAAAGTGGTCAGG | |
| ADAMTS-5 | Forward |
CTGCCACCACACTCAAGAAC | 208 bp |
| | Reverse |
TGGAGGCCATCGTCTTCAAT | |
Western blot analysis
Western blotting was used to detect the protein
expression and phosphorylation of JNK, ERK and p38. Total proteins
were extracted from chondrocytes using RIPA (Beyotime Institute of
Biotechnology) and PMSF (Shanghai Aladdin Biochemical Technology
Co., Ltd) buffers. Lysates were sonicated (sonicated time: 40 sec,
interval time 60 sec, power/frequency: 100 W/16 KHz) three times on
finely-crushed dry ice for 5 min and centrifuged at 119 (x100 g)
for 30 min at 4˚C. Protein concentration was determined using the
BCA protein assay kit. Protein samples (40 µg/lane) was then
separated by SDS-PAGE (10%) and transferred onto PVDF membranes.
The membranes were blocked with 5% non-fat dry milk for 2 h at room
temperature and subsequently washed three times for 5 min in TBS
with 0.1% Tween-20 (TBST). The membranes were incubated
sequentially with primary antibodies (1:1,000) against JNK, p-JNK,
ERK, p-ERK, p38, p-p38 and GAPDH overnight at 4˚C. After washing
three times with TBST for 5 min, the membranes were incubated with
HRP-conjugated secondary antibodies (BA1054, Wuhan Boster
Biological Technology Co., Ltd.; 1:50,000) at room temperature for
2 h. Finally, proteins were visualized using the Immobilon Western
Chemiluminescent HRP substrate (cat. no. WBKLS0500; EMD Millipore),
and then the blots were analyzed using the ChemiDoc XRS system (Bio
Rad Laboratories, Inc.). The density of the protein bands was
quantified using Image Lab 3.0 software (Bio-Rad Laboratories,
Inc.). The relative protein levels of the target protein in each
sample were determined before the levels of phosphorylated protein
were normalized to those of their corresponding total protein.
Statistical analysis
The results are expressed as the mean ± standard
deviation. All assays were performed in triplicate. One-way
analysis of variance followed by the Dunnett's test was used for
multiple comparisons using the GraphPad Prism 8.0 software
(Graphpad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of vicenin 3 on SW1353
chondrocyte cytotoxicity
The potential cytotoxicity of vicenin 3 on SW1353
chondrocytes was tested using CCK-8 assay. Vicenin 3 at the
concentration range of 6.25-25 µM did not exert cytotoxic effects
on the cells. However, significant reductions in cell viability was
observed at concentrations >50 µM (Fig. 1B). In addition, 5 and 20 µM vicenin
3 did not alter chondrocyte viability in combination with
inflammatory stimuli (10 ng/ml IL-1β; Fig. 1C). Therefore, 5 and 20 µM vicenin 3
were chosen for subsequent experiments.
Effect of vicenin 3 on NO and PGE2
production in chondrocytes
The possible effects of vicenin 3 on IL-1β-induced
NO and PGE2 production in SW1353 cells were next investigated.
SW1353 cells were pretreated with vicenin 3 (5 and 20 µM) for 1 h
before subsequent IL-1β (10 ng/ml) stimulation for 24 h. NO
concentration in the cell suspension was then determined using the
Griess reaction whereas PGE2 levels were measured using ELISA. As
shown in Fig. 2, the levels of NO
and PGE2 in the supernatant were significantly increased after
IL-1β treatment, which were significantly reversed by vicenin 3 in
a dose-dependent manner.
Effect of vicenin 3 on expression and
secretion of MMP-1, MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5 in
IL-1β-induced SW1353 chondrocytes
ECM degradation by matrix degrading enzymes, such as
MMPs is a characteristic feature of OA (32). Therefore, the effect of vicenin 3
on the secretion levels of MMP-1, MMP-3 and MMP-13 was evaluated in
IL-1β-induced SW1353 cells. As shown in Fig. 3A and B, IL-1β significantly upregulated the
secretion and mRNA expression levels of MMP-1, MMP-3 and MMP-13,
whilst treatment with the higher dose of vicenin 3 (20 µM) resulted
in the significant reversal of this IL-1β-induced increase in
MMP-1, MMP-3 and MMP-13 mRNA expression and protein secretion.
Although 5 µM vicenin 3 did not significantly affect the secretion
of MMP-3, it did significantly reverse the IL-1β-induced increase
in MMP-1, MMP-3 and MMP-13 mRNA expression and MMP-1 and MMP-13
secretion. In addition, treatment with vicenin 3 significantly
reversed the IL-1β-induced increase in ADAMTS-4 and ADAMTS-5 mRNA
expression (Fig. 3C and D). These results suggest that vicenin 3
reduced the IL-1β-induced expression of matrix degrading enzymes in
SW1353 chondrocytes.
 | Figure 3Effect of vicenin 3 on IL-1β-induced
MMP-1, MMP-3, MMP-13, and ADAMTS-4 and ADAMTS-5 in SW1353 human
chondrocytes. (A) The protein secretion levels of MMP-1, MMP-3 and
MMP-13 were determined using ELISA. The mRNA expression levels of
(B) MMP-1, MMP-3, MMP-13, (C) ADAMTS-4 and (D) ADAMTS-5 were
assayed using reverse transcription-quantitative PCR.
###P<0.001 vs. Control group. *P<0.05,
**P<0.01 and ***P<0.001 vs. IL-1β. IL,
interleukin; MMP, matrix metalloproteinase; ADAMTS, A
disintegrin-like and metalloproteinase with thrombospondin
motifs. |
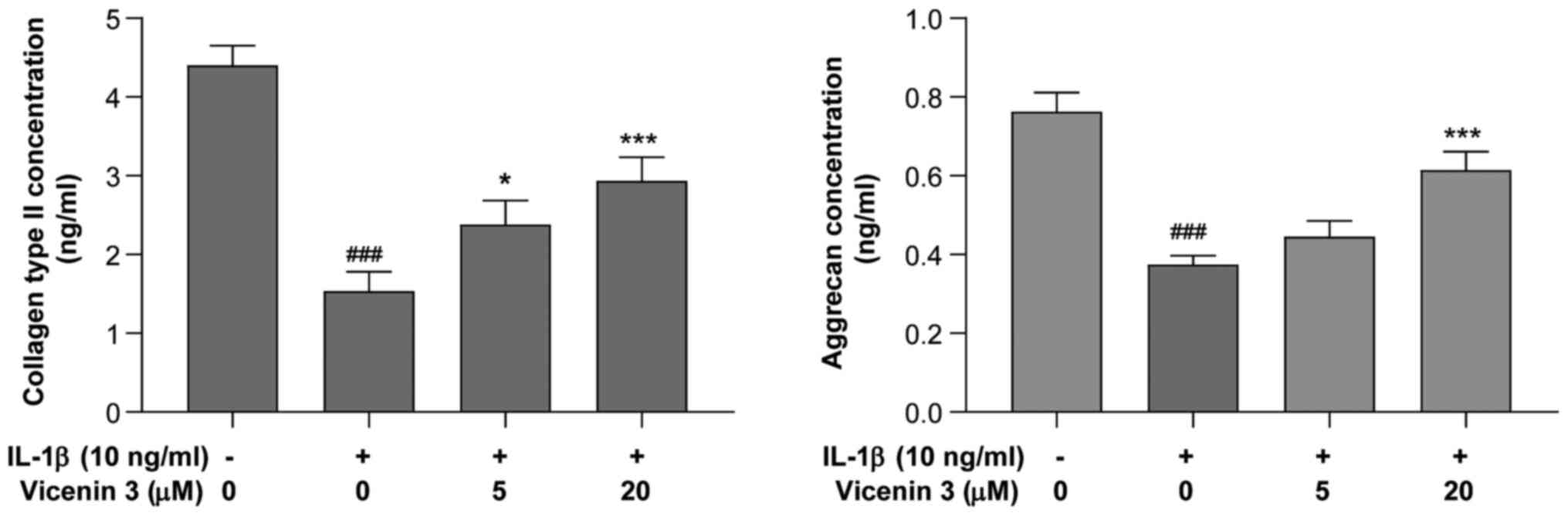
Effect of vicenin 3 on collagen type
II and aggrecan secretion by IL-1β-induced SW1353 chondrocytes
The effects of vicenin 3 on the secretion of
collagen type II and aggrecan, major components of ECM in the
articular cartilage (33), by
IL-1β-stimulated SW1353 chondrocytes was next assessed using ELISA.
As shown in Fig. 4, collagen type
II and aggrecan levels were significantly decreased by IL-1β,
whereas pre-treatment with vicenin 3 (5 and 20 µM) significantly
prevented this IL-1β-induced decrease in the secretion of collagen
type II. In addition, vicenin 3 (20 µM) significantly prevented the
IL-1β-induced decrease in aggrecan expression. These results
suggest that vicenin 3 has potent cartilage matrix-protective
effects by suppressing IL-1β-induced degradation of collagen type
II and aggrecan in SW1353 chondrocytes.
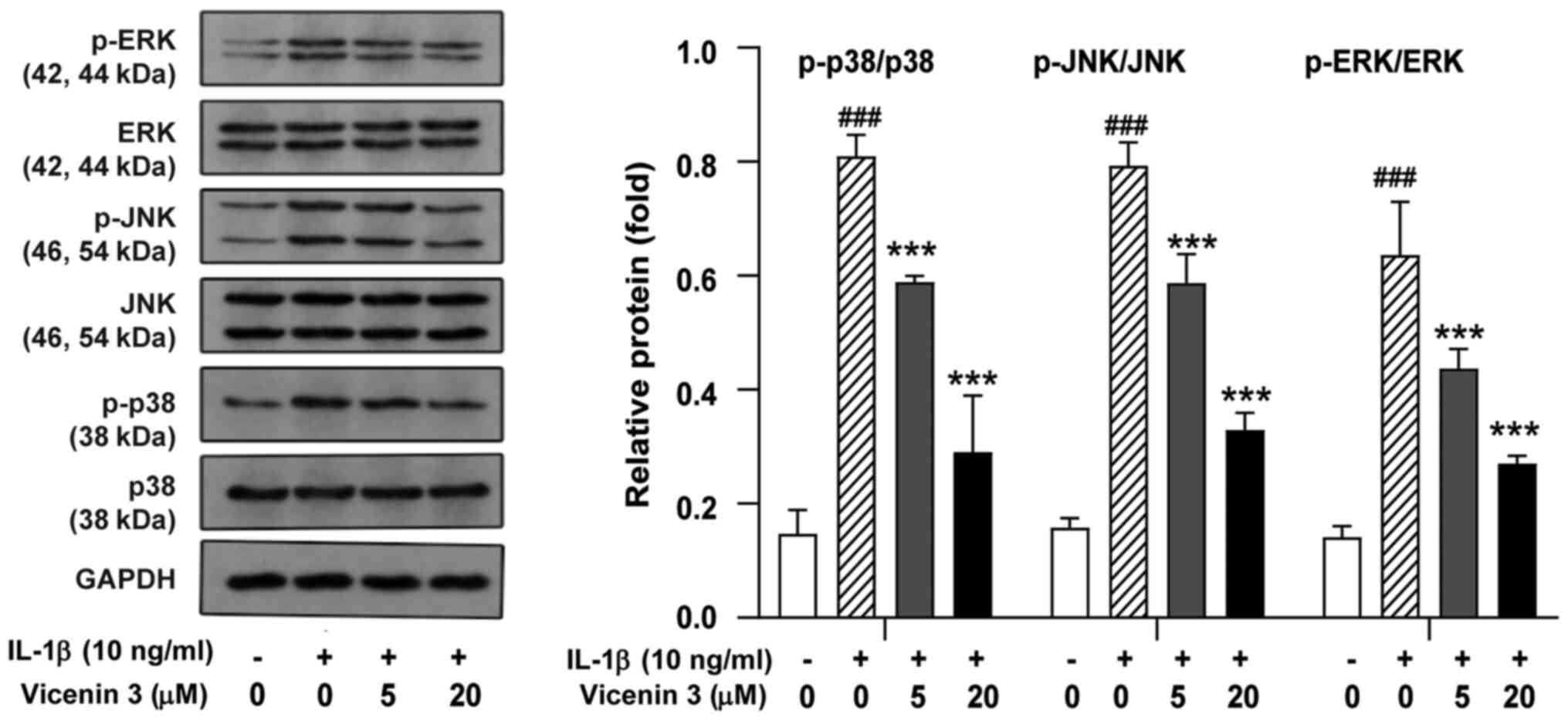
Effect of vicenin 3 on MAPK activation
in IL-1β-induced SW1353 chondrocytes
Since IL-1β-induced MMP expression was previously
found to be mediated by members of the MAPK kinase family, such as
ERK, JNK, and p38 MAPK (34), the
potential effects of vicenin 3 on the activity of these three MAPK
kinases were next examined. In IL-1β-stimulated chondrocytes, the
phosphorylation of ERK, JNK and p38 MAPK was found to be activated
compared with that in the control group but their corresponding
total protein expression levels were not significantly affected
(Fig. 5). However, pretreatment
with vicenin 3 significantly reversed the IL-1β-induced
phosphorylation of ERK, JNK and p38 MAPK (Fig. 5). These results suggest that the
suppressive effects of vicenin 3 on the IL-1β-induced expression of
cartilage degrading enzymes was mediated through MAPK signaling in
SW1353 chondrocytes.
Vicenin 3 ameliorates the degradation
of ECM in IL-1β-treated SW1353 chondrocytes by blocking the p38
signaling pathway
Western blot analysis demonstrated that vicenin 3
exerted a significant inhibitory influence on IL-1β-induced
activation of ERK, JNK and p38 MAPK (Fig. 5). p38 MAPK has been reported to be
associated with OA and has been considered to be a target for
drug-mediated modulation in OA (35,36).
Therefore, it was investigated whether vicenin 3 and the p38MAPK
inhibitor (SB203580) had similar efficacy in IL-1β-stimulated
SW1353 chondrocytes. As shown in Fig.
6, vicenin 3 was also found to exert similar effects compared
with those mediated by SB203580, a p38 inhibitor, on blocking p38
activation in IL-1β-stimulated SW1353 chondrocytes. Subsequently,
in IL-1β-incubated SW1353 chondrocytes, which exhibited
significantly higher protein secretion levels of PGE2, MMP-1, MMP-3
and MMP-13 compared with those in the control group, treatment with
vicenin 3 or SB203580 significantly reduced the secretion of these
enzymes (Fig. 6). By contrast,
analysis of ELISA results revealed that the secretion levels of
collagen type II by SW1353 chondrocytes was significantly decreased
by IL-1β, which was significantly reversed by vicenin 3 or SB203580
treatment (Fig. 6). However,
vicenin 3 exhibited a more potent inhibitory effect on the
secretion of aggrecan compared with that by SB203580 (Fig. 6). Therefore, these results
demonstrated that vicenin 3-related amelioration of ECM degradation
by SW1353 chondrocytes was associated at least in part with the
blocking of p38 MAPK signaling.
Discussion
OA is a degenerative joint disease that is
frequently observed among the elderly and exerts great burden on
the society and economy due to the lack of therapeutic methods
(37). The underlying pathological
mechanism of OA remains to be fully elucidated. However, it is
generally accepted that OA is caused at least in part by
degradation of the cartilaginous matrix because of the
inflammation-induced upregulation of catabolism in chondrocytes
(4). A number of studies have
highlighted the importance of chondrocyte function in the
development of OA, since they are the only type of cells in the
articular cartilage and are responsible for maintaining the
anabolic and catabolic balance in the cartilage ECM (38,39).
In the present study, a protective effect of vicenin 3 on
chondrocyte was found, which was mediated through the inhibition of
various pathological factors affecting OA, including NO-induce
stress, degradation of articular ECM and the expression of
proinflammatory cytokines and mediators. This effect was found to
be due to the inhibition of MAPK signaling. Application of various
p38 inhibitors, including synthetic pyridine imidazole small
molecular compounds, for OA has conferred disappointing results
owing to their long-term toxicities, such as gastrointestinal
disorders, dizziness and rashes (40). Vicenin 3 is a natural flavone
di-C-glycoside that can be purified from Premna fulva Craib
(20). In the present study, it
was found to suppress IL-1β-stimulated MAPK activation in a similar
manner to that exerted by the p38 MAPK inhibitor SB203580. Taken
together, these findings suggest that vicenin 3 may have
therapeutic potential as ap38 MAPK inhibitor for the treatment of
OA.
Inflammation is a potent catabolic inducer of the
cartilage during OA pathogenesis, which apparently disrupts tissue
maintenance and functionality as a result of chondrocyte apoptosis
and hinders the regenerative ability of the joint (41). In particular, IL-1β has been found
to trigger the initiation and acceleration of ECM degradation by
chondrocytes, by stimulating the release of cartilage-degrading
enzymes in the MMP and ADAMTS families and other catabolic factors,
including NO and PGE2(6). This
consequently contributes to chondrocyte dysfunction (6). As a mediator of the inflammatory
response, NO and PGE2 is involved in a number of inflammatory
degenerative diseases, including OA and chronic neuronal diseases
(42,43). In particular, the increase of
inflammatory mediators such as NO and PGE2 by IL-1β alleviate OA
pathogenesis (44). Therefore,
inhibition of these inflammatory mediators would be of therapeutic
potential by assessing the effect of potential anti-inflammatory
drugs on the joint degenerative process induced by inflammation. In
the present study, the production of NO and PGE2 were increased by
IL-1β treatment, but pre-treatment with vicenin 3 reverse this
effect.
MMPs belong to a family of proteolytic enzymes and
can facilitate OA development by mediating the irreversible
degradation of collagen type II and proteoglycan in the cartilage
(4). In particular, MMP-13 is
considered to be a key player in the catabolic processes during OA
because of its ability to degrade collagen type II and proteoglycan
(9). In addition, ADAMTS-4 and -5
are considered to be the main enzymes responsible for the
degradation of aggrecan and associated with OA (10). Therefore, the level of cartilage
matrix components (collagen type II and proteoglycan) can be used
as indicators for assessing the progression of cartilage
destruction. In the present study, vicenin 3 inhibited the
IL-1β-induced secretion and mRNA expression of MMP-1, MMP-3,
MMP-13, ADAMTS-4 and ADAMTS-5 in SW1353 chondrocytes. In addition,
vicenin 3 reversed the IL-1β-induced reduction of collagen type II
and aggrecan secretion. These results suggest that vicenin 3 has a
protective effect on chondrocytes to inhibit the development of OA
following IL-1β-related induction.
Numerous intracellular signaling systems have been
reported to participate in regulating OA (45,46).
Among these, MAPKs have been extensively studied, where they were
reported to be involved in the development and progression of OA
(47). In addition, activation of
p38MAPK has been revealed to be associated with cartilage collagen
degradation, chondrocyte apoptosis and the inflammation process in
OA (35,48). Therefore, p38 MAPK was proposed to
be a therapeutic target for OA (36). In the present study, vicenin 3 was
able to reduce the phosphorylation of MAPKs (ERK1/2, JNK and p38)
in vitro induced by IL-1β stimulation. These results suggest
that vicenin 3 may have the therapeutic potential for the treatment
of OA by blocking MAPK signaling pathways.
In conclusion, IL-1β-induced production of
inflammatory factors NO and PGE2 and cartilage-degrading enzymes
MMP-1, MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5 by SW1353 chondrocytes
were found to be significantly reversed by vicenin 3 (especially at
a concentration of 20 µM) in the present study. Furthermore,
vicenin 3 reversed the reduction in the production of ECM
components collagen type II and aggrecan by SW1353 chondrocytes
following IL-1β treatment. These findings suggest that vicenin 3,
which is a main component of Jian-Gu injection, exerts protective
effects against IL-1β-treated OA development.
Despite determining the discovery of the protective
effects of vicenin 3 and the associated underlying mechanism of
action using an in vitro OA model, the present study also
has limitations. Although comparison to a previous study showed
that SW1353 human chondrosarcoma is commensurate with the in
vitro primary experimental human chondrocyte system (49), it would be more valuable to use
chondrocytes isolated from patients with OA. In addition, further
validation studies, such as the recommended optimal dose for use
in vivo, are also required. Vicenin 3 is one of the five
structurally related flavone C-glycoside components reported to be
present in the Jian-Gu injection (20). Therefore, further studies are
required to clarify the protective effects of other flavone
C-glycoside compounds on chondrocytes.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the Natural
Science Foundation of Guangxi Zhuang Autonomous Region (grant no.
2018GXNSFAA294031), Key-Area Research and Development Program of
Guangdong Province (grant no. 2020B1111110003)and Guangxi
Innovation-Driven Development Special Project (grant no.
GuikeAA18118015).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YYC and DPL designed this study. XJY and XHJ
performed mechanistic and phenotypic experiments, respectively.
XJY, XRY, and YYC analyzed the data and interpreted the results of
the experiments. FLL analyzed the data and contributed to
manuscript revision. DPL and FLL confirmed the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Di Nicola V: Degenerative osteoarthritis a
reversible chronic disease. Regen Ther. 15:149–160. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hunter DJ and Bierma-Zeinstra S:
Osteoarthritis. Lancet. 393:1745–1759. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Blasioli DJ and Kaplan DL: The roles of
catabolic factors in the development of osteoarthritis. Tissue Eng
Part B Rev. 20:355–363. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Guilak F, Nims RJ, Dicks A, Wu CL and
Meulenbelt I: Osteoarthritis as a disease of the cartilage
pericellular matrix. Matrix Biol. 71-72:40–50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zeng L, Rong XF, Li RH and Wu XY: Icariin
inhibits MMP-1, MMP-3 and MMP-13 expression through MAPK pathways
in IL-1β-stimulated SW1353 chondrosarcoma cells. Mol Med Rep.
15:2853–2858. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu-Bryan R and Terkeltaub R: Emerging
regulators of the inflammatory process in osteoarthritis. Nat Rev
Rheumatol. 11:35–44. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Matejova JP, Spakova T, Harvanova D, Lacko
M, Filip V, Sepitka R, Mitro I and Rosocha J: A Preliminary Study
of Combined Detection of COMP, TIMP-1, and MMP-3 in Synovial Fluid:
Potential Indicators of Osteoarthritis Progression. Cartilage: Aug
4, 2020 (Epub ahead of print).
|
|
8
|
Murphy G, Knäuper V, Atkinson S, Butler G,
English W, Hutton M, Stracke J and Clark I: Matrix
metalloproteinases in arthritic disease. Arthritis Res. 4 (Suppl
3):S39–S49. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Burrage PS, Mix KS and Brinckerhoff CE:
Matrix metalloproteinases: Role in arthritis. Front Biosci.
11:529–543. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Majumdar MK, Askew R, Schelling S, Stedman
N, Blanchet T, Hopkins B, Morris EA and Glasson SS: Double-knockout
of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal
animals and prevents the progression of osteoarthritis. Arthritis
Rheum. 56:3670–3674. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dancevic CM and McCulloch DR: Current and
emerging therapeutic strategies for preventing inflammation and
aggrecanase-mediated cartilage destruction in arthritis. Arthritis
Res Ther. 16:429–440. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang Z, Huang C, Jiang Q, Zheng Y, Liu Y,
Liu S, Chen Y, Mei Y, Ding C, Chen M, et al: Guidelines for the
diagnosis and treatment of osteoarthritis in China (2019 edition).
Ann Transl Med. 8:1213. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Latourte A, Kloppenburg M and Richette P:
Emerging pharmaceutical therapies for osteoarthritis. Nat Rev
Rheumatol. 16:673–688. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li Z, Meng D, Li G, Xu J, Tian K and Li Y:
Celecoxib Combined with Diacerein Effectively Alleviates
Osteoarthritis in Rats via Regulating JNK and p38MAPK Signaling
Pathways. Inflammation. 38:1563–1572. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Konijnenbelt-Peters J, van der Heijden C,
Ekhart C, Bos J, Bruhn J and Kramers C: Metamizole (Dipyrone) as an
Alternative Agent in Postoperative Analgesia in Patients with
Contraindications for Nonsteroidal Anti-Inflammatory Drugs. Pain
Pract. 17:402–408. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pharmacopoeia Committee of the Ministry of
Health of the People's Republic of China: Jian gu zhu she ye
(Jian-gu injection). In: Prescription preparation of Chinese
Medicine. Vol 14. Pharmacopoeia Committee of the Ministry of Health
of the People's Republic of China, pp59-60, 1997 (In Chinese).
|
|
17
|
Ma YH, Sun Q, Yang L, Zhang J and Zhai WT:
Effect of Jiangu injection by shoulder injection combined with
arthroscopic subacromial space decompression on shoulder function
recovery in patients with subacromial impingement syndrome. Mod J
Integr Tradi Chin West Med. 27:2415–2418. 2018.(In Chinese).
|
|
18
|
Chen Z, Hao LD and Zhong W: Clinical
efficacy of pain spot injection with Jiangu injection in acute
lumbar sprain. J Guandong Med Coll. 34:295–297. 2016.(In
Chinese).
|
|
19
|
Wang HQ, Huang YR and Qin XL: Treatment of
40 cases of knee osteoarthritis with acupoint injection of Jiangu
injection. J Guangxi Trad Chin Med Univ. 14:23–24. 2011.(In
Chinese).
|
|
20
|
Chen Y, Yan X, Lu F, Jiang X, Friesen JB,
Pauli GF, Chen SN and Li DP: Preparation of flavone di-C-glycoside
isomers from Jian-Gu injection (Premna fulva Craib.) using
recycling counter-current chromatography. J Chromatogr A.
1599:180–186. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xue Y, Liu Y, Xie Y, Cong C, Wang G, An L,
Teng Y, Chen M and Zhang L: Antioxidant activity and mechanism of
dihydrochalcone C-glycosides: Effects of C-glycosylation and
hydroxyl groups. Phytochemistry. 179(112393)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen GL, Fan MX, Wu JL, Li N and Guo MQ:
Antioxidant and anti-inflammatory properties of flavonoids from
lotus plumule. Food Chem. 277:706–712. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xiao J, Capanoglu E, Jassbi AR and Miron
A: Advance on the Flavonoid C-glycosides and Health Benefits. Crit
Rev Food Sci Nutr. 56 (Suppl 1):S29–S45. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Khan MA, Khurana N, Ahmed RS, Umar S, Md
G, Sarwar AH, Alam Q, Kamal MA and Ashraf GM: Chemokines: A
Potential Therapeutic Target to Suppress Autoimmune Arthritis. Curr
Pharm Des. 25:2937–2946. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Garcia EF, de Oliveira MA, Candido LCM,
Coelho FM, Costa VV, Queiroz-Junior CM, Boff D, Amaral FA, de Souza
DG, Teixeira MM, et al: Effect of the Hydroethanolic Extract from
Echinodorus grandiflorus Leaves and a Fraction Enriched in
Flavone-C-Glycosides on Antigen-Induced Arthritis in Mice. Planta
Med. 82:407–413. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Keshet Y and Seger R: The MAP kinase
signaling cascades: a system of hundreds of components regulates a
diverse array of physiological functions. Methods Mol Biol.
661:3–38. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Thalhamer T, McGrath MA and Harnett MM:
MAPKs and their relevance to arthritis and inflammation.
Rheumatology (Oxford). 47:409–414. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang H, Shan XB and Qiao YJ, Wang H, Shan
XB and Qiao YJ: PDK2 promotes chondrogenic differentiation of
mesenchymal stem cells by upregulation of Sox6 and activation of
JNK/MAPK/ERK pathway. Braz J Med Biol Res. 50(e5988)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sun HY, Hu KZ and Yin ZS: Inhibition of
the p38-MAPK signaling pathway suppresses the apoptosis and
expression of proinflammatory cytokines in human osteoarthritis
chondrocytes. Cytokine. 90:135–143. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Feng Z, Zheng W, Li X, Lin J, Xie C, Li H,
Cheng L, Wu A and Ni W: Cryptotanshinone protects against
IL-1β-induced inflammation in human osteoarthritis chondrocytes and
ameliorates the progression of osteoarthritis in mice. Int
Immunopharmacol. 50:161–167. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure, function, and biochemistry. Circ Res. 92:827–839.
2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sharma S, Vazquez-Portalatin N, Calve S
and Panitch A: Biomimetic molecules lower catabolic expression and
prevent chondroitin sulfate degradation in an osteoarthritic ex
vivo model. ACS Biomater Sci Eng. 2:241–250. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yao M, Wang X, Zhao Y, Wang X and Gao F:
Expression of MMPs is dependent on the activity of
mitogen-activated protein kinase in chondrosarcoma. Mol Med Rep.
15:915–921. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kühn K, Shikhman AR and Lotz M: Role of
nitric oxide, reactive oxygen species, and p38 MAP kinase in the
regulation of human chondrocyte apoptosis. J Cell Physiol.
197:379–387. 2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Joos H, Albrecht W, Laufer S and Brenner
RE: Influence of p38MAPK inhibition on IL-1beta-stimulated human
chondrocytes: A microarray approach. Int J Mol Med. 23:685–693.
2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rahmati M, Nalesso G, Mobasheri A and
Mozafari M: Aging and osteoarthritis: Central role of the
extracellular matrix. Ageing Res Rev. 40:20–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Schroeppel JP, Crist JD, Anderson HC and
Wang J: Molecular regulation of articular chondrocyte function and
its significance in osteoarthritis. Histol Histopathol. 26:377–394.
2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Alexopoulos LG, Youn I, Bonaldo P and
Guilak F: Developmental and osteoarthritic changes in
Col6a1-knockout mice: Biomechanics of type VI collagen in the
cartilage pericellular matrix. Arthritis Rheum. 60:771–779.
2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Joos H, Albrecht W, Laufer S and Brenner
RE: Differential effects of p38MAP kinase inhibitors on the
expression of inflammation-associated genes in primary,
interleukin-1β-stimulated human chondrocytes. Br J Pharmacol.
160:1252–1262. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Robinson WH, Lepus CM, Wang Q, Raghu H,
Mao R, Lindstrom TM and Sokolove J: Low-grade inflammation as a key
mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol.
12:580–592. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Woo JH, Lee JH, Kim H, Park SJ, Joe EH and
Jou I: Control of Inflammatory Responses: A New Paradigm for the
Treatment of Chronic Neuronal Diseases. Exp Neurobiol. 24:95–102.
2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Frizziero A, Bonsangue V, Trevisan M, Ames
PRJ and Masiero S: Foot tendinopathies in rheumatic diseases:
Etiopathogenesis, clinical manifestations and therapeutic options.
Clin Rheumatol. 32:547–555. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rai MF and Sandell LJ: Inflammatory
mediators: Tracing links between obesity and osteoarthritis. Crit
Rev Eukaryot Gene Expr. 21:131–142. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Stampella A, Monteagudo S and Lories R:
Wnt signaling as target for the treatment of osteoarthritis. Best
Pract Res Clin Rheumatol. 31:721–729. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sun K, Luo J, Guo J, Yao X, Jing X and Guo
F: The PI3K/AKT/mTOR signaling pathway in osteoarthritis: A
narrative review. Osteoarthritis Cartilage. 28:400–409.
2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chowdhury TT, Salter DM, Bader DL and Lee
DA: Signal transduction pathways involving p38 MAPK, JNK, NFkappaB
and AP-1 influences the response of chondrocytes cultured in
agarose constructs to IL-1β and dynamic compression. Inflamm Res.
57:306–313. 2008.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Masuko-Hongo K, Berenbaum F, Humbert L,
Salvat C, Goldring MB and Thirion S: Up-regulation of microsomal
prostaglandin E synthase 1 in osteoarthritic human cartilage:
Critical roles of the ERK-1/2 and p38 signaling pathways. Arthritis
Rheum. 50:2829–2838. 2004.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Gebauer M, Saas J, Sohler F, Haag J, Söder
S, Pieper M, Bartnik E, Beninga J, Zimmer R and Aigner T:
Comparison of the chondrosarcoma cell line SW1353 with primary
human adult articular chondrocytes with regard to their gene
expression profile and reactivity to IL-1β. Osteoarthritis
Cartilage. 13:697–708. 2005.PubMed/NCBI View Article : Google Scholar
|