Introduction
As the global population ages, and with improvements
in medical and health technologies, there are more elderly patients
and more are requiring surgical interventions and procedures
(1). Globally, 50% of all elderly
individuals are estimated to undergo at least one surgical
procedure (2). Postoperative
cognitive dysfunction (POCD) is a complication of anesthesia and
surgery associated with significant morbidity and even mortality
that is widely considered an important clinical problem,
particularly in elderly patients. In a prospective multicenter
trial performed by ISPOCD (international study of postoperative
cognitive dysfunction) in 1998, the incidence of POCD in patients
undergoing non-cardiac surgery was present in 26% at 1 week, and
approximately 10% of the patients still had cognitive dysfunction
after 3 months (3). Currently,
there is no effective clinical treatment for POCD (4) and an urgent need to develop a novel
treatment strategy to improve the prognosis of this condition
(5).
The risk and precipitating factors of POCD are
multifarious including increasing age, low education level, burden
of illness, pain, anesthesia, repeated surgeries, postoperative
infections and respiratory complications (6). Especially volatile anesthetics, one
class of the most widely used drugs since the 19th century for
general anesthesia is often mentioned as a possible cause of POCD
(7). For instance, isoflurane
(8), sevoflurane (9) and desflurane (10), which are commonly used in clinic,
have been found to cause cognitive impairment. Previous studies
have provided evidence of neurotoxicity caused by volatile
anesthetics (11-13),
and the safety of these anesthetic agents has come under scrutiny
(14). Impairment of memory and
spatial learning were observed in aged rodents receiving volatile
anesthetics including sevoflurane (12). Although the mechanisms underlying
the volatile anesthetic neurotoxicity have not been clarified,
neuroapoptosis (15),
neuroinflammation (16) and
neurodegeneration (17) may be
involved.
Normobaric hyperoxia (NBO) preconditioning (PC) has
been demonstrated to protect against heart and cerebral ischemia as
well as renal ischemia (18-20).
Similarly, NBO-PC also attenuates cognitive impairment in an
Alzheimer's disease mouse model (21). The protective effects of NBO-PC are
attributed to antiapoptotic and anti-inflammatory effects (22). Since apoptosis and inflammation are
involved in the neurotoxicity induced by volatile anesthetics
(15,16), NBO-PC may be an effective and
feasible method to alleviate cognitive impairment related to
volatile anesthetics including sevoflurane.
Thus far, to the best of our knowledge whether
NBO-PC can ameliorate cognitive deficit induced by sevoflurane and
the possible mechanism by which it may exert its effect has not yet
been clarified. We previously found that sevoflurane induces
apoptosis in hippocampal neurons and causes cognitive deficit in
aged rats (15). Based on these
findings, it was hypothesized that NBO-PC may ameliorate cognitive
deficit induced by sevoflurane through inhibiting hippocampal
apoptosis. In the present study, this hypothesis was tested in an
aged rat model of cognitive dysfunction induced by sevoflurane and
the underlying molecular mechanism of this phenomenon was explored.
In brief, if the neuroprection of NBO-PC could be proved, it may
indicate a potential novel target for the treatment of POCD.
Materials and methods
Study design
The present study was a prospective, randomized,
controlled animal study, which started in September 2018 and ended
in October 2019, with the duration of the animal experimentation
for 5 months. It was approved by the Ethics Committee for Animal
Experimentation (Ethical approval no. Guo A2017-026-1), and the
animals were studied at Hebei Medical University (Shijiazhuang,
China).
Animals
A total of 66 male Sprague-Dawley 20-month-old rats
(450-550 g) were divided randomly into 3 groups (n=22 each): i)
Control group (C group); ii) sevoflurane group (S group); and iii)
sevoflurane + NBO-PC group (HO group). To adapt to the animal care
facility for 5 days before the experiment, these rats were housed
in plastic cages under standard conditions, with free access to
food and water, and maintained on a 12/12 h light/dark cycle. The
rational for choosing rats of this age was previous evidence that
sevoflurane inhalation resulted in learning and memory deficits
(23). There was no death in each
group prior to decapitation.
Preconditioning protocol and
experimental procedure
NBO-PC was performed as previously described
(24). Briefly, animals assigned to
the HO group received 4 h of NBO treatment at 1 atm absolute (ATA)
in 95% oxygen each day for 6 consecutive days in environmental
chambers comprised air-tight boxes (65x25x45 cm) with gas inlet and
outlet ports at room temperature (22±1˚C). The C and S groups were
exposed to normobaric normoxia (21% oxygen) according to the same
protocol. After the last preconditioning (the 6th preconditioning
cycle) 24 h later, rats assigned to the S and HO groups were placed
in sealed transparent anesthesia induction chambers with soda lime
at the bottom near the side opening of where the anesthetic
machines were connected. The aforementioned rats received 2.5%
sevoflurane (Maruishi Pharmaceutical Co., Ltd.) via humidified 50%
O2 carrier gas from a calibrated vaporizer for 5 h. Rats
in the C group were also placed in the same chambers for 5 h with
no sevoflurane given. Following 24 h sevoflurane-exposure, rats
were subdivided for the Morris water maze (MWM) test, apoptosis
detection in the hippocampus and cytosolic calcium concentration
measurement of hippocampal cells. The experimental procedure was
shown in Fig. 1.
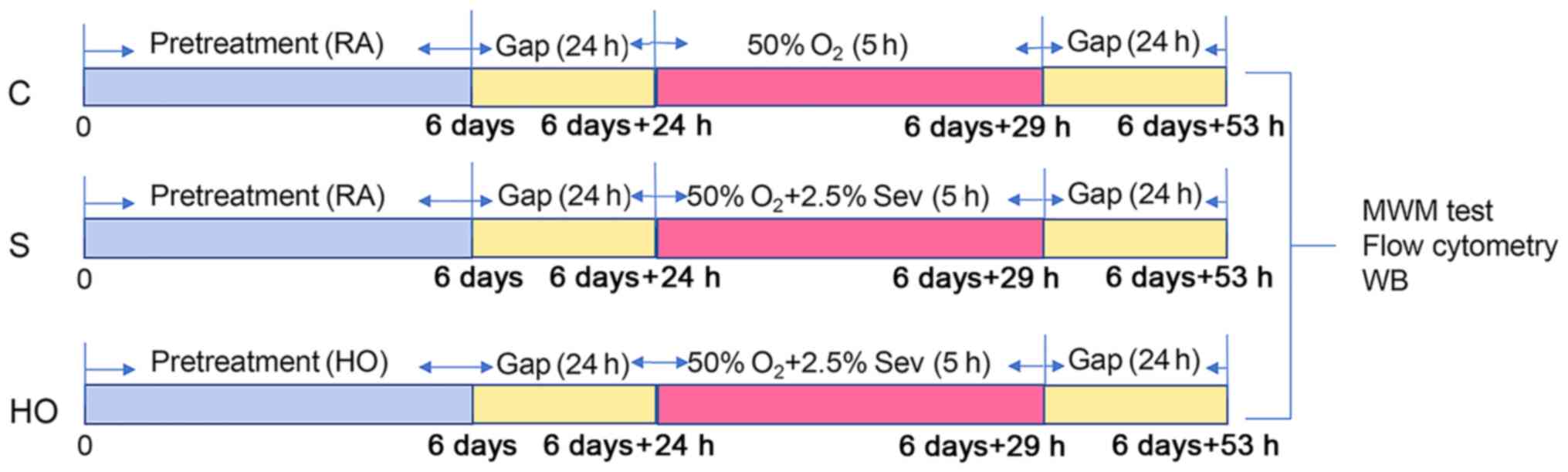 | Figure 1Experimental protocol for the study
of normobaric hyperoxia preconditioning. RA, normoxia; HO,
normobaric hyperoxia; Gap, ordinary air room without any procedure;
d, day; h, hour; Sev, sevoflurane; WB, western blotting; MWM,
Morris water maze; C group, control group; S group, sevoflurane
group; HO group, sevoflurane + NBO-PC group; NBO-PC, normobaric
hyperoxia preconditioning. |
Morris water maze test
Cognitive function of 10 rats randomly selected from
each group was tested using the MWM test 24 h after sevoflurane
exposure. The MWM (Shanghai Jiliang Software Technology Co., Ltd.)
was a circular pool (180 cm diameter, 50 cm depth) with black walls
and a water (23±1˚C) depth of 30 cm, which was divided into 4 equal
quadrants. A hidden round platform (10 cm diameter) was submerged 2
cm below the surface of water, located in one quadrant. The MWM
test consisted of 5 days of place navigation test (a test of
ability to learn the location of the hidden platform) and a probe
trial (a test of ability to remember the previously-learned
location of the escape platform having been removed) on the 6th
day. In the test, rats were given 4 trials/day for 5 consecutive
days. A trial was terminated and recording was stopped when the
animal reached the platform, where it was allowed to remain for 15
sec. If the rat failed to find the target before 90 sec, it was
usually guided to the goal and allowed to stay on the platform for
15 sec with the escape latency being recorded as 90 sec. On the
sixth day, the hidden platform was removed and the rat was placed
in the opposite quadrant. Each rat was allowed to swim freely in
the pool for 90 sec and the platform crossing times were
recorded.
Measurements of apoptosis rate and
cytosolic calcium concentration ([Ca2+]c) in
the hippocampus
Apoptosis rate of the hippocampus was measured by
flow cytometry with an Annexin-V-FITC/propidium iodide (PI)
apoptosis detection kit (Vazyme Biotech Co. Ltd.).
Phosphatidylserine (PS) normally located on the inner side of the
cell membrane, flips to the outer side of the cell membrane at the
early stage of apoptosis (24).
Annexin-V, a Ca2+ dependent phospholipid-binding
protein, with affinity for PS is routinely used to label
externalization of PS (25). PI
(propidium iodide, a nucleic acid dye), which does not permeate
cells with an intact plasma membrane, can penetrate the cell
membrane to make the nucleus red in the late stage of apoptosis and
necrosis (26). Annexin-V and PI
dual staining allows discrimination of apoptotic cells
(Annexin-V+, PI-), late apoptotic/necrotic
cells (both Annexin-V- and PI+) and live
cells (both Annexin-V- and PI-) (27). A total of 6 rats randomly selected
from each group, 24 h after sevoflurane-exposure were anesthetized
with an intraperitoneal injection of 10% chloral hydrate (250
mg/kg) without any signs of peritonitis observed. According to
previous literature (28), the loss
of the righting reflex (LORR, an indicator of hypnosis), ambulation
and voluntary movement were considered to be indicators of the
success of chloral hydrate anesthesia. LORR was assessed by
attempting to place the rat in left lateral recumbency, followed by
dorsal recumbency. If the rat remained on its back for 10 sec, LORR
was considered to be achieved (29). After successful induction of chloral
hydrate, A total of 18 rats from three groups were sacrificed by
decapitation. Brain tissue was immediately removed and the left
hippocampi were separated and cut into blocks. Hippocampal tissue
(~100 mg) was placed on a 100-mesh copper net and cut up with
tweezers and then gently rubbed and filtered. The filtrate was
centrifuged at 167.7 x g for 5 min at room temperature. Cells
collected after centrifugation were resuspended in 500 µl Annexin-V
binding buffer to prepare a single cell suspension
(1-5x105/l). Subsequently, the cells were incubated with
5 µl Annexin-V-FITC at 5˚C for 10 min in dark and then 5 µl PI was
added and the cells were incubated at 5˚C for 10 min in dark. A
flow cytometer (FC500; Beckman Coulter Inc.) was used to detect the
apoptosis rate of early apoptotic cells with a analyzing software
(EXPO32 ADC v1.2; Beckman Coulter Inc.).
Hippocampus cells from the right hippocampi of the
18 aforementioned rats were collected in the way described above
and suspended in 3 ml Dulbecco's modification Eagle's medium (DMEM)
and made into a single cell suspension loaded
(1-5x105/l) to measure the
[Ca2+]c. Next, 3 µl Fluo-3/AM (calcium ion
fluorescence probe; Hangzhou MultiSciences (Lianke) Biotech, Co.,
Ltd.) was added and cells were incubated at 37˚C for 30 min, washed
twice with DMEM, and then resuspended in DMEM at 37˚C for 15 min.
Flow cytometry instrument (FC500; Beckman Coulter Inc.) was used to
measure fluorescence intensity, with the excitation wavelength of
488 nm and emission wavelength of 525 nm with a analyzing software
(EXPO32 ADC v1.2; Beckman Coulter Inc.).
Western blotting
Western blotting was performed to determine
expression of bcl-2, bax and active caspase-3 in the hippocampus.
The proteins were extracted from the hippocampal tissue samples of
the 6 remainder rats of each group. Briefly, the hippocampus was
homogenized in RIPA lysis buffer (Wuhan Servicebio Technology Co.,
Ltd.), and the total protein concentration of the supernatant was
determined using a BCA protein quantification kit (Wuhan Servicebio
Technology Co., Ltd.). Proteins were separated by 12% SDS/PAGE gel
(SDS-PAGE kit; Beijing Zoman Biotechnology Co., Ltd.) with 50 µg
protein loaded per lane and then transferred on to PVDF membranes
(EMD Millipore). After blocking with 5% skimmed milk at 37˚C for 2
h, the membranes were incubated at 4˚C overnight with primary
antibodies: bcl-2 (1:1,000; Proteintech Group Inc.; cat. no.
12789-1-AP), bax (1:1,000; Arigo Biolaboratories Corp.; cat. no.
ARG66247), active caspase-3 (1:2,000; ImmunoWay Biotechnology
Company; cat. no. YM3431). Anti-β-actin (1:1,000; ProteinTech Group
Inc.; cat. no. 66009-1-lg) was also used as a protein loading
control for each sample. Membranes were further incubated with
secondary antibodies (1:10,000; Rockland Immunochemicals Inc.; cat.
no. 36595) for 2 h at room temperature. The signals were detected
using an Odyssey CLx infrared imaging system (LI-COR Biosciences).
ImageJ software (v1.8.0; National Institutes of Health) was used
for quantification of the signals.
Statistical analysis
Statistical analyses were performed with the SPSS
software v.23.0 (IBM Corp), and the data were expressed as mean ±
SD. Three biological repetitions were conducted for each
experiment. Escape latency were analyzed by two-way
repeated-measures ANOVA followed by the post hoc Bonferronis test.
The statistical differences of all the other data were analyzed by
one-way ANOVA without repeated measures followed by the post hoc
Bonferronis test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of NBO-PC on cognitive deficit
induced by sevoflurane
To evaluate the cognitive function of the rats the
MWM test was conducted. All rats had a tendency of reduced escape
latency as training progressed indicating that the rats were
learning from the day by day practice (Fig. 2A and B). The escape latency was significantly
longer and the platform crossings were lower in S group rats
compared with C group rats, indicating significant cognitive
impairment induced by sevoflurane (Fig.
2B-D). Notably, the learning and memory impairment induced by
sevoflurane was ameliorated by NBO-PC, as indicated by shorter
escape latency (Fig. 2B) and
increased platform crossings (Fig.
2C and D). Taken together,
these results imply that NBO-PC can ameliorate the dysfunction of
learning and memory induced by sevoflurane in aged rats.
 | Figure 2Effects of normobaric hyperoxia
preconditioning on sevoflurane-induced learning and memory
impairment by the MWM test. (A) Typical paths during place
navigation test for each group on days, 1, 3 and 5 after
anesthesia. (B) Escape latency (time to find the hidden platform)
plotted against training day. 1, 2, 3, 4 and 5 d means the 1-5 day
after rats were anesthetized. Escape latency in each group
decreased significantly (P<0.05) compared with 1d. (C) A typical
path during the probe trial for each group. (D) Platform crossing
times during the probe trial of the MWM test. Sevoflurane exposure
increased the times of platform crossing compared with the control
condition (*P<0.05). Normobaric hyperoxia
preconditioning attenuated the sevoflurane-induced decrease in the
number of platform crossings (#P<0.05 vs. S group).
(n=10/group). *P<0.05 vs. control group,
#P<0.05 vs. S group, ∆P<0.05 vs. 1d,
▲P<0.05 vs. 2d, ◊P<0.05 vs. 3d,
♦P<0.05 vs. 4d. MWM, Morris water maze; C, control
group; S group, sevoflurane group; HO group, sevoflurane + NBO-PC
group; NBO-PC, normobaric hyperoxia preconditioning. |
Effects of NBO-PC on apoptosis in the
hippocampus
Next, to determine whether the neuroprotection of
NBO pretreatment is associated with the level of apoptosis in the
hippocampus, the apoptosis rate in the hippocampus was measured by
flow cytometry 24 h after sevoflurane-exposure. Compared with C
group, the apoptosis rate was significantly increased in the S and
HO groups (Fig. 3A and B). However, the increase of apoptosis rate
in the HO group was lower compared with that in the S group
(Fig. 3A and B).
 | Figure 3Effects of normobaric hyperoxia
preconditioning on the apoptosisin the hippocampus. (A)
Representative plots of apoptosis detection by flow cytometry in
the hippocampus of aged rats in each group, early apoptotic cells
(B4, Annexin-V+/PI-), late apoptotic or
necrotic cells (B2, Annexin-V+/PI+), live
cells (B3, Annexin-V-/PI-), mechanical
damaged cells (B1, Annexin-V-/PI+). (B)
Apoptosis rate in the hippocampus 24 h after sevoflurane exposure
in each group. (C) Representative western blot image from each
group. (D) Western blot analysis of Bcl-2, Bax and active caspase-3
expression in the hippocampus of each group. *P<0.05
vs. control group, #P<0.05 vs. S group. C, control
group; S group, sevoflurane group; HO group, sevoflurane + NBO-PC
group; NBO-PC, normobaric hyperoxia preconditioning. |
In addition, the protein expression level of bcl-2,
bax and active caspase-3 in the hippocampus were also tested to
estimate the level of apoptosis. Downregulation of antiapoptosis
protein (bcl-2) (30) and
upregulation of pro-apoptotic protein (bax) (31) and apoptosis protein (caspases-3)
(32) are an indication of
apoptosis. Caspase-3 has classically been defined as the main
executioner of apoptosis, and the activated form of caspase-3 is
involved in the execution phase of apoptosis (33). In the present study, the ‘caspase-3’
in Fig. 3C and D represented active caspase-3.
As shown in Fig. 3C
and D, compared with the C group,
the anesthesia with 2.5% sevoflurane (S group) induced significant
increase of bax and active caspase-3 and a decrease of bcl-2 in the
hippocampus. Although similar variations were observed in the HO
group, the change in the HO group was lower compared with the S
group (Fig. 3C and D). All the aforementioned data suggested
that NBO-PC inhibited apoptosis in the hippocampus caused by
sevoflurane.
Effects of NBO-PC on
[Ca2+]c in the hippocampus
It has been confirmed that sevoflurane increases
[Ca2+]c to induce neuroapoptosis (34). To evaluate the role of
Ca2+ in the NBO-PC mediated neuroprotective effect, the
[Ca2+]c in the hippocampus was measured after
sevoflurane anesthesia in the aged rats. Sevoflurane induced an
elevation of [Ca2+]c compared with the C
group (Fig. 4A and B), while NBO-PC (HO group) significantly
decreased the elevation of [Ca2+]c caused by
sevoflurane (Fig. 4A and B). Hence, it was proposed that the
inhibition of apoptosis induced by NBO-PC was mediated by reducing
[Ca2+]c.
Discussion
The present study investigated whether NBO-PC
ameliorates cognitive deficit after sevoflurane anesthesia, and
whether NBO-PC influences the hippocampal apoptosis in aged rats.
The major findings of the present study are as follows: i) NBO-PC
reduced cognitive deficit induced by sevoflurane-exposure; ii)
antiapoptosis protein (bcl-2) expression increased, pro-apoptotic
protein (bax) and apoptosis protein (caspase-3) decreased in the
hippocampus after NBO-PC; and iii) the apoptosis rate and
[Ca2+]c decreased in the hippocampus after
NBO-PC. These results demonstrated that the protective role of
NBO-PC in sevoflurane-induced spatial and learning impairment was
partly related to inhibition of apoptosis in the hippocampus via
reducing [Ca2+]c.
The potential detrimental effects of anesthetics on
aged brains has become a hot topic in recent years due to concerns
about the safety of general anesthesia/anesthetics (35). Although clinical evidence regarding
the association between anesthetic exposures of aged patients and
subsequent cognitive impairments remains unclear, repeated or
consistent exposures to general anesthetics may be a potential
harmful risk in aged brains (36,37).
Sevoflurane with properties of fast onset and rapid recovery
(38) is commonly used as a general
anesthesia drug in various types of surgery (for example,
orthopedic surgery, gynecological surgery and gastrointestinal
surgery).
Notably, numerous in vitro and in vivo
studies have demonstrated that sevoflurane induces neurotoxicity
(12,15,39).
Associated with sevoflurane-induced cell injury and death in
neurocyte (40), sevoflurane also
has been demonstrated to impair the cognitive functions of the aged
animals (12,41). Mechanisms underlying
sevoflurane-induced neurotoxicity have not been clarified (40), nevertheless, neuroapoptosis may be a
key mechanism (15,42). An in vitro study demonstrated
that sevoflurane induces apoptosis of neural stem cells by
activating γ-aminobutyric acid (43). Chen et al (44) found that sevoflurane initiates
endoplasmic reticulum stress mediated apoptosis in hippocampal
neurons of aging rats. The present study revealed that 2.5%
sevoflurane exposure for 5 h decreased the expression of bcl-2,
increased the expression of bax and caspase-3, and increased the
apoptosis rate in the hippocampus, which were consistent with the
changes in cognitive function. Additionally, in the present study
the increased [Ca2+]c in the hippocampus
after sevoflurane-exposure was also found indicating a
calcium-mediated neuroapoptosis. Apart from neuroapoptosis, there
are other mechanisms underlying-induced neurotoxicity, such as
neuroinflammation (12),
neurodegeneration (45).
NBO, in addition to serving as a tool for
enhancement of oxygen delivery, has been demonstrated to provided
neuroprotection in various models, including ischemia-reperfusion
brain injury (18), newborn
hypoxia-ischemia brain injury (46), cerebral hemorrhage (47) and brain trauma (48). Notably, Gao et al (21) found that NBO treatment improves
spatial learning and memory deficits in APP/PS1 transgenic mice,
suggesting that the NBO treatment may have a similar effect on
cognitive impairment caused by exposure to anesthetics. Hence, the
present study, investigated the effect of NBO pretreatment on the
neurotoxicity of sevoflurane in aged rats. In one previous
dose-response study (18),
protective effect against cerebral ischemia-reperfusion injury was
induced by exposing rats to a normobaric hyperoxic environment (95%
O2 exposure for 16 and 24 h consecutively), with no
protective effect of NBO exposure less than 16 h. Simultaneously,
attention should be paid to the toxicity of long-term oxygen
inhalation. It has previously been reported that exposure to 95%
O2 for 24 h resulted in severe pulmonary congestion with
extravasations of red blood cell, edema and alteration in the
alveolar structure (49). Mohammadi
and Bigdeli (24) confirmed that
normobaric hyperoxia preconditioning (exposure to 95% inspired NBO
for 4 h/day for 6 consecutive days) had neuroprotective effect on
rats in the middle cerebral artery occlusion model. Hence, the
regimen for NBO-PC in the present study was based on the current
study. The findings of the present study demonstrated that NBO-PC
was neuroprotective in aged rats exposed to 2.5% sevoflurane for 5
h. The MWM test clearly demonstrated that NBO-PC greatly improved
cognition impairment caused by sevoflurane in aged rats. As
expected, the apoptosis in the hippocampus was in concert with the
changes in cognitive function in the present study. In addition,
the present study demonstrated that antiapoptosis protein (bcl-2)
expression increased, pro-apoptotic protein (bax) and apoptosis
protein (caspases-3) decreased in the hippocampus after NBO-PC, and
the apoptosis rate decreased in the hippocampus after NBO-PC.
Concurrently, it was observed in the present study
that NBO-PC reduced the increase of [Ca2+]c
to sevoflurane in the hippocampus. Intracellular Ca2+,
one of the most widely used intracellular messengers is involved in
controlling almost all cell processes including muscle contraction,
exocytosis, proliferation, differentiation, protein synthesis and
gene expression (50). Disruption
of the intracellular calcium homeostasis, particularly due to a
persistent and excessive increase in the intracellular
Ca2+ can induce cell death by apoptosis (51). In addition, apoptosis cell death
mediated by calcium dysregulation serves an important role in
anesthetic neurotoxicity (52). The
results of the present study implied that NBO-PC may play a
neuroprotective role by reducing the intracellular calcium increase
caused by sevoflurane anesthesia through some molecular mechanisms,
which need further study.
In the present study, a lower chloral hydrate dose
(250 mg/kg) was used to anaesthetize the rats in accordance with
some previous studies (53,54). Some studies (55,56)
use 300-400 mg/kg chloral hydrate to perform surgery or establish
animal models, the process of which requires deep anesthesia and
long duration. However, in the present study just a mild anesthesia
was needed to ensure that the rats were hypnotized without
struggling. In addition, the potential effects of chloral hydrate
on experimental variables should also be taken into consideration
(57). Therefore, in view of the
low requirements for the depth and the duration of anesthesia and
the possible influence of high dose of chloral hydrate on detection
indicators, the lower dose of chloral hydrate was used to
anaesthetize the rats in the present study.
The present study had some limitations. In the
present study, 20-month-old rats were selected as aged rats. Among
the published articles on cognitive dysfunction, the ages of old
rats were very different ranging from 18-24 months (58-65).
According to the aforementioned studies, the rats selected by the
present study are equivalent to the aged. It was reported that 1
rat month is comparable to 3 human years in adulthood (66), which indicates differences in
anatomy, physiology and developmental processes of different months
old rats. In addition, these differences may lead to differences in
research results. Although the precise correlation between age of
laboratory rats and human is still a subject of debate, it has been
accepted that 24 month old rats correspond to 60 year old humans
(66). Hence, the age of the old
rats in this experiment may be relatively young. In addition, some
assays, such as histopathological staining of the hippocampal
sections were not performed to visually reflect the brain damage
caused by sevoflurane due to funding. So future studies must select
much older rats as subjects and optimize the assessment of brain
damage through histopathological staining if funding is
available.
In conclusion, the present study demonstrated that
NBO-PC decreased the hippocampal apoptosis as well as alleviated
the memory deficits in aged rats who were exposed to sevoflurane.
The results of the present study, suggested that neuron apoptosis
inhibited by NBO-PC is in association with a decrease of cytosolic
Ca2+ in the hippocampus, which may at least partially be
the molecular mechanism by which NBO-PC induces
neuroprotection.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81771134),
the Natural Science Foundation of Hebei Province (grant no.
H2018206305), the Hebei Province Technology Innovation guide
Project Science and the Technology Winter Olympics Special Project
(grant no. 19977790D). The Hebei Provincial government funded the
specialty capacity building and specialty leader training
program.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW was responsible for designing the study,
performing the experiment, collecting the data and writing the
manuscript. CPY was responsible for designing the study, performing
the experiment, and collecting the data. YLT and ZJZ were
responsible for collection of experimental specimens and the
extraction of proteins and reviewing the manuscript. ZYH was
responsible for analyzing and interpreting the data. QJW was
responsible for providing experimental ideas and reviewing the
manuscript. YW and QJW were responsible for the confirming the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee for
Animal Experimentation (Ethical approval no. Guo A2017-026-1), and
the animals were studied at Hebei Medical University (Shijiazhuang,
China). All applicable international, national, and/or
institutional guidelines for the care and use of animals were
followed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu X, Hu Y, Yan E, Zhan G, Liu C and Yang
C: Perioperative neurocognitive dysfunction: Thinking from the gut?
Aging. 12:15797–15817. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kotekar N, Shenkar A and Nagaraj R:
Postoperative cognitive dysfunction-current preventive strategies.
Clin Interv Aging. 13:2267–2273. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Moller JT, Cluitmans P, Rasmussen LS, Houx
P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD,
et al: Long-term postoperative cognitive dysfunction in the elderly
ISPOCD1 study ISPOCD investigators. International study of
post-operative cognitive dysfunction. Lancet. 351:857–861.
1998.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu Y and Yin Y: Emerging roles of immune
cells in postoperative cognitive dysfunction. Mediators Inflamm.
2018(6215350)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Berger M, Nadler JW, Browndyke J, Terrando
N, Ponnusamy V, Cohen HJ, Whitson HE and Mathew JP: Postoperative
cognitive dysfunction: Minding the gaps in our knowledge of a
common postoperative complication in the elderly. Anesthesiol Clin.
33:517–550. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vide S and Gambús PL: Tools to screen and
measure cognitive impairment after surgery and anesthesia. Presse
Med. 47:e65–e72. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ologunde R and Ma D: Do inhalational
anesthetics cause cognitive dysfunction? Acta Anaesthesiol Taiwan.
49:149–153. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang F, Zhu ZQ, Liu DX, Zhang C, Gong QH
and Zhu YH: Emulsified isoflurane anesthesia decreases
brain-derived neurotrophic factor expression and induces cognitive
dysfunction in adult rats. Exp Ther Med. 8:471–477. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen Y, Zhang P, Lin X, Zhang H, Miao J,
Zhou Y and Chen G: Mitophagy impairment is involved in
sevoflurane-induced cognitive dysfunction in aged rats. Aging
(Albany NY). 12:17235–17256. 2020.PubMed/NCBI View Article : Google Scholar : (Online ahead of
print).
|
|
10
|
Tojo A, Uchimoto K, Inagawa G and Goto T:
Desflurane impairs hippocampal learning on day 1 of exposure: A
prospective laboratory study in rats. BMC Anesthesiol.
19(119)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang S, Hu X, Guan W, Luan L, Li B, Tang
Q and Fan H: Isoflurane anesthesia promotes cognitive impairment by
inducing expression of β-amyloid protein-related factors in the
hippocampus of aged rats. PLoS One. 12(e0175654)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cui RS, Wang K and Wang ZL: Sevoflurane
anesthesia alters cognitive function by activating inflammation and
cell death in rats. Exp Ther Med. 15:4127–4130. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Istaphanous GK, Howard J, Nan X, Hughes
EA, McCann JC, McAuliffe JJ, Danzer SC and Loepke AW: Comparison of
the neuroapoptotic properties of equipotent anesthetic
concentrations of desflurane, isoflurane, or sevoflurane in
neonatal mice. Anesthesiology. 114:578–587. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Steinmetz J, Christensen KB, Lund T, Lohse
N and Rasmussen LS: ISPOCD Group. Long-term consequences of
postoperative cognitive dysfunction. Anesthesiology. 110:548–555.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu X, Song X, Yuan T, He J, Wang X and
Wang Q: Effects of calpain on sevoflurane-induced aged rats
hippocampal neuronal apoptosis. Aging Clin Exp Res. 28:633–639.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cao L, Li L, Lin D and Zuo Z: Isoflurane
induces learning impairment that is mediated by interleukin 1β in
rodents. PLoS One. 7(e51431)2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liang G, Ward C, Peng J, Zhao Y, Huang B
and Wei H: Isoflurane causes greater neurodegeneration than an
equivalent exposure of sevoflurane in the developing brain of
neonatal mice. Anesthesiology. 112:1325–1334. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bigdeli MR, Rasoulian B and Meratan AA: In
vivo normobaric hyperoxia preconditioning induces different degrees
of antioxidant enzymes activities in rat brain tissue. Eur J
Pharmacol. 611:22–29. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Petrosillo G, Di Venosa N, Moro N,
Colantuono G, Paradies V, Tiravanti E, Federici A, Ruggiero FM and
Paradies G: In vivo hyperoxic preconditioning protects against
rat-heart ischemia/reperfusion injury by inhibiting mitochondrial
permeability transition pore opening and cytochrome c release. Free
Radic Biol Med. 50:477–483. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wahhabaghai H, Heidari R, Zeinoddini A,
Soleyman-Jahi S, Golmanesh L, Rasoulian B, Akbari H, Foadoddoni M
and Esmailidehaj M: Hyperoxia-induced preconditioning against renal
ischemic injury is mediated by reactive oxygen species but not
related to heat shock proteins 70 and 32. Surgery. 157:1014–1022.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gao B, Long Z, Zhao L and He G: Effect of
normobaric hyperoxia on behavioral deficits and neuropathology in
Alzheimer's disease mouse model. J Alzheimers Dis. 27:317–326.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bigdeli MR: Neuroprotection caused by
hyperoxia preconditioning in animal stroke models.
ScientificWorldJournal. 11:403–421. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ma H, Yao L, Pang L, Li X and Yao Q:
Tetrandrine ameliorates sevoflurane-induced cognitive impairment
via the suppression of inflammation and apoptosis in aged rats. Mol
Med Rep. 13:4814–4820. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mohammadi E and Bigdeli MR: Effects of
preconditioning with normobaric hyperoxia on
Na+/Ca²+ exchanger in the rat brain.
Neuroscience. 237:277–284. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Banfalvi G: Methods to detect apoptotic
cell death. Apoptosis. 22:306–323. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Riccardi C and Nicoletti I: Analysis of
apoptosis by propidium iodide staining and flow cytometry. Nat
Protoc. 1:1458–1461. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wlodkowic D, Skommer J and Darzynkiewicz
Z: Flow cytometry-based apoptosis detection. Methods Mol Biol.
559:19–32. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Field KJ, White WJ and Lang CM:
Anaesthetic effects of chloral hydrate, pentobarbitone and urethane
in adult male rats. Lab Anim. 27:258–269. 1993.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Reimer JN, Schuster CJ, Knight CG, Pang
DSJ and Leung VSY: Intraperitoneal injection of sodium
pentobarbital has the potential to elicit pain in adult rats
(Rattus norvegicus). PLoS One. 15(e0238123)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Senichkin VV, Pervushin NV, Zuev AP,
Zhivotovsky B and Kopeina GS: Targeting Bcl-2 family proteins:
What, where, when? Biochemistry (Mosc). 85:1210–1226.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
D'Orsi B, Mateyka J and Prehn JHM: Control
of mitochondrial physiology and cell death by the Bcl-2 family
proteins Bax and Bok. Neurochem Int. 109:162–170. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Snigdha S, Smith ED, Prieto GA and Cotman
CW: Caspase-3 activation as a bifurcation point between plasticity
and cell death. Neurosci Bull. 28:14–24. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chang L, Zhang X, Liu W, Song Y, Gao X,
Ling W and Wu Y: Immunoreactivity of Ki-67/β-tubulin and
immunocolocalization with active caspase-3 in rat dentate gyrus
during postnatal development. J Chem Neuroanat. 46:10–18.
2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhu X, Yao Y, Guo M, Li J, Yang P, Xu H
and Lin D: Sevoflurane increases intracellular calcium to induce
mitochondrial injury and neuroapoptosis. Toxicol Lett. 336:11–20.
2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vlisides P and Xie Z: Neurotoxicity of
general anesthetics: An update. Curr Pharm Des. 18:6232–6240.
2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Armstrong R, Xu F, Arora A, Rasic N and
Syed NI: General anesthetics and cytotoxicity: Possible
implications for brain health. Drug Chem Toxicol. 40:241–249.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Manatpon P and Kofke WA: Toxicity of
inhaled agents after prolonged administration. J Clin Monit Comput.
32:651–666. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ebert TJ, Robinson BJ, Uhrich TD,
Mackenthun A and Pichotta PJ: Recovery from sevoflurane anesthesia:
A comparison to isoflurane and propofol anesthesia. Anesthesiology.
89:1524–1531. 1998.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lv G, Li C, Wang W, Li N and Wang K:
Silencing SP1 alleviated sevofurane-induced pocd development via
cholinergic anti-infammatory pathway. Neurochem Res. 45:2082–2090.
2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yang L, Shen Q, Xia Y, Lei X and Peng J:
Sevoflurane-induced neurotoxicity is driven by OXR1
post-transcriptional downregulation involving has-miR-302e. Mol Med
Rep. 18:4657–4665. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Fei X, Wang JX, Wu Y, Dong N and Sheng ZY:
Sevoflurane-induced cognitive decline in aged mice: Involvement of
toll-like receptors 4. Brain Res Bull. 165:23–29. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yang X, Zheng YT and Rong W: Sevoflurane
induces apoptosis and inhibits the growth and motility of colon
cancer in vitro and in vivo via inactivating Ras/Raf/MEK/ERK
signaling. Life Sci. 239(116916)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Qiu J, Shi P, Mao W, Zhao Y, Liu W and
Wang Y: Effect of apoptosis in neural stem cells treated with
sevoflurane. BMC Anesthesiol. 15(25)2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chen G, Gong M, Yan M and Zhang X:
Sevoflurane induces endoplasmic reticulum stress mediated apoptosis
in hippocampal neurons of aging rats. PLoS One.
8(e57870)2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Jiang J and Jiang H: Effect of the inhaled
anesthetics isoflurane, sevoflurane and desflurane on the
neuropathogenesis of Alzheimer's disease (Review). Mol Med Rep.
12:3–12. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kelestemur T, Beker MC, Caglayan AB,
Caglayan B, Altunay S, Kutlu S and Kilic E: Normobaric oxygen
treatment improves neuronal survival functional recovery and axonal
plasticity after newborn hypoxia-ischemia. Behav Brain Res.
379(112338)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
You P, Lin M, Li K, Ye X and Zheng J:
Normobaric oxygen therapy inhibits HIF-1α and VEGF expression in
perihematoma and reduces neurological function defects.
Neuroreport. 27:329–336. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Donkin JJ and Vink R: Mechanisms of
cerebral edema in traumatic brain injury: Therapeutic developments.
Curr Opin Neurol. 23:293–299. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Al-Motabagani MA: Histological changes in
the alveolar structure of the rat lung after exposure to hyperoxia.
Ital J Anat Embryol. 110:209–223. 2005.PubMed/NCBI
|
|
50
|
Berridge MJ, Bootman MD and Roderick HL:
Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev
Mol Cell Biol. 4:517–529. 2003.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Orrenius S, Zhivotovsky B and Nicotera P:
Regulation of cell death: The calcium-apoptosis link. Nat Rev Mol
Cell Biol. 4:552–565. 2003.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Yang M and Wei H: Anesthetic
neurotoxicity: Apoptosis and autophagic cell death mediated by
calcium dysregulation. Neurotoxicol Teratol. 60:59–62.
2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Silverman J and Muir WW III: A review of
laboratory animal anesthesia with chloral hydrate and chloralose.
Lab Anim Sci. 43:210–216. 1993.PubMed/NCBI
|
|
54
|
Uhlig C, Krause H, Koch T, Gama de Abreu M
and Spieth P: Anesthesia and monitoring in small laboratory mammals
used in anesthesiology, respiratory and critical care research: A
systematic review on the current reporting in top-10 impact factor
ranked journals. PLoS One. 10(e0134205)2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Sjakste N, Baumane L, Meirena D, Lauberte
L, Dzintare M and Kalviņs I: Drastic increase in nitric oxide
content in rat brain under halothane anesthesia revealed by EPR
method. Biochem Pharmacol. 58:1955–1959. 1999.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Grinchii D, Paliokha R, Tseilikman V and
Dremencov E: Inhibition of cytochrome P450 by proadifen diminishes
the excitability of brain serotonin neurons in rats. Gen Physiol
Biophys. 37:711–713. 2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Herbst LS, Gaigher T, Siqueira AA, Joca
SRL, Sampaio KN and Beijamini V: New evidence for refinement of
anesthetic choice in procedures preceding the forced swimming test
and the elevated plus-maze. Behav Brain Res.
368(111897)2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Templer VL, Wise TB and Heimer-McGinn VR:
Social housing protects against age-related working memory decline
independently of physical enrichment in rats. Neurobiol Aging.
75:117–125. 2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Sun J, Zhang S, Zhang X, Dong H and Qian
Y: IL-17A is implicated in lipopolysaccharide-induced
neuroinflammation and cognitive impairment in aged rats via
microglial activation. J Neuroinflammation. 12(165)2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
La Spina M, Sansevero G, Biasutto L,
Zoratti M, Peruzzo R, Berardi N, Sale A and Azzolini M:
Pterostilbene improves cognitive performance in aged rats: An in
vivo study. Cell Physiol Biochem. 52:232–239. 2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Nishigaki A, Kawano T, Iwata H, Aoyama B,
Yamanaka D, Tateiwa H, Shigematsu-Locatelli M, Eguchi S, Locatelli
FM and Yokoyama M: Acute and long-term effects of haloperidol on
surgery-induced neuroinflammation and cognitive deficits in aged
rats. J Anesth. 33:416–425. 2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Yang N, Li Z, Han D, Mi XN, Tian M, Liu T,
Li Y, He J, Kuang C, Cao Y, et al: Autophagy prevents hippocampal
α-synuclein oligomerization and early cognitive dysfunction after
anesthesia/surgery in aged rats. Aging (Albany NY). 12:7262–7281.
2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Cao Y, Li Z, Ma L, Yang N and Guo XY:
Isoflurane-induced postoperative neurovascular and cognitive
dysfunction is associated with VEGF overexpression in aged rats. J
Mol Neurosci. 69:215–223. 2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Terrando N, Yang T, Wang X, Fang J, Cao M,
Andersson U, Erlandsson HH, Ouyang W and Tong J: Systemic HMGB1
neutralization prevents postoperative neurocognitive dysfunction in
aged rats. Front Immunol. 7(441)2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Fonken LK, Frank MG, D'Angelo HM, Heinze
JD, Watkins LR, Lowry CA and Maier SF: Mycobacterium vaccae
immunization protects aged rats from surgery-elicited
neuroinflammation and cognitive dysfunction. Neurobiol Aging.
71:105–114. 2018.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Sengupta P: The laboratory rat: Relating
its age with human's. Int J Prev Med. 4:624–630. 2013.PubMed/NCBI
|


















